Abstract
Objective
This study investigated the associations of plasma leptin levels with insulin resistance (IR) and prediabetes in relatively lean, rural Chinese men and women.
Design and methods
This study included 574 subjects aged 21–45 years from a community-based twin cohort. Plasma leptin concentrations were measured by sandwich immunoassays using flowemetric xMAP technology. Prediabetes was defined based on fasting plasma glucose and 75g oral glucose tolerance test. Multivariate linear and logistic regression analyses were used to investigate gender-specific associations of leptin with IR measures and prediabetes, adjusting for intra-twin correlation, measures of adiposity, and other pertinent covariates.
Results
The body mass index(BMI) is 22.3±2.7 kg/m2 in men and 22.5±2.7 kg/m2 in women. Leptin levels were positively associated with IR. Individuals with higher tertiles of leptin also had increased risk of prediabetes with OR of 2.6 (95%CI: 1.4–5.1) and 4.3 (95%CI: 2.1–8.7) in men; OR of 1.1 (95%CI: 0.6–2.1) and 3.1 (95%CI 1.5–6.2) in women for 2nd and 3rd tertile, respectively. These associations were attenuated after further adjusting for adiposity measurements only in men. The Leptin-prediabetes associations disappeared after adjusting for the homeostatic model assessment of insulin resistance (HOMA-IR) in both genders.
Conclusion
In this sample of relatively lean rural Chinese adults, plasma leptin levels were associated with IR and prediabetes in a dose-response fashion, which were not totally explained by adiposity. Our data underscored that prediabetes is not all about obesity, and leptin may be an additional biomarker for screening individuals at high risk for prediabetes in this population.
Keywords: Leptin, insulin resistance, prediabetes, Chinese
Introduction
Prediabetes, characterized by impaired fasting glucose (IFG), impaired glucose tolerance(IGT), or a combined IFG/IGT(1) reflects glucose dysregulation intermediate between normaglycemia and type 2 diabetes mellitus. Prediabetes, often asymptomatic and under-detected, is likely fueling the epidemics of type 2 diabetes and its consequences worldwide (2). Cowie et al (3) reported that the crude prevalence of prediabetes was almost 30% in US adult population. While obesity is a well-recognized risk factor for prediabetes and type 2 diabetes in heavy western populations, there are paradox findings in relatively lean Asian populations. For example, 33% of US Asian Indian had prediabetes(4). Our own data showed that prevalence of prediabetes is 26% in middle-aged, rural, relatively lean (mean BMI 21.6kg/m2 in men 22.1kg/m2 in women) Chinese population. The high prevalence of prediabetes in relatively lean populations raised a possibility that prediabetes is not all about obesity. A better understanding the role of other important determinants of prediabetes may provide new insight into pathogenic mechanism of prediabetes and type 2 diabetes and offer a window of opportunity for early detection and prevention of prediabetes and type 2 diabetes.
Leptin, an adipokine encoded by the obese(ob) gene(5), is primarily produced by white adipose cells. It has a variety of important central and peripheral actions, related to regulation of food intake, energy balance and metabolism(6,7). Leptin levels in human increase with increasing body fat and leptin resistance has been postulated to present in obese individuals. Independent of the relationship to body fat, leptin levels are also positively correlated with fasting insulin levels in human (8). While the association between leptin and insulin has not been fully elucidated, several studies indicated that leptin inhibits insulin synthesis and secretion, while insulin stimulates leptin secretion. This bidirectional hormonal feedback loop between insulin and leptin has been named the adipoinsular axis (9). To date, few data is available on the role of leptin in prediabetes, especially, in relatively lean Chinese population.
This study was to examine whether leptin is associated with insulin resistance (IR) and prediabetes and whether these associations can be explained by measures of adiposity in relatively lean, rural Chinese adults.
Subjects and methods
Study population and Procedures
This report utilized data from an ongoing NIH funded study of metabolic syndrome in a large community-based Chinese twin cohort in Anqing, Anhui province in China during 2005–2006. All the study subjects completed a questionnaire interview, standardized physical examination, dual-energy X-ray absorptiometry (DEXA) scan and oral glucose tolerance test (OGTT) after at least 10 hours of overnight fasting. We utilized a nested case-control design which included all prediabetic cases and normal glucose tolerance controls matched by age and gender. A total of 574 subjects (290 males, 284 females) aged 21–45 years were included in this analysis. The study protocol was approved by the Institutional Review Boards of Children’s memorial Hospital and the Biomedical Institute, Anhui Medical University in Hefei, China. All participants gave written consent.
Physical Activity
Physical activity was assessed using the short version of the international physical activity questionnaire (IPAQ-Short) (http://www.ipaq.ki.se). Participants were asked information about 3 types of physical activity (vigorous, moderate and walking) across various physical activity domains (i.e., leisure time, occupational or school, domestic, and transport), using the “last 7 days” as the reference period. Using both the total volume of activity and the number of activity days/sessions per week, the IPAQ generates a categorical indicator (low, moderate, and high) of regular physical activity.
Anthropometric parameters
Height and weight were measured using standard protocols, without shoes and outerwear. Height was measured to the nearest 0.1 cm on a portable stadiometer and weight to the nearest 0.1 kg. Waist circumference(WC) was measured at the horizontal circumference midway between the lowest rib margin and the iliac crest. Body mass index (BMI) was calculated as weight (kg)/height squared (m2). Blood pressure(BP) was measured by trained nurses according to a standard procedure. A standard whole-body scan was performed by DEXA (DPX- GE-lunar MD, USA) to measure total body fat and trunk fat using a standard software calculation(10). Percentage body fat(PBF) and percentage trunk fat(PTF) were calculated as (total body fat in kg/ weight in kg) × 100 and (trunk fat in kg/ total body fat in kg) × 100, respectively.
Laboratory methods
Plasma was separated from blood cell in the field within 30 min after the blood was drawn and kept refrigerated. Plasma glucose was measured within 2 hours by a glucose oxidase method (Hitachi 7020 Automatic Analyzer, Japan). Standard quality control procedures were performed each day with standard samples that came with the reagents (the coefficient of variation, CV<8%). Triglycerides and cholesterol were measured by enzymatic methods (Boehringer Mannheum, Germany) and high density lipoprotein-cholesterol (HDL-c) by the same enzymatic method after precipitation with dextran sulfate/magnesium chloride. Serum insulin was measured by electrochemiluminescence (ECL) method on an Elecsys 2010 system (Roche, Switzerland). Duplicate analyses were also conducted daily using samples collected from study participants (CV<10%, mean=3%). Plasma leptin concentrations were determined using a sandwich immunoassays based flowemetric xMAP technology on Luminex 200 machines (luminex muti-analyte profiling system, Luminex, Corp., Austin, TX, USA). Immunoassays kit is commercially available from Millipore Corporation. Each sample was duplicated and intra-assay CV was <5.1%.
Definition of Insulin Resistance and Prediabetes
All the study subjects completed a standard 75g glucose OGTT after at least 10 hours of overnight fasting. Blood samples were obtained at 0 and 120 minutes after glucose administration, for glucose and insulin measurements. Insulin resistance was estimated using fasting insulin, 2h post-load insulin(2h PI) and the homeostatic model assessment of insulin resistance (HOMA-IR). HOMA-IR was calculated using the following formulae: HOMA-IR=fasting insulin concentration (µU/mL)×fasting glucose concentration (mmol/L)/22.5(11).
Prediabetes was defined as either IFG (i.e. fasting plasma glucose was between 5.6mmol/L–6.9mmol/L), and/or IGT (i.e. 2h post-load glucose was between 7.8mmol/L–11.0mmol/L).
Exclusion criteria for this analysis included any of the followings: previous diagnosis of diabetes by a physician, plasma glucose concentration ≥7.0mmol/L after a 10 hours fast, or plasma glucose concentration ≥11.1mmol/L 2h after a 75-g oral glucose tolerance test, or taking any anti-diabetic medication.
Statistical analysis
The distribution of fasting insulin, 2h PI, HOMA-IR and leptin was right skewed and a logarithmic transformation was used to normalize the data for subsequent statistical analyses. In addition to analyzing leptin as a continuous variable, we also divided leptin into three tertiles so that we can assess its functional relationship and dose-response associations with IR and prediabetes. We first examined the associations between tertiles of leptin and IR measures using gender-specific multiple linear regression models, with adjustment for age, education (primary school minor or plus), occupation (farmer or non-farmer), active smoking and passive smoking, alcohol consumption (yes or no) and physical activity (Model A). Since the sample contains 187 twin pairs, we also accounted for intra-twin pair correlation using generalized estimation equation. We further evaluated if these associations could be modified by components of metabolic syndrome (Model B) and different adiposity measures (BMI, PBF, PTF, and WC for Model C to F). We also performed similar analysis among two subgroups: subjects with normal glucose tolerance (NGT) and subjects with prediabetes. To examine explanatory contribution of leptin to IR measures, partial R2 was calculated. Finally, we performed gender-specific multiple logistic regression analyses to assess the associations between leptin tertiles and prediabetes, with the adjustment for the same covariates as listed above plus HOMA-IR (Model G). A two-sided p-value<0.05 was regarded as statistically significant. We also used Bonferroni correction to address potentially inflated type I error (false positive) due to multiple comparisons (i.e. 4 independent tests), with corrected p-value cutoff of 0.0125. All analyses were performed using SAS version 9.1 (SAS Institute, Cary. NC).
Results
Demographic, anthropometric and clinical characteristics
This study includes 290 males, BMI 22.3±2.7 kg/m2 and 284 females, BMI 22.5±2.7 kg/m2 and 280 prediabetes cases and 294 normal glucose tolerance (NGT) controls. Table 1 displays the characteristics of NGT and prediabetes subjects by gender. Smoking status, education level and occupation are compatible between prediabetes cases and NGT controls. As expected, leptin concentrations, plasma glucose, insulin measurements, and leptin levels were significantly higher in prediabetes subjects compared to NGT subjects in men and women (p<0.05). Also as expected, adiposity measurements and triglycerides were significantly higher in prediabetes subjects compared to NGT subjects, most notably in men. There were no differences in BP, and plasma HDL-c concentrations between NGT and prediabetes groups. Moreover, the leptin levels were much higher in women than men in both NGT and prediabetes subjects.
Table 1.
Demographic and clinical characteristics of Chinese men and women aged 21–45 years
| Variables | Men |
Women |
||
|---|---|---|---|---|
| NGT | PDM | NGT | PDM | |
| N | 147 | 143 | 147 | 137 |
| Age(years) | 36.4±6.9 | 34.6±7.3* | 38.1±5.4 | 36.3±5.6# |
| Education (% primary school minor) | 84.4 | 83.2 | 96.6 | 99.3 |
| Occupation (% farmer) | 76.9 | 67.1 | 70.8 | 70.1 |
| Smoke status (% yes) | ||||
| Active smoking | 67.4 | 59.4 | 3.4 | 4.4 |
| Passive smoking | 70.8 | 73.4 | 76.9 | 70.1 |
| Alcohol consumption (%yes) | 39.5 | 43.4 | 4.8 | 2.9 |
| Physical activity | ||||
| Low | 29(19.7) | 17(11.9) | 24(16.3) | 30(21.9) |
| Moderate | 21(14.3) | 28(19.6) | 27(18.4) | 19(13.9) |
| High | 70(47.6) | 73(51.1) | 76(51.7) | 65(47.5) |
| Unknow | 27(18.4) | 25(17.5) | 20(13.6) | 23(16.8) |
| BMI(kg/m2) | 21.7±2.3 | 22.8±2.9# | 22.3±2.5 | 22.8±2.8 |
| PBF(%) | 13.3±6.8 | 16.9±8.0# | 28.2±5.8 | 29.3±6.2 |
| PTF(%) | 53.3±6.1 | 55.9±6.0# | 50.7±4.5 | 51.4±4.9 |
| WC(cm) | 73.2±7.3 | 78.1±9.0# | 72.9±7.2 | 74.2±8.6 |
| SBP(mmHg) | 108.7±14.3 | 113.2±12.9 | 108.4±16.4 | 107.6±12.5 |
| DBP(mmHg) | 73.4±11.9 | 75.8±10.4 | 69.9±12.5 | 70.4±9.3 |
| Triglyceride(mmol/L) | 1.0±0.6 | 1.3±0.8# | 1.0±0.7 | 1.0±0.5 |
| Cholesterol(mmol/L) | 4.1±0.7 | 4.4±0.9 | 4.1±0.7 | 4.2±0.8 |
| HDL-c(mmol/L) | 1.7±0.5 | 1.8±0.6 | 1.6±0.5 | 1.7±0.5 |
| FPG(mmol/L) | 5.1±0.3 | 5.8±0.3# | 5.1±0.3 | 5.8±0.4# |
| 2h PG(mmol/L) | 4.8±1.3 | 5.7±1.7# | 5.8±1.1 | 6.8±1.6# |
| FSI (µU/mL) | 4.8±2.8 | 7.1±5.0* | 6.0±2.6 | 8.2±5.2* |
| 2hPI (µU/mL) | 21.6±19.9 | 30.8±33.8* | 30.1±16.6 | 45.9±36.5* |
| HOMA-IR | 1.1±0.7 | 1.8±1.3* | 1.4±0.6 | 2.1±1.5* |
| Leptin(ng/mL) | 1.9±1.9 | 3.4±3.4* | 6.8±4.0 | 9.5±6.8* |
Data are presented as percent and mean±SD;
p<0.01 for t test compared with NGT groups;
p<0.05 for t test based on log transformed variables;
NGT, normal glucose tolerance; PDM, prediabetes (including impaired fasting glucose and impaired glucose tolerance); BMI, body mass index; PBF, percentage body fat; PTF, percentage trunk fat; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-c, high density lipoprotein-cholesterol; FPG, fasting plasma glucose; 2h PG, 2h post-load glucose; FSI, fasting serum insulin; 2hPI, 2h post-load insulin; HOMA-IR, homeostasis modeling accessible of insulin resistance.
Association of plasma leptin with insulin resistance
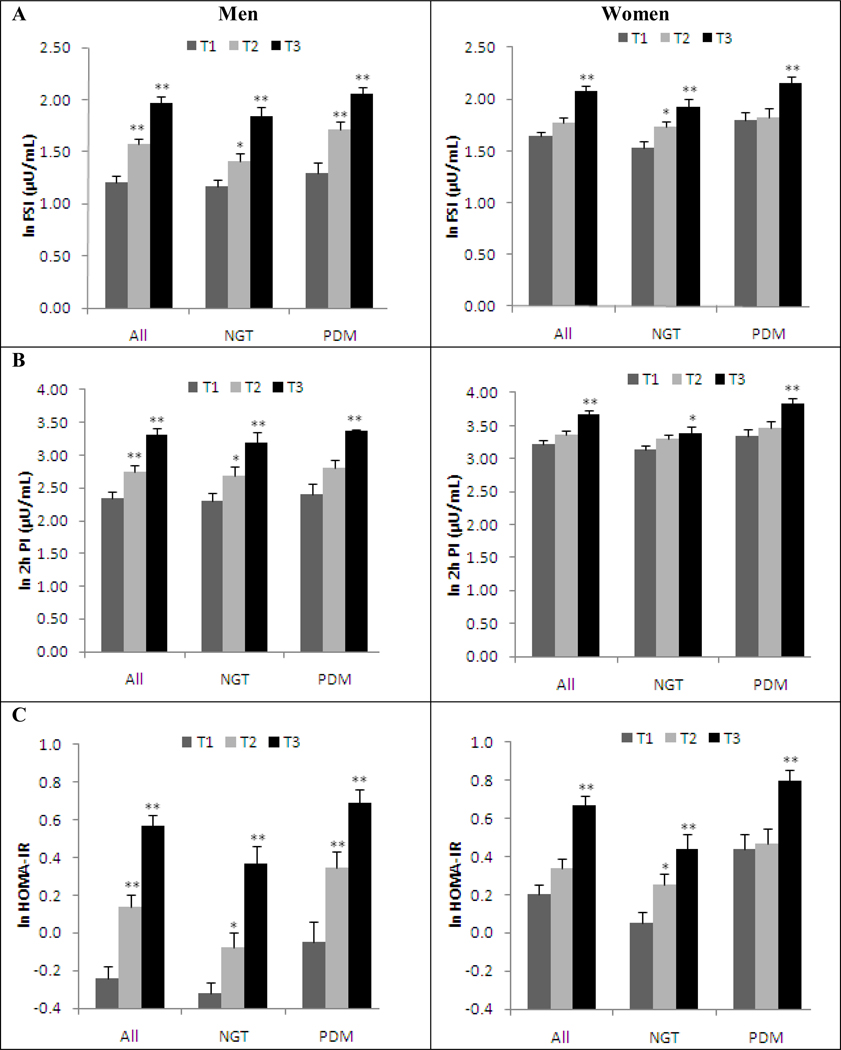
In both gender, we observed a dose-response relation between the tertiles of leptin and fasting serum insulin(FSI), 2h PI and HOMA-IR among all the subjects as well as among the subgroups: subjects with NGT and subjects with prediabetes (Figure 1). Table 2 showed that higher tertiles of leptin were positively associated with FSI, 2h PI and HOMA-IR in both genders. For example, comparing with the lowest tertile of leptin, the 2nd and 3rd tertiles of leptin were associated with elevated HOMA-IR with β and SE estimates of 0.32 ± 0.08 and 0.71 ± 0.09 in men, when adjusting for ages, education, occupation, active smoking and passive smoking, alcohol consumption and physical activity (Model A). The corresponding estimates for women were 0.15 ± 0.07 and 0.50 ± 0.08. These associations didn’t change after adjusting blood pressure, lipid profile (Model B), while they became attenuated but remained significant after adjusting for adiposity measurements (Model C to F). The explanatory contribution of leptin to HOMA-IR was 21% in both gender, which was attenuated to 12% and 13% in men and women, respectively after adjusting for blood pressure and lipid profile. It was further attenuated to 2%–10% after adjustment for adiposity measures. Similar patterns were observed for FSI and 2h PI levels.
Figure 1.
The distribution of insulin resistance measurs classified by leptin tertiles, grouped for all subjects (all); subjects with normal fasting glucose (NGT); subjects with prediabetes (PDM).
A: log transformed mean fasting serum insulin levels for each tertile of plasma leptin concentrations, *p<0.05 compared with 1st leptin tertile; **p<0.01 compared with 1st leptin tertile. FSI, fasting serum insulin
B: log transformed mean 2h post-load insulin levels for each tertile of plasma leptin concentrations; *p<0.05 compared with 1st leptin tertile; **p<0.01 compared with 1st leptin tertile. 2h PI, oral glucose tolerant 2h postload insulin
C: log transformed mean HOMA-IR levels for each tertile of plasma leptin concentrations, *p<0.05 compared with 1st leptin tertile; **p<0.01 compared with 1st leptin tertile. HOMA-IR, homeostasis modeling accessible of insulin resistance.
Table 2.
Association between leptin tertiles and insulin resistance measurements in Chinese men and women aged 21–45 years.
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| 2nd tertile | 3rd tertile | lnleptin | 2nd tertile | 3rd tertile | lnleptin | |
| β(se) | β(se) | Partial R2 | β(se) | β(se) | Partial R2 | |
| HOMA-IR | ||||||
| Model A | 0.32(0.08)& | 0.71(0.09)& | 0.2084 | 0.15(0.07)* | 0.50(0.08)& | 0.2062 |
| Model B | 0.29(0.08)& | 0.56(0.09)& | 0.1181 | 0.09(0.07) | 0.41(0.08)& | 0.1342 |
| Model C | 0.20(0.07)# | 0.36(0.09)& | 0.0369 | 0.03(0.07) | 0.28(0.09)# | 0.0560 |
| Model D | 0.15(0.08) | 0.29(0.11)# | 0.0201 | 0.03(0.07) | 0.26(0.08)& | 0.0532 |
| Model E | 0.29(0.08)& | 0.57(0.09)& | 0.0996 | 0.07(0.07) | 0.34(0.08)& | 0.0887 |
| Model F | 0.17(0.07)* | 0.32(0.09)& | 0.0305 | 0.03(0.07) | 0.24(0.08)# | 0.0392 |
| FSI | ||||||
| Model A | 0.30(0.07)& | 0.66(0.08)& | 0.1989 | 0.14(0.07)* | 0.47(0.07)& | 0.2062 |
| Model B | 0.27(0.07)& | 0.52(0.08)& | 0.1116 | 0.07(0.06) | 0.37(0.07)& | 0.1269 |
| Model C | 0.19(0.07)# | 0.34(0.08)& | 0.0342 | 0.02(0.06) | 0.25(0.08)& | 0.0510 |
| Model D | 0.14(0.08) | 0.26(0.10)# | 0.0161 | 0.02(0.06) | 0.24(0.08)# | 0.0462 |
| Model E | 0.28(0.07)& | 0.53(0.08)& | 0.0961 | 0.06(0.06) | 0.31(0.07)& | 0.0828 |
| Model F | 0.17(0.07)* | 0.30(0.09)& | 0.0287 | 0.02(0.06) | 0.22(0.07)# | 0.0338 |
| 2h PI | ||||||
| Model A | 0.36(0.13)# | 0.75(0.14)& | 0.1129 | 0.12(0.07) | 0.48(0.09)& | 0.1042 |
| Model B | 0.36(0.14)# | 0.68(0.16)& | 0.0908 | 0.08(0.08) | 0.40(0.10)& | 0.0664 |
| Model C | 0.30(0.14)* | 0.59(0.19)# | 0.0549 | 0.10(0.08) | 0.43(0.11)& | 0.0595 |
| Model D | 0.10(0.13) | 0.11(0.19) | 0.0054 | 0.06(0.08) | 0.33(0.11)# | 0.0219 |
| Model E | 0.33(0.13)* | 0.58(0.17)& | 0.0569 | 0.09(0.08) | 0.39(0.10)& | 0.0512 |
| Model F | 0.31(0.14)* | 0.56(0.19)# | 0.0504 | 0.09(0.08) | 0.42(0.10)& | 0.0521 |
p<0.05;
p<0.01;
p<0.001 compared with 1st leptin tertile; HOMA-IR, homeostasis modeling accessible of insulin resistance; FSI, fasting serum insulin; 2h PI, 2h post-load insulin.
Model A, adjusted for age, education, occupation, active and passive smoking, alcohol consumption, and physical activity;
Model B, Model A plus Triglyceride, Cholesterol, high density lipoprotein-cholesterol(HDL-c), systolic blood pressure(SBP), diastolic blood pressure(DBP).
Model C, Model B plus body mass index(BMI);
Model D, Model B plus percentage body fat;
Model E, Model B plus percentage trunk fat;
Model F, Model B plus waist circumference.
Association of leptin with prediabetes
As showed in table 3, compared to individuals with the lowest tertiles of leptin, those with higher leptin tertiles had increased risk of prediabetes (OR=2.6, 95%CI: 1.4–5.1 and OR=4.3, 95%CI:2.1–8.7 for 2nd and 3rd tertiles in men, respectively; OR=1.1, 95%CI: 0.6–2.1 and OR=3.1, 95%CI 1.5–6.2 for 2nd and 3rd tertiles in women, respectively) after adjusting age, education, occupation, smoking and alcohol drinking status and physical activity (Model A). The associations remained after adjusting for blood pressure, and lipid profile(Model B). With further adjustment for adiposity measures in the model: BMI (model C), PBF (model D), PTF (model E), WC (model F), the associations of leptin with prediabetes were attenuated in men. Adjustment for adiposity measures had no impact on leptin-prediabetes associations in women. Finally, the leptin-prediabetes associations disappeared after adjusting for HOMA-IR (model G) in both genders.
Table 3.
Association between leptin levels and the risk of prediabetes in Chinese men and women aged 21–45 years.
| Leptin tertiles | N | OR (95%CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (ng/ml) | NGT | PDM | Model A | Model B | Model C | Model D | Model E | Model F | Model G |
| Men | |||||||||
| 1st tertile(low) | 67 | 29 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2nd tertile | 45 | 52 | 2.6(1.4–5.1)# | 2.5(1.3–4.9)# | 2.3(1.2–4.7)* | 2.4(1.2–4.9)* | 2.3(1.1–4.7)* | 2.0(1.0–4.0) | 1.7(0.9–3.4) |
| 3rd tertile(high) | 35 | 62 | 4.3(2.1–8.7)& | 3.6(1.7–7.8)& | 3.4(1.4–8.2)# | 3.4(1.3–8.9)* | 3.1(1.3–7.2)# | 2.2(0.9–5.3) | 2.0 (0.9–4.7) |
| P trend | <0.0001 | 0.0009 | 0.0056 | 0.0120 | 0.0073 | 0.0652 | 0.0878 | ||
| Women | |||||||||
| 1st tertile(low) | 57 | 37 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2nd tertile | 55 | 40 | 1.1(0.6–2.1) | 1.1(0.6–2.1) | 1.1(0.6–2.3) | 1.3(0.6–2.6) | 1.1(0.6–2.2) | 1.1(0.6–2.1) | 1.0(0.5–1.9) |
| 3rd tertile(high) | 35 | 60 | 3.1(1.5–6.2)# | 3.1(1.5–6.3)# | 3.2(1.4–7.3)# | 3.8(1.6–9.3)# | 2.9(1.4–6.3)# | 3.0(1.3–6.8)# | 1.9(0.9–4.0) |
| P trend | 0.0014 | 0.0022 | 0.0056 | 0.0029 | 0.0048 | 0.0102 | 0.0993 | ||
p<0.05;
p<0.01;
p<0.001 compared with 1st leptin tertile; NGT, normal glucose tolerance; PDM, prediabetes;
Model A, adjusted for age, education, occupation, active and passive smoking, alcohol consumption, and physical activity;
Model B, Model A plus triglyceride, cholesterol, high density lipoprotein-cholesterol, systolic blood pressure, diastolic blood pressure
Model C, Model B plus body mass index(BMI);
Model D, Model B plus percentage body fat;
Model E, Model B plus percentage trunk fat;
Model F, Model B plus waist circumference;
Model G, Model B plus homeostasis modeling accessible of insulin resistance(HOMA-IR).
Of note, we evaluated the associations between leptin and IR measures, leptin and prediabetes stratified by gender, and thus the significant findings may be false due to the multiple comparisons (i.e. 4 independent tests). To address this concern, we used Bonferroni correction to address potentially inflated type I error (false positive). Our results showed that the majority of the associations remained statistically significant even after Bonferroni correction, using a cutoff of p<0.0125.
Discussions
This is the first study that assessed gender-specific plasma leptin levels in relation to prediabetes in relatively lean middle-aged rural Chinese subjects. We found significant associations between higher leptin levels and increased risk of prediabetes in both men and women in a dose-response fashion. The leptin-prediabetes associations were independent of BP and lipid levels. Furthermore, the leptin-prediabetes associations can not be totally explained by comprehensive measures of adiposity, because further adjusting for adiposity measurements only attenuated these associations in men, and had little effect in women. Our findings reinforce the notion that prediabetes is not all about obesity. If confirmed in future studies, letpin may be an additional biomarker for identifying individuals at high risk of prediabetes in this lean Chinese population.
The biological mechanism by which leptin affects the risk of prediabetes is not completely clear. Notably, the leptin-prediabetes association did disappear after adjusting for HOMA-IR in both genders, suggesting that IR may be a possible biological pathway by which leptin affects prediabetes. The relationship between leptin and insulin appears to be bidirectional, that is, leptin inhibits insulin synthesis and secretion while insulin stimulates leptin secretion(9). The effect of leptin also appears to vary by species (mice vs. human) and by clinical conditions. Although leptin improves glucose homeostasis and insulin resistance in humans with lipodystrophy or congenital leptin deficiency(12,13), results in humans with ‘typical’ obesity were disappointing in this regard(14). In the majority of cases of obesity, despite both an intact leptin receptor and high circulating leptin levels, leptin fails to induce weight loss and IR improvement. This diminished response to the anorexigenic and insulin-sensitizing effects of leptin is called “leptin resistance”. Moreover, attenuation of leptin sensitivity in the brain leads to excess triglyceride accumulation in adipose tissue, as well as in muscle, liver, and pancreas, resulting in impaired insulin sensitivity and secretion(15). Consequently, this could be a sign of decreased inhibition of insulin secretion, and the resulting hyperinsulinemia will further stimulate leptin production. This scenario could lead to what we have observed, that is, high leptin is associated with hyperinsulinemia and prediabetes.
Our finding is consistent with a recent study in which serum leptin level was higher in those with prediabetes than in the controls in Saudi subjects(16). It is also consistent with previous longitudinal studies on the effects of increased leptin concentrations on diabetes in Caucasian (17) and Japanese American populations(18). In addition, Nowak et al (19) found that leptin may antagonize some functions of insulin by the attenuation of insulin receptor capacity in liver.
While leptin was independently associated with IR measures and prediabetes in both genders, our data did show some gender differences. First, we found higher leptin levels in females than in males, as reported in previous studies(20). However, this difference was attenuated after adjusting for PBF (data not shown), which is consistent with previous report that gender difference disappeared when leptin values were compared across similar body fat percentage(21). This is consistent with the concept that women have a higher body fat content at a given body mass index and leptin levels are affected by total adiposity. Second, our data suggest that adiposity mediated a proportion of the association between leptin and IR measures in both genders and between leptin and prediabetes only in men. One possible explanation for the gender difference is different distribution of fat mass by gender, e.g. more abdominal visceral adipose tissue in men and more subcutaneous adipose tissue mass in women(22). An alternative explanation for the gender difference is that leptin may have differential effects in men and women.
Our study has several strengths. First, we were able to examine a wide array of adiposity measures including BMI, WC, and DEXA-based measurements of body fat, which can more accurately reflect body composition than BMI and WC. Second, this study included a relatively homogenous population, in which most of them were farmers, had similar lifestyle and physical activity levels, and dietary intake. The most important feature of our study is we focused on prediabetes instead of diabetes. This minimizes several sources of confounding, such as drug treatment effect, un-measured metabolic alterations or other subclinical pathology as a result of diabetes, and decreased β cell function within diabetic individuals that might obscure secretion and effects of leptin. As such, our study was unlikely confounded by above mentioned factors. Finally, this population had relative low plasma insulin levels, which would minimize the confounding of high insulin level on the association between leptin levels and prediabetes.
Our study also has limitations. This cross-sectional analysis only allows for the establishment of an association and not a temporal and causal relationship between leptin and prediabetes. The study sample was nested within a large twin cohort, but we limited to one twin per family for majority of the sample. For the small number of twin pairs (n=187) included in the analysis, we used General Estimating Equations to account for intra-pair correlation. Of note, the selected subjects were quite comparable with those excluded from this study with regards to age, gender, adiposity measures. Furthermore, this community-based twin population is comparable to local general population as demonstrated in our previous report(23). Although this is among the largest study of this kind in Chinese population, the sample size of our study is modest. We performed a power analysis using program QUANTO (http://hydra.usc.edu/gxe) and demonstrated that we have reasonable power (>85%) to detect an association with ORs of 2.5 and above (based on the estimates in Table 3, and frequency of exposure to higher leptin levels in normal controls: 0.3 – 0.4). Lastly, our findings have not been replicated in independent study samples.
In summary, this study demonstrated significant dose-response association between plasma leptin level and IR meaures and risk of prediabetes in this relatively lean, middle-aged rural Chinese population. Leptin appeared to offer additional information beyond adiposity on the risk of prediabetes in this non-obese Chinese sample. Continued follow-up of these subjects will help evaluate the utility of leptin as a useful predictor of prediabetes and future development of type 2 diabetes.
Acknowledgement
We would like to thank the study participants and their families, and the faculty and staff of Anhui Medical University.
Funding
This work was supported in part by the National Institute of Health grant R01 HD049059, R01 HL0864619, and R01 AG032227.
Footnotes
Declaration of interest
All authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- 1.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 3.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra R, Patel T, Kotha P, Raji A, Ganda O, Banerji M, Shah V, Vijay K, Mudaliar S, Iyer D, Balasubramanyam A. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications. 2009 doi: 10.1016/j.jdiacomp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 6.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 7.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53 Suppl 1:S152–S158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MW, Prigeon RL, Kahn SE, Nicolson M, Moore J, Morawiecki A, Boyko EJ, Porte D., Jr Evidence that plasma leptin and insulin levels are associated with body adiposity via different mechanisms. Diabetes Care. 1997;20:1476–1481. doi: 10.2337/diacare.20.9.1476. [DOI] [PubMed] [Google Scholar]
- 9.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2000;278:E1–E14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 10.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271:E941–E951. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 13.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 15.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 16.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 17.Welsh P, Murray HM, Buckley BM, de Craen AJ, Ford I, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Sattar N. Leptin predicts diabetes but not cardiovascular disease: results from a large prospective study in an elderly population. Diabetes Care. 2009;32:308–310. doi: 10.2337/dc08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeely MJ, Boyko EJ, Weigle DS, Shofer JB, Chessler SD, Leonnetti DL, Fujimoto WY. Association between baseline plasma leptin levels and subsequent development of diabetes in Japanese Americans. Diabetes Care. 1999;22:65–70. doi: 10.2337/diacare.22.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Nowak KW, Mackowiak P, Nogowski L, Szkudelski T, Malendowicz LK. Acute leptin action on insulin blood level and liver insulin receptor in the rat. Life Sci. 1998;63:1347–1352. doi: 10.1016/s0024-3205(98)00398-1. [DOI] [PubMed] [Google Scholar]
- 20.Al-Daghri NM, Al-Attas OS, Al-Rubeaan K, Mohieldin M, Al-Katari M, Jones AF, Kumar S. Serum leptin and its relation to anthropometric measures of obesity in pre-diabetic Saudis. Cardiovasc Diabetol. 2007;6:18. doi: 10.1186/1475-2840-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 22.Demerath EW, Reed D, Choh AC, Soloway L, Lee M, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obesity (Silver Spring) 2009;17:2060–2066. doi: 10.1038/oby.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Kumar R, Venners S, Pongracic J, Wang B, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, Xu X, Wang X. Age and gender specific lung function predictive equations provide similar predictions for both a twin population and a general population from age 6 through adolescence. Pediatr Pulmonol. 2007;42:631–639. doi: 10.1002/ppul.20631. [DOI] [PubMed] [Google Scholar]