Abstract
Epidemiologic, preclinical, and clinical trials suggest that green tea (GT) consumption may prevent prostate cancer via the action of green tea polyphenols including (-)-epigallocatechin-3-gallate (EGCG). In order to study the metabolism and bioactivity of green tea polyphenols in human prostate tissue, men with clinically localized prostate cancer consumed 6 cups of GT (n=8) daily or water (n=9) for 3-6 weeks prior to undergoing radical prostatectomy. Using high performance liquid chromatography 4″-O-methyl EGCG (4″-MeEGCG) and EGCG were identified in comparable amounts, and (-)-epicatechin-3-gallate (ECG) in lower amounts in prostatectomy tissue from men consuming GT (38.9 ± 19.5, 42.1 ± 32.4, and 17.8 ± 10.1 pmol/g tissue, respectively). The majority of EGCG and other green tea polyphenols were not conjugated. Green tea polyphenols were not detected in prostate tissue or urine from men consuming water preoperatively. In the urine of men consuming GT, 50-60% of both (-)-epigallocatechin (EGC) and (-)-epicatechin were present in methylated form with 4′-O-MeEGC being the major methylated form of EGC. When incubated with EGCG LNCaP prostate cancer cells were able to methylate EGCG to 4″-MeEGCG. The capacity of 4″-MeEGCG to inhibit proliferation and NF-κB activation and induce apoptosis in LNCaP cells was decreased significantly compared to EGCG. In summary, methylated and non-methylated forms of EGCG are detectable in prostate tissue following a short-term GT intervention and the methylation status of EGCG may potentially modulate its preventive impact on prostate cancer, possibly based on genetic polymorphisms of catechol O-methyltransferase.
Keywords: Green tea polyphenols, methylation, catechol-O-methyltransferase
Introduction
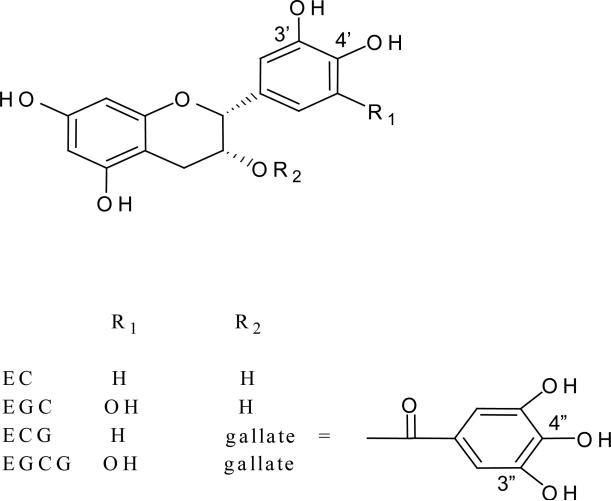
The consumption of green tea, brewed from the leaves of Camellia sinensis, may be protective for a number of chronic diseases including cardiovascular disease, neurodegenerative disease, osteoporosis, and cancer (1-4). The active phytochemicals in green tea are polyphenols, also known as flavan-3-ols, including (-)-epigallocatechin (EGC), (-)-epigallocatechin-3-gallate (EGCG), (-)-epicatechin (EC), and (-)-epicatechin-3-gallate (ECG) (Figure 1), with EGCG being the most abundant and possibly the most bioactive of the green tea polyphenols (GTPs) (5).
Figure 1.
Chemical structure of green tea polyphenols.
In addition to their antioxidant activity, GTPs selectively induce apoptosis and cell cycle arrest in cancer cells without affecting normal cells (6-8). In LNCaP prostate cancer cells the induction of apoptosis by GTPs is regulated through the activation of specific caspases, particularly caspase 3, and modulation of apoptosis-related genes, via down-regulation of Bcl-2 and up-regulation of Bax (9) . Additionally, GTPs exert chemopreventive and anti-inflammatory activity by modulating cell signaling pathways such as the nuclear factor kappa B (NF-κB) pathway, mitogen-activated protein kinase pathway, epidermal growth factor receptor mediated pathway, and the insulin-like growth factor mediated pathway (10, 11).
The chemopreventive and anticancer effects of green tea and GTPs have been demonstrated in cell culture studies and in various animal models (12-14). The majority of epidemiological studies support an association between green tea consumption and a reduced risk of prostate cancer (15-17). In a clinical case control trial, Bettuzzi et al. (18) demonstrated a reduced incidence of prostate cancer (CaP) in men with prostatic intraepithelial neoplasia after a one-year green tea supplement intervention compared to a group of men receiving placebo. Likewise, in a single-arm pre-prostatectomy trial of a green tea supplement, McLarty et al. (19) demonstrated a decrease in serum prostate-specific antigen levels and decreased prostate tissue vascular endothelial growth factor and hepatocyte growth factor concentrations.
It has been proposed that the limited bioavailability of GTPs and their conversion in vivo into less active metabolites could affect their chemopreventive potential (20). The majority of EGCG in the human blood circulates in the free form and only a small amount occurs in the glucuronidated or sulfated form (21, 22). Methylation of EGCG by catechol-O-methyltransferase (COMT) has been described in vitro and in animal studies (22, 23). However to date the biotransformation in human tissue in vivo has not been studied. The present study was carried out both to determine the degree of methylation of EGCG in human prostate glands obtained in men consuming green tea for up to six weeks prior to prostatectomy, and to determine the potential impact of methylation of EGCG on its preventive actions on prostate cancer cells.
Materials and Methods
Green tea intervention in men diagnosed with CaP
The following protocol was approved by the UCLA and Veterans Administration human subjects’ committees and informed consent was signed by all study subjects. In 2008 a randomized intervention trial was initiated in which men with clinically localized prostate cancer were randomized to 6 cups daily of green tea, black tea, or water for 3 to 6 weeks prior to undergoing radical prostatectomy. We report here on results from the first 17 subjects randomized to the green tea and water groups. Subjects randomized to the green tea group received detailed instructions to prepare the tea using 1 tea bag in 240 ml of boiling water to be brewed for 5 min. Green tea bags (authentic green tea) were generously provided by Celestial Seasonings, (Boulder CO). All tea bags were from the same production lot number. The control group was instructed to consume 6 cups of water daily. A tea consumption diary was kept to record compliance. A first voided urine was collected at baseline, one to two times during the intervention, and at the end of the intervention. Fasting blood was collected before the intervention and within 5 days of the surgery to measure liver function tests and prostate-specific antigen (PSA) concentration. Liver function tests (aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) were measured by the UCLA clinical laboratory. Serum PSA concentration was analyzed by competitive chemiluminescent immunoassay using the DPC Immulite analyzer (Siemens Healthcare Diagnostics, Dallas, TX) available in our laboratory according to the manufacturer's instruction. Fresh prostate tissue obtained from the prostatectomy was wrapped in foil, frozen in liquid nitrogen, and stored at -70 °C.
Tissue and urine sample extraction and HPLC detection
For tissue GTP analysis, 300 mg of prostate tissue in 200 μl of 2% ascorbic acid solution was homogenized with (-)-catechingallate as an internal standard. The homogenates were buffered with 300 μl of 0.4 M of phosphate solution (pH 6.5) incubated without enzyme, or with 1,000 units of β-glucuronidase (G7896, Sigma Chemicals, St Louis, MO) alone, or 1,000 units of β-glucuronidase and 40 units of sulfatase (S-9754, Sigma Chemicals) at 37°C for 45 min for detection of free, glucuronidated , or total form of GTPs. The sulfated GTPs were calculated by subtraction of free and glucuronidated forms from the total form. After incubation, the mixture was adjusted to pH 3 with trifluoroacetic acid and centrifuged at 3000 rpm for 5min. The pellets were washed twice with 300 μl of 0.05 M of phosphate buffer (pH 3.0) and the supernatants were combined and solid phase extraction (SPE) was performed using preconditioned HLB cartridges (Waters Corporation, Milford, MA). The cartridges were washed (1 ml of water, 1 ml of 5% methanol) and analytes eluted (70% dimethylformamide in methanol), dried in vacuum, and reconstituted for detection by HPLC-CoulArray electrochemical detection system (ESA, Chelmsford, MA) as previously described (24). The detection limit was 0.2 pmol/g tissue.
The method for urinary GTP detection was described previously (21) with minor modifications. Briefly, an aliquot of 250 μl urine sample was incubated without enzyme, or with 50 units of β-glucuronidase alone, or 50 units of β-glucuronidase and 20 units of sulfatase buffered with 100 μl of ascorbic acid- EDTA solution at 37°C for 2h for detection of free, glucuronidated or total form. The analytes were separated and detected using HPLC after SPE with the conditions as described above. The detection limit was 1 μg/L GTPs in urine. The identities of the tea polyphenols and their metabolites found in the prostate tissues and urine samples were confirmed using mass spectrometry (LC/MS/MS). The GTP concentrations were adjusted by urinary creatinine concentration.
Cell line and cell culture
The human prostate adenocarcinoma LNCaP cell line was obtained from ATCC (Chicago, IL), and cultured in RPMI 1640 medium with glutamine supplemented with 10% (v:v) of fetal bovine serum (FBS) (USA Scientific, Ocala, FL), 100 IU/ml of penicillin and 100 μg/ml of streptomycin (Invitrogen Inc, Carlsbad, CA) at 37 °C in a 5% CO2 incubator. At pH above 6.5 EGCG undergoes autoxidation and dimerization and forms hydrogen peroxide (H2O2) (25). To minimize the H2O2 effect 50 units/ml of catalase was added to the medium prior to EGCG (Sigma Chemicals) and 4″-O-methyl EGCG (4″-MeEGCG) (Nacalai USA, Inc., San Diego, CA).
Cellular absorption of EGCG and 4″-MeEGCG
LNCaP cells were allowed to grow to 60-70 percent confluency in 100 mm Petri dishes. In one experiment, cells were incubated with fresh serum-complete medium containing 40 or 80 μmol/L EGCG for 2, 24, 48, or 72h, respectively. In the other experiment, cells were incubated with 80 μmol/L of EGCG or 4″-MeEGCG for 2 or 24h. The procedures for cell harvest was described by Lambert et al (26) with some modifications. Briefly, the medium was removed and the dishes were washed with 10 ml of PBS for 3 times. The dishes were placed on ice and 100 μL of 2% ascorbic acid in water was added. Cells were scraped and collect for homogenization. The homogenate was centrifuged at 10,000 rpm for 15min and the supernatant was transfer with a little left for detection of cytosolic protein concentration. Equal volume of cold methanol was added to the transferred supernatant to precipitate proteins. The mixture was centrifuged at 14,000 rpm for 15 min and the supernatant was transferred, dried under nitrogen flow, and reconstituted for HPLC-CoulArray detection. Cytosolic EGCG and 4″-MeEGCG concentrations were normalized by cytosolic protein determined by the Bio-Rad protein assay according to the manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA). All the experiments were repeated three times.
Inhibition of cell proliferation by GTPs
LNCaP cells were seeded into 96-well plates at a density of 1×104 per well with RPMI 1640 serum-complete medium and treated with the following: vehicle control (NT), EGCG or 4″-MeEGCG at 40 and 80 μmol/L for 24, 48, or 72h. 50 units/ml of catalase was added to the medium prior to EGCG and 4″-MeEGCG. Cell viability was determined by trypan blue dye exclusion assay. Triplicate wells of viable cells for each concentration were counted on a hemacytometer after trypsinization. Each well had three repeats of counting. The experiment was repeated three times.
Inhibition of NF-κB activation by GTPs
LNCaP cells were allowed to grow to 70-80 percent confluency in 6-well plates and incubated with 40 or 80 μmol/L of EGCG or 4″-MeEGCG in fresh complete RPMI 1640 medium for 24h. 10 ng/ml of tumor necrosis factor (TNF)-α (R&D systems, Minneapolis, MN) was added to the medium to incubate with the cells for 15 min. After that, the medium was aspirated and the cells were washed twice with cold PBS. The cells were lysed in cold lysis buffer (25 mM HEPES, 150 mmol/L sodium chloride, 1% (v:v) Triton 100, 1% (v:v) protease inhibitor (Sigma Chemicals), 5 mmol/L EDTA) for 5 min on ice and collected in a microfuge tube. The crude lysate was passed through 26 ½ G needle and cleared by centrifugation at 14,000g for 10 min at 4°C. The protein concentration was measured by the Bio-Rad protein assay according to the manufacturer's protocol (Bio-Rad Laboratories).
For the Western blot analysis, 50 μg of protein was loaded onto a 15% Tris-HCl gel. Separated proteins were electrotransferred to PVDF membranes and blocked in Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk for 1 hour at room temperature. Membranes were incubated with rabbit anti-human NF-κB inhibitor protein κB (IκB)-α (sc-203) (Santa Cruz, CA) at a dilution of 1:500 overnight at 4°C. Goat anti-rabbit IgG-Horseradish Peroxidase (sc 2030) (Santa Cruz, CA) was used as the second antibody. Protein was visualized and analyzed using a ChemiDoc XRS (Bio-Rad Laboratories) chemiluminescent detection and imaging system. After striping the membrane, monoclonal antibody to β-actin (ab 6276) (Abcam Inc., Cambridge, MA) was applied as loading control.
Induction of apoptosis by GTPs
To compare the capacity of 4″-MeEGCG to EGCG in inducing apoptosis, LNCaP cells were allowed to grow to 50-60 percent confluency in 6-well plates and treated with 40 or 80 μmol/L of EGCG or 4″-MeEGCG in fresh complete RPMI 1640 medium for 24, 48, or 72h. 50 units/ml of catalase was added to the medium prior to EGCG and 4″-MeEGCG. Following treatment, the cells were harvested and Western blot performed as described for NF-κB using the rabbit anti-human cleaved caspase-3 (Cat. No 9661, Santa Cruz, CA) which targets the activated caspase-3 fragments at a dilution of 1: 200.
Statistical analysis
SPSS (Version 17.0, Chicago, IL) was used for statistical analyses. Mean value, median, and standard deviation (SD) were calculated using descriptive statistics. Comparison of means was performed by two independent samples t-test, or one-way analysis of variance with Tukey's posttest when data were normally distributed. Otherwise, the Kruskal– Wallis test or Wilcoxon rank-sum test were used to compare the differences if data were not normally distributed. Differences were considered significant if P < 0.05.
Results
Clinical Characteristics and Compliance
There were no significant differences between the green tea and control groups in regards to mean Gleason score (6.6 ± 0.5 and 6.9 ± 0.3, respectively) and serum baseline prostate-specific antigen concentration (7.2 ± 5.1 vs. 8.8 ± 5.7 μg/L, respectively). The mean duration of the intervention in the green tea and the control group was 25 and 27 days, respectively, with an average compliance of 94.5 ± 3.0% and 93.4% for the tea and water control groups. No adverse events were reported in the green tea or control group and there was no liver toxicity as measured by pre and post-intervention serum levels of alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase (data not shown).
GTPs and metabolites in human prostate tissue and urine
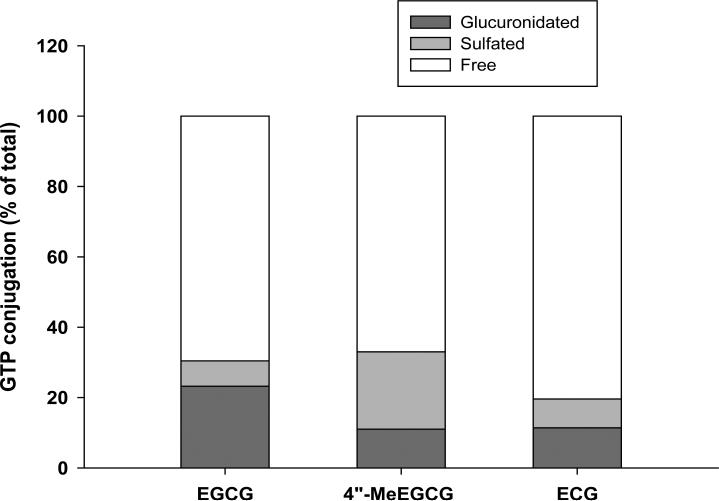
EGCG was the most abundant polyphenol in the green tea leaves and brewed green tea preparations used in the clinical trial (Table 1). The recovery rates for tissue analysis using solid phase extraction for EGC, EC, EGCG, 4″-MeEGCG, and ECG were 65%, 69%, 56%, 56%, and 52%, respectively and in urine 82%, 86%, 74%, 71%, and 75%, respectively. GTPs were found in prostate tissue of all the subjects in the green tea group but with considerable individual variation in the concentration (Table 2). An average of 48% of total EGCG was found in methylated form (4″-MeEGCG) in the prostate of subjects in the green tea group (Tables 2). No GTPs were detected in the control group either in prostate tissue or in urine (data not shown). In the prostate tissue, EGCG was mainly found in the free form (> 60%) along with a small portion being glucuronidated and/or sulfated (Figure 2). EGC and methyl EGC were found in urine throughout the study in all of the 8 participants in the green tea group (Table 3). An average of 61% of EGC was found in methylated form in the urines collected at the end of the study (Table 3). Sulfated and a small amount of glucuronidated methyl EC was found in the urine by LC/MS/MS analysis with peak areas similar to that of EC. The identities of the methylated EC will be addressed when methyl EC standards are available. In addition 100% of urinary EGC was found in conjugated form (data not shown).
Table 1.
Tea polyphenol content of the green tea used for the clinical intervention trial
| EGC* | EGCG | EC | ECG | Catechin | |
|---|---|---|---|---|---|
| Tea water (mg/L) | 202.3 ± 10.9 | 396.8 ± 28.3 | 52.1 ± 4.7 | 62.1 ± 4.6 | 7.9 ± 1.7 |
| Tea leaf (mg/g) | 24.1 ± 0.2 | 47.2 ± 1.4 | 6.2 ± 0.3 | 7.4 ± 0.3 | 0.9 ± 0.2 |
The tea was brewed using 1 tea bag in 240 ml of boiling water for 5 min.
Table 2.
Green tea polyphenol and methyl-metabolite concentration in the prostate tissues of men in the green tea intervention group (n=8)
| GTPs (pmol/g tissue) |
|||
|---|---|---|---|
| Mean ± SD | Median | Range | |
| EGCG | 42.1 ± 32.4* | 26.4 | 9.6 ~ 104.8 |
| 4”-MeEGCG | 38.9 ± 19.5* | 35.8 | 5.9 ~ 69.7 |
| ECG | 17.8 ± 10.1* | 17.2 | 0.2‡ ~ 31.9 |
Non-detectable values are represented by detection limit.
Compared with the control group, P < 0.01. In the control group GTP and methyl-metabolite concentrations were below detection limit.
Figure 2.
Glucuronide- and sulfate-conjugation rate of EGCG, 4”-MeEGCG, and ECG in prostate tissue of men in the tea intervention group expressed as percent of total of each GTP (means of 8 participants).
Table 3.
Concentration of tea polyphenols and methyl-metabolites in urine of participants in the green tea group (n=8)
| Concentration (μmol/g creatinine)‡ |
|||
|---|---|---|---|
| Time | EGC | EC | 4'-MeEGC |
| Baseline | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Week 1 | 8.2 ± 3.9* | 7.6 ± 3.8* | 9.3 ± 4.3* |
| Week 2 | 5.2 ± 4.2* | 3.4 ± 2.4* | 7.4 ± 3.7* |
| Week 3 | 7.8 ± 3.9* | 6.9 ± 3.4* | 12.4 ± 5.9* |
Data are presented as mean ± standard error.
Compared with either the control group or the baseline levels, P < 0.05.
Methylation of EGCG in LNCaP prostate cancer cells
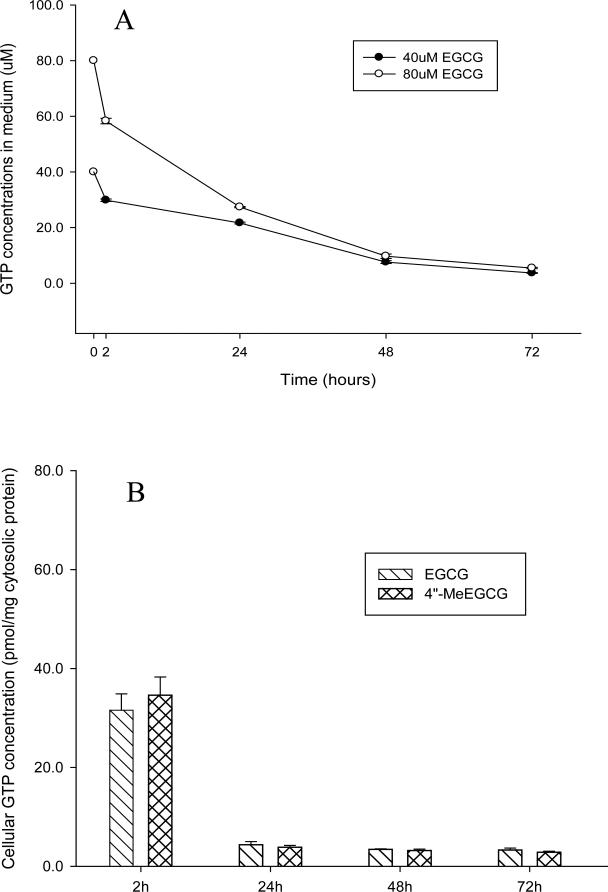
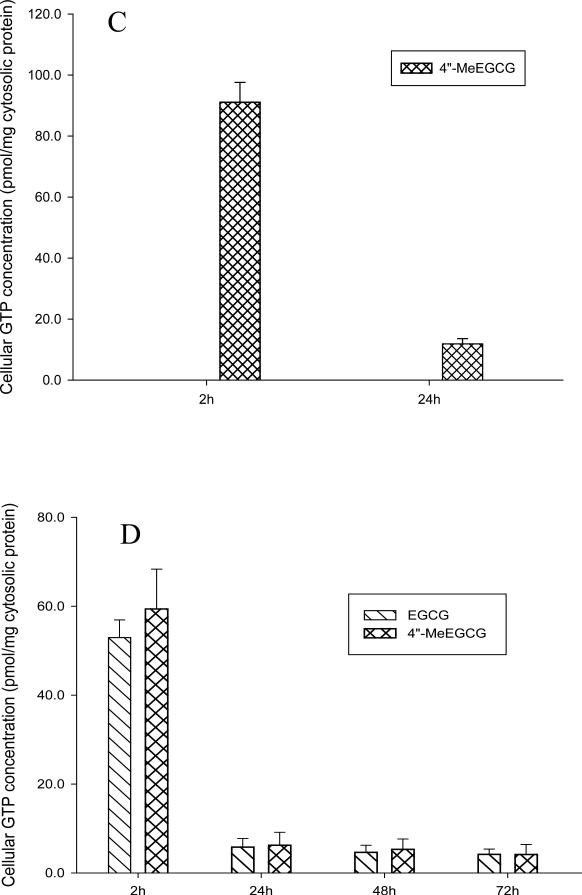
EGCG degraded quickly (> 25%) in cell culture medium during the first two hours (Figure 3A). 4″-MeEGCG showed the same degradation pattern in cell culture medium as EGCG (data not shown). When LNCaP cells were treated with EGCG alone, both EGCG and its metabolite 4″-MeEGCG were detected in the cells in equal concentrations (Figure 3B & C) and trace amounts of 4′, 4″-DiMeEGCG was detected by LC/MS/MS. The cellular content of GTPs decreased by 90% between 2 and 24 h, but remained stable between 24 and 72 h (Figure 3B & C). A dose-dependent increase of cellular EGCG and 4″-MeEGCG concentration was observed when the cells were treated with 40 and 80 μmol/L of EGCG (Figure 3B & C). When LNCaP cells were treated with 4″-MeEGCG alone there was no EGCG detectable in the cells at 2 and 24 h (Figure 3D) and an equivalent amount of 4″-MeEGCG was absorbed compared to the amount of intracellular EGCG following the EGCG treatment (Figure 3C & D).
Figure 3.
Cellular uptake and metabolism of EGCG in LNCaP cells. EGCG concentration in medium after treatment of LNCaP cells with 40 or 80 μmol/L of EGCG (A). Intracellular concentration of EGCG and 4”-MeEGCG after treatment with 40 μmol/L of EGCG (B), 80 μmol/L of EGCG (C) or 80 μmol/L of 4”-MeEGCG (D).
Inhibition of cell growth by EGCG or 4″-MeEGCG treatment
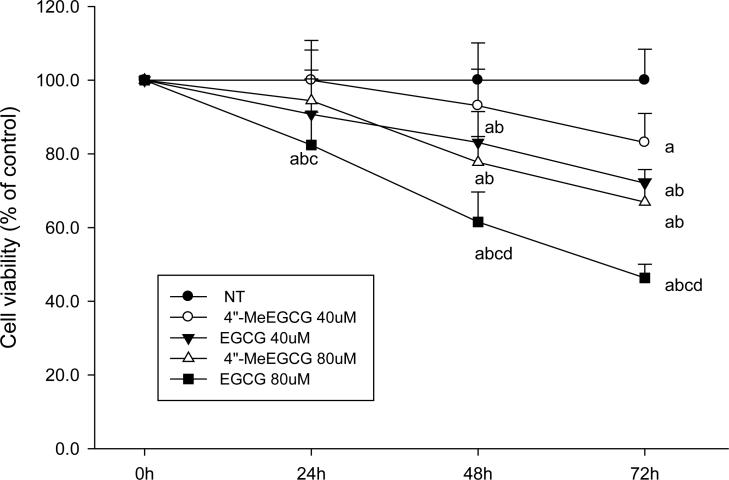
EGCG and 4″-MeEGCG treatment of LNCaP cells was associated with a dose- and time-dependent decrease of cell viability as compared to the vehicle treated control group (P < 0.05), Relative to the vehicle treated control group, at 72 hours the cell proliferation was inhibited by 16.9 ± 7.9% (in mean ± SD) after treatment with 40 μmol/L of 4″-MeEGCG compared to 28.2 ± 3.7% after treatment with the same concentration of EGCG, and an inhibition of 33.4 ± 6.3% compared to 53.6 ± 3.7% was observed after treatment with 80 μmol/L of 4″-MeEGCG and EGCG, respectively (Figure 4).
Figure 4.
Cell viability was inhibited by EGCG or 4”-MeEGCG. LNCaP cells were incubated with EGCG or 4”-MeEGCG (40 or 80 μmol/L) for different time points. Cell viability was detected by trypan blue exclusion assay. The superscript letters represent significant difference between groups (P<0.05): a compared to vehicle control; b compared to 40 μmol/L of 4”-MeEGCG treatment; c compared to 40 μmol/L EGCG treatment; d compared to 80 μmol/L 4”-MeEGCG treatment. Error bars represent standard deviation.
Inhibition of NF-κB activation
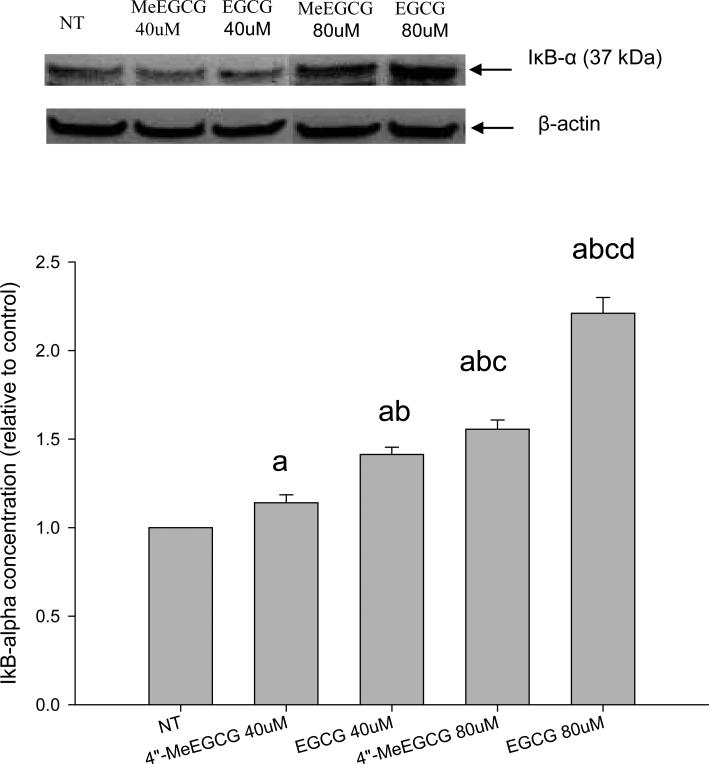
The activation of NF-κB, represented by decreased levels of IκB-α protein, was significantly inhibited by both EGCG and 4″-MeEGCG; however, the inhibition was significantly lower when using 4″-MeEGCG (P < 0.05). Relative to the vehicle treated control group, NF-κB activation was inhibited by 14.1 ± 4.5% (in mean ± SD) after treatment with 40 μmol/L of 4″-MeEGCG compared to 41.3 ± 4.0% after treatment with the same concentration of EGCG, and an inhibition of 55.5 ± 5.3% compared to 121.1 ± 8.9% was observed after treatment with 80 μmol/L of 4″-MeEGCG and EGCG, respectively (Figure 5).
Figure 5.
Inhibition of NF-κB activation by EGCG or 4”-MeEGCG. LNCaP cells were incubated with 40 or 80 μmol/L of EGCG or 4”-MeEGCG for 24h. NF-κB was stimulated by treatment with 10 ng/mL of TNF-α for 15 min. The IκB-α protein was determined by Western blotting. The superscript letters represent significant difference between groups (P<0.05): a compared to vehicle control; b compared to 40 μmol/L of 4”-MeEGCG treatment; c compared to 40 μmol/L of EGCG treatment; d compared to 80 μmol/L of 4”-MeEGCG treatment. Error bars represent standard deviation.
Induction of apoptosis
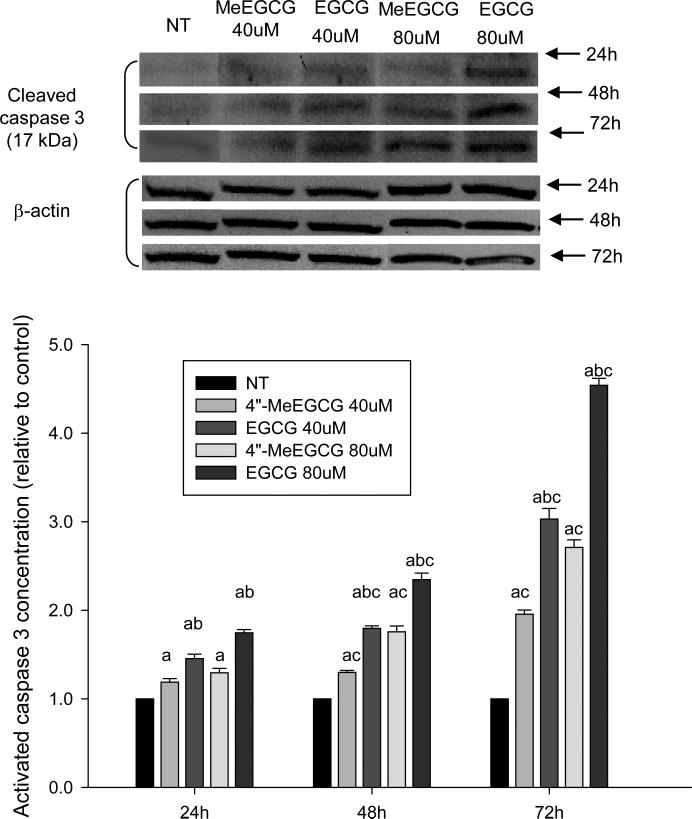
Both EGCG and 4″-MeEGCG induced apoptosis in LNCaP cells in a dose- and time-dependent manner as determined by caspase 3 protein expression. Relative to the control group, at 72 hours caspase 3 protein expression was induced by 95.6 ± 4.7% (in mean ± SD) after treatment with 40 μmol/L of 4″-MeEGCG compared to 203.2 ± 12.1% by the same concentration of EGCG, and an induction of 171.0 ± 8.5% compared to 353.9 ± 7.9% was observed after treatment with 80 μmol/L of 4″-MeEGCG and EGCG, respectively (Figure 6).
Figure 6.
Induction of apoptosis by EGCG or 4”-MeEGCG. LNCaP cells were incubated with 40 and 80 μmol/L of EGCG or 4”-MeEGCG for different time points. The protein concentration of activated caspase 3 was determined by Western blotting. The superscript letters represent significant difference between groups (P<0.05): a compared to vehicle control; b compared to 4”-MeEGCG treatment at the same dose level; c compared to the previous time point. Error bars represent standard deviation.
Discussion
The bioavailability and biotransformation of green tea appears to play a key role in the biological activity of GTPs in target tissues (22, 27). The major biotransformation affecting EGCG appears to be O-methylation by COMT (23). Prior studies from our laboratory demonstrated that GTPs are present in the human prostate after the consumption of 6 cups of tea daily (28). However our current study is the first to demonstrate that in prostate tissue collected from participants consuming 6 cups of green tea daily, 48% of EGCG was present in the methylated form as 4″-MeEGCG with a majority of both EGCG and 4″-MeEGCG in the free form (non-glucuronidated/sulfated). A different methylated EGCG metabolite (4′, 4″-DiMeEGCG) was found in rat and mouse tissue by Meng et al. after green tea or EGCG treatment (22), which may reflect a species variation. In addition the presence of 4′, 4″-DiMeEGCG may depend on the concentration of EGCG since we only found it in trace amounts in cell culture extracts but not in human prostate tissue extracts. In the current study none of the non-gallated GTPs including EGC and EC or their metabolites were found in the prostate tissue. This may be due to the extensive glucuronidation and sulfation of the non-gallated GTPs during intestinal absorption and liver metabolism leading to enhanced urinary excretion (21, 29). Our findings confirmed that the majority of GTPs excreted in urine were present in conjugated form. In addition about equal amounts of urinary GTPs were found in methylated form in the present study supporting other publications (30), which may affect the total methylation status of the body.
The findings of a considerable amount of methyl EGCG in the human prostate led us to investigate whether EGCG methylation occurs in the prostate or whether EGCG is absorbed in methylated form. Studies of other investigators using liver homogenates demonstrated in vitro that EGCG was methylated by liver cytosolic COMT to 4″-MeEGCG and 4', 4″-diMeEGCG and EGC was methylated to 4'-MeEGC (23). However, only trace amounts of 4″-MeEGCG has been reported in the plasma after green tea consumption (22, 31), which may not constitute a major source for 4″-MeEGCG in tissues. In the present study we found that treatment of cultured prostate cancer cells with EGCG resulted in methylation of 50 percent of EGCG within cells after 2h. However no demethylation of 4″-MeEGCG was observed in cultured LNCaP cells. These results indicate that methylation of EGCG may occur in prostate tissue by cytosolic COMT.
The catechol structure of polyphenols plays a critical role in their antioxidant activity (20). Therefore, it is of concern that conjugation of the catechol hydroxyl groups of tea polyphenols may result in altered chemopreventive activities. In the current study, we examined whether the anti-carcinogenic activity of EGCG was influenced by methylation at the 4″-position of EGCG. The results revealed a significantly decreased activity of 4″-MeEGCG in inhibiting LNCaP cell viability. In addition, methylation of EGCG reduced the ability of EGCG to induce apoptosis significantly. EGCG has been shown to be an effective inhibitor of NF-κB (32, 33). Extracellular stimuli, such as reactive oxygen species and proinflammatory cytokine TNF-α, can trigger the activation of NF-κB by dissociation and degradation of the NF-κB inhibitor protein IκB (34). Once released NF-κB migrates to the nucleus to initiate the transcription of many genes promoting inflammation and suppressing apoptosis (35-37). In the present study, the methylated form of EGCG decreased TNF-α induced NF-κB activation significantly compared to unmethylated EGCG. Similarly, other investigators demonstrated that methylation of EGCG and ECG decreased the proteasome-inhibitory activity in human leukemic Jurkat T cells, which may reduce their chemopreventive effect (38).
Our observation that methylation decreased the anti-carcinogenic activity of EGCG provides a potential explanation for the outcome of an epidemiological study in Asian-American women that demonstrated a significantly decrease in breast cancer risk only among those tea drinkers possessing at least one low-activity COMT allele (39). Common polymorphisms result in slow methylation and increased bioactivity of EGCG from green tea. Future green tea intervention studies will benefit from the evaluation of interindividual variations in methylation and conjugation processes, which may impact on the chemopreventive effects of tea polyphenols in prostate cancer and possibly other malignancies.
In summary, we have demonstrated that the predominant green tea polyphenols present in human prostate tissue following a green tea intervention are EGCG and methylated EGCG, and to a lesser extent EGC. Due to their similarity to human prostate tissue in performing methylation processes LNCaP prostate cancer cells provide an appropriate in vitro model to study GTP chemopreventive activity. Future tea intervention studies will need to address interindividual variation in methylation and conjugation processes, which may potentially modulate the chemopreventive effects of tea polyphenols in prostate cancer and possibly in other cancers based on common polymorphisms of the gene coding for COMT.
Acknowledgments
Grant support: NIH Grant RO1 CA116242 (S.M. Henning), NIH P50CA092131 (W.J. Aronson)
Footnotes
Conference Presentation: Presented at the American Association for Cancer Research (AACR) 2009 annual meeting held in Denver, CO.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- 1.Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail. 2007;9:152–9. doi: 10.1016/j.ejheart.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Kalfon L, Youdim MB, Mandel SA. Green tea polyphenol (-) -epigallocatechin-3-gallate promotes the rapid protein kinase C- and proteasome-mediated degradation of Bad: implications for neuroprotection. J Neurochem. 2007;100:992–1002. doi: 10.1111/j.1471-4159.2006.04265.x. [DOI] [PubMed] [Google Scholar]
- 3.Shen CL, Wang P, Guerrieri J, Yeh JK, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int. 2008;19:979–90. doi: 10.1007/s00198-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 4.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120:1344–50. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 5.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 6.Yokozawa T, Cho EJ, Hara Y, Kitani K. Antioxidative activity of green tea treated with radical initiator 2, 2'-azobis(2-amidinopropane) dihydrochloride. J Agric Food Chem. 2000;48:5068–73. doi: 10.1021/jf000253b. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Della-Fera M, Baile C. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes Res. 2005;13:982–90. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- 8.Hsu S, Farrey K, Wataha J, et al. Role of p21WAF1 in green tea polyphenol-induced growth arrest and apoptosis of oral carcinoma cells. Anticancer Res. 2005;25:63–7. [PubMed] [Google Scholar]
- 9.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–9. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 11.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohishi T, Kishimoto Y, Miura N, et al. Synergistic effects of (-)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett. 2002;177:49–56. doi: 10.1016/s0304-3835(01)00767-4. [DOI] [PubMed] [Google Scholar]
- 13.Jia XD, Han C. Chemoprevention of tea on colorectal cancer induced by dimethylhydrazine in Wistar rats. World J Gastroenterol. 2000;6:699–703. doi: 10.3748/wjg.v6.i5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao H, Hao X, Simi B, et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–9. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 15.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–5. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi N, Ohmori K, Shimazu T, et al. No association between green tea and prostate cancer risk in Japanese men: the Ohsaki Cohort Study. Br J Cancer. 2006;95:371–3. doi: 10.1038/sj.bjc.6603230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–7. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 18.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 19.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila Pa) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 20.Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol. 2009;83:11–21. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JS, Luo H, Wang P, et al. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46:232–40. doi: 10.1016/j.fct.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X, Sang S, Zhu N, et al. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol. 2002;15:1042–50. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab Dispos. 2003;31:572–9. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 24.Henning SM, Niu Y, Lee NH, et al. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–64. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 25.Yang GY, Liao J, Li C, et al. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–9. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 26.Lambert JD, Kwon SJ, Ju J, et al. Effect of genistein on the bioavailability and intestinal cancer chemopreventive activity of (-)-epigallocatechin-3-gallate. Carcinogenesis. 2008;29:2019–24. doi: 10.1093/carcin/bgn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–51. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 28.Henning SM, Aronson W, Niu Y, et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J Nutr. 2006;136:1839–43. doi: 10.1093/jn/136.7.1839. [DOI] [PubMed] [Google Scholar]
- 29.Lee MJ, Maliakal P, Chen L, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 30.Sang S, Lee MJ, Yang I, Buckley B, Yang CS. Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data-dependent acquisition. Rapid Commun Mass Spectrom. 2008;22:1567–78. doi: 10.1002/rcm.3546. [DOI] [PubMed] [Google Scholar]
- 31.Stalmach A, Troufflard S, Serafini M, Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53(Suppl 1):S44–53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 32.Chen PC, Wheeler DS, Malhotra V, Odoms K, Denenberg AG, Wong HR. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 2002;26:233–41. doi: 10.1023/A:1019718718977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui IA, Shukla Y, Adhami VM, et al. Suppression of NFkappaB and its regulated gene products by oral administration of green tea polyphenols in an autochthonous mouse prostate cancer model. Pharm Res. 2008;25:2135–42. doi: 10.1007/s11095-008-9553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldwin AS., Jr. Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syed DN, Afaq F, Kweon MH, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–82. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- 36.Ishida I, Kohda C, Yanagawa Y, Miyaoka H, Shimamura T. Epigallocatechin gallate suppresses expression of receptor activator of NF-kappaB ligand (RANKL) in Staphylococcus aureus infection in osteoblast-like NRG cells. J Med Microbiol. 2007;56:1042–6. doi: 10.1099/jmm.0.47029-0. [DOI] [PubMed] [Google Scholar]
- 37.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 38.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, Dou QP. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213:252–60. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 39.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–9. [PubMed] [Google Scholar]