Abstract
Sustained release systems have been developed for the use of growth factors in tissue engineering applications. However, many of these systems continue to have limitations associated with low loading efficiencies and reduced biological activity after release. In this paper, we utilized a lipid-based microtube system for the sustained release of BMP-2. The lipid microtubes were fabricated using a self-assembly method, in order to avoid the use of harsh organic solvents that may damage the protein. BMP-2 was loaded into the microtubes by rehydrating dried microtubes in the protein solution. The loading efficiency and release kinetics of BMP-2 in the microtubes were measured using in vitro immunoassays. Loading efficiency was found to be dependent on microtube concentration. The potential for this system to deliver biologically active BMP-2 was assessed using the alkaline phosphatase assay and von Kossa staining on human mesenchymal stem cell cultures. The results demonstrate that the lipid microtube system is able to provide sustained delivery of biologically active BMP-2 and thereby induce osteogenic differentiation.
Keywords: BMP-2, Microtubes, Sustained release
1. Introduction
Grafting procedures are presently the most common treatment used to restore function to damaged or degenerated tissue [1,2]. However, these procedures are limited by the amount of graft material, and have resulted in high rates of failure as well as donor site morbidity [1,2]. As a result, there is a great need for new tissue engineering strategies that aim to restore, maintain or remodel natural tissues at damaged or degenerated sites [3-5]. Tissue engineered constructs that include cell, protein or gene delivery have shown potential to serve as graft substitutes.
Protein or growth factor therapies have shown promise because they have highly specific actions [2,3]. However, these protein therapies often require frequent injections of high doses to overcome the poor stability of the protein, making these treatments very expensive and more likely to cause side effects in the patient [2,4]. To address these limitations, a sustained delivery system that is able to optimally control the rate and location of growth factor release while also maintaining a blood concentration level within therapeutic limits is needed [4]. The existing growth factor delivery systems include lipid-based and polymeric vehicles. However, the fabrication processes of many of these systems often involve the use of harsh organic solvents, which can denature and deactivate sensitive proteins, resulting in low efficiencies [4,6,7].
Bone morphogenetic proteins (BMPs) have been utilized widely in bone tissue engineering because of their ability to initiate and maintain the entire bone formation cascade – chemotaxis, proliferation and differentiation [2-4,8-12]. However, delivery of BMPs alone is suboptimal because of the extremely high doses needed to overcome the short half-life. Sustained BMP-2 activity has previously been achieved by incorporating the protein in a degradable carrier and co-delivery with other factors to promote bone formation. The current carrier materials include collagen, tricalcium phosphate, demineralized bone matrix, hydrogels and synthetic polymers [3,4,8,13]. The delivery of BMP-2 in an absorbable collagen sponge is FDA approved for use in long bone fractures and spinal fusions. However, this system still requires large doses of BMP-2 at high costs and has unpredictable pharmacokinetic profiles [8,13]. Therefore, development of carrier systems that are able to reduce cost and have predictable release kinetics is needed. BMP-2 has been shown to have temporal expression during fracture healing, and with higher concentrations after chondrogenesis has occurred [10,11,14]. However, the exact period when BMP-2 is most effective is still unclear. A well-designed carrier system could provide sustained delivery of BMP-2 within the therapeutic limits throughout the course of tissue regeneration, ensuring that BMP-2 is present during the optimal time for activity [13,15].
The overall goal of this research was to develop a sustained release system for bone tissue engineering applications, using a lipid-based microtube vehicle previously described for delivery of neural growth factor [6], transforming growth factor-β [16] and brain-derived neurotrophic factor [17]. The self-assembly method used to fabricate this carrier system avoids the use of organic solvents, heat and other harmful procedures [6,18]. These microtubes are also beneficial because they can be injected for minimally invasive procedures and are unlikely to be engulfed by cells due to their size [6,17]. We hypothesized that the lipid microtubes will provide sustained release of BMP-2, enhancing bone regeneration. To test this hypothesis, the system was analyzed using in vitro assays to monitor the loading efficiency, release kinetics and the bioactivity of the released BMP-2.
2. Materials and methods
2.1 Preparation of lipid microtubes
Lipid microtubes were prepared using a self-assembly method described by Meilander et al. [6]. First, 10 mg of DC8,9PC lipid (Avanti Polar Lipids, Alabaster, AL, USA) was dissolved in 10 ml of 70% ethanol at 55°C. The lipid solution was held at 55°C for 6 h and then slowly cooled to 25°C in a microprocessor-controlled refrigerated water bath (Isotemp Refrigerated Circulator, Fisher Scientific, Waltham, MA, USA). The microtubes were then stored at room temperature until further use.
Before using the microtubes, the cryoprotectant trehalose (Sigma, St. Louis, MO, USA) was added to the solution at a concentration of 50 mM to preserve the tubular structure during the drying process. The microtubes were then centrifuged (1500 g, 10 min) and the supernatant was removed. Any remaining ethanol solution in the microtubes was removed by lyophilization.
2.2 Microtube characterization
An aliquot of microtubes was placed on a glass slide with a cover slip to create a single layer of microtubes. The microtubes were imaged using a Zeiss Axiovert light microscope connected to an AxioCam digital camera (Carl Zeiss, Inc., Oberkochen, Germany) and desktop computer running AxioVision software. The lengths of microtubes in at least five fields of view were traced from the captured images using the microscope software. The yield of microtubes was calculated from a known dilution of microtubes counted on a hemocytometer.
To monitor the degradation, microtubes were incubated at 37°C in 500 μl of phosphate-buffered saline (PBS) or minimal essential medium (α-MEM; Invitrogen, Carlsbad, CA, USA) with 15% fetal calf serum (FBS; Atlanta Biologicals, Atlanta, GA, USA), 2 mM L-glutamine, 100 units of penicillin and 100 μg of streptomycin (Invitrogen). At various time points microtube samples were collected and theit lengths measured using the light microscope.
2.3 Protein loading
The microtubes were loaded with recombinant human BMP-2 (R&D Systems, Minneapolis, MN, USA) by rehydrating the dried microtubes in 40 μl of protein solution per mg of lipid. The microtubes were allowed to incubate in the protein solution overnight. Unencapsulated growth factor was collected by centrifugation (1500 g, 10 min).
To measure the loading efficiency, microtubes were counted on a hemocytometer and diluted to known concentrations. The microtubes were then incubated overnight in BMP-2 solution at a concentration of 2 μg ml–1. The unencapsulated protein was collected by centrifugation and assayed with an enzyme-linked immunosorbent assay (ELISA; R&D Systems).
To monitor the release kinetics, BMP-2 loaded microtubes were incubated in 500 μl of PBS. The microtubes were then separated by centrifugation and the supernatant was collected and stored at -20°C until assay. Microtubes were resuspended in 500 μl of fresh PBS at each time point. The amount of BMP-2 released was measured using an ELISA.
2.4 Bioactivity assay
The bioactivity of released BMP-2 was analyzed by determining its ability to increase alkaline phosphatase activity in human mesenchymal stem cells (hMSCs). Human mesenchymal stem cells, kindly provided by Tulane University, were harvested from bone marrow aspirates. Cells were passaged twice in α-MEM containing 15% FBS, 2 mM L-glutamine, 100 units of penicillin and 100 μg streptomycin. The hMSCs were subcultured at a low density (500 cells cm–2) and not allowed to expand past 70% confluency. The hMSCs were then trypsinized, counted, and seeded in 24-well tissue culture treated plates at a density of 10,000 cells cm–2. The cells were allowed to reach confluency, usually within 1-2 days. The cells were then differentiated in medium supplemented with 6 mM β-glycerophosphate, 50 μg ml–1 ascorbic acid 2-phosphate, 1 μM dexamethasone and 50 ng ml–1 thyroxine (Sigma), and control or BMP-2 loaded microtubes were added to the medium. Cells layers were collected and analyzed at 1 and 4 weeks for alkaline phosphatase (ALP) activity. ALP activity was quantified using the p-nitrophenylphosphate method. Briefly, cell layers were washed with PBS, lysed with 0.1% TritonX-100/PBS and repeatedly frozen and thawed to disrupt the cell membranes. Subsequently, 50 μl of the supernatant was incubated with 50 μl of p-nitrophenylphosphate solution (Sigma) for 30 min to 2 h at 37°C. The reaction was stopped by adding 100 μl of 1 M NaOH and the absorbance was read at 405 nm using a colorimetric plate reader. ALP activity was normalized to total DNA content. DNA content was quantified using Quant-iT™ Pico Green dsDNA Assay Kit (Invitrogen).
Cell layers were also analyzed for phosphate deposits with the von Kossa staining technique at 4 weeks. Cells were fixed in 70% ethanol and incubated under ultraviolet light with 5% silver nitrate (Sigma) solution for 10 min.
2.5 Statistical analysis
Statistical analysis was done using Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA). Groups were compared using an analysis of variance. p-values less than 0.05 were considered significant. The data presented is the mean ± SEM.
3. Results
3.1 Microtube characterization
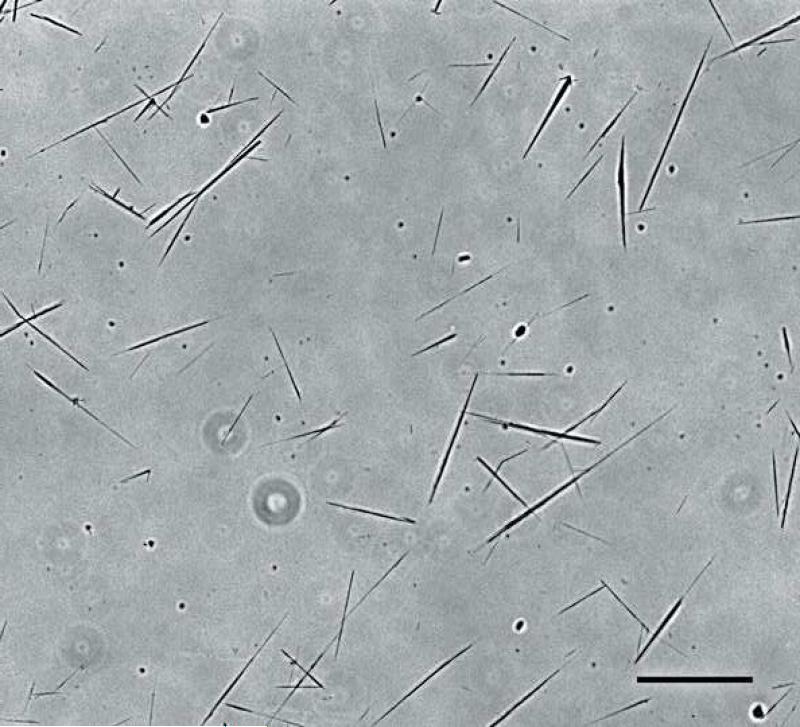
The microtubes fabricated using the self-assembly method described (Fig. 1) had an average length of 30±15 μm. The majority of the microtubes were below 50 μm; however, some microtubes reached lengths over 100 μm (Fig. 2). Typically 6×108 microtubes were fabricated from 10 mg of DC8,9PC lipid.
Fig. 1.
Light microscope image of DC8,9PC lipid microtubes fabricated using the self-assembly method described by Meilander et al. [6]. Scale bar = 50 μm.
Fig. 2.
Representative length distribution of lipid microtubes measured from light microscope images. The average length was 30±15 μm.
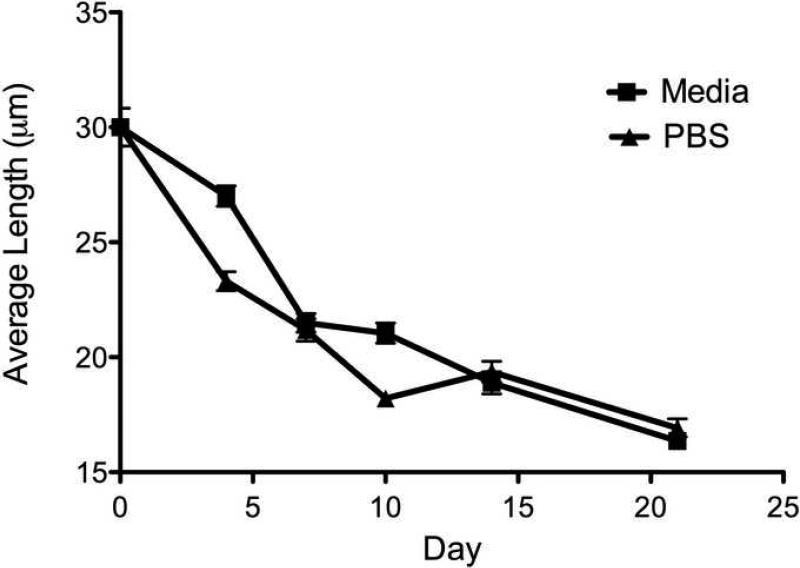
Degradation occurred from the ends of the microtubes, as observed in the decrease in average length from 30±15 to 17±10 μm after 21 days (Fig. 3). There was no difference in degradation when incubated in PBS or cell culture media; however, the microtubes had a tendency to aggregate in cell culture media.
Fig. 3.
Lipid microtube degradation as measured by the decrease in lengths. Lengths decreased by approximately 50% over the course of 3 weeks. Data shown are the average length ± SEM.
3.2 Protein loading
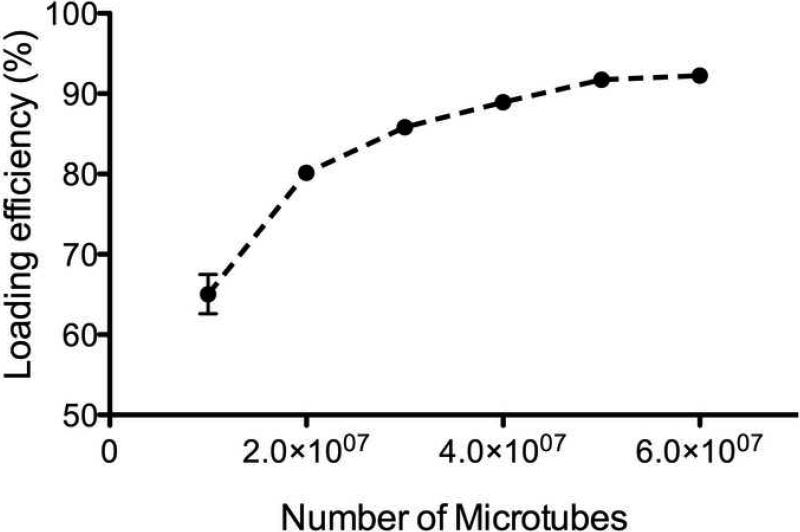
BMP-2 was effectively loaded into the microtubes. Loading efficiency showed a correlation with the amount of microtubes. The amount of encapsulated protein increased with an increase in microtube concentration (Fig. 4). The efficiencies reached a maximum of about 90% at concentrations above approximately 4×107 microtubes. The percent encapsulated was calculated as a ratio of the amount of protein encapsulated to the total amount of protein initially added to the microtubes.
Fig. 4.
Loading efficiency in relation to amount of microtubes. Data shown are the average ± SEM.
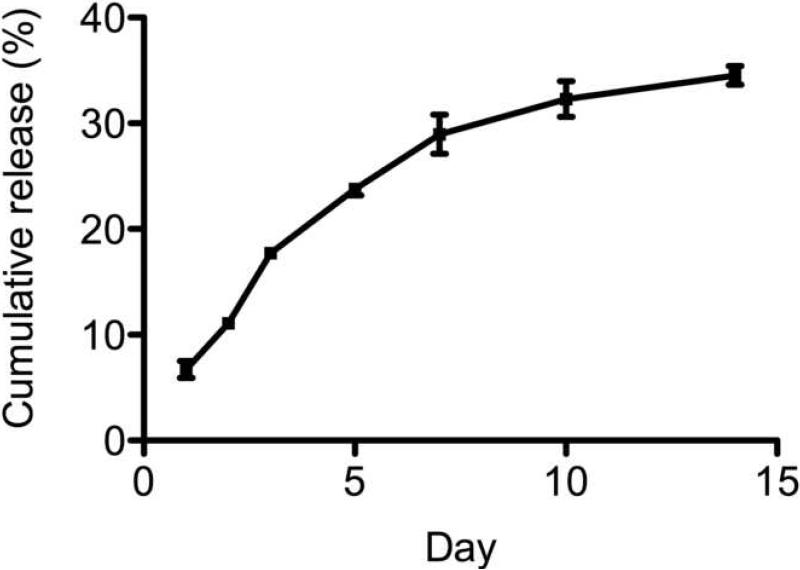
Protein was released from microtubes for a period of 2 weeks without a large initial burst release (Fig. 5). Approximately 40-50% of the loaded protein was released over this time. After 10-14 days the amount of protein released into the media approached the lower end of the detectable range of the ELISA and therefore no further measurements were done. The cumulative release was calculated as a ratio of the initially loaded protein.
Fig. 5.
Release kinetics of BMP-2 measured with ELISA. Cumulative release calculated as the total amount released divided by the initially loaded BMP-2. Data shown are the average ± SEM.
3.3 Post-release bioactivity assay
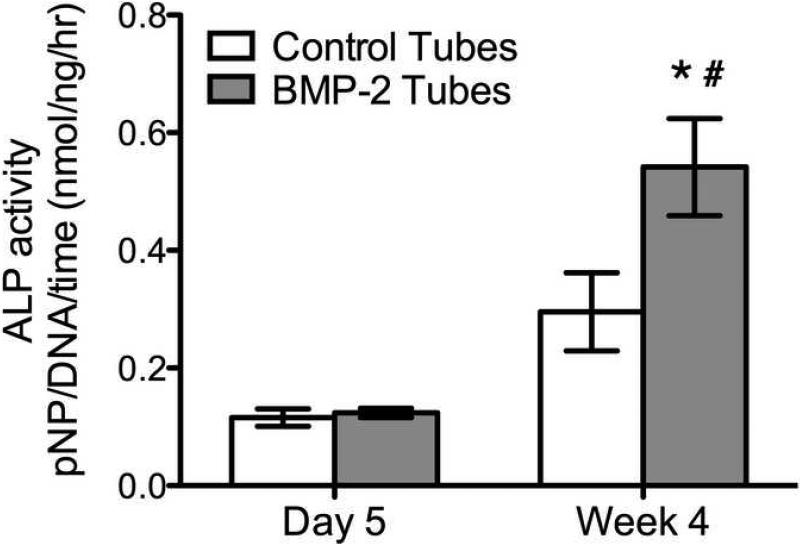
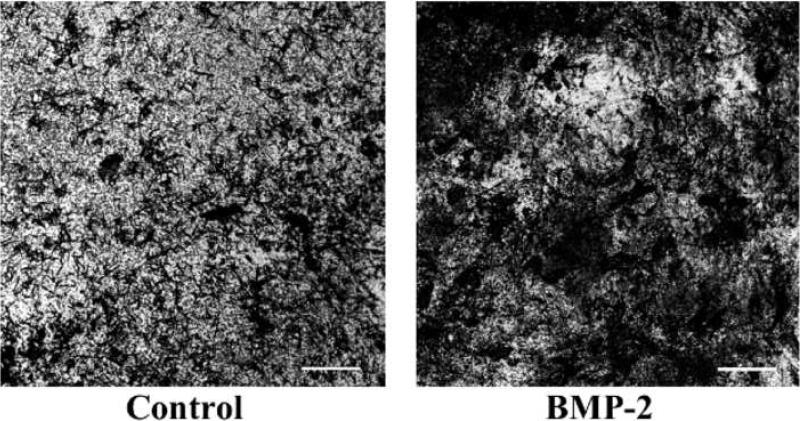
To ensure the BMP-2 released from the microtubes remained biologically active, the ALP activity of hMSCs was measured. The results show that hMSCs in the presence of microtubes releasing BMP-2 had a significant increase in ALP activity in hMSCs after 4 weeks (Fig. 6). The control PBS-loaded microtubes did not significantly increase the ALP activity in hMSCs after 4 weeks. These results were also confirmed by the von Kossa staining at 4 weeks, showing more phosphate deposits on hMSCs treated with BMP-2 loaded microtubes (Fig. 7).
Fig. 6.
Alkaline phosphatase activity in hMSCs in response to BMP-2-loaded microtubes. Statistical difference between BMP-2 and control tubes at 4 weeks (*p<0.05), and BMP-2 tubes at 5 days and 4 weeks (#p<0.05). Data shown are the average ± SEM (n=5).
Fig. 7.
Von Kossa stain of hMSCs treated with control or BMP-2 microtubes after 4 weeks. Scale bar = 50 μm.
4. Discussion
Although some protein therapies have been approved for clinical use, there are still many limitations with these systems [8,13]. Critical issues for effective therapeutic protein delivery include the stability of the proteins and the effective dose. This has led researchers to develop sustained and controlled delivery systems [4]. However, the fabrication processes used for many of these carriers are extremely harsh and unfavorable for protein structure and function [4,6,7]. Meilander et al. [6] were able to characterize the lipid microtube system for growth factor delivery applications, specifically for the delivery of neural growth factor. However, the efficacy of these microtubes as a delivery vehicle may be growth factor dependent. Therefore, in this paper we have described the use of these lipid microtubes for the sustained release of BMP-2 for bone tissue engineering applications.
Lipid microtubes were successfully fabricated using the self-assembly method previously described [18,19]. The microtubes degraded from the ends as expected, as shown by the decrease in average length. This degradation can be delayed or reduced by cross-linking the lipid molecules [20]. The microtubes formed aggregates in the cell culture media as they degraded due to the calcium ions in the media acting on the negatively charged fatty acids produced during hydrolysis [18]. BMP-2 was effectively loaded into the microtubes by rehydrating the dried microtubes in the protein solution. As the microtubes rehydrate, the protein is drawn into the microtubes by capillary action. This led to our hypothesis that the loading efficiency of the microtubes is directly correlated to the concentration of microtubes. Our results showed that the total amount of encapsulated BMP-2 correlated with the total number of microtubes used, reaching over 90% loading efficiencies at higher microtube concentrations. The dose of BMP-2 encapsulated was similar to the doses used in in vivo models; therefore these results will be applicable to clinically relevant models. Many other sustained release systems, such as nanoparticles and microparticles, have reported much lower loading efficiencies, of only 20-40% [21-26]. The loading efficiency at all microtube concentrations exceeded the values typically reported in other systems. Our results confirmed the hypothesis and showed that the efficiency increased as the number of microtubes increased due to more available volume for protein loading.
The lipid microtubes were able to provide sustained release of BMP-2, as shown by the release profile. The release profile seemed to follow first-order kinetics, which is fairly easy to characterize and model. Preclinical research has anticipated that sustained release at low doses will be more effective than a large bolus dose, and will pose a lower risk of side effects [1]. The lipid microtube system described in this paper is able to provide sustained levels of BMP-2 for at least 2 weeks without a large initial burst release.
We have been able to show that BMP-2 released from this microtube system remains biologically active. The ALP assay is a well-established bioassay for BMP-2 activity, as an early marker of osteogenic differentiation [27-32]. The BMP-2 released was still able to enhance ALP activity after 4 weeks in culture, indicating that the microtubes provided sustained release of biologically active BMP-2. There were no obvious effects of the microtubes on cell survival and morphology. Previous studies showed that these microtubes have no adverse effects on cells in vitro and do not cause a significant inflammatory response when implanted in rodents [6,17,33].
The results obtained in these experiments with regard to loading efficiency and release kinetics will be used in future experiments to predict the dose and release rate following implantation into bone defects [34]. This information will help us to develop an effective delivery system that we can tune for specific experimental parameters, such as dose and length of release. The various parameters that can be modified to control the release profile include: average microtube length, degradation rate, loading concentration and the concentration of microtubes. By careful design of this microtube system, BMP-2 or other growth factors may be delivered in a sustained manner for tissue regeneration applications.
5. Conclusions
In this study, we have characterized BMP-2 release and shown that biological activity is retained during sustained release of BMP-2. In addition, the characterization of the microtubes will allow us to predict and control certain parameters of the sustained release system. These results indicate that lipid microtubes can be used to provide sustained release of BMP-2 for bone tissue engineering.
Acknowledgements
This research was funded by NIH grants AR051336 (REG) and NS44409 (RVB), NIH Biotechnology Training Grant (T32-GM008433), and the Georgia Tech/Emory Center for the Engineering of Living Tissues (GTEC) NSF Grant EEC-9731643.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hollinger JO, Uludag H, Winn SR. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv Drug Deliv Rev. 1998;31(3):303–318. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 2.Oest ME, et al. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25(7):941–50. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 3.Boontheekul T, Mooney DJ. Protein-based signaling systems in tissue engineering. Curr Opin Biotechnol. 2003;14(5):559–65. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ. Cytokine delivery and tissue engineering. Yonsei Med J. 2000 doi: 10.3349/ymj.2000.41.6.704. [DOI] [PubMed] [Google Scholar]
- 5.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9(Suppl 1):S5–15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 6.Meilander NJ, et al. Lipid-based microtubular drug delivery vehicles. Journal of Controlled Release. 2001;71(1):141–52. doi: 10.1016/s0168-3659(01)00214-0. [DOI] [PubMed] [Google Scholar]
- 7.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta biomaterialia. 2005;1(4):461–70. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Boden SD. The ABCs of BMPs. Orthopaedic Nursing. 2005;24(1):49–52. doi: 10.1097/00006416-200501000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Riley EH, J.M.L., Urist MR, Lyons KM, Lieberman JR. Bone morphogenetic protein-2: biology and applications. Clinical Orthopaedics and Related Research. 1996;324:39–46. [PubMed] [Google Scholar]
- 10.Riley EH, et al. Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat Res. 1996 [PubMed] [Google Scholar]
- 11.Sakou T. Bone morphogenetic proteins: from basic studies to clinical approaches. Bone. 1998;22(6):591–603. doi: 10.1016/s8756-3282(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 12.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1(4):267–80. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone biology and clinical applications. The Journal of Bone and Joint Surgery. 2002 doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11–25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Winn SR, H.U., Hollinger JO. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Advanced Drug Delivery Reviews. 1998;31:303–318. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 16.Spargo BJ, et al. Controlled release of transforming growth factor-beta from lipid-based microcylinders. J Microencapsul. 1995 doi: 10.3109/02652049509010293. [DOI] [PubMed] [Google Scholar]
- 17.Jain A, et al. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27(3):497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Yager P, Schoen PE. Formation of tubules by a polymerizable surfactant. Molecular Crystals and Liquid Crystals. 1984 [Google Scholar]
- 19.Meilander NJ, et al. Sustained release of plasmid DNA using lipid microtubules and agarose hydrogel. Journal of Controlled Release. 2003;88(2):321–31. doi: 10.1016/s0168-3659(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 20.Carlson PA, Gelb MH, Yager P. Zero-order interfacial enzymatic degradation of phospholipid tubules. Biophys J. 1997;73(1):230–8. doi: 10.1016/S0006-3495(97)78063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi F, Wu H, Jia GL. Formulation and characterization of poly (d, l-lactide-co-glycolide) nanoparticle containing .... Journal of Clinical Pharmacy & Therapeutics. 2006 doi: 10.1111/j.1365-2710.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 22.Zambaux MF, et al. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. Journal of Controlled Release. 1998;50(1-3):31–40. doi: 10.1016/s0168-3659(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Ooi CP. Hydrolytic degradation and drug release properties of ganciclovir-loaded biodegradable microspheres. Acta biomaterialia. 2008 doi: 10.1016/j.actbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum D, Brannon-Pepas L. Drug Delivery Systems in Cancer Therapy. 2003:390. [Google Scholar]
- 25.Cohen H, et al. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000;7(22):1896–905. doi: 10.1038/sj.gt.3301318. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Feng SS. Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. International journal of pharmaceutics. 2007;342(1-2):208–14. doi: 10.1016/j.ijpharm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Datta N, et al. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of .... Biomaterials. 2005 doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, et al. The effect of crosslinking heparin to demineralized bone matrix on mechanical strength and specific binding to human bone morphogenetic protein-2. Biomaterials. 2008;29(9):1189–1197. doi: 10.1016/j.biomaterials.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Takada T, et al. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278(44):43229–35. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 30.Zhao B, et al. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281(32):23246–53. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 31.Hosseinkhani H, et al. Bone regeneration through controlled release of bone morphogenetic protein-2 from 3-D tissue engineered nano-scaffold. Journal of Controlled Release. 2007;117(3):380–6. doi: 10.1016/j.jconrel.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Jeon O, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun. 2008;369(2):774–80. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Bellamkonda RV. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng. 2003;9(3):421–30. doi: 10.1089/107632703322066606. [DOI] [PubMed] [Google Scholar]
- 34.Guo L, et al. Heterogeneous and Anomalous Diffusion inside Lipid Tubules. Journal of Physical Chemistry B. 2007 doi: 10.1021/jp076562n. [DOI] [PubMed] [Google Scholar]