Abstract
Background
Several cardiovascular disease (CVD) biomarkers sensitive to tobacco exposure have been identified, but how tobacco use cessation impacts them is less clear. We sought to investigate the effects of a smoking cessation program with an exercise intervention on CVD biomarkers in sedentary women.
Methods
This is a cohort study on a subsample of a 2×2 factorial randomized controlled trial (RCT) (exercise setting: home vs. facility; level of exercise counseling: prescription only vs. prescription and adherence counseling) conducted January 2004 through December 2007. The analyses were completed in October 2010. In the greater Boston area, 130 sedentary female smokers aged 19–55 completed a 15-week program. All participants received nicotine replacement therapy (transdermal patch) and brief behavioral counseling for 12 weeks. They all received an exercise prescription on a moderate intensity level. All exercise interventions lasted for 15 weeks, from 3 weeks precessation until 12 weeks postcessation. Main outcome measures were selected CVD biomarkers hypothesized to be affected by smoking cessation or exercise measured at baseline and 12 weeks postcessation.
Results
Independent of tobacco abstinence, improvement was seen in inflammation (white blood cells [WBC]), prothrombotic factor (red blood cells [RBC]), and cardiovascular fitness level (maximum oxygen consumption [Vo2max]). This suggests that even if complete abstinence is not achieved, reduction in tobacco exposure and increase in exercise can improve the cardiovascular risk profile. A significant decrease was seen for total cholesterol and the total cholesterol high-density lipoprotein cholesterol (HDL-C): ratio only among the abstainers. The heart rate was reduced among all participants, but this decrease was more profound among abstainers. A significant weight gain and body mass index (BMI) increase were observed among abstainers and those who relapsed. We also found an increase in hemoglobin A1c (Hb A1c), although significant only when the groups were combined.
Conclusions
A smoking cessation intervention including exercise reduced tobacco-induced cardiovascular damage selectively within 3 months.
Introduction
Tobacco use is a causal risk factor for cardiovascular disease (CVD), including acute myocardial infarction (MI), sudden cardiac death, stroke, aortic aneurysm, and peripheral vascular diseases. In addition, it adversely affects other risk factors, such as lipids, glucose metabolism, prothrombotic factors, and markers of infection and inflammation.1 Quitting reduces the risk of many diseases, especially coronary heart disease (CHD), a type of CVD that is reduced by 25%–50% within 1–2 years, followed by gradual risk reduction to that of nonsmokers 10–15 years after cessation.1 Compared to cancer morbidity and mortality, the risk reduction in CVD is relatively fast after cessation. However, morbidity is still a very distal outcome. This is a primary reason why identifying more proximal, reliable CVD biomarkers related to tobacco use has become increasingly important.2
Smoking produces a transient increase in the hemodynamic factors heart rate (HR) and blood pressure (BP) through activation of the sympathetic nervous system.3–5 Smoking cessation decreased BP in the first week postcessation in one study,6 and another study reported a significant decrease in BP and HR 12 weeks postcessation.7 Smoking enhances the prothrombotic state via increased blood fibrinogen concentration,8 platelet activity,9 hematocrit levels,10 red blood cell (RBC) mass,2 and mean corpuscular volume (MCV).7 C-reactive protein (CRP)2 and white blood cell (WBC) count,2,11,12 biomarkers of inflammation and infection, are significantly increased in smokers compared to nonsmokers.13 Further, although smoking may cause unfavorable lipid profiles, smoking cessation has not been shown consistently to impact the lipid profile, with the exception of an increase in high-density lipoprotein cholesterol (HDL-C).14
Sedentary lifestyle and inactivity are associated with many cardiovascular biomarkers such as decreased HDL-C and elevated low-density lipoprotein cholesterol (LDL-C), triglycerides, BP, and HR. Also, glucose metabolism is impacted by higher glycosylated hemoglobin (Hb A1c) and glucose. Prothrombotic fibrinogen and CRP also are reported to be high among sedentary individuals.15–17 Similar to smoking cessation, CVD risk decreases with increasing physical activity.15 Exercise decreases resting HR,18 BP,19 total cholesterol,20 LDL-C, total/HDL-C ratio,21 and triglycerides22 and increases HDL-C. It also promotes decrease in the hypercoagulative state and prothrombotic factors, such as hematocrit, RBC count,23 platelet activity,24 and fibrinogen.25,26 Glucose metabolism has been reported to improve by increased insulin sensitivity.27
A combination of smoking cessation and exercise should have a favorable impact on CVD biomarkers, although little is still known about how fast any of these changes can be observed. This study aimed to explore how a 15-week smoking cessation program containing an exercise intervention impacted the CVD risk profile among initially sedentary female smokers. We investigated whether CVD biomarkers changed between the baseline at 3 weeks precessation and the end of treatment (EOT) 12 weeks postcessation among women who abstained from smoking and those who relapsed. We hypothesized that within the 15-week program, significant improvement in several CVD biomarkers would be demonstrated.
Materials and Methods
Research design
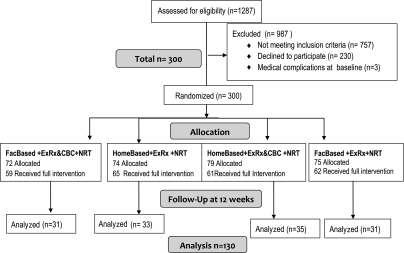
This was a cohort study on a subsample of a randomized controlled trial (RCT) sponsored by the National Institute of Drug Abuse (NIDA). This trial was approved by the Harvard Medical School Office for Research Subject Protection (August 2003) and by the Boston University School of Medicine Human Research Committee (May 2003). All participants provided informed consent. The study had four arms: (1) home-based exercise+exercise prescription only, (2) home-based exercise+exercise prescription+behavioral adherence counseling, (3) facility-based exercise+exercise prescription only, and (4) facility-based exercise+prescription+behavioral adherence counseling. The overall study design is illustrated in Figure 1, including recruitment, enrollment, and attrition. Data were collected between January 2004 and December 2007. The analyses for this study were conducted between 2008 and 2010.
FIG. 1.
Flow diagram of the study. CBC, cognitive-behavioral counseling; ExRx, exercise prescription; FacBased, facility-based exercise intervention; Home Based, home-based exercise intervention; NRT, nicotine replacement therapy.
Participants
Participants qualifying for the study (sedentary female, nonpregnant, nonexercising smoker 18–55 years of age and free of CVD) were recruited from the greater Boston area using radio, newspaper, subway, bulletin board, and television advertising. Female smokers were invited to participate in a smoking cessation study including free nicotine replacement therapy (NRT) (Nicoderm® patch), brief counseling, exercise prescription, plus additional treatment should they qualify. NRT was provided at no cost, and a payment of $25 was given to participants on completion of the week 12 postcessation visit. The main exclusion criteria were current involvement in exercise of at least moderate intensity more than once per week, an average consumption rate of fewer than five cigarettes per day (CPD), active and severe psychiatric illness, a history of serious CVD or insulin-dependent diabetes mellitus (IDDM), or skin condition contraindicating patch use. Information about exclusion criteria was obtained from a telephone medical screen and a baseline clinical diagnostic graded exercise test (GXT).
A total of 300 participants were randomized into the study at baseline. Fifty-four (18%) did not complete their prequit visit, which took place 3 weeks after baseline. The prequit visit included detailed information on the quitting protocol and starting with NRT, plus assessment of prequit cotinine and carbon monoxide (CO) as well as basic vital signs, such as HR and BP. Of the remaining 247 (82.3% of 300) participants 117 (47.4% of 247) had dropped out or did not come to the 12-week postcessation (i.e. EOT) visit and are not included in our current analysis. These analyses were based on the 130 women who came to the prequit visit, completed the treatment, and came to their EOT assessment.
Intervention
NRT and brief behavioral counseling
All participants were provided with a standard treatment for tobacco dependence (NRT and brief behavioral counseling) as recommended by the clinical guidelines.28 NRT guidance included detailed instructions on how to administer the patch and wearing a patch for the first 8 weeks that delivers 21 mg of nicotine over 24 hours. Participants wore 14-mg/24-hour patches during weeks 9 and 10 and 7-mg/24-hour patches in weeks 11 and 12, being instructed to continue the NRT despite any smoking slips. Brief counseling, including practical advice, social support, and guidance for dealing with withdrawal symptoms, was conducted in the standard manner for all participants for approximately 10 minutes at each visit. This included a total of seven sessions during the 15-week period.
Exercise
As a behavioral adjunct all participants were given an exercise prescription (intensity, duration, frequency, and modality of exercise) based on their baseline GXT results using a Bruce protocol.29 The maximum oxygen consumption (Vo2max) derived from indirect calorimetry served as our primary measure of aerobic power and common marker of cardiorespiratory fitness. Values obtained from the GXT were used to define the intensity of the exercise prescription (moderate intensity exercise=60%–80% of maximal HR). The main modality recommended was walking or running. In two study arms, the participants were assigned to additional exercise adherence counseling (as a combination with home-based or facility-based exercise), whereas in two other arms, participantes were assigned to the exercise prescription only (again as a combination with home-based or facility-based exercise).
Measures
Outcome variables
Blood biomarkers
Altogether, 13 biomarkers were analyzed: CRP (mg/L), WBC (1000/μL), and RBC counts (10/μL), MCV(fL), mean corpuscular hemoglobin (MCH) (pg), platelets (1000/μL), fibrinogen (mg/dL), Hb A1c(%) and lipids (mg/dL), including triglycerides, total cholesterol, HDL-C, LDL-C, and total/HDL-C ratio. These biomarkers were measured from blood drawn at baseline and week 12. The blood samples were sent to Quest Diagnostics, Inc. for analysis.
Anthropometric measures
Body weight measured at each visit and height measured at the baseline visit were used for calculation of BMI.
Hemodynamic measures
HR and BP were measured by the DynaPulse® 5000AUTO.30 BP was always measured before any engagement to exercise. Three readings were taken while the participant was sitting; the first reading was disregarded, and the mean of the second and third readings was recorded.
Cardiorespiratory fitness
We assessed aerobic capacity (Vo2max) with GXT at the baseline and at 12 weeks postcessation.
Tobacco abstinence and use
Abstinence from cigarettes with biochemical verification was assessed immediately after quitting, and then at 1, 2, 4, 8, and 12 weeks postcessation. We used the prolonged abstinence with grace period (a period immediately after the quit date in which continued smoking is not counted as a failure) as the definition of relapse (treatment failure), as suggested by the smoking cessation literatue.31 Relapse was defined as any smoking on 7 consecutive days or smoking at least once each week over 2 consecutive weeks; the latter part of this definition is included to count those who smoke on a regular but less than daily basis as a failure. Self-reported abstinence was biochemically verified by CO determination, a reliable measure of tobacco exposure.32 The CO levels were measured at all precessation and postcessation visits. At postcessation visits, participants with CO values (expired−ambient) >8 ppm were classified as smokers.33 In addition, we recorded self-reported cigarette consumption during the 12-week postcessation period.
Intervention adherence
Adherence to exercise was assessed by the Godin Leisure-Time Exercise Questionnaire34 and reported as a continuous variable (frequency of weekly exercise). Adherence to NRT was assessed by recording the number of days each participant reported wearing a patch for a minimum of 16 hours.
Baseline characteristics
Most of these data were obtained using a semistructured questionnaire commonly applied in cessation trials, such as our previously published RCT studies.35–37 Among sociodemographics, we included age, race, education, income level, and marital status, which are known predictors of successful smoking cessation.36,38 Age of initiation, years of smoking, number of pack-years, number of quit attempts, longest quit attempt, number of other smokers in the household, and nicotine dependence measured by the Fagerström Test for Nicotine Dependence (FTND)39 and by prequit CO level were assessed as smoking-related variables. Finally, because accelerated nicotine metabolism in women is caused by estrogen,40,41 we considered menopausal status and use of hormone replacement therapy (HRT) or use of hormonal contraceptives.
Statistical analyses
The analyses were performed using Statistica version 6.1 for Windows.42 First, in order to assess any differences between the 130 participants included in the study vs. the 170 not included, we compared the variables related to CVD health assessed at baseline. Second, differences in baseline characteristics between the abstainers and relapsers were tested by chi-square tests for categorical variables and t tests for continuous variables. Third, we examined the effects of tobacco abstinence status and the time engaged in a smoking cessation program combined with NRT and exercise on CVD biomarkers at 12 weeks postcessation. All the outcome variables were continuous; hence, we conducted a series of a mixed design (abstinence status by time) analyses of variance (ANOVAs). We first conducted the ANOVAs without any covariates (unadjusted results) and then ran the same analysis, first by adding nicotine intake (NRT adherence and number of cigarettes smoked during the 12 weeks of postcessation) and second by adding exercise adherence as a covariate. The p values for main effects and interaction effect were set at p=0.05. When post-hoc analyses were needed, we used the Tukey Honestly Significant Difference (HSD) for unequal sample sizes.
Results
Dropout analysis
To assess potential bias caused by dropout from the 12-week follow-up study, the results of an analysis comparing the outcome variables assessed at baseline among the 130 women included in the study vs. the 170 not included are shown in Table 1. No significant differences in the means were seen between the groups.
Table 1.
Comparison of Baseline Biomarker Values Between Subjects Included in Study and Those Excluded Because of Dropout from Follow-Up
| |
Included in study |
Excluded from study |
|
||
|---|---|---|---|---|---|
| Blood biomarker | n | Mean | n | Mean | p valuea |
| CRP (mg/L) | 121 | 3.5±0.8 | 156 | 2.8±0.7 | 0.20 |
| WBC (1000/μL) | 125 | 7.5±0.4 | 157 | 7.7±0.3 | 0.50 |
| RBC (106/μL) | 125 | 4.4±0.1 | 157 | 4.5±0.1 | 0.89 |
| MCV (fL) | 125 | 90±1.1 | 157 | 90.8±1.0 | 0.29 |
| MCH (pg) | 125 | 31±0.4 | 157 | 30.9±0.3 | 0.72 |
| Platelets (1000/μL) | 123 | 265±11 | 156 | 272±9.7 | 0.37 |
| Fibrinogen (mg/dL) | 120 | 309±12 | 153 | 310±11 | 0.90 |
| Hemoglobin A1c (%) | 123 | 5.42±0.2 | 158 | 5.41±0.1 | 0.94 |
| Triglycerides (mg/dL)b | 125 | 132±15 | 159 | 129±13 | 0.78 |
| Cholesterol (mg/dL) | 125 | 196±7.2 | 159 | 193±6.4 | 0.60 |
| HDL-C (mg/dL) | 125 | 56.4±2.5 | 159 | 56.7±2.2 | 0.87 |
| LDL-C (mg/dL) | 125 | 113±6.5 | 157 | 111±5.9 | 0.60 |
| Total/HDL-C (ratio) | 124 | 3.73±0.2 | 159 | 3.64±0.2 | 0.58 |
| Other measures | |||||
| Weight (kg) | 130 | 75.8±3.1 | 170 | 75.2±2.7 | 0.78 |
| BMI (kg/m2) | 130 | 28.9±1.1 | 170 | 28.8±1.0 | 0.86 |
| Heart rate (times/min) | 128 | 78.7±2.1 | 115 | 77.6±2.3 | 0.47 |
| BP, systolic (mg/dL) | 128 | 114±2.8 | 115 | 116±2.9 | 0.38 |
| BP, diastolic (mg/dL) | 128 | 74.1±1.6 | 115 | 74.2±1.7 | 0.92 |
| Vo2 max (ml/kg/min) | 129 | 24.8±1.5 | 166 | 24.6±1.1 | 0.85 |
p value determined by t test.
One case was excluded (no. 5156 change=−430).
BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein-cholesterol; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; RBC, red blood cell; Vo2max, maximum oxygen consumption; WBC, white blood cell.
Characteristics by abstinence
Of the 130 participants, 58 (44.6%) were abstinent, and 72 (55.4%) had relapsed. The distributions of baseline and intervention-related variables by abstinence status assessed at 12-week follow-up are presented in Table 2. There were no significant differences in sociodemographic characteristics, hormonal status, or intervention assignment between abstinent and relapsed women. Concerning smoking-related variables, the relapsed women had more pack-years of smoking, higher nicotine dependence (by FTND), and higher prequit CO levels, whereas the abstainers had better adherence to NRT.
Table 2.
Distributions of Selected Baseline or Prequit Variables and Intervention Variables by Smoking Status, Assessed at 12-Week Postcessation Follow-Up
| |
|
Abstainers n=58 |
Relapsers n=72 |
|
|---|---|---|---|---|
| Baseline variables | n | % or mean±SD | p | |
| Sociodemographics | ||||
| Age | 130 | 39.5±10.5 | 41.5±9.7 | 0.27a |
| Race | 0.78b | |||
| White | 100 | 75.9 | 77.8 | |
| Black | 25 | 19.0 | 19.4 | |
| Other | 5 | 5.2 | 2.8 | |
| Education | 128 | 0.21c | ||
| % of college graduates | 56.1 | 45.1 | ||
| Marital status | 0.31b | |||
| Single | 63 | 48.3 | 49.3 | |
| Married | 35 | 32.8 | 22.5 | |
| Separated/widowed | 31 | 19.0 | 28.2 | |
| Income | 126 | 0.81c | ||
| % of <$35,000/year | 36.8 | 34.8 | ||
| Employment | 128 | 0.10c | ||
| % employed | 80.7 | 67.6 | ||
| Hormonal status | 0.52b | |||
| Menopausal status and hormone use | ||||
| Premenopausal without contraceptives | 76 | 63.2 | 56.4 | |
| Premenopausal with contraceptives | 17 | 14.0 | 12.7 | |
| Menopausal without HRT | 33 | 22.8 | 28.2 | |
| Menopausal with HRT | 2 | – | 2.8 | |
| Intervention assignment | ||||
| % in facility-based exercise intervention | 130 | 56.9 | 40.3 | 0.06c |
| % in intensive exercise adherence counseling | 130 | 42.3 | 52.8 | 0.61c |
| Smoking-related variables | ||||
| Cigarettes per day | 130 | 15.3±6.6 | 17.2±6.8 | 0.12a |
| Age of smoking initiation | 130 | 18.0±5.9 | 17.3±4.5 | 0.41a |
| Total years of smoking | 130 | 19.8±10.4 | 23.1±10.1 | 0.07a |
| Pack-years | 130 | 16.2±12.8 | 21.0±13.1 | 0.04a |
| Carbon monoxide (CO) (ppm) (prequit) | 130 | 21.3±10.7 | 27.1±15.9 | 0.02a |
| Fagerström Test for Nicotine Dependence score (0–11) | 127 | 4.02±2.1 | 4.87±2.4 | 0.04a |
| Number of other smokers in household | 129 | 1.34±0.6 | 1.44±0.7 | 0.44a |
| Longest quit attempt <90 days | 130 | 34.5% | 45.8% | 0.19c |
| Additional baseline variables | ||||
| Body mass index (kg/m2) | 130 | 28.4±6.2 | 29.3±7.0 | 0.46a |
| Vo2max (mL/kg/min) | 130 | 25.4±7.2 | 24.6±7.6 | 0.54a |
| Confidence to quit score (0–4) | 129 | 3.10±0.8 | 3.11±0.9 | 0.95a |
| Treatment adherence variables | ||||
| Adherence to exercise (times/week) | 130 | 3.50±2.2 | 3.50±1.9 | 0.85a |
| Adherence to NRT (number of days) | 130 | 60.2±16.3 | 53.7±18.0 | 0.03a |
| Days smoked during treatment | 130 | 1.2±1.55 | 43.5±28.5 | 0.00a |
| Cigarettes smoked during treatment | 130 | 2.05±3.38 | 305±379.42 | 0.00a |
p determined by t test.
p determined by chi-square test.
p determined by Fisher's exact test.
HRT, hormone replacement therapy; NRT, nicotine replacement therapy.
Changes in cardiovascular biomarkers
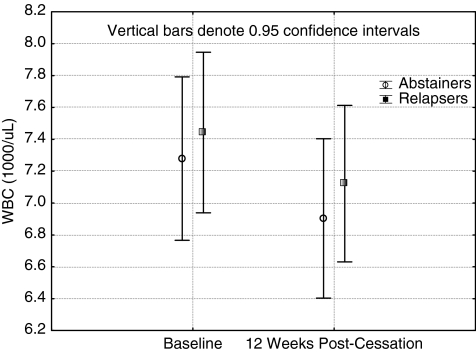
Tables 3 and 4 show the unadjusted means and standard deviations and the main and interaction effects for analysis without and with covariates (nicotine intake or exercise adherence adjusted, although not simultaneously). Among the inflammation biomarkers CRP and WBC, the only significant finding was an unadjusted time effect in WBC. During the treatment period, the WBC values fell significantly. Regarding prothrombotic factors, a time effect was found for RBC for unadjusted values, and it showed a decrease. Interestingly, MCV and platelets had a significant time effect only when nicotine intake was added as a covariate. The direction of the change was opposite what was hypothesized; that is, it did increase. MCH and fibrinogen did not show any significant results, with the exception that nicotine intake (number of cigarettes smoked during treatment) was a significant covariate. Hb A1c showed a consisted time effect in all three analysis. It increased with time, with the smallest effect in a condition when exercise adherence was included as a covariate. Tukey HSD for unequal sample sizes indicated that there was no statistically significant increase (time effect) for either the abstainers or relapsers alone (p=0.21 and 0.18, respectively), only when the groups were combined.
Table 3.
Changes in Cardiovascular Biomarkers During Exercise and Smoking Cessation Intervention by Abstinence Status, Including Time and Abstinence Main Effects and Time×Abstinence Interactions
| |
|
Abstainers (n=58) |
Relapsers (n=72) |
|
|
|
||
|---|---|---|---|---|---|---|---|---|
| N | Baseline | Week 12 | Baseline | Week 12 | Time effect | Abstinence effect | Time×abstinence interaction | |
| CRP (mg/L) | 114 | 2.2±3.1 | 2.7±3.1 | 4.4±6.8 | 3.7±6.7 | Unadjusted | ||
| F(1,112)=0.1, p=0.73 | F(1,112)=2.8, p=0.09 | F(1,112)=3.7, p=0.06 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=0.2, p=0.67/F(1,110)=0.8, p=0.36) | ||||||||
| F(1,110)=0.3, p=0.56 | F(1,110)=3.5, p=0.06 | F(1,110)=2.3, p=0.13 | ||||||
| Adjusted for exercise adherence: (F(1,111)=3.3, p=0.07) | ||||||||
| F(1,111)=0.6, p=0.43 | F(1,111)=2.4, p=0.12 | F(1,111)=3.5, p=0.06 | ||||||
| WBC (1000/μL) | 114 | 7.3±1.9 | 6.9±1.7 | 7.4±2.0 | 7.1±2.0 | Unadjusted | ||
| F(1,112)=5.8, p=0.02 | F(1,112)=0.3, p=0.56 | F(1,112)=0.04, p=0.85 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=0.1, p=0.78/F(1,110)=0.2, p=0.67) | ||||||||
| F(1,110)=0.1, p=0.81 | F(1,110)=0.04, p=0.84 | F(1,110)=0.6, p=0.45 | ||||||
| Adjusted for exercise adherence: (F(1,111)=0.0, p=0.98) | ||||||||
| F(1,111)=0.0, p=0.94 | F(1,111)=0.3, p=0.56 | F(1,111)=0.0, p=0.94 | ||||||
| RBC (106/μL) | 114 | 4.4±0.3 | 4.3±0.3 | 4.5±0.5 | 4.4±0.4 | Unadjusted | ||
| F(1,112)=20, p<0.001 | F(1,112)=0.4, p=0.54 | F(1,112)=0.2, p=0.63 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=4.6, p=0.03/F(1,110)=1.1, p=0.29) | ||||||||
| F(1,110)=0.2, p=0.66 | F(1,110)=0.5, p=0.49 | F(1,110)=0.2, p=0.63 | ||||||
| Adjusted for exercise adherence: (F(1,111)=0.5, p=0.47) | ||||||||
| F(1,111)=0.7, p=0.39 | F(1,111)=0.3, p=0.58 | F(1,111)=0.1, p=0.73 | ||||||
| MCV (fL) | 114 | 90.5±4.3 | 90.1±4.9 | 89.4±9.3 | 90.3±6.0 | Unadjusted | ||
| F(1,112)=0.2, p=0.62 | F(1,112)=0.2, p=0.66 | F(1,112)=1.7, p=0.20 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=2.7, p=0.10/F(1,110)=7.2, p=0.008) | ||||||||
| F(1,110)=5.5, p=0.02 | F(1,110)=0.6, p=0.43 | F(1,110)=0.4, p=0.53 | ||||||
| Adjusted for exercise adherence: (F(1,111)=3.5, p=0.06) | ||||||||
| F(1,112)=0.6, p=0.45 | F(1,112)=0.1, p=0.76 | F(1,112)=1.5, p=0.21 | ||||||
| MCH (pg) | 114 | 30.7±1.7 | 30.7±1.8 | 30.7±2.6 | 30.8±2.4 | Unadjusted | ||
| F(1,112)=0.4, p=0.50 | F(1,112)=0.0, p=0.90 | F(1,112)=0.1, p=0.71 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=2.3, p=0.12/F(1,110)=5.0, p=0.03) | ||||||||
| F(1,110)=0.7, p=0.39 | F(1,110)=0.1, p=0.76 | F(1,110)=0.7, p=0.41 | ||||||
| Adjusted for exercise adherence: (F(1,110)=3.3, p=0.07) | ||||||||
| F(1,112)=0.7, p=0.39 | F(1,112)=0.1, p=0.79 | F(1,112)=0.1, p=0.74 | ||||||
| Platelets (1000/μL) | 112 | 264±66 | 266±65 | 259±59 | 264±70 | Unadjusted | ||
| F(1,110)=0.6, p=0.45 | F(1,110)=0.1, p=0.76 | F(1,110)=0.1, p=0.77 | ||||||
| Adjusted for nicotine intakea: (F(1,108)=0.04, p=0.84/F(1,108)=0.04, p=0.95) | ||||||||
| F(1,108)=4.2, p=0.04b | F(1,108)=0.1, p=0.72 | F(1,108)=0.02, p=0.90 | ||||||
| Adjusted for exercise adherence: (F(1,109)=1.8, p=0.18) | ||||||||
| F(1,109)=0.1, p=0.79 | F(1,109)=0.04, p=0.84 | F(1,109)=0.1, p=0.73 | ||||||
| Fibrinogen (mg/dL) | 109 | 303±65 | 304±64 | 312±73 | 298±62 | Unadjusted | ||
| F(1,07)=0.7, p=0.40 | F(1,107)=0.1, p=0.80 | F(1,107)=0.8, p=0.36 | ||||||
| Adjusted for nicotine intakea: (F(1,105)=0.04, p=0.84/F(1,105)=0.04, p=0.95) | ||||||||
| F(1,05)=0.01, p=0.90 | F(1,105)=0.00, p=0.95 | F(1,105)=1.5, p=0.22 | ||||||
| Adjusted for exercise adherence: (F(1,106)=0.01, p=0.91) | ||||||||
| F(1,06)=0.2, p=0.65 | F(1,106)=0.06, p=0.80 | F(1,106)=0.8, p=0.36 | ||||||
| Hemoglobin A1c (%) | 115 | 5.4±0.7 | 5.5±0.6 | 5.4±0.7 | 5.6±1.0 | Unadjusted | ||
| F(1,113)=7.8, p=0.006 | F(1,113)=0.04, p=0.83 | F(1,113)=0.0, p=0.98 | ||||||
| Adjusted for nicotine intakea: (F(1,111)=6.5, p=0.01/F(1,111)=0.0, p=0.98) | ||||||||
| F(1,111)=9.0, p=0.003 | F(1,111)=0.1, p=0.76 | F(1,111)=2.0, p=0.16 | ||||||
| Adjusted for exercise adherence: (F(1,112)=5.6, p=0.02) | ||||||||
| F(1,112)=4.2, p=0.04 | F(1,112)=0.0, p=0.96 | F(1,112)=0.0, p=0.98 | ||||||
| Triglycerides (mg/dL)c | 115 | 126±75 | 127±72 | 127±79 | 137±76 | Unadjusted | ||
| F(1,113)=0.6, p=0.43 | F(1,113)=0.2, p=0.63 | F(1,113)=0.5, p=0.47 | ||||||
| Adjusted for nicotine intakea: (F(1,111)=0.6, p=0.43/F(1,111)=0.0, p=0.96) | ||||||||
| F(1,113)=1.9, p=0.17 | F(1,113)=0.1, p=0.77 | F(1,113)=0.1, p=0.79 | ||||||
| Adjusted for exercise adherence: (F(1,112)=3.3, p=0.07) | ||||||||
| F(1,113)=4.2, p=0.04d | F(1,113)=0.1, p=0.71 | F(1,113)=0.4, p=0.54 | ||||||
| Cholesterol (mg/dL) | 116 | 200±39 | 194±39 | 187±37 | 191±40 | Unadjusted | ||
| F(1,114)=0.3, p=0.60 | F(1,114)=1.5, p=0.23 | F(1,114)=4.6, p=0.03 | ||||||
| Adjusted for nicotine intakea: (F(1,112)=0.6, p=0.45/F(1,112)=0.9, p=0.36) | ||||||||
| F(1,112)=0.3, p=0.60 | F(1,112)=2.0, p=0.16 | F(1,112)=2.6, p=0.11 | ||||||
| Adjusted for exercise adherence: (F(1,113)=0.2, p=0.66) | ||||||||
| F(1,114)=0.4, p=0.52 | F(1,114)=1.5, p=0.22 | F(1,114)=4.3, p=0.04 | ||||||
| HDL-C (mg/dL) | 116 | 57.4±15 | 59.1±14 | 55.8±14 | 56.1±15 | Unadjusted | ||
| F(1,114)=1.3, p=0.25 | F(1,114)=0.8, p=0.37 | F(1,114)=0.6, p=0.45 | ||||||
| Adjusted for nicotine intakea: (F(1,112)=4.1, p=0.04/F(1,112)=0.4, p=0.53) | ||||||||
| F(1,114)=0.7, p=0.39 | F(1,114)=0.0, p=0.96 | F(1,114)=0.1, p=0.76 | ||||||
| Adjusted for exercise adherence: (F(1,113)=8.8, p=0.004) | ||||||||
| F(1,114)=0.5, p=0.49 | F(1,114)=0.5, p=0.47 | F(1,114)=0.4, p=0.51 | ||||||
| LDL-C (mg/dL) | 114 | 117±38 | 110±36 | 105±34 | 106±34 | Unadjusted | ||
| F(1,112)=2.3, p=0.13 | F(1,112)=1.6, p=0.20 | F(1,112)=2.8, p=0.09 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=0.1, p=0.78/F(1,110)=1.9, p=0.17) | ||||||||
| F(1,112)=2.6, p=0.11 | F(1,112)=3.3, p=0.07 | F(1,112)=1.4, p=0.23 | ||||||
| Adjusted for exercise adherence: (F(1,110)=0.8, p=0.36) | ||||||||
| F(1,112)=0.08, p=0.78 | F(1,112) 1.8, p=0.18 | F(1,112)=2.7, p=0.10 | ||||||
| Total HDL-C (ratio) | 114 | 3.9±1.5 | 3.5±1.1 | 3.6±1.2 | 3.7±1.4 | Unadjusted | ||
| F(1,112)=2.0, p=0.16 | F(1,112)=0.01, p=0.91 | F(1,112)=5.5, p=0.02 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=3.0, p=0.08/F(1,110)=0.3, p=0.56) | ||||||||
| F(1,112)=0.6, p=0.43 | F(1,112)=0.5, p=0.46 | F(1,112)=3.0, p=0.09 | ||||||
| Adjusted for exercise adherence: (F(1,110)=4.9, p=0.03) | ||||||||
| F(1,112)=0.3, p=0.61 | F(1,112)=0.1, p=0.79 | F(1,112)=5.0, p=0.03 | ||||||
Adherence to NRT during treatment/total number of cigarettes smoked during treatment.
Time×NRT adherence interaction (F=5.5, p=0.02).
One case was excluded (no. 5156 change=−430).
Time×exercise adherence interaction (F=3.7, p=0.05).
Table 4.
Changes in Other Cardiovascular Risk Factors During Exercise and Smoking Cessation Intervention by Abstinence Status, Including Time and Abstinence Main Effects and Time×Abstinence Interactions
| |
|
Abstainers (n=58) |
Relapsers (n=72) |
|
|
|
||
|---|---|---|---|---|---|---|---|---|
| N | Baseline | Week 12 | Baseline | Week 12 | Time effect | Abstinence effect | Time×abstinence interaction | |
| Weight (kg) | 130 | 75.0±19 | 76.3±19 | 76.3±19 | 77.9±19 | Unadjusted | ||
| F(1,28)=21.4, p<0.001 | F(1,128)=0.2, p=0.66 | F(1,128)=0.3, p=0.61 | ||||||
| Adjusted for nicotine intakea: (F(1,110)=0.2, p=0.67/F(1,110)=0.8, p=0.36) | ||||||||
| F(1,110)=0.3, p=0.56 | F(1,110)=3.5, p=0.06 | F(1,110)=2.3, p=0.13 | ||||||
| Adjusted for exercise adherence: (F(1,111)=3.3, p=0.07) | ||||||||
| F(1,111)=0.6, p=0.43 | F(1,111)=2.4, p=0.12 | F(1,111)=3.5, p=0.06 | ||||||
| BMI (kg/m2) | 130 | 28.4±6.2 | 28.9±6.2 | 29.3±7.0 | 29.9±7.2 | Unadjusted | ||
| F(1,128)=10.7, p=0.001 | F(1,128)=1.2, p=0.28 | F(1,128)=0.4, p=0.53 | ||||||
| Adjusted for nicotine intakea: (F(1,126)=0.1, p=0.73/F(1,126)=0.6, p=0.45) | ||||||||
| F(1,126=0.2, p=0.67 | F(1,126)=0.5, p=0.46 | F(1,126)=2.7, p=0.10 | ||||||
| Adjusted for exercise adherence: (F(1,127)=4.1, p=0.04) | ||||||||
| F(1,127)=4.0, p=0.05 | F(1,127)=0.6, p=0.45 | F(1,127)=0.2, p=0.64 | ||||||
| Heart rate (times/min) | 128 | 79.7±11 | 73.6±9.5 | 78.3±14 | 76.7±12 | Unadjusted | ||
| F(1,126)=16.8, p<0.001 | F(1,126)=0.2, p=0.65 | F(1,126)=5.6, p=0.02 | ||||||
| Adjusted for nicotine intakea: (F(1,124)=0.1, p=0.72/F(1,124)=0.6, p=0.45) | ||||||||
| F(1,124)=0.2, p=0.67 | F(1,124)=0.5, p=0.46 | F(1,124)=2.7, p=0.10 | ||||||
| Adjusted for exercise adherence: (F(1,125)=1.9, p=0.17) | ||||||||
| F(1,125)=1.0, p=0.32 | F(1,125)=0.2, p=0.62 | F(1,125)=5.5, p=0.02 | ||||||
| BP, systolic (mg/dL) | 123 | 114±11 | 112±12 | 114±14 | 113±13 | Unadjusted | ||
| F(1,121)=2.6, p=0.11 | F(1,121)=0.1, p=0.78 | F(1,121)=0.4, p=0.44 | ||||||
| Adjusted for nicotine intakea: (F(1,119)=0.2, p=0.65/F(1,119)=0.0, p=0.98) | ||||||||
| F(1,119)=0.5, p=0.47 | F(1,119)=0.02, p=0.88 | F(1,119)=0.3, p=0.57 | ||||||
| Adjusted for exercise adherence: (F(1,118)=7.1, p=0.009) | ||||||||
| F(1,118)=0.0, p=0.92 | F(1,118)=0.0, p=0.88 | F(1,118)=0.5, p=0.47 | ||||||
| BP, diastolic (mg/dL) | 123 | 73.4±7.6 | 71.3±9.5 | 73.0±9.8 | 72.5±8.6 | Unadjusted | ||
| F(1,121)=3.5, p=0.06 | F(1,121)=0.1, p=0.80 | F(1,121)=1.5, p=0.22 | ||||||
| Adjusted for nicotine intakea: (F(1,119)=0.2, p=0.65/F(1,119)=0.0, p=0.99) | ||||||||
| F(1,119)=0.01, p=0.90 | F(1,119)=0.02, p=0.89 | F(1,119)=1.4, p=0.24 | ||||||
| Adjusted for exercise adherence: (F(1,20)=8.5, p=0.004) | ||||||||
| F(1,120)=0.4, p=0.53 | F(1,120)=0.01, p=0.92 | F(1,120)=1.4, p=0.23 | ||||||
| Vo2 max (mL/kg/min) | 122 | 25.7±7.2 | 27.8±8.0 | 24.6±7.7 | 26.0±8.5 | Unadjusted | ||
| F(1,119)=10.7, p=0.001 | F(1,119)=1.2, p=0.28 | F(1,119)=0.4, p=0.53 | ||||||
| Adjusted for nicotine intakea: (F(1,117)=0.2, p=0.63/F(1,117)=2.1, p=0.15) | ||||||||
| F(1,117)=0.02, p=0.89 | F(1,117)=0.05, p=0.80 | F(1,117)=0.5, p=0.49 | ||||||
| Adjusted for exercise adherence: (F(1,118)=3.6, p=0.06) | ||||||||
| F(1,118)=3.6, p=0.06 | F(1,18)=0.9, p=0.34 | F(1,18)=0.4, p=0.52 | ||||||
Adherence to NRT during treatment/total number of cigarettes smoked during treatment.
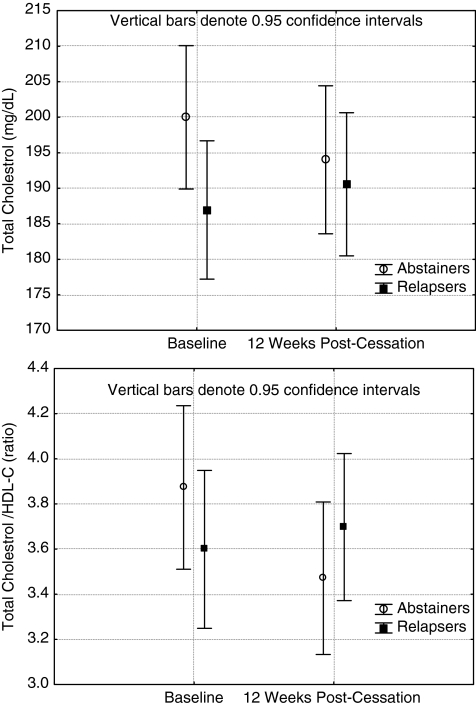
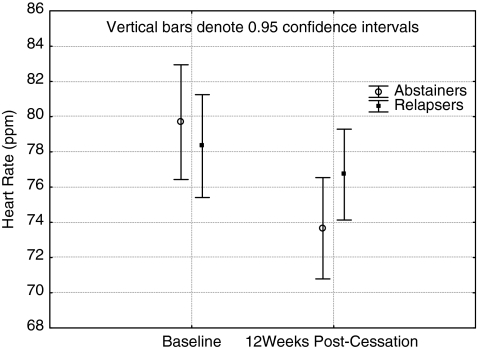
Regarding lipids, triglycerides increased, but this was only statistically significant when adjusting for exercise adherence. Abstinence status×time interaction effects were observed consistently in the analysis for total cholesterol and total cholesterol/HDL-C ratio. When adjusted for nicotine intake, the p values only approached significance (p-0.11 and 0.09, respectively. The interaction shows a favorable decrease among abstainers and an increase among relapsers (Figs. 2 and 3). Among other biomarkers, a time effect was observed in weight and BMI. They both increased significantly. Post hoc tests indicated that this increase was significant for both abstainers (p=0.04 for weight and p=0.03 for BMI) and for relapsers (p value for weight and BMI<0.001) (Table 4). When controlled for nicotine intake or exercise adherence (weight only), however, these effects where no longer significant. For hemodynamic variables, there were consistent abstinence status×time interaction effects for HR, reflecting a more profound decrease in HR among the abstinent participants (Fig. 4). Although no significant time or abstinence status interactions were observed for systolic and diastolic BP, exercise adherence had significant direct effects on them (Table 4). Finally, when assessing the cardiovascular fitness level, a significant time effect was observed when not adjusting for nicotine intake.
FIG. 2.
Changes in white blood cells (WBC) from baseline to week 12 among abstainers and relapsers.
FIG. 3.
Changes in total cholesterol and total cholesterol/high-density lipoprotein cholesterol (HDL-C) from baseline to week 12 among abstainers and relapsers.
FIG. 4.
Changes in heart rate from baseline to week 12 among abstainers and relapsers.
Discussion
This study aimed to explore how a 15-week smoking cessation program containing an exercise intervention and nicotine transdermal patch impacted the cardiovascular risk profile among initially sedentary female smokers. As in many previous treatment studies, both the attrition and relapse rates were high. Nearly 20% dropped out before their prequit visit, and about half of the participants who came to their prequit visit and made a quit attempt had relapsed by the end of the treatment. Clearly, there is a need for more effective treatments for female smokers.
The smoking cessation and exercise program of 15 weeks in duration had a favorable impact on several CVD risk factors. Independent of tobacco abstinence, improvement was seen in inflammation (WBC), prothrombotic factor (RBC), and cardiovascular fitness level (Vo2max). This suggests that even if complete abstinence is not achieved, reduction in tobacco exposure and increase in exercise can improve the cardiovascular risk profile. Regarding lipids, significant results were observed for total cholesterol and the total cholesterol/HDL-C ratio; a significant decrease was seen among abstainers, whereas a slight nonsignificant increase was detected among relapsers. For other lipids, no significant smoking status or time effects were found. Interestingly, heart rate was generally reduced among all, but this decrease was more profound among abstainers. As expected, there was a significant weight gain and BMI increase among all. We also observed a significant increase in Hb A1c.
Some earlier smoking cessation studies have shown a decrease in the inflammatory markers. In a study with NRT, the abstainers and smoking reducers had a greater reduction in WBC count after 6 months.43,44 In line with those earlier findings, we also observed a significant decrease in WBC count. Interestingly, the WBC count decreased in both abstainers and relapsers, and these results did not change even when controlled for nicotine intake or exercise adherence, suggesting that the combination of the cessation program that led to either complete abstinence or reduction in smoking had a beneficial effect on this biomarker. CRP has gained attention as a significant biomarker for cardiac events, and we did find an impact for tobacco abstinence status. There is clear evidence that lifetime tobacco exposure impacts postcessation CRP levels,45,46 although this biomarker may need more time to normalize than 3 months of abstinence, which was our follow-up. Additional reasons may be that although our smokers were sedentary, smoked an average of 16 cigarettes per day, and had relatively high BMI (mean 28.9), the average CRP at baseline (3.3) was not clearly elevated. It is also reflective of healthy middle-aged women with similar BMI and activity levels in the Boston area.47
Previous research has suggested that smoking reduction or cessation leads to decreased levels in the biomarkers of a hypercoagulable state or prothrombotic factors.7,48 We did not find strong evidence to support this among any of our biomarkers. It may be that our follow-up period was not long enough to detect these changes, as some of them were observed at 6 months postcessation.44
Among hemodynamic factors, HR decreased among the participants, but it was more profound among the abstainers than among those who relapsed. Our results support previous findings indicating HR normalization even if NRT is being used.49 Although no significant time or abstinence effects were observed for systolic and diastolic BP, exercise seemed to have important effects on both. Diastolic BP decreased almost significantly (p=0.11), the average decrease being 2.1 units for abstainers and 0.5 units for those relapsed. More importantly, when adjusting for exercise adherence, which had a significant impact on diastolic BP, the time effect for BP change became clearly nonsignificant. This means that exercise adherence had an important role here. A clinically important issue is that earlier literature reported that smoking cessation was associated with an increased incidence of hypertension.50 In relation to this evidence, our decreasing trend of BP, partly explained by exercise effect, seems to be encouraging.
Previous research on smoking cessation and lipid profile has consistently found an increase in HDL-C and triglycerides.44 We observed some increase in HDL-C among abstainers and an increase among relapsers in triglycerides. These differences did not reach statistical significance. However, both the total cholesterol and, even more promising, the total/HDL-C ratio improved among abstainers only, even after adjustment for exercise adherence or nicotine intake. This suggests that both smoking cessation and exercise had a favorable impact.
Hb A1c is a good indicator of glucose metabolism, reflecting processes of glucose tolerance for several weeks. In our analysis, no statistically significant increase was found among either abstainers or those who relapsed. This is encouraging, as immediate effects of smoking cessation may increase the risk for type 2 diabetes by elevated glucose and triglycerides levels.51 Unfortunately, we did not measure fasting glucose; because fasting glucose is a more sensitive measure,51 it is possible that we could have seen changes at follow-up if we had that outcome measured.
We observed a significant increase in weight and BMI, but this weight increase was relatively small, actually only on average 1.3 kg for abstainers and 1.6 kg for those relapsed. This is clinically clearly less than the average weight increase of 3.8 kg reported in women after smoking cessation.28 When adjusting the time effect for total nicotine intake during the treatment, such as NRT adherence, the time effect disappeared but the abstinence effect became significant. This leads us to suggest that NRT adherence among the abstainers was an important confounder. As a matter of fact, there is evidence that NRT can prevent weight gain to some extent.52 A similar trend was evident with BMI, in that although the time effect was significant, the average BMI increase was only 0.5 units in abstainers and 0.6 among those who relapsed. Here again, adjusting for nicotine intake attenuated the time effect. Further, the effect of exercise adherence seems to be important because time effect was attenuated toward less significance when adjusting for exercise. This leads us to acknowledge the fact that muscle mass weighs more than fat mass. Thus, it is possible that those abstainers who had high adherence to NRT and exercise gained some weight that was mostly due to increase of muscle mass. Unfortunately, we did not measure muscle mass in this study. It remains a question of longer follow-up to see if sustained abstinence or continued exercise or their interaction is responsible for long-term development in weight and BMI after smoking cessation.
Chiolero et al.53 reviewed the relationships among smoking, body weight, and insulin resistance. According to these authors, nicotine increases energy expenditure in the short term and could reduce appetite, explaining why smokers tend to have lower body weight than do nonsmokers and why smoking cessation is frequently followed by weight gain. However, the review suggests that smokers who smoke high number of cigarettes daily actually tend to have greater body weight than do nonsmokers or smokers who smoke only a few cigarettes daily. This is likely to reflect a clustering of risky behaviors conducive to weight gain, such as a low degree of physical activity, poor diet, and smoking. Other factors, such as weight cycling, could also be involved. In the context of the worldwide obesity epidemic and a high prevalence of smoking, greater risk of central obesity and insulin resistance among smokers, particularly female smokers in menopause, is a matter of major concern. Moreover, the previously mentioned risk factors are of high relevance in the target group of this intervention, as all women participating in this trial had two major risk factors, daily smoking and a sedentary lifestyle.
There are several limitations to our study. At baseline, every participant was engaged in at least two health-compromising behaviors identified as CVD risk factors.54,55 Hence, we are dealing with a subgroup of relatively high-risk women in terms of their CVD risk profile, and our intervention results can be generalized only among adult women who initially are daily cigarette smokers and physically inactive. As this trial recruited women from the Boston community and surroundings, however, our sample quite accurately represented sedentary female smokers as well middle-aged women in general in that metropolitan area.43
We recognize that our participants were exposed to nicotine via NRT during abstinence and via tobacco if relapsed. One may ask if NRT contributed to the results where we did not observe improvement among abstainers compared to relapsers. This question may be justified by the fact that nicotine may increase oxidative stress.56 However, nicotine has a very complex pharmacology.57 Long-term use of nicotine as a medication could have adverse cardiovascular effects, such as promoting angiogenesis and arteriogenesis,58 inducing endothelial cell dysfunction, and possibly inducing insulin resistance,57 as well as promoting lipolysis, which could contribute to adverse lipid profiles.59 Although nicotine may adversely affect cardiac output and its related components, its role in atherosclerosis remains controversial. Studies have reported that nicotine alone has caused no change, a decrease, or an increase in nitric oxide levels and that levels commonly found in a smoker have only a minor effect on initiating or proliferating lesion formation.60 Moreover, in our trial, exposure to NRT was for 3 months, a relatively short time in comparison to years of tobacco exposure.
There were 54 participants who completed the baseline visit but did not complete the prequit assessment. Attrition is a critical problem in clinical trials for substance use and dependence; high attrition rates are commonplace and often expected.37,61–63 Pretreatment attrition among smokers has begun to receive attention only recently.61,63 Factors associated with early attrition include younger age, lower socioeconomic status, and history of psychotropic medication use. Low motivation, weight concerns, and affective vulnerabilities, such as depression, also have been reported to predict early attrition. We examined these factors in our earlier exercise studies among women. In our first trial, we found that of the modifiable factors, feelings of guilt and weight concerns predicted attrition.37,61 In the present trial, we conducted similar analysis and found that depression was one of the strongest predictors of an early dropout (unpublished observations). The nonmodifiable factors, such as race (minority), lower education, and number of children at home, remain significant in both studies.
Finally, we investigated only how tobacco abstinence impacted these select CVD biomarkers. We did adjust for treatment adherence and nicotine intake but, given the small sample size, did not examine, for example, BMI changes, caloric intake, or other prospective mediating or moderating factors. Similarly, we assessed the change only at the end of the treatment, that is, 3 months postcessation. Additional studies are needed to measure biomarkers at more frequent intervals, with longer follow-up assessing patterns of changes.
In summary, our results suggest that in as short a time as 3 months, abstinence from or reduced consumption of cigarettes combined with an exercise intervention can reduce immediate tobacco-induced cardiovascular damage measured by such cardiovascular biomarkers as cholesterol and HR. In addition, the current program resulted in relatively low weight gain and triglyceride increases and a significant cardiovascular fitness level increase. Although it may not be clear how exercise or abstinence affects one particular biomarker, it is clear that overall, the impact of this smoking cessation program was more favorable that that of many other reported programs. Given that there is an increasing number of reports where smoking cessation may temporarily elevate the risk for type 2 diabetes,51 even smaller changes can be clinically significant and serve as a motivator for the participants.64
Acknowledgments
This work was supported by following grants: NIDA-12503 (T. Kinnunen), M01 RR00533/GCRC (Boston University Clinical and Translational Science Institute), the Paavo Nurmi Foundation (T. Korhonen), and the Office for Enrichment Programs at Harvard Medical School (A.G.). We thank Dr. Karen Freund for her medical and scientific advice and physicians at the Women's Health Unit and Women's Health Interdisciplinary Research Center, Boston University School of Medicine, for overseeing the graded exercise testing. We further thank Dr. Frank M. Perna, Ms. Donna Medaglia Terwal, and other STOP&STEP professionals for delivering the intervention and collecting the exercise-related data as well as all the women who volunteered in the study.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Bullen C. Impact of tobacco smoking and smoking cessation on cardiovascular risk and disease. Expert Rev Cardiovasc Ther. 2008;6:883–895. doi: 10.1586/14779072.6.6.883. [DOI] [PubMed] [Google Scholar]
- 2.Hatsukami DK. Benowitz NL. Rennard SI. Oncken C. Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine Tobacco Res. 2006;8:169–191. doi: 10.1080/14622200600576628. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB. Kannel C. Paffenbarger RS., Jr Cupples LA. Heart rate and cardiovascular mortality: The Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 4.Franklin SS. Larson MG. Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE. Haymond MW. Santiago JV. Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 6.Ward KD. Bliss RE. Vokonas PS. Garvey AJ. Effects of smoking cessation on blood pressure. Am J Cardiol. 1993;72:979–981. doi: 10.1016/0002-9149(93)91121-w. [DOI] [PubMed] [Google Scholar]
- 7.Hatsukami DK. Kotlyar M. Allen S, et al. Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest. 2005;128:2528–2537. doi: 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB. D'Agostino RB. Belanger AJ. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: Insights from the Framingham Study. Am H J. 1987;113:1006–1010. doi: 10.1016/0002-8703(87)90063-9. [DOI] [PubMed] [Google Scholar]
- 9.Fusegawa Y. Goto S. Handa S. Kawada T. Ando Y. Platelet spontaneous aggregation in platelet-rich plasma is increased in habitual smokers. Thromb Res. 1999;93:271–278. doi: 10.1016/s0049-3848(98)00184-4. [DOI] [PubMed] [Google Scholar]
- 10.Grines CL. Topol EJ. O'Neill WW, et al. Effect of cigarette smoking on outcome after thrombolytic therapy for myocardial infarction. Circulation. 1995;91:298–303. doi: 10.1161/01.cir.91.2.298. [DOI] [PubMed] [Google Scholar]
- 11.Horne BD. Anderson JL. John JM, et al. Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 12.Rohde LE. Hennekens CH. Ridker PM. Survey of C-reactive protein and cardiovascular risk factors in apparently healthy men. Am J Cardiol. 1999;84:1018–1022. doi: 10.1016/s0002-9149(99)00491-9. [DOI] [PubMed] [Google Scholar]
- 13.Yanbaeva DG. Dentener MA. Creutzberg EC. Wesseling G. Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K. Noguchi Y. Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: A meta-analysis. Prev Med. 2003;37:283–290. doi: 10.1016/s0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 15.Mora S. Cook N. Buring JE. Ridker PM. Lee IM. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillard TR. Sherman WM. Devor ST. Kirby TE. Osei K. Importance of aerobic fitness in cardiovascular risks in sedentary overweight and obese African-American women. Nurs Res. 2007;56:407–415. doi: 10.1097/01.NNR.0000299851.67676.34. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera de Leon A. Rodriguez-Perez Mdel C. Rodriguez-Benjumeda LM, et al. Sedentary lifestyle: Physical activity duration versus percentage of energy expenditure. Rev Espan Cardiol. 2007;60:244–250. [PubMed] [Google Scholar]
- 18.Foster C. Central circulatory adaptations to exercise training in health and disease. Clin Sports Med. 1986;5:589–604. [PubMed] [Google Scholar]
- 19.Fagard RH. Prescription and results of physical activity. J Cardiovasc Pharmacol. 1995;25(Suppl 1):S20–27. doi: 10.1097/00005344-199525001-00006. [DOI] [PubMed] [Google Scholar]
- 20.Halbert JA. Silagy CA. Finucane P. Withers RT. Hamdorf PA. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: A meta-analysis of randomized, controlled trials. Eur J Clin Nutr. 1999;53:514–522. doi: 10.1038/sj.ejcn.1600784. [DOI] [PubMed] [Google Scholar]
- 21.Fontana L. Villareal DT. Weiss EP, et al. the Washington University School of Medicine CALERIE Group. Calorie restriction or exercise: Effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol.Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 22.Kraus WE. Houmard JA. Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 23.Branch JD., 3rd Pate RR. Bourque SP. Convertino VA. Durstine JL. Ward DS. Exercise training and intensity does not alter vascular volume responses in women. Aviat Space Environ Med. 1999;70:1070–1076. [PubMed] [Google Scholar]
- 24.Wang JS. Jen CJ. Chen HI. Effects of exercise training and deconditioning on platelet function in men. Arterioscl Thromb Vasc Biol. 1995;15:1668–1674. doi: 10.1161/01.atv.15.10.1668. [DOI] [PubMed] [Google Scholar]
- 25.Imhof A. Koenig W. Exercise and thrombosis. Cardiol Clin. 2001;19:389–400. doi: 10.1016/s0733-8651(05)70224-1. [DOI] [PubMed] [Google Scholar]
- 26.Myint PK. Luben RN. Wareham NJ. Welch AA. Bingham SA. Khaw KT. Physical activity and fibrinogen concentrations in 23,201 men and women in the EPIC-Norfolk population-based study. Atherosclerosis. 2008;198:419–425. doi: 10.1016/j.atherosclerosis.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Mayer-Davis EJ. D'Agostino R., Jr Karter AJ, et al. Intensity and amount of physical activity in relation to insulin sensitivity: The Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–674. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 28.Fiore M. United States. Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence. Rockville, Md: U.S. Dept. of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- 29.Franklin BA. Whaley MH. Howley ET. Balady GJ. American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 6th. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 30.Brinton TJ. Walls ED. Chio SS. Validation of pulse dynamic blood pressure measurement by auscultation. Blood Pressure Monitoring. 1998;3:121–124. [PubMed] [Google Scholar]
- 31.Hughes JR. Keely JP. Niaura RS. Ossip-Klein DJ. Richmond RL. Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tobacco Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 32.Berlin I. Radzius A. Henningfield JE. Moolchan ET. Correlates of expired air carbon monoxide: Effect of ethnicity and relationship with saliva cotinine and nicotine. Nicotine Tobacco Res. 2001;3:325–331. doi: 10.1080/14622200110050400. [DOI] [PubMed] [Google Scholar]
- 33.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 34.Godin G. Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 35.Doherty K. Militello FS. Kinnunen T. Garvey AJ. Nicotine gum dose and weight gain after smoking cessation. J Consult Clin Psychol. 1996;64:799–807. [PubMed] [Google Scholar]
- 36.Garvey AJ. Kinnunen T. Nordstrom BL, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine Tobacco Res. 2000;2:53–63. doi: 10.1080/14622200050011303. [DOI] [PubMed] [Google Scholar]
- 37.Kinnunen T. Leeman RF. Korhonen T, et al. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine Tobacco Res. 2008;10:689–703. doi: 10.1080/14622200801979043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broms U. Silventoinen K. Lahelma E. Koskenvuo M. Kaprio J. Smoking cessation by socioeconomic status and marital status: The contribution of smoking behavior and family background. Nicotine Tobacco Res. 2004;6:447–455. doi: 10.1080/14622200410001696637. [DOI] [PubMed] [Google Scholar]
- 39.Heatherton TF. Kozlowski LT. Frecker RC. Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 40.Pauly JR. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci. 2008;13:505–516. doi: 10.2741/2696. [DOI] [PubMed] [Google Scholar]
- 41.Benowitz NL. Lessov-Schlaggar CN. Swan GE. Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Hill T. Lewicki P. Statistics methods and applications. Tulsa OK: StatSoft; 2007. [Google Scholar]
- 43.Eliasson B. Hjalmarson A. Kruse E. Landfeldt B. Westin A. Effect of smoking reduction and cessation on cardiovascular risk factors. Nicotine Tobacco Res. 2001;3:249–255. doi: 10.1080/14622200110050510. [DOI] [PubMed] [Google Scholar]
- 44.Haustein KO. Krause J. Haustein H. Rasmussen T. Cort N. Changes in hemorheological and biochemical parameters following short-term and long-term smoking cessation induced by nicotine replacement therapy (NRT) Int J Clin Pharmacol Ther. 2004;42:83–92. doi: 10.5414/cpp42083. [DOI] [PubMed] [Google Scholar]
- 45.Crook MA. Scott DA. Stapleton JA. Palmer RM. Wilson RF. Sutherland G. Circulating concentrations of C-reactive protein and total sialic acid in tobacco smokers remain unchanged following one year of validated smoking cessation. Eur J Clin Invest. 2000;30:861–865. doi: 10.1046/j.1365-2362.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 46.Hastie CE. Haw S. Pell JP. Impact of smoking cessation and lifetime exposure on C-reactive protein. Nicotine Tobacco Res. 2008;10:637–642. doi: 10.1080/14622200801978722. [DOI] [PubMed] [Google Scholar]
- 47.Mora S. Lee I-M. Buring JE. Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 48.Van Tiel E. Peeters PH. Smit HA, et al. Quitting smoking may restore hematological characteristics within five years. Ann Epidemiol. 2002;12:378–388. doi: 10.1016/s1047-2797(01)00282-4. [DOI] [PubMed] [Google Scholar]
- 49.Lewis MJ. Balaji G. Dixon H. Syed Y. Lewis KE. Influence of smoking abstinence and nicotine replacement therapy on heart rate and QT time-series. Clin Physiol Funct Imaging. 2010;30:43–50. doi: 10.1111/j.1475-097X.2009.00902.x. [DOI] [PubMed] [Google Scholar]
- 50.Janzon E. Hedblad B. Berglund G. Engstrom G. Changes in blood pressure and body weight following smoking cessation in women. J Intern Med. 2004;255:266–272. doi: 10.1046/j.1365-2796.2003.01293.x. [DOI] [PubMed] [Google Scholar]
- 51.Yeh HC. Duncan BB. Schmidt MI. Wang NY. Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: A cohort study. Ann Intern Med. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nordström BL. Kinnunen T. Utman CH. Garvey AJ. Long-term effects of nicotine gum on weight gain after smoking cessation. Nicotine Tobacco Res. 1999;1:259–268. doi: 10.1080/14622299050011381. [DOI] [PubMed] [Google Scholar]
- 53.Chiolero A. Faeh D. Paccaud F. Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Department of Health and Human Services (USDHHS) The Surgeon General's report on physical activity and health. U.S. Department of Health and Human Services, Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 55.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 56.Catanzaro DF. Zhou Y. Chen R, et al. Potentially reduced exposure cigarettes accelerate atherosclerosis: Evidence for the role of nicotine. Cardiovasc Toxicol. 2007;7:192–201. doi: 10.1007/s12012-007-0027-z. [DOI] [PubMed] [Google Scholar]
- 57.Benowitz NL. Basic cardiovascular research and its implications for the medicinal use of nicotine. J Am Coll Cardiol. 2003;41:497–498. doi: 10.1016/s0735-1097(02)02819-x. [DOI] [PubMed] [Google Scholar]
- 58.Heeschen C. Weis M. Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41:489–496. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 59.Hellerstein MK. Benowitz NL. Neese RA, et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest. 1994;93:265–272. doi: 10.1172/JCI116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell SC. Moffatt RJ. Stamford BA. Smoking and smoking cessation—The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis. 2008;201:225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 61.Leeman RF. Quiles ZN. Molinelli LA, et al. Attrition in a multi-component smoking cessation study for females. Tobacco Induced Dis. 2006;3:59–71. doi: 10.1186/1617-9625-3-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy DE. Piasecki TM. Lawrence DL. Jorenby DE. Shiffman S. Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacPherson L. Stipelman BA. Duplinsky M. Brown RA. Lejuez CW. Distress tolerance and pre-smoking treatment attrition: Examination of moderating relationships. Addict Behav. 2008;33:1385–1393. doi: 10.1016/j.addbeh.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reichert V. Xue X. Bartscherer D, et al. A pilot study to examine the effects of smoking cessation on serum markers of inflammation in women at risk for cardiovascular disease. Chest. 2009;136:212–219. doi: 10.1378/chest.08-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]