Abstract
Objective
Simultaneous analysis of the protein composition of biological fluids is now possible. Such an approach can be used to identify biological markers of disease and to understand the pathophysiology of disorders that have eluded classification, diagnosis, and treatment. The purpose of this study was to analyze the differences in protein composition in amniotic fluid of patients in preterm labor.
Study Design
Amniotic fluid was obtained by amniocenteses from three groups of women with preterm labor and intact membranes: (1) women without intra-amniotic infection/inflammation (IAI) who delivered at term; (2) women without intra-amniotic IAI who delivered a preterm neonate; and (3) women with IAI. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was defined as an elevated amniotic fluid interleukin (IL)-6 (≥2.3 ng/mL). Two-dimensional (2D) chromatography was used for analysis. The first dimension separated proteins by isoelectric point, while the second, by the degree of hydrophobicity. 2D protein maps were generated using different experimental conditions (reducing agents as well as protein concentration). The maps were used to discern subsets of isoelectric point/hydrophobicity containing differentially expressed proteins. Protein identification of differentially expressed fractions was conducted with mass spectrometry. ELISA immunoassays as well as surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS)--based on-chip antibody capture immunoassay were also used for confirmation of a specific protein that was differentially expressed.
Results
1) Amniotic fluid protein composition can be analyzed using a combination of 2D liquid chromatography and mass spectrometry for the identification of proteins differentially expressed in patients in preterm labor; 2) While total insulin-like growth factor-binding protein-1 (IGFBP-1) concentration did not change, IGFBP-1 fragments at about 13.5 kDa were present in patients with intra-amniotic IAI; 3) proteins which were over-expressed in group 1 included Von Ebner gland protein precursor, IL-7 precursor, apolipoprotein A1, tropomyosin sk1 (TPMsk1) fragment, ribosomal protein S6 kinase alpha-3 and alpha-1-microglobulin/bikunin precursor (AMBP); 4) proteins which were over-expressed in group 3 included fibrinopeptide B, transferrin, (MHC) class 1 chain-related A antigen fragment, transcription elongation factor A, sex-determining region Y (SRY) box 5 protein, Down syndrome critical region 2 protein (DSCR2), and human peptide 8 (HP8); and 5) one protein, retinol binding protein, was over-expressed in women who delivered preterm, regardless of the presence of IAI.
Conclusions
A combination of techniques involving 2D chromatography, mass spectrometry, and immunoassays allows identification of proteins that are differentially regulated in amniotic fluid of patients with preterm labor. Specifically, the amount of the IGFBP-1 fragments at approximately 13.5 kDa was found to be increased in patients with IAI, while the amount of the intact form of IGFBP-1 was decreased.
Keywords: Preterm birth, intra-amniotic infection/inflammation, chorioamnionitis, IGFBP-1, MALDI-TOF, LC-MS/MS, SELDI-TOF MS, two-dimensional chromatography
INTRODUCTION
High-dimensional biology (HDB) refers to the simultaneous study of the genetic variants (DNA variants), transcription [messenger RNA (mRNA)], peptides and proteins, as well as metabolites of an organ, tissue, or an organism in health and disease. HDB is expected to assist with the development of new diagnostic, prognostic and therapeutic tools in medicine[1-13].
One approach in proteomics is to separate proteins in a biological fluid and/or tissue followed by protein identification of peptides or proteins which are differentially expressed in the conditions of clinical interest[14,15]. In the past, separation has been conducted with two-dimensional gel electrophoresis, followed by mass spectrometry (MS) analysis of differentially expressed proteins for identification[14,16].
Two-dimensional chromatography (in which proteins and peptides are separated as a function of hydrophobicity and isoelectric point in a liquid phase and this information displayed in protein maps corresponding to the conditions of interest) has emerged as an alternative strategy to two-dimensional gel electrophoresis for identifying novel proteins[16,17]. In this technology, separation by reversed phase chromatography is a convenient and well-proven method of separation by hydrophobicity that is “orthogonal” to the first axis of isoelectric point (which is shared with 2D gel electrophoresis), and thus provides good mixture simplification. Thus, we applied this approach followed by MS to determine if such an approach could be useful in identifying differentially expressed proteins in different clinical groups of patients with preterm labor.
MATERIAL AND METHODS
Study Design
A cross-sectional study was conducted by searching our clinical database and bank of biologic samples. This study included women who presented with preterm labor and intact membranes (PTL) between 20 to 34 weeks of gestation, that were subdivided into the following categories: Group 1: preterm labor who delivered at term with a negative amniotic fluid culture for microorganisms and without intra-amniotic inflammation (IAI) (n=86); Group 2: preterm labor who delivered preterm (<37 weeks) without IAI(n=86); and Group 3: preterm delivery with IAI (n=86). Pools for use in the proteomics studies were generated for each group by combining 125 μL aliquots of amniotic fluid samples from individual patients corresponding to each group.
Preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least 2 every 10 minutes and some degree of cervical change before 37 completed weeks of gestation. All patients were diagnosed to have preterm labor by their physicians and were admitted to the hospital. All patients provided written informed consent to donate amniotic fluid for research purposes according to protocols at the institutions at which the studies were conducted. Amniocenteses were performed for clinical indications at the discretion of the attending physician to assess the microbiologic state of the amniotic cavity and/or fetal lung maturity. Amniotic fluid not required for clinical purposes was centrifuged at 4oC for 10 minutes and stored at -70°C. A sample of amniotic fluid was transported to the laboratory for aerobic, anaerobic, and genital Mycoplasmas fluid cultures. Amniotic fluid white blood cell (WBC) count, red blood cell (RBC) count and glucose concentrations were performed. The results of these tests were used for subsequent clinical management. Patients with amniotic fluid RBC count of more than 100 cells were excluded.
Intra-amniotic inflammation was defined as amniotic fluid concentration of interleukin-6 (IL-6) higher than 2.3 ng/ml. This cut-off of IL-6 concentration was derived from applying a receiver-operating characteristic curve to amniotic fluid concentrations of IL-6 of patients with spontaneous preterm labor included in this study for the identification of patients who delivered within 7 days of amniocentesis. Many of these samples have been used previously in several studies focusing on term and preterm parturition, cytokines, chemokines, arachidonic acid metabolites, and other biological markers of disease.
Amniotic fluid preparations for proteomics studies
Three different methods for preparing amniotic fluid samples for proteomic investigation via 2D separation chromatography were investigated. We found that these methods had a profound impact on the ability to detect, characterize and quantify differential protein expression in amniotic fluid, and describe here each method and its strengths and limitations.
For details of amniotic fluid preparations and analytical methods, the reader is referred to the Appendix section. A brief description of the methods is provided as follows:
Reduced amniotic fluid
In a first set of experiments, amniotic fluid samples were treated with reducing agents to improve solubilization. This method was successful only in identifying high-abundance proteins.
Untreated amniotic fluid
In a second set of experiments, untreated amniotic fluid was buffer-exchanged and directly injected into the chromatofocussing column.
Concentrated amniotic fluid
In a third set of experiments, protein from large volumes of amniotic fluid from groups 1 and 3 was concentrated by centrifugation with a molecular weight cutoff filter (30 kDa). The retentate was treated with a reducing agent and refiltered. The filtrate was buffer-exchanged as in the case of untreated amniotic fluid and subjected to 2D liquid chromatography separation.
Analytical methods
Two-dimensional chromatographic fractionation (2D-CF) and analysis
2D fractionation employed chromatofocusing (CF) to fractionate proteins based upon a narrow range of isoelectric points (pI) in a first dimensional separation, followed by nonporous Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) separation of each of the individual pI fractions according to hydrophobicity in the second dimensional separation as first described by Lubman et al.[18] Using ultraviolet (UV) detection, an image is generated in software of either the intact expressed proteins using ProteoVue or their differential expression using DeltaVue (ProteoVue and DeltaVue software programs are commercially available from Beckman Coulter, Inc., Fullerton, CA, USA). Thus, the 2D-CF method may be regarded as a combination of a preparative (for mixture simplification) and an analytic (by visualizing the two dimensional protein distribution) separation technique.
Gel electrophoresis and mass spectrometry analysis
Chromatographic fractions of amniotic fluid samples that discriminated among groups were selected for protein identification using (MS). Corresponding fractions were combined and analyzed by sulfate--polyacrylamide gel electrophoresis (SDS-PAGE) following the method of Laemmli.[19] Selected gel plugs from the SDS-PAGE gel were trypsin digested as described in the Appendix for mass spectrometric analyses. This method is used to produce cleaner digests for mass spectrometric analysis.
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) analysis
Amniotic fluid samples were spotted onto a Matrix Assisted Laser Desorption Ionization (MALDI) target, using a ProMS-robot (Genomic Solutions Ltd, Huntingdon, UK) with ZipTips (Millipore, Billerica, MA USA). MALDI/MS data were acquired on a Voyager DE-STR mass spectrometer (Applied Biosystems, Foster City, CA, USA) and the observed m/z values were used for peptide mass fingerprint protein identification using ProFound software (The Rockefeller University, New York, NY, USA).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
Samples that proved inconclusive following MALDI-TOF were reanalyzed by nano-LC-MS/MS on a Micromass Quadrupole Time of Flight (Q-TOF) mass spectrometer (Waters Corp., Milford, MA USA). MS/MS spectra were searched against SwissProt using Mascot software (www.matrixscience.com) to determine peptide sequences.
Liquid-phase (direct) mass spectrometry analysis
Chromatographic fractions of amniotic fluid samples that discriminated among groups were also analyzed directly from the liquid phase by trypsin digestion followed by MALDI-TOF/MS and electrospray ionization-ion trap mass spectrometry (ESI-IT-MS).
SELDI-TOF MS ProteinChip Immunoassays
Insulin-like Growth Factor Binding Protein -1 (IGFBP-1) and IGFBP-1 fragments were analyzed in the amniotic fluid pools of groups 1 and 3 using a ProteinChip Array-based immunoassay and Surface-Enhanced Laser Desorption/Ionization Time-of-Flight (SELDI-TOF) mass spectrometry.[20] Affinity-purified goat anti-human IGFBP-1 capture antibody (R&D Systems, Minneapolis, MN) and goat IgG (Sigma-Aldrich, St. Louis, MO) for a negative control were used to assess relative concentrations of IGFBP-1 and its fragments in these groups.
Immunoassays for IGFBP-1
An ELISA assay for IGFBP-1 was used to determine absolute concentration of immunoreactive protein. ELISA kits were obtained from Diagnostics Systems Laboratories, Inc. (Webster, TX, USA). The IGFBP-1 ELISA was validated for human amniotic fluid in our laboratory. The calculated inter- and intra-assay coefficients of variation (CV) for IGFBP-1 immunoassays in our laboratory were 7.4% and 3.7%, respectively. The sensitivity for IGFBP-1 was 1.38 ng/ml.
RESULTS
Clinical and demographic characteristics
Table 1 describes the clinical and demographic characteristics of patients in the three study groups used in this work. There were no significant differences in the median maternal age and ethnic groups and other variables among the patients who contributed amniotic fluid to the pools used for proteomic analysis. Patients with PTL with IAI had a significantly lower gestational age at amniocentesis, shorter amniocentesis-to-delivery interval, lower gestational age at delivery, lower neonatal birthweight and higher amniotic fluid IL-6 concentration than the other two groups. This is consistent with previous observation which document the lower the gestational age, the higher the risk of IAI[21]. Among patients with IAI, nineteen (22%) had a positive amniotic fluid culture for one or more microorganisms. Table 2 presents the microorganisms isolated from amniotic fluid.
Table 1.
Demographic and clinical characteristics of women with spontaneous preterm labor and intact membranes
| Group 1: Preterm labor without infection/inflammation and term delivery N = 86 | Group 2: Preterm labor without infection/inflammation and preterm delivery N = 86 | Group 3: Preterm labor with infection/inflammation and preterm delivery N = 86 | P-value | |
|---|---|---|---|---|
| Maternal age (years: mean ± SD) | 23.7 ± 6.6 | 22.9 ± 5.3 | 24.6 ± 6.2 | NS |
| Gestational age at amniocentesis (weeks: mean ± SD) | 30.3 ± 3.2 | 29.0 ± 4.0 | 26.9 ± 4.0 | <0.05 |
| Gestational age at delivery (weeks: mean ± SD) | 38.4 ± 1.3 | 33.6 ± 2.8 | 30.3 ± 3.2 | <0.05 |
| Birth weight (grams: mean ± SD) | 3041 ± 418 | 2154 ± 609 | 1283 ± 695 | <0.05 |
| Amniotic fluid IL-6 concentration (pg/ml: mean ± SD) | 749 ± 596 | 892 ± 539 | 71,837 ± 106,763 | <0.05 |
SD- standard deviation
Table 2.
Organisms isolated from amniotic fluid culture from patients with intra-amniotic infection who delivered preterm (Group 3).
| Organisms | Number of cases |
|---|---|
| Fusobacterium | 7 |
| Ureaplasma urealyticum | 4 |
| Gardnerella vaginalis | 3 |
| Peptostreptococcus | 2 |
| Capnocytophaga | 1 |
| Acinetobacterium | 1 |
| Prevotella bivia | 1 |
| Staphylococcus | 1 |
| Streptococcus agalactia | 1 |
| Lactobacillus | 1 |
| Porphyromonas | 1 |
| Bacteroides | 1 |
2D chromatography and MS analysis
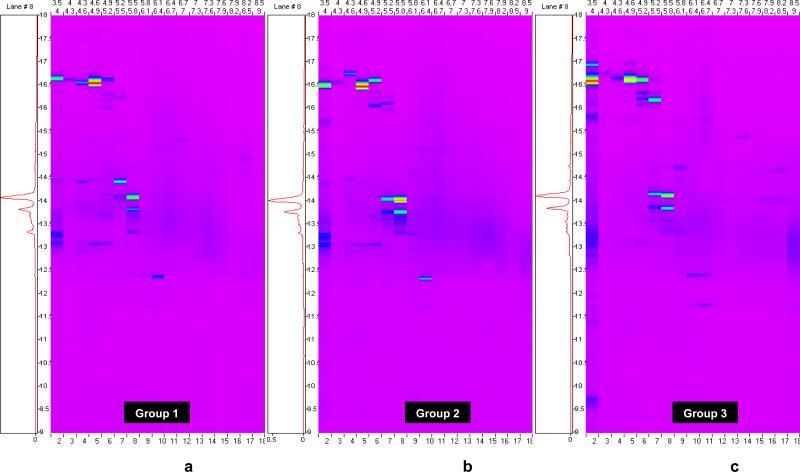
Figure 1 shows the three 2D chromatographic ProteoVue protein maps obtained from amniotic fluid, which had been treated using reducing agents with the purpose of improving protein solubility which facilitates liquid-phase separation of these molecules in 2D chromatography. The maps, generated by measuring total protein concentration with a UV detector as each pI fraction elutes from the reversed phase column in the 2D-LC apparatus, display the intact protein distributions in the amniotic fluid of women with preterm labor who delivered at term (Figure 1a), preterm labor who delivered preterm without IAI (Figure 1b) and preterm labor with IAI (Figure 1c). ProteoVue maps are useful for locating regions in the pI/hydrophobicity plane that contain significant amounts of protein, and by visually comparing such maps from two or more samples, determining regions of potential differential expression.
Figure 1.
ProteoVue Maps of the three pools of amniotic fluid after treatment with reducing agents. 1a) patients with preterm labor without infection/inflammation who delivered at term (group 1); 1b) patients who delivered preterm without intra-amniotic infection/inflammation (group 2); 1c) patients who delivered preterm with intra-amniotic infection/inflammation (group 3).
Using the ProteoVue maps we selected 25 fractions of amniotic fluid which have been separated by 2D chromatography as potentially containing proteins that are stereotypic to the groups. These fractions were subjected to SDS-PAGE to separate the proteins present in each liquid fraction by molecular weight.
SDS-PAGE gels yield 42 spots which were excised and subjected to liquid chromatography and tandem mass spectrometry (LC-MS/MS) for protein identification. The proteins identified in the three clinical groups are displayed in Table 3. Human retinol binding protein appeared to be expressed in group 2 and 3 but not in group 1 suggesting that this protein is a feature which characterizes the amniotic fluid of women destined to deliver preterm regardless of the presence of infection/inflammation. Other proteins such as fibrinopeptide B, β-2-microglobulin, and transferrin were only identified in the amniotic fluid of women with intra-amniotic infection/inflammation. Many of the fractions submitted to SDS-PAGE and LC-MS/MS did not yield protein identifications suggesting that this sample preparation technique yielded identities only for high-abundance proteins that appeared to be differentially expressed.
Table 3.
Protein identification from the first and second sets of experiments.
| Iso-electric point | Retention time | Protein identification | Protein name | Identification Method |
|---|---|---|---|---|
| Over-expressed in group 1 (Preterm Labor – Term delivery) | ||||
| 4.9 – 5.2 | 11.8 – 12.4 | P08833 | Insulin-like growth factor binding protein 1 | MALDI-TOF/MS |
| Over-expressed in group 2 (Preterm Labor – Preterm delivery – No intra-amniotic infection/inflammation) | ||||
| 5.5 – 5.8 | 13 – 14.3 | P02753 | Retinol Binding Protein | MALDI-TOF/MS |
| Over-expressed in group 3 (Preterm Labor – Preterm delivery – Intra-amniotic infection/inflammation) | ||||
| 5.2 – 5.5 | 16 – 16.75 | P02753 | Retinol Binding Protein | MALDI-TOF/MS |
| 5.2 – 5.5 | 16 – 16.75 | P02675 | Fibrinopeptide B | LC-MS/MS |
| 5.5 – 5.8 | 13 – 14.3 | P02787 | Transferrin | LC-MS/MS |
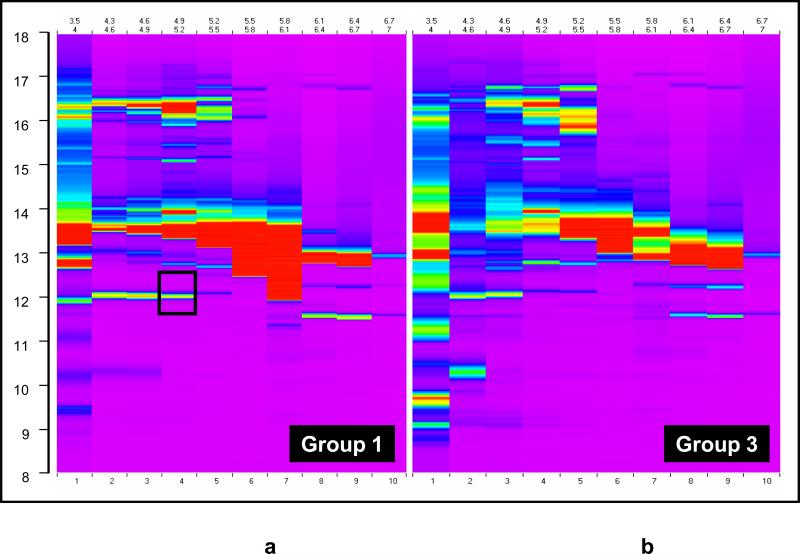
To address this issue, a second set of 2D liquid fractionation experiments were performed with untreated amniotic fluid (not treated with reducing agents). Figure 2 displays the resulting two ProteoVue maps in amniotic fluid of women with preterm labor who delivered at term (Figure 2a) and preterm labor with IAI (Figure 2b). It is clear that the amount of expressed protein observed in the ProteoVue maps is much greater than in Figure 1 (note that the same concentration dynamic range is represented in both figures). These results suggest that treatment of amniotic fluid with reducing agents interfered with the ability to detect and identify proteins. It is possible that the re-formation in the chromatofocussing column of albumin disulfide bonds broken by the use of reducing agents causes irreversible adsorption on the column, trapping many other proteins and thereby preventing their detection. Thus, sample processing plays an important role in global protein fractionation and analysis.
Figure 2.
ProteoVue Maps of the two pools of untreated amniotic fluid in the second set of experiments. (a) Group 1: patients with preterm labor without infection/inflammation who delivered at term (b)Group 3: patients who delivered preterm with intra-amniotic infection/inflammation. The region indicated by the box in (a) contains the fractions from which IGFBP-1 was subsequently identified.
ProteoVue and DeltaVue software analysis was used to select 18 fractions of differentially expressed areas in the map for protein identification. DeltaVue is especially useful for subtractively comparing the 2D-LC distributions obtained from two samples under similar conditions. Selected fractions were subjected to SDS-PAGE and analyzed by MALDI-TOF/MS and LC-MS/MS.
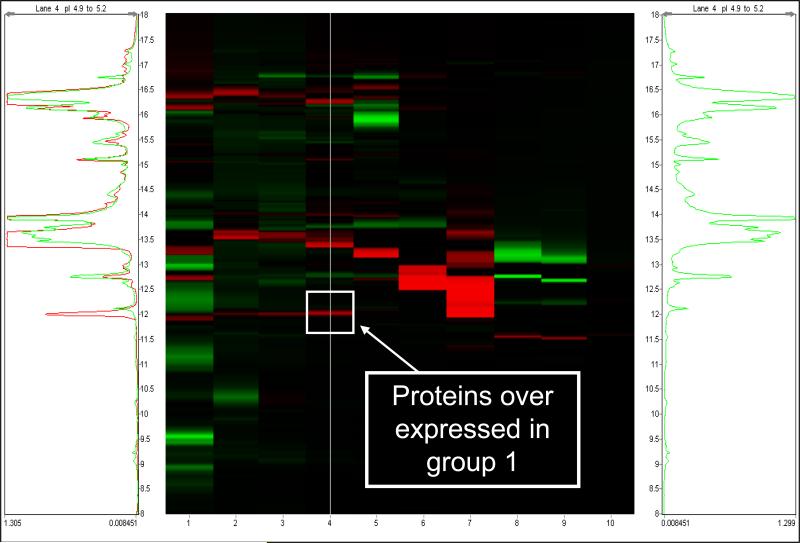
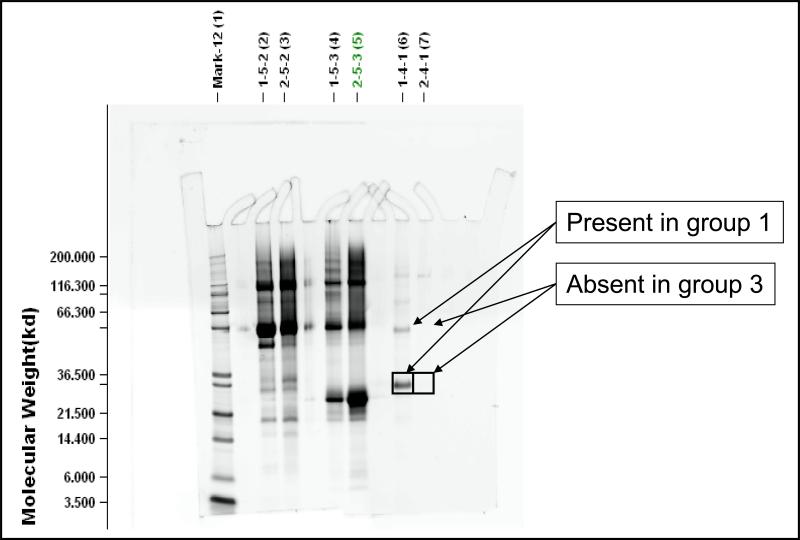
Table 3 summarizes the results of the two experiments in which 2D chromatography was used to identify biomarkers in amniotic fluid from patients with premature labor. IGFBP-1 was found to be differentially expressed in a set of fractions having pI's in the range of 4.9 - 5.2. Figure 3 shows the DeltaVue map highlighting the group 1 and 3 sample comparison for these fractions showing a protein band that is highly expressed in group 1 compared to group 3 (no differential expression was observed that was specific to group 2). Figure 4 shows the SDS-PAGE image for the amniotic fluid from the corresponding fractions of patients in groups 1 and 3. Proteins with approximate molecular weights of 30 kDa and 60 kDa were observed in group 1 but not in group 3. These gel bands were excised, digested and analyzed by LC-MS/MS. Both bands were identified as IGFBP-1 in monomeric (30 kDa) and dimeric (60 kDa) forms. Such forms have been previously reported[22]. Although transthyretin, beta-2 microglobulin, albumin and albumin precursors (high abundance proteins) were also identified, we interpret their presence as reflecting background proteins.
Figure 3.
DeltaVue map for untreated amniotic fluid, overlaying the protein concentration of patients with preterm labor without infection/inflammation who delivered at term (group1 - in red) and patients who delivered preterm with intra-amniotic infection/inflammation (group 3 - in green). The center image is the difference map for proteins, with the colored bands indicating protein over-expression from the respective sample. The green and red chromatograms (to the left and right of the center image, respectively) are the protein profiles for the pI 4.9 – 5.2 CF fraction of each group. The arrow and the square indicate one fraction that was collected for identification of a protein that was over expressed in group 1 compared to group 3.
Figure 4.
One dimensional SDS-Page for untreated amniotic fluid, comparing several fractions of patients with preterm labor without infection/inflammation who delivered at term (group1) and patients who delivered preterm with intra-amniotic infection/inflammation (group 3). In the third fraction analyzed (identified as 1.4.1 and 2.4.1 and corresponding to the RT fractions with an over expression of proteins of group 1 into the PI 4.9-5.2 CF fractions). Two spots at an approximate molecular weight of 30 Kda and 60 Kda were identified in group 1 but not in group 3. Those two spots were excised and IGFBP-1 was subsequently identified by LC-MS/MS in both. No protein was identified in the two corresponding spots of group 3.
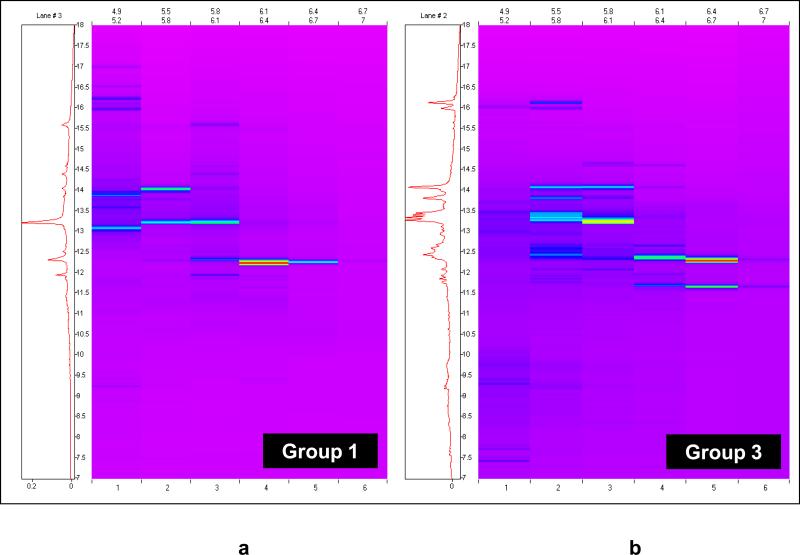
We undertook the generation of a third set of 2D chromatography maps from amniotic fluid in order to increase the likelihood of identifying lower abundance biomarkers. The approach was designed to address the issue of the albumin interference on the chromatofocussing column (second dimension). Several modifications were introduced to increase the likelihood of differential protein detection and identification. First, we increased the total volume of amniotic fluid so that the total amount of protein would be larger. Second, the protein in untreated amniotic fluid was concentrated by using centrifugation and a molecular weight filter which excluded molecules below 30 kDa (concentration step). Third, the retentate was treated with reducing agents to release low molecular weight proteins from albumin and re-filtered. The resulting filtrate was subjected to 2D liquid phase chromatography. Fourth, we eliminated the SDS-PAGE step because previous experiments showed that SDS-PAGE after 2D liquid chromatography resulted in a loss of sensitivity in the detection and identification of proteins. Further, this experiment was limited to groups 1 and 3 due to the requirement for large volumes (80 cc) of amniotic fluid for each group. We elected not to generate a pool of the second group because this group of patients is very difficult to obtain and would exhaust our bank of amniotic fluid for this particular clinical subgroup (group 2). This approach was successful in identifying some low-abundance differentially expressed proteins.
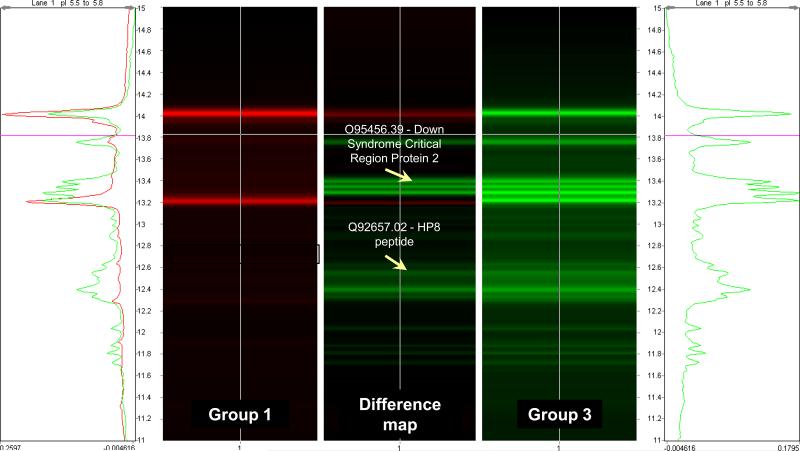
Figure 5 displays the 2D chromatographic ProteoVue maps representing differentially expressed protein in amniotic fluid of women with preterm labor who delivered at term (Figure 5a) and preterm labor with IAI (Figure 5b). From these ProteoVue maps, 22 fractions were selected for analysis by MALDI-TOF and ESI-IT MS. Figure 6 shows an example of a DeltaVue map for one particular fraction corresponding to the proteins with an isoelectric point between 5.5 and 5.8 and reversed phase HPLC retention time ranging between 11 and 15 minutes. The location of two proteins differentially expressed and subsequently identified as Down syndrome critical region protein 2 and Human peptide 8 (HP8) are indicated in the figure.
Figure 5.
ProteoVue Maps of the two pools of reducing solubilization buffer-treated retentates from concentrated amniotic fluid (experiment 3). (a) Patients with preterm labor without infection/inflammation who delivered at term (group 1).(b) Patients who delivered preterm with intra-amniotic infection/inflammation (group 3).
Figure 6.
DeltaVue map overlaying the proteins from the pI 5.5-5.8 CF fractions of patients with preterm labor without intra-amniotic infection/inflammation who delivered at term (group 1 - in red) and patients who delivered preterm with intra-amniotic infection/inflammation (group 3 - in green) according to the hydrophobicity. The center image is the difference map of proteins concentrations between the two groups. The arrows indicate two fractions in which proteins were over expressed in group 3, identified as Down Syndrome Critical Region Protein 2 and human peptide 8 (HP8).
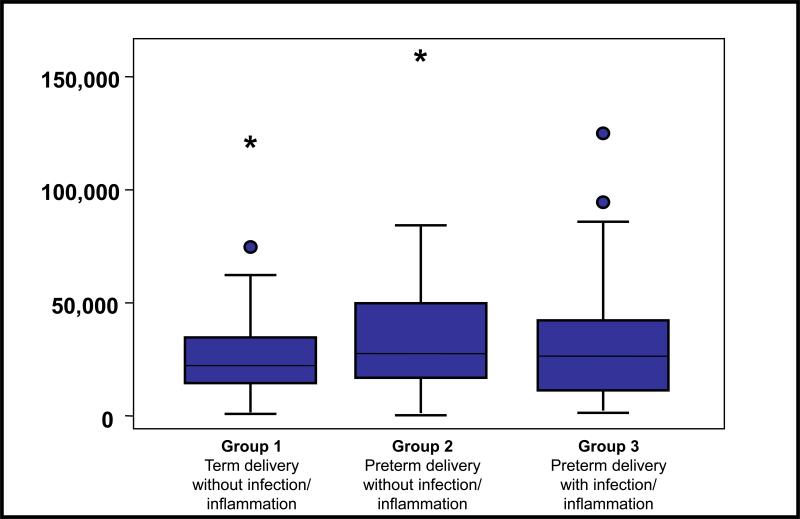
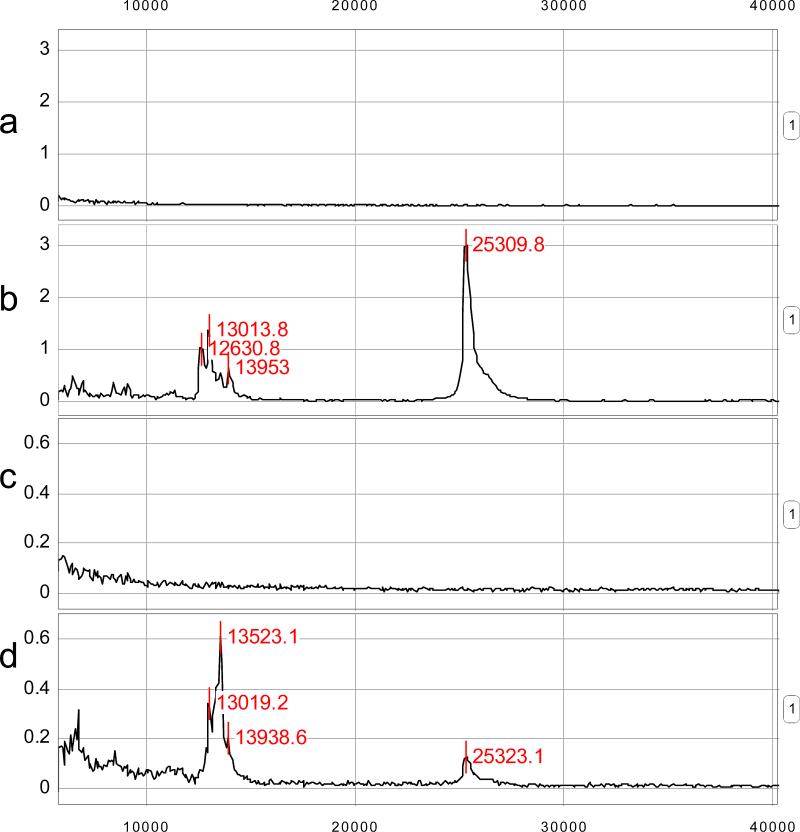
Table 4 lists the proteins differentially expressed using ProteoVue and DeltaVue and subsequently identified with MS. It should be noted that an over expression of IGFBP-1 was identified in group 1 but not in group 3. To confirm this over-expression of IGFBP-1 in amniotic fluid of group 1, the concentrations were measured in all three groups using ELISA. However, there was no difference in the mean amniotic fluid concentration of IGFBP-1 between group 1 (31,079 ng/ml± 22,077) and group 2 (37,736 ng/ml± 28,701) or group 3 (34,042 ng/ml± 26,953) (Figure 7). A ProteinChip Array-based immunoassay using SELDI-TOF demonstrated that IGFBP-1 was largely in a full-length form in the amniotic fluid pool of group 1 but significantly degraded in the amniotic fluid pool of group 3 (Figure 8). From these results it is clear that available antibodies used for ELISA measurements of the levels of IGFBP-1 do not discriminate between intact IGFBP-1 and IGFBP-1 fragments. This has important implications in how IGFBP-1 ELISA based assays are used in interpreting the biology of IGFBP-1 in group 1 and group 3 patients.
Table 4.
Protein identification from the third set of experiments: concentrated AF
| Iso-electric point | Retention time | Protein identification | Protein name | Identification Method |
|---|---|---|---|---|
| Protein ID from fractions with over expression in group 1 (Preterm Labor – Term delivery) | ||||
| 4.3 – 4.6 | 12.0 – 13.0 | P08833 | Insulin-like growth factor binding protein 1 precursor (Placental protein 12) | MALDI-TOF/MS |
| 4.3 – 4.6 | 13.0 – 14.1 | Q8TCG4 | TPMsk1 (Tropomyosin sk1 fragment) | MALDI-TOF/MS |
| 4.6 – 4.9 | 13.7 – 14.3 | P31025 | Von Ebner's gland protein precursor (Tear lipocalin) | MALDI-TOF/MS, Nanospray-ESI-IT |
| 4.3 – 4.6 | 12.0 – 13.0 | P13232 | Interleukin-7 precursor | Nanospray-ESI-IT |
| 4.9 – 5.2 | 13.7 – 14.3 | P02760 | AMBP (Alpha-1-microglobulin/bikunin precursor) | MALDI-TOF/MS |
| 5.2 – 5.5 | 13.9 – 14.3 | P51812 | Ribosomal protein S6 kinase alpha 3 | MALDI-TOF/MS |
| 5.5 – 5.8 | 15.8 – 16.4 | P02647 | Apolipoprotein A1 | MALDI-TOF/MS, Nanospray-ESI-IT |
| Protein ID from fractions with over expression in group 3 (Preterm Labor – Preterm delivery – Intra-amniotic infection/inflammation) | ||||
| 5.5 – 5.8 | 8.8 – 9.9 | 098237 | MHC class I chain-related protein A (Fragment) | MALDI-TOF/MS |
| 5.5 – 5.8 | 8.8 – 9.9 | Q8TD37 | Transcription elongation factor A Protein 2 | MALDI-TOF/MS |
| 5.5 – 5.8 | 8.8 – 9.9 | Q8N1D9 | SRY (Sex determining region Y)-box 5 | MALDI-TOF/MS |
| 5.5 – 5.8 | 12.2 – 12.8 | Q92657 | HP8 (Human peptide 8) | MALDI-TOF/MS |
| 5.5 – 5.8 | 12.8 – 13.7 | 095456 | Down Syndrome Critical Region Protein 2 | Nanospray-ESI-IT |
Figure 7.
Amniotic Fluid IGFBP-1 concentration determined by ELISA in the three groups of patients with preterm labor. No significant differences in the mean concentrations of IGFBP-1 among the three groups of patients were detected.
Figure 8.
SELDI-TOF MS ProteinChip immunoassays of amniotic fluid. (a) The IgG negative control for group 1 (preterm labor without infection/inflammation who deliver at term) (b) The IGFBP-1 capture antibody assay for the same amniotic fluid. (c) The IgG negative control for group 3 (preterm labor with intra-amniotic infection/inflammation). (d) The IGFBP-1 capture antibody assay for the same amniotic fluid (group 3). Peaks near 13 kDa correspond to proteolytic fragments of IGFBP-1, while the peak near 25 kDa correspond to the intact IGFBP-1 protein. Comparison of Figures (b) and (d) reveals evidence for proteolytic cleavage occurring in amniotic fluid from women with intra-amniotic infection/inflammation (group 3), but not in those without it (group 1).
DISCUSSION
Principal findings of this study
1) Amniotic fluid protein composition can be analyzed using a combination of 2D liquid chromatography and MS for the identification of proteins differentially expressed in patients in preterm labor; 2) experimental data suggest that IGFBP-1 is degraded in patients with IAI resulting in an increased concentration of a fragment of IGFBP-1; 3) proteins which were over-expressed in amniotic fluid of women without IAI who delivered at term included Von Ebner gland protein precursor, IL-7 precursor, apolipoprotein A1, TPMsk1 fragment, ribosomal protein S6 kinase alpha-3 and alpha-1-microglobulin/bikunin precursor (AMBP); 4) proteins which were over-expressed in amniotic fluid of women with IAI who delivered preterm included fibrinopeptide B, transferrin, MHC class 1 chain-related A antigen fragment, transcription elongation factor A, sex-determining region Y (SRY) box 5 protein, Down syndrome critical region 2 protein, and HP8; and 5) one protein, retinol binding protein, was over-expressed in women who delivered preterm, regardless of the presence of IAI.
The use of two-dimensional chromatography in the analysis of amniotic fluid
Amniotic fluid proteomic profile has been the subject of extensive research[3,6,7,9,10,23-31]. We undertook the use of 2D chromatographic technique as a means of comprehensive profiling of amniotic fluid samples with the goal of “mining” for biomarkers to identify patients with spontaneous preterm labor who have no evidence of inflammation and deliver at term, as well as those with IAI. The most important advantage of this 2D approach is that the amniotic fluid sample complexity is reduced through orthogonal fractionation and “mapping” of the expressed proteins (like a 2D gel based approach) with the fractionated proteins intact and in the liquid phase for easy isolation and analysis with other analytical techniques such as mass spectrometry, and Western blot analysis[32-40]. Indeed, 2D chromatography analysis has been recently used in amniotic fluid by Michaels et al[41], resulting in the identification of 118 non-redundant proteins with high confidence.
Intact protein isolation and analysis has potential advantages over the traditional strategy requiring digestion of the protein before mass spectrometry (“bottom up” proteomics). Direct access to the intact expressed proteins has potential advantages because the proteins are preserved in their original state rather than digested[42-44]. The intact protein can be analyzed through a number of techniques such as Western Blot analyses and other immunoassays which can not be applied to the digestion products.
A cluster of fragments of IGFBP-1 is present in the amniotic fluid of patients with preterm delivery in the presence of IAI
We identified IGFBP-1 via both MALDI-TOF and ESI-MS/MS and determined it to be differentially expressed in both the untreated amniotic fluid sample and in the molecular weight exclusion experiments. No significant difference in the amniotic fluid concentrations of IGFBP-1 in groups 1, 2 and 3 were detected by ELISA. On the other hand, SELDI-TOF MS immunoassays revealed that IGFPB-1 is significantly degraded in group 3 as compared to group 1. All data together suggest that the total amount of IGFBP-1 does not change, but that inflammation/infection leads to increased proteolytic degradation of IGFBP-1.
IGFBP-1 and IGFBP-1 fragments have been previously observed as potential biomarkers of infection/inflammation in the amniotic fluid[30]. Gravett et al.[30] detected by MS, and confirmed using Western Blot analysis, that the concentration of intact IGFBP-1 (reported by these investigators as having a mass of 30 kDa) in the control group was comparable to its concentration in patients with IAI[30]. Their Western Blot analysis also indicated the presence of a previously unreported proteolytic IGFBP-1 fragment (at approximately 11 kDa) in the infection group that was undetected in the control group[30]. Lee et al.[45] made a similar observation of preferential production of IGFBP-1 fragments in the amniotic fluid of patients with IAI. Furthermore, they proved that these fragments were the result of proteolytic degradation of IGFBP-1 by matrix metalloproteinases (MMPs), and different MMPs generated fragments of IGFBP-1 of different masses[45].
Using antibody capture SELDI-TOF MS immunoassay, we found evidence of proteolytic degradation of IGFBP-1 in patients with infection/inflammation. Such evidence was that (a) in patients with no IAI the peak corresponding to the intact IGFBP-1 protein was greater in amplitude than the cluster of peaks corresponding to the immunoreactive proteolytic fragments of IGFBP-1, and in contrast, (b) in patients with IAI the amplitude of these peaks was reversed (Figure 8). Thus, our observations are consistent in substance with those of Gravett et al.[30] and Lee et al[45]. However, the SELDI-TOF antibody capture immunoassay provides a more accurate measurement of the masses of both the intact protein as well as the degradation products.
Interestingly, the cervical-vaginal fluid proteome profile was recently studied[46-48]. Indeed, Pereira et al[46] reported the analysis of cervico-vaginal fluid of: 1) women with preterm labor who delivered before term; 2) women with preterm labor who delivered at term; and 3) asymptomatic pregnant women, showed two IGFBP-1 fragments (~11 kDa and ~16 kDa) in cervical-vaginal fluid of women with preterm birth but not in the two other groups[46]. It is possible that these two fragments correspond to the proteolytic fragments of IGFBP-1 observed in amniotic fluid by Gravett et al.[30], Lee et al.[45] and us.
IGFBP-1 is a physiologic constituent of amniotic fluid and has been implicated in several pathophysiological states including sepsis[49], AIDS[50], excessive alcohol consumption[51], and diabetes[52]. IGFBP-1 seems to play a role in the development of the decidua.[53] Its primary mode of action is in the regulation of insulin-like growth factors (IGFs). Posttranslational modifications of IGFBP-1 alter the affinity of IGFBP-1 to IGFs, including phosphorylation and dephosphorylation[54,55], proteolysis[56,57], and polymerization[58,59].
Wang et al.[60] recently purified IGFBP-1-specific protease activity from the urine of an individual with multiple myeloma. The proteolytic fraction contained azurocidin, a cationic antimicrobial protein which was also observed to be differentially present in IAI by Gravett et al.[30] and by us (unpublished data from ITRAQ-based LC-MS/MS studies), but has not heretofore been causally-linked with IAI-dependent proteolysis of IGFBP-1. Wang et al.[60] determined that this protease cleaves IGFBP-1 at Ile130-Ser131 in both phosphorylated and unphosphorylated forms. The resulting fragment theoretical masses are consistent with both the 11 kDa fragment reported by Gravett et al.[30] (N-terminal fragment) and the 13.5 kDa fragment observed in the current study (C-terminal fragment).
Both the present results and those of Gravett et al.[30] and Lee et al.[45] suggest a role for proteolytic regulation of IGFBP-1 biological activity in IAI. Collectively, these results seem to point toward the possibility that proteolysis of IGFBP-1 in amniotic fluid is an important regulatory mechanism in the context of IAI. Indeed, the present results suggest that the dominant modulatory mechanism of IGFBP-1 due to IAI is not apparently due to transcriptional regulation of IGFBP-1 expression, but rather by proteolytic modification of a relatively tightly regulated pool of protein.
Other proteins over-expressed in amniotic fluid of patients with IAI
Of interest, we identified two proteins that have not been reported in amniotic fluid. Down syndrome critical region protein 2 (DSCR2) is a protein that has no homology to any known protein and that is encoded by a 5-10 Mb region of the long arm of the human chromosome 21, called the Down syndrome critical region. While its function in human biology remains unknown, expression of the DSCR2 gene is predominant in the cells and tissues that have a high rate of proliferation such as the adult testis and Jurkat cells[61]. Jurkat cells are a line of human leukaemic T lymphocyte cells capable of producing IL-2[62]. DSCR2 gene expression has been demonstrated through all stages of embryonic development in mouse[63]. The high homology between human and mouse proteins suggests a conservation in evolution and consequently an important role of DSCR2 for development or survival of mammalian cells[64,65]. The role of this protein in pregnancy, and specifically in amniotic fluid, remains to be elucidated.
Transcription elongation factor SII was discovered in amniotic fluid of women with infection/inflammation. Transcription elongation factors are implicated in the regulation of RNA chain elongation by RNA polymerase II[66-69] and may play a role in the host response to infection[70] or in deploying an inflammatory response.
Human peptide 8 was also identified in the amniotic fluid of women with infection/inflammation. This nucleoprotein, originally cloned because of its strong expression during the acute phase response observed in patients with pancreatitis, is a High Mobility Group (HMG) I/Y-like protein that has been implicated in the regulation of transcription[71,72]. Interestingly, expression of peptide 8 mRNA in several organs has been showed to increase after lipopolysaccharide administration[73].
The precise role in pregnancy, parturition and inflammation for the new proteins identified in this study requires further investigation. Specifically, differential expression among groups needs to be demonstrated with other methods and their biological functions defined.
Other proteins over-expressed in amniotic fluid of patients without IAI
We identified Von Ebner's gland protein precursor (tear lipocalin) in patients with preterm labor without evidence of inflammation. Antibodies for immunoassays are not available to the authors to pursue this line of investigation at this time. Similarly, retinol binding protein, another member of the lipocalin family has been reported in amniotic fluid[74,75]. Lipocalins are proteins that regulate transfer[76,77], immunological[78-80] and developmental processes[81], and are also involved in the response of organisms to stress factors[82-86] and in signal transduction[87-91]. While Von Ebner's gland protein precursor was found from amniotic fluid of women who delivered at term, retinol binding proteins were more abundant in infected amniotic fluid. These findings are in agreement with those of Gravett et al.[30] who reported a novel protein similar to mouse von Ebner gland protein from women with no infection and neutrophil gelatinase-associated lipocalin in infected amniotic fluid.
Conclusion
The study reported herein utilized a combination of techniques involving two-dimensional chromatography, mass spectrometry, and immunoassays to characterize pooled amniotic fluid samples from patients with spontaneous preterm labor. This approach was successful in identifying proteins that are differentially regulated in amniotic fluid of patients with preterm labor. Specifically, the amount of the IGFBP-1 fragments at approximately 13.5 kDa was found to be increased in patients with intra-amniotic infection/inflammation, while the amount of the intact form of IGFBP-1 was decreased.
Acknowledgment
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Evans GA. Designer science and the “omic” revolution. Nat.Biotechnol. 2000;18:127. doi: 10.1038/72480. [DOI] [PubMed] [Google Scholar]
- 2.Gracey AY, Cossins AR. Application of microarray technology in environmental and comparative physiology. Annu.Rev.Physiol. 2003;65:231–259. doi: 10.1146/annurev.physiol.65.092101.142716. [DOI] [PubMed] [Google Scholar]
- 3.Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Bienvenut WV, Lemery D, Quadroni M, Dastugue B, et al. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3:1521–1525. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- 4.Mehta T, Tanik M, Allison DB. Towards sound epistemological foundations of statistical methods for high-dimensional biology. Nat.Genet. 2004;36:943–947. doi: 10.1038/ng1422. [DOI] [PubMed] [Google Scholar]
- 5.Strange K. The end of “naive reductionism”: rise of systems biology or renaissance of physiology? Am.J.Physiol Cell Physiol. 2005;288:C968–C974. doi: 10.1152/ajpcell.00598.2004. [DOI] [PubMed] [Google Scholar]
- 6.Thadikkaran L, Crettaz D, Siegenthaler MA, Gallot D, Sapin V, Iozzo RV, Queloz PA, Schneider P, Tissot JD. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin.Chim Acta. 2005;360:27–36. doi: 10.1016/j.cccn.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Wang TH, Chang YL, Peng HH, Wang ST, Lu HW, Teng SH, Chang SD, Wang HS. Rapid detection of fetal aneuploidy using proteomics approaches on amniotic fluid supernatant. Prenat.Diagn. 2005;25:559–566. doi: 10.1002/pd.1186. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, Mazaki-Tovi S, Than NG, Hassan S, Tromp G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006;113(Suppl 3):118–35. 118–135. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford JT, Pereira L, Buckmaster J, Gravett MG, Tolosa JE. Amniocentesis results and novel proteomic analysis in a case of occult candidal chorioamnionitis. J Matern.Fetal Neonatal Med. 2006;19:667–670. doi: 10.1080/14767050600738289. [DOI] [PubMed] [Google Scholar]
- 10.Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, Fountoulakis M. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–4419. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- 11.Hampton T. Comprehensive “proteomic profile” of amniotic fluid may aid prenatal diagnosis. JAMA. 2007;298:1751. doi: 10.1001/jama.298.15.1751. [DOI] [PubMed] [Google Scholar]
- 12.Vascotto C, Salzano AM, D'Ambrosio C, Fruscalzo A, Marchesoni D, di LC, Scaloni A, Tell G, Quadrifoglio F. Oxidized transthyretin in amniotic fluid as an early marker of preeclampsia. J Proteome.Res. 2007;6:160–170. doi: 10.1021/pr060315z. [DOI] [PubMed] [Google Scholar]
- 13.Nagalla SR, Canick JA, Jacob T, Schneider KA, Reddy AP, Thomas A, Dasari S, Lu X, Lapidus JA, Lambert-Messerlian GM, et al. Proteomic analysis of maternal serum in down syndrome: identification of novel protein biomarkers. J Proteome.Res. 2007;6:1245–1257. doi: 10.1021/pr060539h. [DOI] [PubMed] [Google Scholar]
- 14.Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- 15.Phizicky E, Bastiaens PI, Zhu H, Snyder M, Fields S. Protein analysis on a proteomic scale. Nature. 2003;422:208–215. doi: 10.1038/nature01512. [DOI] [PubMed] [Google Scholar]
- 16.O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol.Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald T, Sheng S, Stanley B, Chen D, Ko Y, Cole RN, Pedersen P, Van Eyk JE. Expanding the subproteome of the inner mitochondria using protein separation technologies: one- and two-dimensional liquid chromatography and two-dimensional gel electrophoresis. Mol.Cell Proteomics. 2006;5:2392–2411. doi: 10.1074/mcp.T500036-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Lubman DM, Kachman MT, Wang H, Gong S, Yan F, Hamler RL, O'Neil KA, Zhu K, Buchanan NS, Barder TJ. Two-dimensional liquid separations-mass mapping of proteins from human cancer cell lysates. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;782:183–196. doi: 10.1016/s1570-0232(02)00551-2. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Diamond DL, Kimball JR, Krisanaprakornkit S, Ganz T, Dale BA. Detection of beta-defensins secreted by human oral epithelial cells. J.Immunol.Methods. 2001;256:65–76. doi: 10.1016/s0022-1759(01)00442-2. [DOI] [PubMed] [Google Scholar]
- 21.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Sakai K, Busby WHJ, Clarke JB, Clemmons DR. Tissue transglutaminase facilitates the polymerization of insulin-like growth factor-binding protein-1 (IGFBP-1) and leads to loss of IGFBP-1's ability to inhibit insulin-like growth factor-I-stimulated protein synthesis. J Biol Chem. 2001;276:8740–8745. doi: 10.1074/jbc.M008359200. [DOI] [PubMed] [Google Scholar]
- 23.Tsangaris GT, Kolialexi A, Karamessinis PM, Anagnostopoulos AK, Antsaklis A, Fountoulakis M, Mavrou A. The normal human amniotic fluid supernatant proteome. In Vivo. 2006;20:479–490. [PubMed] [Google Scholar]
- 24.Cho CK, Shan SJ, Winsor EJ, Diamandis EP. Proteomics analysis of human amniotic fluid. Mol.Cell Proteomics. 2007;6:1406–1415. doi: 10.1074/mcp.M700090-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Michaels JE, Dasari S, Pereira L, Reddy AP, Lapidus JA, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, Jr., et al. Comprehensive proteomic analysis of the human amniotic fluid proteome: gestational age-dependent changes. J Proteome.Res. 2007;6:1277–1285. doi: 10.1021/pr060543t. [DOI] [PubMed] [Google Scholar]
- 26.Michel PE, Crettaz D, Morier P, Heller M, Gallot D, Tissot JD, Reymond F, Rossier JS. Proteome analysis of human plasma and amniotic fluid by Off-Gel isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis. 2006;27:1169–1181. doi: 10.1002/elps.200500680. [DOI] [PubMed] [Google Scholar]
- 27.Ruetschi U, Rosen A, Karlsson G, Zetterberg H, Rymo L, Hagberg H, Jacobsson B. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J.Proteome.Res. 2005;4:2236–2242. doi: 10.1021/pr050139e. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay R. Biomarkers for preterm birth. J Proteome.Res. 2005;4:1900. doi: 10.1021/pr050532k. [DOI] [PubMed] [Google Scholar]
- 29.Klein LL, Freitag BC, Gibbs RS, Reddy AP, Nagalla SR, Gravett MG. Detection of intra-amniotic infection in a rabbit model by proteomics-based amniotic fluid analysis. Am J Obstet Gynecol. 2005;193:1302–1306. doi: 10.1016/j.ajog.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–469. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson S, Ramstrom M, Palmblad M, Axelsson O, Bergquist J. Explorative study of the protein composition of amniotic fluid by liquid chromatography electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. J Proteome.Res. 2004;3:884–889. doi: 10.1021/pr0499545. [DOI] [PubMed] [Google Scholar]
- 32.Mann M. Quantitative proteomics? Nat.Biotechnol. 1999;17:954–955. doi: 10.1038/13646. [DOI] [PubMed] [Google Scholar]
- 33.Keough T, Lacey MP, Fieno AM, Grant RA, Sun Y, Bauer MD, Begley KB. Tandem mass spectrometry methods for definitive protein identification in proteomics research. Electrophoresis. 2000;21:2252–2265. doi: 10.1002/1522-2683(20000601)21:11<2252::AID-ELPS2252>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Haynes PA, Yates JR., III Proteome profiling-pitfalls and progress. Yeast. 2000;17:81–87. doi: 10.1002/1097-0061(20000630)17:2<81::AID-YEA22>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naaby-Hansen S, Waterfield MD, Cramer R. Proteomics--post-genomic cartography to understand gene function. Trends Pharmacol.Sci. 2001;22:376–384. doi: 10.1016/s0165-6147(00)01663-1. [DOI] [PubMed] [Google Scholar]
- 36.Chakravarti DN, Chakravarti B, Moutsatsos I. Informatic tools for proteome profiling. Biotechniques. 2002;(Suppl):4–10. 12–5, 4–5. [PubMed] [Google Scholar]
- 37.Ong SE, Pandey A. An evaluation of the use of two-dimensional gel electrophoresis in proteomics. Biomol.Eng. 2001;18:195–205. doi: 10.1016/s1389-0344(01)00095-8. [DOI] [PubMed] [Google Scholar]
- 38.Spandidos A, Rabbitts TH. Sub-proteome differential display: single gel comparison by 2D electrophoresis and mass spectrometry. J Mol.Biol. 2002;318:21–31. doi: 10.1016/S0022-2836(02)00052-9. %19. [DOI] [PubMed] [Google Scholar]
- 39.Hammack BN, Owens GP, Burgoon MP, Gilden DH. Improved resolution of human cerebrospinal fluid proteins on two-dimensional gels. Mult.Scler. 2003;9:472–475. doi: 10.1191/1352458503ms954oa. [DOI] [PubMed] [Google Scholar]
- 40.Desrosiers RR, Beaulieu E, Buchanan M, Beliveau R. Proteomic analysis of human plasma proteins by two-dimensional gel electrophoresis and by antibody arrays following depletion of high-abundance proteins. Cell Biochem.Biophys. 2007;49:182–195. doi: 10.1007/s12013-007-0048-z. [DOI] [PubMed] [Google Scholar]
- 41.Michaels JE, Dasari S, Pereira L, Reddy AP, Lapidus JA, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, Jr., et al. Comprehensive proteomic analysis of the human amniotic fluid proteome: gestational age-dependent changes. J.Proteome.Res. 2007;6:1277–1285. doi: 10.1021/pr060543t. [DOI] [PubMed] [Google Scholar]
- 42.Nemeth-Cawley JF, Tangarone BS, Rouse JC. “Top Down” characterization is a complementary technique to peptide sequencing for identifying protein species in complex mixtures. J Proteome.Res. 2003;2:495–505. doi: 10.1021/pr034008u. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs JM, Mottaz HM, Yu LR, Anderson DJ, Moore RJ, Chen WN, Auberry KJ, Strittmatter EF, Monroe ME, Thrall BD, et al. Multidimensional proteome analysis of human mammary epithelial cells. J Proteome.Res. 2004;3:68–75. doi: 10.1021/pr034062a. [DOI] [PubMed] [Google Scholar]
- 44.Millea KM, Krull IS, Cohen SA, Gebler JC, Berger SJ. Integration of multidimensional chromatographic protein separations with a combined “top-down” and “bottom-up” proteomic strategy. J Proteome.Res. 2006;5:135–146. doi: 10.1021/pr050278w. [DOI] [PubMed] [Google Scholar]
- 45.Lee SE, Han HD, Park IS, Romero R, Yoon BH. Evidence supporting proteolytic cleavage of insulin-like growth factor binding protein-1 (IGFBP-1) protein in amniotic fluid. Journal of Perinatal Medicine. 2008 doi: 10.1515/JPM.2008.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira L, Reddy AP, Jacob T, Thomas A, Schneider KA, Dasari S, Lapidus JA, Lu X, Rodland M, Roberts CTJ, et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res. 2007;6:1269–1276. doi: 10.1021/pr0605421. [DOI] [PubMed] [Google Scholar]
- 47.Dasari S, Pereira L, Reddy AP, Michaels JE, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, Jr., Gravett MG, et al. Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome.Res. 2007;6:1258–1268. doi: 10.1021/pr0605419. [DOI] [PubMed] [Google Scholar]
- 48.Gravett MG, Thomas A, Schneider KA, Reddy AP, Dasari S, Jacob T, Lu X, Rodland M, Pereira L, Sadowsky DW, et al. Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome.Res. 2007;6:89–96. doi: 10.1021/pr060149v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.FernÃindez-CelemÃ-n L, Thissen JP. Interleukin-6 stimulates hepatic insulin-like growth factor binding protein-4 messenger ribonucleic acid and protein. Endocrinology. 2001;142:241–248. doi: 10.1210/endo.142.1.7903. [DOI] [PubMed] [Google Scholar]
- 50.Congote LF. Monitoring insulin-like growth factors in HIV infection and AIDS. Clin Chim Acta. 2005;361:30–53. doi: 10.1016/j.cccn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Dal Maso L, La Vecchia C, Augustin LSA, Mantzoros CS, Kendall CWC, Franceschi S. Relationship between a wide range of alcohol consumptions, components of the insulin-like growth factor system and adiponectin. Eur J Clin Nutr. 2007;61:221–225. doi: 10.1038/sj.ejcn.1602519. [DOI] [PubMed] [Google Scholar]
- 52.Katz LEL, Jawad AF, Ganesh J, Abraham MÃ, Murphy K, Lipman TH. Fasting c-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007;8:53–59. doi: 10.1111/j.1399-5448.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 53.Rutanen EM, Menabawey M, Isaka K, Bohn H, Chard T, Grudzinskas JG. Synthesis of placental protein 12 by decidua from early pregnancy. J Clin Endocrinol Metab. 1986;63:675–679. doi: 10.1210/jcem-63-3-675. [DOI] [PubMed] [Google Scholar]
- 54.Hoeck WG, Mukku VR. Identification of the major sites of phosphorylation in IGF binding protein-3. J Cell Biochem. 1994;56:262–273. doi: 10.1002/jcb.240560220. [DOI] [PubMed] [Google Scholar]
- 55.Jones JI, D'Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci U S A. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanzaki S, Hilliker S, Baylink DJ, Mohan S. Evidence that human bone cells in culture produce insulin-like growth factor-binding protein-4 and -5 proteases. Endocrinology. 1994;134:383–392. doi: 10.1210/endo.134.1.7506211. [DOI] [PubMed] [Google Scholar]
- 57.Sakai K, Iwashita M, Takeda Y. Profiles of insulin-like growth factor binding proteins and the protease activity in the maternal circulation and its local regulation between placenta and decidua. Endocr J. 1997;44:409–417. doi: 10.1507/endocrj.44.409. [DOI] [PubMed] [Google Scholar]
- 58.Brinkman A, Kortleve DJ, Zwarthoff EC, Drop SL. Mutations in the C-terminal part of insulin-like growth factor (IGF)-binding protein-1 result in dimer formation and loss of IGF binding capacity. Mol Endocrinol. 1991;5:987–994. doi: 10.1210/mend-5-7-987. [DOI] [PubMed] [Google Scholar]
- 59.Busby WH, Hossenlopp P, Binoux M, Clemmons DR. Purified preparations of the amniotic fluid-derived insulin-like growth factor-binding protein contain multimeric forms that are biologically active. Endocrinology. 1989;125:773–777. doi: 10.1210/endo-125-2-773. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Shafqat J, Hall K, StÃ¥hlberg M, Wivall-Helleryd IL, Bouzakri K, Zierath JR, Brismar K, Jörnvall H, Lewitt MS. Specific cleavage of insulin-like growth factor-binding protein-1 by a novel protease activity. Cell Mol Life Sci. 2006;63:2405–2414. doi: 10.1007/s00018-006-6248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vidal-Taboada JM, Sanz S, Egeo A, Scartezzini P, Oliva R. Identification and characterization of a new gene from human chromosome 21 between markers D21S343 and D21S268 encoding a leucine-rich protein. Biochem Biophys Res Commun. 1998;250:547–554. doi: 10.1006/bbrc.1998.9352. [DOI] [PubMed] [Google Scholar]
- 62.Koizumi T, Nakao Y, Matsui T, Katakami Y, Katakami N, Fujita T. Inhibitors of IL-2 production and IL-2 receptor expression in human leukemic T-cell line, Jurkat. Cell Immunol. 1986;103:469–475. doi: 10.1016/0008-8749(86)90107-3. [DOI] [PubMed] [Google Scholar]
- 63.Vidal-Taboada JM, Lu A, Pique M, Pons G, Gil J, Oliva R. Down syndrome critical region gene 2: expression during mouse development and in human cell lines indicates a function related to cell proliferation. Biochem.Biophys.Res.Commun. 2000;272:156–163. doi: 10.1006/bbrc.2000.2726. [DOI] [PubMed] [Google Scholar]
- 64.Vesa J, Brown Y, Greenfield D, Korenberg JR. Molecular and cellular characterization of the Down syndrome critical region protein 2. Biochem.Biophys.Res.Commun. 2005;328:235–242. doi: 10.1016/j.bbrc.2004.09.226. [DOI] [PubMed] [Google Scholar]
- 65.Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS, Toyoda A, Ishii K, Totoki Y, Choi DK, et al. The DNA sequence of human chromosome 21. Nature. 2000;405:311–319. doi: 10.1038/35012518. [DOI] [PubMed] [Google Scholar]
- 66.Reines D, Ghanouni P, Gu W, Mote J, Jr., Powell W. Transcription elongation by RNA polymerase II: mechanism of SII activation. Cell Mol.Biol Res. 1993;39:331–338. [PubMed] [Google Scholar]
- 67.Shilatifard A, Conaway JW, Conaway RC. Mechanism and regulation of transcriptional elongation and termination by RNA polymerase II. Curr.Opin.Genet.Dev. 1997;7:199–204. doi: 10.1016/s0959-437x(97)80129-3. [DOI] [PubMed] [Google Scholar]
- 68.Wind M, Reines D. Transcription elongation factor SII. Bioessays. 2000;22:327–336. doi: 10.1002/(SICI)1521-1878(200004)22:4<327::AID-BIES3>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomes NP, Bjerke G, Llorente B, Szostek SA, Emerson BM, Espinosa JM. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev. 2006;20:601–612. doi: 10.1101/gad.1398206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamamoto Y, Hirashima S, Natori S, Yamamoto N, Kobayashi N. Augmentation and stable expression of a novel transcription factor SII in CD4-positive cells on infection with human immunodeficiency virus type-1 (HIV-1). Biochem.Biophys.Res.Commun. 1988;155:1346–1352. doi: 10.1016/s0006-291x(88)81289-0. [DOI] [PubMed] [Google Scholar]
- 71.Encinar JA, Mallo GV, Mizyrycki C, Giono L, Gonzalez-Ros JM, Rico M, Canepa E, Moreno S, Neira JL, Iovanna JL. Human p8 is a HMG-I/Y-like protein with DNA binding activity enhanced by phosphorylation. J Biol Chem. 2001;276:2742–2751. doi: 10.1074/jbc.M008594200. [DOI] [PubMed] [Google Scholar]
- 72.Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem. 1997;272:32360–32369. doi: 10.1074/jbc.272.51.32360. %19. [DOI] [PubMed] [Google Scholar]
- 73.Jiang YF, Vaccaro MI, Fiedler F, Calvo EL, Iovanna JL. Lipopolysaccharides induce p8 mRNA expression in vivo and in vitro. Biochem Biophys Res Commun. 1999;260:686–690. doi: 10.1006/bbrc.1999.0953. [DOI] [PubMed] [Google Scholar]
- 74.Sklan D, Shalit I, Lasebnik N, Spirer Z, Weisman Y. Retinol transport proteins and concentrations in human amniotic fluid, placenta, and fetal and maternal sera. Br.J Nutr. 1985;54:577–583. doi: 10.1079/bjn19850144. [DOI] [PubMed] [Google Scholar]
- 75.Wallingford JC, Milunsky A, Underwood BA. Vitamin A and retinol-binding protein in amniotic fluid. Am J Clin.Nutr. 1983;38:377–381. doi: 10.1093/ajcn/38.3.377. [DOI] [PubMed] [Google Scholar]
- 76.Ottonello S, Petrucco S, Maraini G. Vitamin A uptake from retinol-binding protein in a cell-free system from pigment epithelial cells of bovine retina. Retinol transfer from plasma retinol-binding protein to cytoplasmic retinol-binding protein with retinyl-ester formation as the intermediate step. J Biol Chem. 1987;262:3975–3981. [PubMed] [Google Scholar]
- 77.Grzyb J, Latowski D, Strzalka K. Lipocalins - a family portrait. J Plant Physiol. 2006;163:895–915. doi: 10.1016/j.jplph.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Logdberg L, Wester L. Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim.Biophys.Acta. 2000;1482:284–297. doi: 10.1016/s0167-4838(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 79.Kim J, Yang P, Suraokar M, Sabichi AL, Llansa ND, Mendoza G, Subbarayan V, Logothetis CJ, Newman RA, Lippman SM, et al. Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Res. 2005;65:6189–6198. doi: 10.1158/0008-5472.CAN-04-4439. [DOI] [PubMed] [Google Scholar]
- 80.Helliwell RJ, Keelan JA, Marvin KW, Adams L, Chang MC, Anand A, Sato TA, O'Carroll S, Chaiworapongsa T, Romero RJ, et al. Gestational age-dependent up-regulation of prostaglandin D synthase (PGDS) and production of PGDS-derived antiinflammatory prostaglandins in human placenta. J Clin Endocrinol Metab. 2006;91:597–606. doi: 10.1210/jc.2005-1982. [DOI] [PubMed] [Google Scholar]
- 81.Pagano A, Giannoni P, Zambotti A, Randazzo N, Zerega B, Cancedda R, Dozin B. CALbeta, a novel lipocalin associated with chondrogenesis and inflammation. Eur.J Cell Biol. 2002;81:264–272. doi: 10.1078/0171-9335-00243. [DOI] [PubMed] [Google Scholar]
- 82.Winkler MF, Gerrior SA, Pomp A, Albina JE. Use of retinol-binding protein and prealbumin as indicators of the response to nutrition therapy. J Am Diet.Assoc. 1989;89:684–687. [PubMed] [Google Scholar]
- 83.Lichtlen P, Wang Y, Belser T, Georgiev O, Certa U, Sack R, Schaffner W. Target gene search for the metal-responsive transcription factor MTF-1. Nucleic Acids Res. 2001;29:1514–1523. doi: 10.1093/nar/29.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taba Y, Miyagi M, Miwa Y, Inoue H, Takahashi-Yanaga F, Morimoto S, Sasaguri T. 15-deoxy-delta 12,14-prostaglandin J2 and laminar fluid shear stress stabilize c-IAP1 in vascular endothelial cells. Am J Physiol Heart Circ.Physiol. 2003;285:H38–H46. doi: 10.1152/ajpheart.01037.2002. [DOI] [PubMed] [Google Scholar]
- 85.Ulivi V, Tutolo G, Mallein-Gerin F, Daga A, Cancedda R, Cancedda FD. A common pathway in differentiation and inflammation: p38 mediates expression of the acute phase SIP24 iron binding lipocalin in chondrocytes. J Cell Physiol. 2006;206:728–737. doi: 10.1002/jcp.20511. [DOI] [PubMed] [Google Scholar]
- 86.Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J Radiat.Res.(Tokyo) 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- 87.Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- 88.Numata A, Shimoda K, Kamezaki K, Haro T, Kakumitsu H, Shide K, Kato K, Miyamoto T, Yamashita Y, Oshima Y, et al. Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway. J Biol Chem. 2005;280:12621–12629. doi: 10.1074/jbc.M408442200. [DOI] [PubMed] [Google Scholar]
- 89.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 90.Trivedi SG, Newson J, Rajakariar R, Jacques TS, Hannon R, Kanaoka Y, Eguchi N, Colville-Nash P, Gilroy DW. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc.Natl.Acad.Sci U.S.A. 2006;103:5179–5184. doi: 10.1073/pnas.0507175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ost A, Danielsson A, Liden M, Eriksson U, Nystrom FH, Stralfors P. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 2007;21:3696–3704. doi: 10.1096/fj.07-8173com. [DOI] [PubMed] [Google Scholar]