The baboon is a natural model of idiopathic (inherited) generalized epilepsy in humans, with affected animals exhibiting spontaneous generalized myoclonic and tonic-clonic seizures, predominantly in the morning (Killam et al., 1967). Similar to humans, scalp EEG studies in the baboon demonstrate generalized ictal (during seizures) and interictal (between seizures) epileptic discharges (IEDs) (Killam et al., 1967; Szabó et al., 2005). These studies also confirmed earlier findings of photosensitivity of the epileptic baboon, implying that visual stimuli, especially flashing lights, can induce seizures and IEDs. Based upon these electroclinical features, the epilepsy of the baboon closely resembles a common epilepsy syndrome in humans, namely juvenile myoclonic epilepsy (JME).
While idiopathic generalized epilepsies are diagnosed in almost half of people with epilepsy (www.epilepsyfoundation.org/about/statistics.cfm), their underlying pathophysiologies are still largely unknown. Functional MRI studies in humans and functional PET studies in the baboon have successfully mapped cerebral blood flow (CBF) changes during photic stimulation (Szabó et al., 2007; Moeller et al., 2009). CBF changes are multiregional and cortical, but these findings did not clarify whether structural or electrophysiological abnormalities underlie the functional abnormalities. Structural MRI studies have not demonstrated gross structural abnormalities in humans or baboons with IGE (Bernasconi et al., 2003; Natsume et al., 2003; Szabó et al., 2007). Additionally, studies that analyzed regional differences in gray matter volume and concentration were discordant with respect to the presence or absence of gray matter changes, the location of gray matter changes, and whether or not they were increased or decreased (Woermann et al., 1999; Betting et al., 2005; Ciumas and Savic, 2006; Kim et al., 2007; Roebling et al., 2009). Therefore, the link between gray matter volume or concentration and epilepsy remains elusive.
Other MRI morphometric techniques may yield more robust and reliable markers of acquired or developmental structural abnormalities affecting the cerebral cortex. Sulcal area measurements provide a useful tool for the evaluation of morphometric changes related to development or aging in humans and baboon (Kochunov et al., 2005). Recently published MRI studies in a baboon pedigree housed at the Southwest Foundation for Biomedical Research (SFBR) demonstrated a high degree of heritability of sulcal areas (Kochunov et al., 2007; Rogers et al., 2007), particularly of the central, external calcarine, inferior occipital, principal and superior temporal sulci (Kochunov et al., 2010). Due to a high prevalence of epilepsy in this pedigree, many of the baboons evaluated with MRI were also independently characterized by scalp EEG (Szabó et al., 2005). With the availability of these large MRI and EEG databases, our group sought to retrospectively evaluate MRI volumetric and morphometric differences of baboons with IEDs on EEG (IED+) and baboons with normal EEG studies (IED−).
Methods
Animal selection
The neuroimaging database included measurements of total brain volume and sulcal areas in 180 baboons with structural MRI studies, while the EEG database contained almost 700 baboons that had scalp EEG studies (both acquired at the University of Texas Health Science Center at San Antonio, UTHSCSA). Seventy-seven baboons were identified that had both scalp EEG and structural MRI studies. Thirty-nine baboons (IED+) had EEG studies demonstrating IEDs (22 F/17 M, mean age 16 +/− 5 years) and thirty-eight animals did not have IEDs on scalp EEG (22 F/16 M, mean age 18 +/−4 years). Of the 39 IED+ baboons, only 6 had a history of witnessed spontaneous and 12 had suspected seizures. Twenty-two had myoclonic seizures and 20 were photosensitive by EEG. Only four IED+ animals did not have a history of witnessed or suspected seizures, recorded seizures or photosensitivity. Only a subset of the MRI scans were evaluated volumetrically, and included 29 IED+ baboons (13 F/16 M, mean age 17+/−3 years) and 19 IED− baboons (7 F/12 M, mean age 15+/−4 years).
All of the baboons were housed and treated in accordance with the “Guide for the Care and Use of Laboratory Animals” (Grossblatt, 1997). The EEG and neuroimaging studies were both approved by the Institutional Animal Care and Use Committees of the UTHSCSA, SFBR and Southwest National Primate Research Center (SNPRC).
MRI Studies
Handling, anesthesia and imaging protocols used in this study are detailed elsewhere (Kochunov et al., 2007; Rogers et al., 2007). In short, high-resolution (isotropic 500 μm) T1-weighted MR images of cerebrum were obtained using a 3D IRTurboFlash sequence optimized for anatomical imaging of the baboon brain. An adiabatic inversion recovery (IR) contrast pulse with linear phase encoding was employed primarily to render a uniform tissue contrast across the imaging volume. A motion-corrected, high gray matter/white matter contrast protocol (~25%), high-signal-to-noise ratio (~25) was developed for a Siemens TRIO 3 Tesla scanner (Siemens, Erlangen, Germany) using an 8-channel primate head coil.
Image processing and analysis
The image processing steps were assembled into an image processing pipeline consisting of the following: removal of non-brain tissue, correction for radiofrequency inhomogeneity and global spatial normalization to a population-wise template to reduce global variability in brain size and orientation (Rogers et al., 2007). Next, object-based-morphology (OBM) methods were used for quantification of gray matter and white matter volumes, and characterization of individual cortical variability (Kochunov et al., 2005). OBM uses a recognition model that parcelates cortical landscape into objects representing the regional in-folding (sulci) and out-folding (gyri). OBM allows for the characterization of the nature of gyral and sulcal variations by parcelation of cortical landscape into simple 2D surfaces. During OBM processing pial and gray matter/white matter interfaces are reconstructed from the tissue-segmented image (Kochunov et al., 2005). A “crevasse detector” is used to delineate 3-D shape of the individual sulcal surfaces based on topological criteria. Topologically, a sulcus is defined as the medial surface between the two opposing gyral banks that span from the most internal portion of sulcal fold (the fundus) and the intersection with the regionally convex hull of the “brain envelop” surface (the top ridge).
Measurements of sulcal area
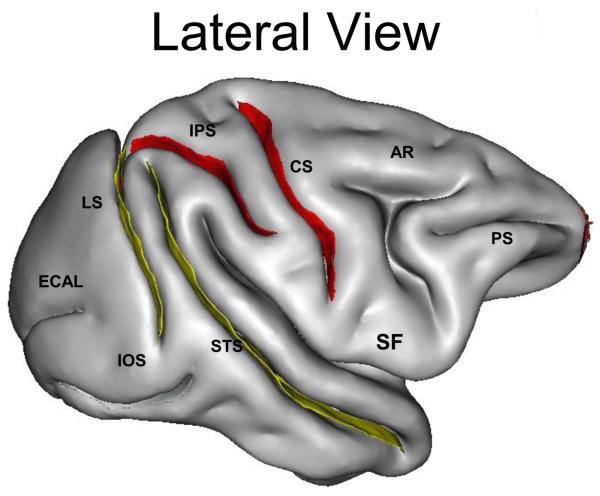
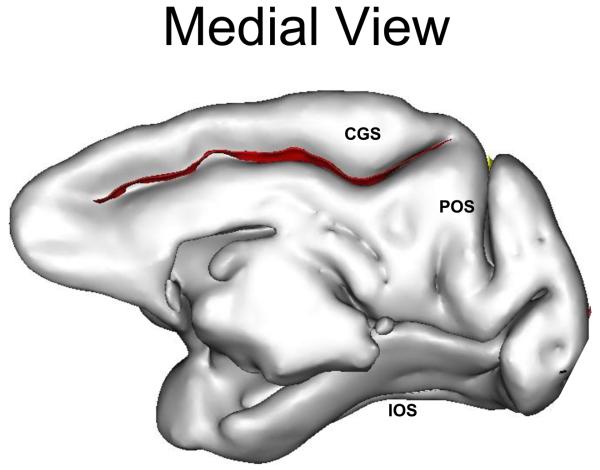
One composite measurement (surface area) and two linear measurements (sulcal length and depth) were made for each sulcus using the methods described elsewhere (Cykowski et al., 2008). Eleven sulci were measured, including central (cs), superior temporal (sts), arcuate (ar; precentral), intraparietal (ips; postcentral), external calcarine (ecal), inferior occipital (ios), principal (ps), cingulate (cgs), parieto-occipital (pos), lunate (ls) and occipitotemporal sulci (ots) (Figure 1). The Sylvian and calcarine fissures were not included as their morphology varied and was anatomically difficult to define.
Figure 1. Affected Sulcal Areas in IED+ Baboons.
To the left a lateral view, and medial view on the right, of the baboon brain. The red labels indicate sulci with statistically significant reductions of sulcal area, whereas the yellow labels reflect marginally significant reductions of sulcal area, in the IED+ baboons. Ps denotes principal sulcus, ar arcuate sulcus, cs central sulcus, ips intraparietal sulcus, sts superior temporal sulcus, lu lunate sulcus, ecal external calcarine sulcus, ios inferior occipital sulcus, cgs cingulate sulcus, pos parieto-occipital sulcus, ots occipitotemporal sulcus (Modified from Kochunov et al., 2005)
Measurement of Gyrification
Cerebral gyrification was measured using the gyrification index (GI), the ratio of the buried to the exposed cortex. The classical 2-D GI methodology (Zilles et al., 1989) for 3-D surfaces was applied by defining the GI as the ratio between the area of a gyrated surface and the area of its convex hull.
EEG Studies
For the one-hour scalp EEG study, all but four infant baboons were restrained in a primate chair. A low dose (5-8 mg/kg) of ketamine was administered intramuscularly to transfer the baboon to and from the primate chair. Interictal epileptic discharges (IED) were only classified once ketamine's effect had resolved. IED+ animals had generalized IEDs, while IEDs were not identified in IED− baboons. The reliability and reproducibility of the EEG studies, EEG instrumentation, and the classification of the studies were described previously (Szabó et al., 2005). The EEG studies were interpreted and classified by a single investigator, who is board-certified in clinical neurophysiology. This investigator was blinded to the animals' previous history.
Statistical Analysis
SPSS Statistics for Windows 17.0 was used for data analysis. The IED+ and IED− groups were compared for age and total brain volume. Sulcal areas, average across hemispheres, were compared across groups and within groups using General Linear Modeling. A multivariate analysis of covariance (MANCOVA) and linear regression analysis (LRA) was used to evaluate age-related structural changes. The omnibus MANCOVA was used to examine age-related trends in sulcal width and depth as a function of IED group status, with age as a continuous covariate. LRA was used to assess multivariate effects obtained in the MANCOVA (if any). This approach was designed to minimize type one error rates.
Results
There were no significant differences between the IED− and IED+ groups with respect to age, total brain, gray or white matter volumes, and gyrification indices (Table 1), The Hotelling's T-Square multivariate test of overall differences among groups was statistically significant [F(2,11) = 30.75, p=0.075] The effect size of this relationship was moderate as indicated by a partial eta-squared of 0.29. IED+ baboons had consistently smaller sulci than the IED− animals, with the exception of the inferior occipital sulcus. Univariate between-subjects test showed statistically significant differences in the intraparietal (ips, p=0.002), central (cs, p=0.03), and cingulate sulci (cgs, p=0.02), and marginal differences involving the lunate (ls, p=0.07) and superior temporal (sts, p=0.08) sulci (Figure 1 and Table 1).
Table 1. Sulcal Areas and Brain Volumes in IED+ and IED− Baboons.
All volumes in mm3 and areas are in mm2 (standardized), IED interictal epileptic discharges, M(ale), F(emale), () standard deviation.
| Attributes and Structures | IED− | IED+ |
|---|---|---|
| Total Brain Volumes | ||
| Number | 19 | 29 |
| Age | 15 (4) | 17 (3) |
| Gender | 7 F/12 M | 13 F/16 |
| Total Brain Volume | 184521 (3395) | 183809 (2543) |
| Total Gray Matter Volume | 102288 (3396) | 102601 (2681) |
| Total White Matter Volume | 82233 (3914) | 81208 (2840) |
| Sulcal Areas (Mean of Left and Right Sides) | ||
| Number | 38 | 39 |
| Age | 16 (5) | 18 (4) |
| Gender | 22F/16M | 22F/15M |
| Gyrification Indices | 1.85 (0.12) | 1.87 (0.13) |
| Principal | 1397 (223) | 1338 (289) |
| Arcuate | 2394 (315) | 2251 (381) |
| Cingulate | 3000 (356) | 2803 (417) |
| Central | 3217 (349) | 3082 (284) |
| Intraparietal | 4571 (589) | 4121 (498) |
| Parieto-occipital | 2753 (685) | 2699 (702) |
| Lunate | 3950 (498) | 3734 (289) |
| Inferior Occipital | 1877 (474) | 1919 (402) |
| External Calcarine | 1335 (451) | 1160 (387) |
| Superior Temporal | 7610 (456) | 7459 (507) |
| Occipito-temporal | 1660 (494) | 1517 (479) |
The sulcal areas were not correlated with age in IED+ nor IED− baboons.
Discussion
This is the first study evaluating volumetric and morphometric differences associated with a natural model of generalized photosensitive epilepsy of the baboon. By combining datasets of brain MRI and scalp electroencephalography (EEG) studies performed in pedigreed baboons, this retrospective analysis compared MRI volume and morphometric changes between animals with interictal epileptic discharges (IED+) and animals without them (IED−). Similar to MRI volumetric and morphometric studies in human generalized epilepsies (Bernasconi et al., 2003; Natsume et al., 2003), IED+ and IED− baboons did not differ with respect to total brain, gray or white matter volumes. In the IED+ baboons, however, a widespread, thought marginally significant, decrease in sulcal areas was encountered. Furthermore, areas of some sulci were significantly decreased in IED+ compared to IED− baboons, including the intraparietal, central and cingulate sulci, and to a less significant degree, the lunate and superior temporal sulci. The gyrification indices did not differ between the groups, but similar to gray and white matter volumes, these indices reflect global, and not regional variations, i.e. decreased gyrification in one area may be masking increased gyrification in another brain region.
The results of this study are preliminary, limited by its retrospective analysis and inclusion of a relatively small number of animals. It is also important to point out that IED+ phenotype does not imply the individual baboon is photosensitive or epileptic, but that it carries an EEG trait indicating a predisposition to spontaneous seizures. As ketamine can lower the threshold for interictal and ictal discharges, particularly at the doses employed in this study, interictal epileptic discharges may not represent as reliable a phenotypic marker as photosensitivity or other clinical symptoms for morphometric analyses. Larger numbers of animals, that are well characterized phenotypically, may demonstrate morphometric variation even within the IED+ group.
Nonetheless, the preliminary findings indicate that there are regional differences in morphometry, and their distribution is not unexpected. The perirolandic and cingulate sulci conform to the brain regions that maximally express ictal and IEDs in the baboon, with or without photic activation (Fischer-Williams et al., 1968). These are also the areas demonstrating the greatest cerebral blood flow changes during photoparoxysmal responses in some humans (Moeller et al., 2009) and baboons (Szabó et al., 2007). The intraparietal sulcus, on the other hand, which divided the parietal lobe of humans and baboons, had the most significantly decreased sulcal areas in the IED+ baboons. Cortical regions associated with the intraparietal sulcus are consistently activated during functional neuroimaging studies of photoparoxysmal responses in humans (Moeller et al., 2009), and along with the perirolandic cortices, its activation is closely correlated with ILS-induced CBF increases in the occipital lobes of photosensitive baboons (Szabó et al., 2009). The decreased sulcal areas in the parietal lobes of IED+ baboons, including the lunate and superior temporal sulci, which extend into the parietal lobe, reflect structural changes in brain regions not implicated by earlier investigators, who proposed that the frontal regions alone were the generators of the ictal and interictal epileptic discharges in this model (Naquet et al., 1975). This paradigm shift was reported in the WAG/Rij model of absence epilepsy, in which the parietal lobes, primarily the barrel somatosensory cortices, were identified as the putative generators of spontaneous ictal and interictal epileptic discharges (Meeren et al., 2002).
While there appears to be general concordance across species that particular cortical areas may be implicated in the generation of generalized ictal and IEDs, it is unclear whether the morphometric abnormalities reflect a structural predisposition or secondary damage related to seizure generation or propagation. Morphometric studies in humans have not shown decreases in cortical gray matter concentration or volume in patients in association with age, seizure frequency or epilepsy duration in idiopathic generalized epilepsies (Woermann et al., 1999; Kim et al., 2007). These authors suggest that alterations, particularly increases, of cortical gray matter volumes or concentrations are likely to be developmental in etiology. While variations in sulcal areas were not correlated with the age of the baboon at the time of imaging, it will be necessary to correlate imaging changes with seizure type, frequency or duration, in order to evaluate potential seizure-related cortical damage. In addition to rigorous histopathological examination of epileptic baboon brains, serial scanning starting at presentation with a first seizure, as well as MRI heritability studies looking at brain development and gyrification in baboons, may be helpful in identifying potential ontogenetic mechanisms underlying the differences of sulcal areas in animals with IEDs (Rogers et al., 2007; Kochunov et al., 2010).
In summary, there are widespread alterations of sulcal areas in baboons with a risk for epilepsy. While it is necessary that these preliminary findings be confirmed in a prospective study involving larger numbers of baboons, these morphometric changes may be markers of networks implicated in the expression of ictal and interictal epileptic discharges. Genetic and pathological studies are needed to determine the etiology of these morphometric changes, while serial MRI studies of baboons in utero and early postnatal development may further clarify the ontogenetic factors underlying the generalized photosensitive epilepsy of the baboon.
Acknowledgements
This study was approved by the Institutional Animal Care and Use Committees of UTHSCSA, SFBR, and the SNPRC and supported by the National Institutes of Health (P51 RR013986 to JR and CAS, R21 NS065431-01 to CAS and R01 NS047755-01 to JTW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernasconi N, Andermann F, Arnold DL, Bernasconi A. Entorhinal cortex: MRI assessment in temporal, extratemporal and idiopathic generalized epilepsy. Epilepsia. 2003;44:1070–1074. doi: 10.1046/j.1528-1157.2003.64802.x. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Li LM, Lopes-Cendes I, Guerreiro MM, Guerreiro CAM, Cendes F. Voxel-based morphometry in patients with idiopathic generalized epilepsies. NeuroImage. 2006;32:498–502. doi: 10.1016/j.neuroimage.2006.04.174. [DOI] [PubMed] [Google Scholar]
- Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67:683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Coulon O, Kochunov PV, Amunts K, Lancaster JL, Laird AR, Glahn DC, Fox PT. The central sulcus: An observer-independent characterization of sulcal landmarks and depth asymmetry. Cereb Cortex. 2007;18:1999–2009. doi: 10.1093/cercor/bhm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Williams M, Poncet M, Riche D, Naquet R. Light-induced epilepsy in the baboon Papio papio: Cortical and depth recordings. Electroencephalogr Clin Neurophysiol. 1968;25:557–569. doi: 10.1016/0013-4694(68)90235-6. [DOI] [PubMed] [Google Scholar]
- Grossblatt N, editor. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington D.C.: 1997. [Google Scholar]
- Killam EK, Stark LG, Killam KF. Photic stimulation in three species of baboons. Life Sci. 1967;6:1569–1574. doi: 10.1016/0024-3205(67)90165-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JK, Koh S-B, Lee S-A, Lee J-M, Kim SI, Kang JK. Regional grey matter abnormailities in juvenile myoclonic epilepsy: A voxel-based morphometry study. NeuroImage. 2007;37:1132–1137. doi: 10.1016/j.neuroimage.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Kochunov PV, Glahn D, Saleem K, Lancaster JL, Fox PT, Shelledy W, Zilles K, Thompson P, Blangero DJ, Rogers J. A genetic study of cerebral sulcation. Does genetics offer a new way of sulcal classification? A study of heritability of sulcal length, depth and area in an extended pedigree of baboons. Hum Brain Mapp. 2007;28:576–583. [Google Scholar]
- Kochunov P, Glahn DC, Fox PT, Lancaster JL, Saleem K, Shelledy W, Zilles K, Thompson PM, Coulon O, Mangin JF, Blangero J, Rogers J. Genetics of primary cerebral gyrification: Heritability of length, depth, and area of primary sulci in an extended pedigree of Papio baboons. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2009.12.045. doi:10.1016/j.neuroimage.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren HKM, Pijn JPM, van Luijtelaar ELJM, Coenen AML, da Silva FHL. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neuroscience. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Ahlgrimm N, Wolff S, Muhle H, Granert O, Boor R, Gotman J, Stephani U, Siniatchkin M. fMRI activation during spike and wave discharges evoked by photic stimulation. NeuroImage. 2009;48:682–695. doi: 10.1016/j.neuroimage.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Naquet R, Catier J, Menini C. Neurophysiology of photically induced epilepsy in Papio papio. Advances in Neurology. 1975;10:107–118. [PubMed] [Google Scholar]
- Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–1300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- Roebling R, Scheerer N, Uttner I, Gruber O, Kraft E, Lerche H. Evalaution of cognition, structural, and functional MRI in juvenile myoclonic epilepsy. Epilepsia. 2009;50:2456–2465. doi: 10.1111/j.1528-1167.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kochunov PV, Lancaster J, Shelledy W, Glahn D, Blangero J, Fox PT. Heritability of brain volume, surface area and shape: an MRI study in an extended pedigree of baboons. Hum Brain Mapp. 2007;28:576–583. doi: 10.1002/hbm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó CÁ, Leland MM, Knape KD, Elliott JJ, Haines VL, Williams JT. Clinical and EEG phenotypes of epilepsy in the baboon (Papio hamadryas spp) Epilepsy Res. 2005;65:71–80. doi: 10.1016/j.eplepsyres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Narayana S, Kochunov PV, Franklin C, Knape KD, Davis MD, Fox PT, Leland MM, Williams JT. PET imaging in the photosensitive baboon: case-controlled study. Epilepsia. 2007;48:245–253. doi: 10.1111/j.1528-1167.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Narayana S, Franklin C, Knape KD, Leland MM, Williams JT, Fox PT. Covariance analysis of occipital lobe CBF in the photosensitive baboon. 2009 [Google Scholar]
- Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structures in juvenile myoclonic epilepsy demonstrated by voxel-based analysis of MRI. Brain. 1999;122:2101–2108. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–50. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]