Abstract
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in young children. Premature infants, immunocompromised individuals and the elderly exhibit the highest risk for the development of severe RSV-induced disease. Murine studies demonstrate that CD8 T cells mediate RSV clearance from the lungs. Murine studies also indicate that the host immune response contributes to RSV-induced morbidity as T-cell depletion prevents the development of disease despite sustained viral replication. Dendritic cells (DCs) play a central role in the induction of the RSV-specific adaptive immune response. Following RSV infection, lung-resident DCs acquire viral antigens, migrate to the lung-draining lymph nodes and initiate the T-cell response. This article focuses on data generated from both in vitro DC infection studies and RSV mouse models that together have advanced our understanding of how RSV infection modulates DC function and the subsequent impact on the adaptive immune response.
Keywords: chemokine, cytokine, dendritic cell, respiratory syncytial virus, T cell, vaccine
Respiratory syncytial virus (RSV) is a single-stranded negative-sense RNA paramyxovirus, which is the leading cause of hospitalizations for lower respiratory tract infection in young children [1]. By the first year of life, approximately two-thirds of children have been infected with RSV at least once, and 90% of children have been infected by their second year of life [2]. Approximately 30 out of 1000 RSV-infected children require hospitalization [3]. In total, it is estimated that RSV causes 34 million acute lower respiratory tract infections annually worldwide in children under the age of 5 years, resulting in up to 196,000 yearly fatalities [4]. Evidence suggests that the host immune response contributes to RSV-induced disease, as previously reviewed [5]. RSV mouse models have been used extensively to examine the role of the immune response in mediating viral clearance as well as the development of immunopathology.
Studies in the murine model have demonstrated that CD8 T cells are required for efficient viral clearance following acute RSV infection [6,7]. However, despite their vital role in mediating viral clearance, murine studies have demonstrated that CD8 T cells can also contribute to RSV-induced disease [6]. In addition to CD8 T cells, CD4 T cells also contribute to RSV-induced disease [6]. Several murine studies have associated Th2 responses with increased RSV pulmonary pathology. Depletion of the Th2-associated cytokine IL-4 prior to acute RSV infection led to decreased lung inflammation and mucus production [8,9]. By contrast, Th1 responses are associated with decreased pulmonary pathology [8,10,11]. Depletion of the Th1 polarizing cytokine IL-12 resulted in increased production of the Th2-associated cytokine IL-13, along with increased airway resistance, pulmonary inflammation and mucus production [10]. Moreover, mice deficient in the interferon (IFN)-induced transcription factor STAT1 exhibit delayed viral clearance and increased production of Th2-associated cytokines [11]. STAT1-deficient mice infected with RSV also exhibit increased pulmonary inflammation and mucus production in an IL-4-dependent manner [8,11]. Thus, in murine models, increased Th2 responses during RSV infection are associated with increased airway resistance and mucus production. It is important to note that although the results of several studies are consistent with the notion that the host immune response can induce immunopathology in RSV-infected humans, other studies suggest that RSV-induced pathology may not be immune mediated, as previously reviewed in detail by Collins and Graham [5]. These data indicate that mouse models may not faithfully reproduce all aspects of RSV-induced disease in humans.
Dendritic cells (DCs) are sentinel cells present in the lung during steady-state conditions. DCs constantly monitor the lungs for foreign pathogens or antigens. Upon RSV infection, DCs acquire viral antigen either through direct infection or indirectly from dying infected cells. DCs that have acquired viral antigen undergo maturation and migrate to the lung-draining lymph nodes (LNs) where they present antigen to naive T cells [12,13]. Murine lung DCs can be divided into two major subsets: CD11chiMHC class IIhi conventional DCs (cDCs) and CD11cintB220+ plasmacytoid DCs (pDCs) [12,13]. In addition, cDCs can be further divided into CD11b+CD103- cDCs (CD11b+ cDCs) and CD11b-CD103+ cDCs (CD103+ cDCs) [12,13]. CD11b+ cDCs and CD103+ cDCs are located in different parts of the lung tissue and exhibit diverse functions [12]. CD103+ cDCs are present in the basal lamina where they extend dendrites into the airway lumen, allowing them to sample the airway for potential foreign pathogens [12,13]. In contrast, CD11b+ cDCs are located within the lung parenchyma where they promote the recruitment of leukocytes through the production of proinflammatory chemokines following infection [12,13]. Unlike CD103+ cDCs and CD11b+ cDCs, the precise anatomical location of pDCs within the lung is currently unknown. Upon viral infection, pDCs are an important source of type I IFNs [12,13]. Human lung DCs consist primarily of CD11chi cDCs, and CD11cdim cDCs with each of these subsets functioning similar to their mouse counterparts [14]. Recent work has focused on understanding the role of DCs following RSV infection. Studies have examined RSV infection of DCs in vitro, as well as tracking DC recruitment and activation following RSV infection in vivo. This review will focus on our current understanding of how DCs modulate the RSV-specific adaptive immune response.
RSV infection of DCs
Respiratory syncytial virus can directly infect murine and human DCs in vitro [15–24]. RSV infects DCs in vitro at a low frequency (i.e., 4%) that can be increased to up to 25% at high multiplicities of infection (i.e., at multiplicity of infection [MOI] ≥20) [17,20,21]. Although RSV infection of DCs results in active viral replication and the production of infectious virions, the level of virus production in RSV-infected DCs is substantially lower than the amount of virus released by infected epithelial cells [16–18].
RSV-induced DC maturation
Respiratory syncytial virus infection of human DCs in vitro results in the maturation of both infected DCs as well as neighboring uninfected DCs [15,19–22,24]. In addition, RSV infection of DCs induces the upregulation of both MHC class I and class II molecules as well as the costimulatory molecules CD83 and CD86 [15,19,20,22,25]. Studies examining in vitro RSV infection of murine DCs have also been reported. RSV infection of murine pDCs increases cell surface expression of MHC class II as well as the costimulatory molecules CD80 and CD86 [23]. Importantly, DC maturation requires the presence of replicating virus, as ultraviolet (UV)-inactivated RSV failed to induce the upregulation of costimulatory molecules or increase the expression of either MHC class I or class II [16,26].
RSV-induced DC cytokine production
In vitro RSV-infection of human monocyte-derived DCs (moDCs) induces the production of proinflammatory cytokines such as IL-1β, IL-6, IL-12, IFN-γ and TNF-α [17,20,21]. Infected moDCs also exhibit increased production of numerous chemokines, including CCL2, CCL3, CCL4, CCL5, CXCL8 and CXCL10 [20,21]. Murine bone marrow-derived DCs (BMDCs) infected in vitro with RSV exhibit increased production of the antiviral cytokines IFN-α and IFN-β, which are required for the upregulation of costimulatory molecules in BMDCs [27].
Human pDCs isolated from peripheral blood mononuclear cells are also susceptible to RSV infection. RSV-infected human pDCs secrete a similar cytokine and chemokine profile to that of RSV-infected moDCs [17]. Human pDCs infected with the Long strain of RSV produced large amounts of the antiviral cytokine IFN-α. IFN-α secretion required replicating virus as UV-inactivated RSV did not induce IFN-α production [28]. Another study demonstrated that whereas the Long strain of RSV induced IFN-α secretion, infection with the A2 strain of RSV failed to induce IFN-α production [29]. By contrast, Guerrero-Plata et al. demonstrated that human pDCs infected with the A2 strain of RSV exhibit increased production of IFN-α [17]. However, one major difference between these two studies is the manner in which RSV was propagated. Guerrero-Plata et al. propagated RSV in HEp-2 cells [17] whereas RSV was grown in Vero cells in the former study [29]. Recent work has demonstrated that RSV virions propagated in Vero cells express a truncated attachment glycoprotein and exhibit a decreased ability to infect human airway epithelial cells [30]. Given that pDC production of IFN-α requires infection with RSV [28], it is possible that RSV propagated in Vero cells may be unable to infect pDCs efficiently enough to stimulate IFN-α production. Similar to human pDCs, murine pDCs produce IFN-α following in vitro RSV infection [23]. Taken together, these studies demon strate that RSV infection of DCs induces the production of proinflammatory cytokines and chemokines.
DC activation of naive T cells
Despite the induction of proinflammatory cytokine production, RSV infection of DCs reduces their capacity to stimulate naive T cells and induce T-cell proliferation [15–17,22,26,31]. Currently, two models have been described to account for the suppression of T-cell activation by RSV-infected DCs. In the first model, suppression of T-cell activation is mediated by the secretion of a soluble factor by RSV-infected DCs [16,31]. In support of this model, CD4 T-cell suppression is induced in a dose-dependent manner when cultured with virus-free supernatants from RSV-infected DCs [31]. Suppression was not dependent on the immunosuppressive cytokines IL-10 and TGF-β [16,31]. In addition, no role for T regulatory cells was found in the suppression of CD4 T-cell proliferation or activation [16]. IFN-α, IFN-γ and IL-1RA protein levels were elevated in the supernatants of RSV-infected DCs. Moreover, antibody-mediated neutralization of each of these cytokines individually failed to restore CD4 T-cell proliferation [16,31]. However, Chi et al. reported that antibody-mediated neutralization of at least two out of three receptors – IFNAR2, IL-10R2 or IL-28R – restored naive CD4 T-cell proliferation [31]. Taken together these data suggest that a soluble factor is released into the supernatant of RSV-infected DCs that is able to suppress the proliferation of naive CD4 T cells.
In addition to the soluble factor model, González et al. demonstrate that RSV infection of DCs disrupts the proper formation of an immune synapse between DCs and naive T cells [26]. González et al. show that naive T cells polarized in the presence of RSV-infected DCs become nonresponsive to restimulation (i.e., IL-2 production and proliferation) with anti-CD3 antibodies, suggesting that the T cells have become anergic [26]. Furthermore, naive CD4 T cells stimulated with RSV-infected DCs failed to polarize their Golgi to the cell surface, suggesting that the DC–T-cell immune synapse is not properly formed [26]. In contrast, the Golgi properly polarized to the immune synapse in naive CD4 T cells stimulated with DCs incubated with UV-inactivated RSV, resulting in efficient proliferation and stimulation of the naive T cells [26]. These data indicate that the impairment of naive T-cell proliferation and stimulation by RSV-infected DCs is the result of inadequate DC-T-cell immune synapse formation. It is worth noting that although González et al. demonstrate that the addition of supernatant from RSV-infected DCs did not alter the ability of naive T cells to produce IL-2 upon CD3 stimulation, the authors did not examine T-cell proliferation in this particular assay, which was the primary readout for the soluble factor studies mentioned previously [16,26,31]. Furthermore, exogenous addition of IL-2 to the RSV-infected DC supernatants had no effect on T-cell proliferation [16,31]. Thus, it is possible that these two models are not mutually exclusive, since cytokines and chemokines are able to modulate DC–T-cell synapse formation [32].
Stimulation of naive T cells with RSV-infected DCs results in decreased production of IL-2, IL-4, IFN-γ and TNF-α [15,16,22]. However, memory T cells stimulated with RSV-infected DCs showed no alteration in effector cytokine production, demonstrating that RSV inhibits the stimulation of naive but not memory T cells [22]. In addition to a decreased capacity to induce T-cell proliferation, RSV-infected DCs also exhibit a reduced capacity to stimulate effector cytokine production by polarized T cells. Taken together, RSV-infected DCs demonstrate a decreased capacity to stimulate naive T cells, either due to the secretion of a soluble factor by infected DCs or the disruption of proper immune synapse formation between infected DCs and naive T cells.
Respiratory DC migration following RSV infection
Several in vivo studies using the RSV mouse model have examined the kinetics and function of respiratory DCs following infection. There is an increase in the total number of DCs in the lung and lung-draining LNs following RSV infection [33]. Importantly, pulmonary DCs express elevated cell surface levels of MHC class II and the costimulatory molecule CD86 [33]. However, in contrast to in vitro studies these lung-derived DCs were able to efficiently stimulate naive T-cell proliferation ex vivo [33]. It should be noted that in the in vitro studies DCs were directly infected with RSV, whereas it is unknown if the DCs in the RSV-infected animals were directly infected or indirectly matured by the presence of proinflammatory cytokines. Regardless, these data suggest that DCs are recruited into the lung and activated upon RSV infection. More recent studies have examined the recruitment and maturation pattern of both cDCs and pDCs following RSV infection [25,34]. The number of cDCs in the lung increased approximately threefold by day 8 postinfection. Although pDC numbers also increase approximately threefold, in contrast to cDC, pDC numbers peak at 72 h postinfection [25,34]. Interestingly, pDC numbers remain elevated for up to 30 days postinfection [23,35]. One potential explanation for the sustained increase in pDC numbers is that pDCs are long-lived cells compared to cDCs, which turnover more rapidly [36]. As discussed earlier, cDCs can be further divided into two subpopulations: the subepithelial CD11b+ cDCs and parenchymal CD103+ DCs [34]. These two distinct populations exhibit very different kinetics in the lung following acute RSV infection. Whereas the total number of CD11b+ cDCs increased in the lung following RSV infection, the total number of CD103+ cDC decreased by 50% during the first 48 h and remained low during the course of the RSV infection [34]. These studies demonstrate that, following RSV infection, CD11c+ cDCs and pDCs are recruited to the lungs, where they are in a prime position to modulate the adaptive immune response. In support of this notion, cDCs isolated from RSV-infected lungs exhibit increased expression of the costimulatory molecules CD80 and CD86 [25].
Upon viral infection, DCs migrate from the site of infection to the draining LNs, where they present viral antigen to naive T cells [37]. The total numbers of cDCs in the lung-draining LNs are increased up to threefold, peaking at day 7 postinfection [25,35]. CD11b+ cDCs and CD103+ cDCs exhibit different recruitment patterns to the LN [34]. During the first 48 h postinfection, CD11b+ and CD103+ cDCs migrate from the lung into the lung-draining LNs at equivalent numbers. After 48 h postinfection, however, CD103+ cDC numbers in the lung-draining LN remain constant. In contrast, CD11b+ cDCs continue to migrate into the LNs, peaking at day 4 postinfection and maintaining increased numbers for at least 7 days postinfection [34]. When compared to resident LN DCs, the migrating cDCs exhibit a mature phenotype, demonstrating elevated cell surface expression of CD40, CD80 and CD86 [34]. In addition, migrating CD11b+ and CD103+ cDCs contain viral RNA in contrast to LN-resident cDCs. Consistent with the increased expression of costimulatory molecules and the presence of virus-derived RNA, migrating cDCs were significantly better at stimulating IFN-γ production by effector CD4 and CD8 T cells compared to LN resident cDCs [34]. Both CD11b+ and CD103+ cDCs exhibit a similar capacity to stimulate IFN-γ production by CD4 and CD8 T cells. Taken together, these studies demonstrate that, following RSV infection, lung cDCs acquire viral antigen, mature and subsequently migrate to the lung-draining LNs to stimulate naive T cells.
Similar to cDCs, pDCs also migrate to the LN following acute RSV infection [35]. An eight-fold increase in the total number of pDCs is observed in the lung-draining LNs following RSV infection [35]. Similar to the lung, pDC numbers remain elevated in the LN for 30 days postinfection [35]. However, the function of these migrating pDCs is currently unclear. Consistent with the idea that pDCs are poorly stimulated, RSV-infected pDCs do not induce the proliferation of naive T cells in vitro [23]. Similar to RSV infection, following ovalbumin (OVA) sensitization pDCs pick up OVA antigen and migrate to the lung-draining LN [38]. Although depletion of pDCs did not affect the total number of LN T cells, an increased production of effector cytokines by LN T cells was observed [38]. Furthermore, adoptive transfer of pDCs prior to OVA sensitization decreased T-cell production of effector cytokines, suggesting that although pDCs do not stimulate naive T cells, they can regulate T-cell effector function [38]. Although studies have demonstrated the importance of pDCs in regulating T-cell effector functions (discussed later), the exact role of migrating pDCs is unclear and requires further investigation.
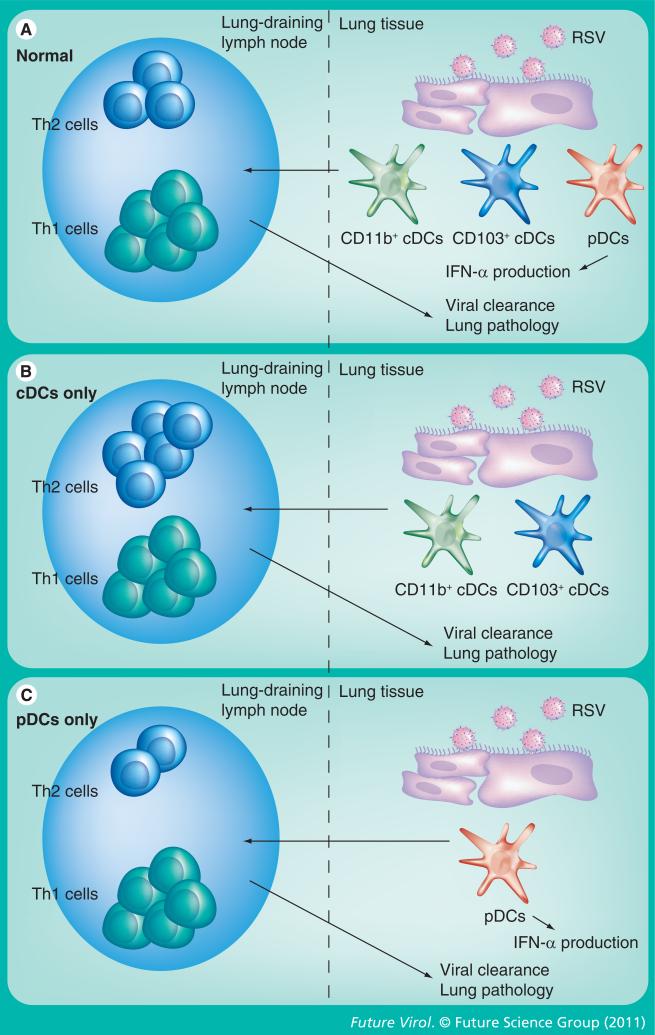
The migration of DCs has also been examined in RSV-infected children. Nasal washes from children with RSV infection display increased levels of both cDCs and pDCs, and increased DC recruitment continues for up to 8 weeks following acute infection [39,40]. In addition to the increased numbers of DCs in the nasal passages, a decrease in the number of DCs in the blood was observed in children with acute RSV infection, indicating that DCs are recruited out of the blood to the site of infection. Furthermore, DCs present in the nasal mucosa contained RSV protein, however it is currently unknown if these DCs are infected or if they acquired viral antigens indirectly from dying RSV-infected cells. In addition, the maturation status of these nasal DCs or their functional capacity remains to be examined. However, correlations between the number of cDCs and the level of proinflammatory cytokines such as IL-6 and MIP-1α suggest that cDCs may represent an important source of inflammatory cytokines in the nasal cavity after RSV infection [39,40]. Overall, it is apparent that upon RSV infection, both cDCs and pDCs are recruited to the site of infection where they obtain viral antigen, resulting in maturation and increased production of proinflammatory cytokines that help modulate the immune response (Figure 1).
Figure 1. Dendritic cells after respiratory syncytial virus infection.
Upon RSV infection of lungs in normal conditions (A), CD11b+ and CD103+ cDCs as well as pDCs are matured and migrate to the lung-draining lymph nodes. These migrating DCs prime both Th1 and Th2 cells, which migrate to the lung and promote viral clearance with little lung pathology (i.e., airway hypersensitivity and histology). RSV infection of pDC-depleted mice (B) results in maturation and migration of cDCs to the lung-draining lymph nodes. Although cDCs prime Th1 cells, in the absence of pDCs, cDCs also promote Th2 activation. These Th2 cells migrate to the lungs, resulting in decreased viral clearance and increased lung pathology. RSV infection of mice that lack cDC recruitment to the lungs presumably results in pDC activation and migration into the lung-draining lymph nodes. (C) These pDCs activate Th1 cells but inhibit the expansion of Th2 cells. Migration of these T cells, as well as IFN-α production by pDCs, results increased viral clearance and decreased lung pathology.
cDC: Conventional dendritic cell; DC: Dendritic cell; pDC: Plasmacytoid dendritic cell; RSV: Respiratory syncytial virus.
Origin of lung DC after RSV infection
CD11c+CD31+Ly6C+ preimmunocytes are present at low levels in the bone marrow and peripheral blood but increase in numbers after inflammatory stimulus [41]. These preimmunocytes possess the capacity to differentiate into mDCs in the presence of GM-CSF in vitro [41]. A significant amount of GM-CSF is present in the lungs of RSV-infected animals the first 48 h after infection [42] that could potentially induce the differentiation of blood preimmunocytes that are recruited to the lungs following RSV infection. However, it remains to be determined if these CD11c+CD31+Ly6C+ preimmunocytes are recruited to the lungs following RSV infection, and if so, what fraction of the increased DC numbers in the lung is due to differentiation of DCs from precursors. In a series of elegant experiments, Wang et al. demonstrate that a CD11c+MHC class II– precursor population is present in the lungs of naive mice [42]. In the presence of GM-CSF, these CD11c+MHC class II– precursor cells differentiate into CD11b+ cDCs [42]. Importantly, this population of progenitor cells decreased following RSV infection. In addition, CD11c+ cDCs from RSV-infected mice demonstrate upregulated Ki-67 expression, suggesting that the CD11c+MHC class II– precursor population may contribute to the increase in the number of cDCs following acute RSV infection. Although pDCs also significantly increase in number following RSV infection, the origin of these pDCs has not been extensively studied, in large part because the origin and lineage of pDCs is controversial and not completely understood [43].
Differential regulation of the immune response by cDCs & pDCs
In murine models, pathology in the lungs following acute RSV infection has been correlated with increased Th2-associated responses that result in mucus production in the lung airways and increased airway hyper-reactivity [8,9]. Signals from DCs largely impact the fate of the T-cell response [37], therefore much work has focused on examining the role of DC subsets in regulating T-cell-mediated pulmonary immuno pathology. Depletion of pDCs prior to RSV infection results in increased airway hyper-reactivity, pulmonary inflammation and mucus production as well as decreased viral clearance [23,35]. Lung T cells isolated from pDC-depleted mice infected with RSV exhibit increased mRNA and protein levels of the Th2-associated cytokines IL-4, IL-5 and IL-13, as well as the Th1 cytokine IFN-γ, upon in vitro restimulation with anti-CD3 [35]. In addition, levels of IFN-α in the lungs of pDC-depleted animals did not increase after RSV infection [35]. In contrast, lungs from control RSV-infected mice contained significant amounts of IFN-α, indicating that pDCs are the primary source of IFN-α after RSV infection [35]. Exogenous addition of IFN-α during RSV infection of pDC-depleted animals resulted in decreased viral titers [35]. However, pDC-depleted animals administered IFN-α still displayed increased airway hypersensitivity and lung-draining LN T cells from these animals exhibited increased levels of IL-4, IL-5, IL-13 and IFN-γ [35]. These data suggest that although pDC secretion of IFN-α is important for mediating efficient viral clearance (Figure 1), pDCs regulate T-cell effector functions via IFN-α-independent mechanisms. To further demonstrate the role of pDCs in the regulation of T-cell responses, Flt3L-mediated expansion of both pDCs and cDCs was found to decrease airway hyper-reactivity, pulmonary mucus production and Th2-associated cytokine production from lung-draining LN T cells after RSV infection [44]. pDCs in Flt3L-treated animals preferentially upregulated the costimulatory molecules CD80 and CD86 after RSV infection compared to cDCs [44]. The pDCs isolated from Flt3L-treated animals also secreted large amounts of IFN-α after RSV infection [44]. In addition, although CD4 T-cell frequencies were unaltered, increased frequencies of CD8 T cells as well as IFN-γ-producing CD8 T cells were present in RSV-infected animals that were pre-treated with Flt3L [44]. However, the selective depletion of pDCs in the Flt3L-treated animals resulted in a reduced number of CD8 T cells and IFN-γ-producing CD8 T cells comparable with RSV-infected wild-type control mice [44]. Furthermore, Flt3L-treated animals depleted of pDCs exhibit an increased number of T cells in the lung-draining LNs after RSV infection compared to both RSV-infected control Flt3L-treated mice as well as RSV-infected wild-type mice. Taken together, these data suggest that pDCs have two major functions during RSV infection: secretion of IFN-α that promotes viral clearance from the lung; and regulation of effector T-cell responses, most notably Th2 responses, thereby limiting airway hyper-reactivity and pulmonary mucus production (Figure 1).
Upon inflammation in the lungs, cDCs utilize CCR6 binding to its ligand CCL20 in order to migrate into the lungs [45]. Upon RSV infection, increased CCL20 levels in the lungs are observed, and CCR6-positive cDCs are recruited to the lungs [46]. By contrast, recruited pDCs are CCR6-negative. In addition there is no recruitment of cDCs to the lungs after RSV infection of CCR6-deficient mice, whereas pDC recruitment is unaltered [46]. CCR6-deficient mice exhibit increased viral clearance, decreased pulmonary mucus production and decreased levels of Th2-associated cytokines in the lungs and lung-draining LNs [46]. These observations indicate that recruited CCR6+ cDCs promote Th2-associated responses upon RSV infection. Further supporting this conclusion, reconstitution of lungs with either wild-type or CCR6-deficient cDCs immediately prior to RSV infection resulted in increased levels of mucus and Th2-associated cytokines as compared to nonreconstituted RSV-infected CCR6-deficient mice. This study, along with the studies discussed previously, suggest that whereas cDCs promote a Th2-associated T-cell response after RSV infection, pDCs inhibit the development of aberrant Th2-associated T-cell responses and promote viral clearance (Figure 1). Further substantiating the role of pDCs in regulating Th2-associated T-cell responses during RSV infection in humans, children who develop asthma after RSV infection, which is thought to result from the overproduction of Th2-associated cytokines, exhibit lower levels of peripheral blood pDCs as compared to children that do not develop asthma [47].
Although the exact mechanism by which pDCs inhibit Th2-associated T-cell responses is unknown, one potential mechanism is through the expression of notch ligands. DCs can direct helper T-cell differentiation during infection through the expression of notch ligands that interact with notch receptors expressed on T cells [48]. For example, DCs that express the notch ligand Delta-like 4 (dll4) promote Th1 responses and inhibit the induction of Th2 responses, whereas expression of Jagged1 on DCs promotes the generation of Th2 responses [48,49]. BMDCs upregulate cell surface expression of dll4 upon RSV infection in vitro, resulting in increased IFN-γ production by RSV-specific CD4 T cells that is blocked in the presence of γ-secretase, an antibody that blocks notch activation [50]. Furthermore, mice treated with anti-dll4 antibody during RSV infection exhibit increased airway hyper-reactivity, pulmonary mucus production and T-cell production of Th2-associated cytokines in both the lungs and lung-draining LNs [51]. These observations suggest that DCs regulate Th2 responses in a dll4-dependent manner. However, the relative expression of dll4 on pDCs and cDCs was not examined, and therefore it is currently unclear if either pDCs or cDCs preferentially regulate Th2 responses during RSV infection. Given the importance of pDCs in regulating Th2-associated responses during RSV infection, and the observation that pDCs have been shown to regulate the effector function of T cells after OVA sensitization [38], it is possible that, following RSV infection, pDC preferentially upregulate dll4 expression and traffic to the lung-draining LNs where they regulate and/or inhibit the generation of Th2 responses following RSV infection (Figure 1).
Role of DCs in RSV vaccine development
Establishment of a suitable antiviral immune response is a stepwise process that is critically dependent on the functions of DCs. DCs contribute to early host defense through the production of type I IFNs [52,53], but are also responsible for shaping the subsequent adaptive immune response. Recent work has demonstrated that nonstructural proteins 1 and 2 (NS1 and NS2) of RSV can inhibit type I IFN production and IFN regulatory factor 3 activation, which has been demonstrated to induce DC maturation [27,54,55]. In addition, infection of human DCs with a recombinant RSV containing NS1 and NS2 mutations resulted in enhanced DC maturation [21]. Antigen presentation to T cells by cDCs is diminished for several weeks following RSV infection [25]. Taken together, these data suggest that RSV can disrupt the development of an adequate adaptive immune response.
Development of RSV vaccines must consider these issues in order to prime an immune response that is both protective and safe. Although inactivated RSV vaccines are no longer virulent, they are typically less immunogenic and often require one or more booster immunizations in order to provide sufficient immunity. A recent study demon strated that decreased DC maturation due to poor Toll-like receptor (TLR) signaling following the administration of UV-inactivated RSV to mice results in exacerbated disease upon RSV challenge [56]. Supplementation of UV-inactivated RSV with poly(I:C) and lipopolysaccharide (LPS), TLR3 and TLR4 agonists, respectively, enhanced the humoral response and prevented exacerbated disease following RSV challenge. This suggests that RSV vaccine-enhanced disease may be the result of a poor humoral response resulting in the production of non-neutralizing antibodies that can form harmful immune complexes. Thereby, incorporation of a TLR agonist may be necessary for inactivated RSV vaccines to enhance DC maturation, thus inducing neutralizing antibodies that protect against disease enhancement upon subsequent RSV challenge. However, it is important to note that not all TLRs are sufficient to prevent vaccine-enhanced disease, as TLR7 and TLR8 stimulation at the time of formalin-inactivated (FI)-RSV immunization did not diminish vaccine-enhanced disease [57]. Adjuvants can also boost the immunogenicity of inactivated vaccines. Careful selection is required, as common alum-based adjuvants have been shown to promote a Th2-associated response [58,59] that is frequently associated with RSV vaccine-enhanced disease symptoms [60,61]. A promising adjuvant, monophosphoryl lipid A, is a TLR4 agonist that increases immunogenicity by inducing DC maturation, but also stimulates a Th1-skewed response that may prevent disease exacerbation [62].
Live-attenuated RSV vaccines are usually more immunogenic in comparison with inactivated vaccines, and may prevent disease associated with primary RSV infection early in life. However, efforts to develop a live-attenuated RSV vaccine that does not induce disease symptoms and still provides sufficient immunogenicity to prevent reinfection have yet to succeed [63,64]. Most work involving RSV subunit vaccines has also been hindered due to potential disease exacerbation similar to that observed in children administered a formalin-inactivated RSV [65–67]. Thus, development and testing of future RSV vaccines should evaluate the DC composition following vaccination. Whereas an adequate DC response is necessary to mediate activation of the adaptive immune response, the DC subset(s) activated will ultimately impact overall immunity and potential disease severity. Thus, future RSV vaccine development must consider the DC response induced to not only promote the appropriate adaptive response to protect against infection, but also to prime a response that does not lead to exacerbated disease upon natural infection.
Conclusion & future perspective
Dendritic cells are sentinel cells that are strategically located in the lungs allowing them to detect foreign antigen and subsequently stimulate adaptive immune responses. Much research has focused on examining the role of DCs following RSV infection as well as the effect of RSV infection on DC maturation and function. RSV is able to infect both human and murine DCs in vitro [17,20,21]. Although RSV-infected DCs mature, they exhibit an inability to stimulate naive T cells [15–17,22,26,31]. However the exact mechanism by which RSV is able to inhibit the capacity of DCs to stimulate naive T cells is currently unknown. Recent studies suggest that either soluble factors [16,31] or disruption of the immune synapse [26] are responsible. Future studies should focus on determining what soluble factors are secreted and whether they are RSV-derived or DC-derived. Furthermore, the exact mechanism of how RSV disrupts the formation of the immune synapse between DCs and naive T cells remains unknown. Importantly, it is unclear whether or not RSV-mediated inhibition of DC activation of naive T cells occurs during in vivo RSV infection. Migrating murine cDCs contain viral protein and are able to efficiently stimulate RSV-specific effector/memory T cells, but their ability to stimulate naive T cells has not been evaluated [34]. In addition, human DCs in the nasal cavity of RSV-infected children contain viral antigen [39], but it remains to be determined whether these DCs exhibit a defect in their capacity to stimulate naive T cells.
pDCs are important in promoting viral clearance following acute RSV infection through the production of IFN-α [35]. In addition, pDCs appear to play a major role in regulating Th2-associated responses following RSV infection, however, the exact mechanism is currently unclear [23,35,44,47]. This review highlights studies that also demonstrate the importance of the notch ligand dll4 in regulating Th2-associated responses following acute RSV infection [51]. A recent study examining splenic pDCs and liver pDCs demonstrated that splenic pDCs preferentially expressed the notch ligand dll4, and in turn inhibited T-cell production of the Th2-associated cytokine IL-4 compared to liver pDCs [68]. Therefore, it would be worthwhile to examine the expression of the notch ligand dll4 on pDCs compared to cDCs, which induce Th2-associated responses after RSV infection [46], to determine if pDCs inhibit Th2-associated responses through the interaction of T cells with notch ligands expressed by the DCs.
Up to this point, no licensed RSV vaccines have been developed. This is due in part to an incomplete understanding of the underlying immunological basis for the enhanced disease exhibited by children immunized with a FI-RSV vaccine [69]. Given the importance of pDCs in regulating the Th2-associated responses during acute RSV infection, we believe that it would be of great interest to examine the role of pDCs versus cDCs following FI-RSV immunization as well as after subsequent RSV challenge. Kruijsen et al. have shown that after RSV challenge of FI-RSV-immunized mice, an increased number of both CD11b+ and CD103+ cDCs are recruited to the lung as compared to RSV-infected mock-immunized mice [70]; however, the function or role of these cDCs, as well as pDCs, during FI-RSV immunization has not been examined. Therefore, DC composition should strongly be considered when generating new vaccinations in order to protect against the generation of detrimental Th2-associated responses and ensure the formation of a protective Th1 response, thus allowing for the production of a safe and effective vaccine.
Executive summary.
Background
■ Respiratory syncytial virus (RSV) is an important human pathogen that can cause serious respiratory tract disease in multiple populations.
■ Lung dendritic cells (DCs) play a critical role in the recognition of viral antigen and initiating adaptive immune responses.
RSV infection of DCs
■ Infection of DCs with RSV in vitro induces their maturation and the maturation of neighboring DCs.
■ RSV-infected DCs produce multiple antiviral cytokines and inflammatory chemokines, which are responsible for the maturation of neighboring DCs.
■ RSV-infected DCs exhibit a reduced capacity to stimulate naive T-cell proliferation due to either secretion of a soluble factor that inhibits T-cell activation or improper immune synapse formation.
Respiratory DC migration following RSV infection
■ Lung CD11b+ conventional DCs and plasmycytoid DCs upregulate maturation markers and increase in number following acute RSV infection.
■ CD103+ and CD11b+ cDCs and pDCs are recruited to the lung-draining lymph nodes (LNs) following RSV infection.
■ Migrating CD103+ and CD11b+ cDCs carry viral protein to the lung-draining LNs and are efficient at stimulating naive CD4 and CD8 T cells.
Differential regulation of the immune response by conventional dendritic cells & plasmacytoid dendritic cells
■ Depletion of plasmacytoid DCs results in increased Th2-associated responses after RSV infection that correlates with increased airway hypersensitivity and pulmonary mucus production as well as decreased viral clearance.
■ Prevention of cDC recruitment during acute RSV infection results in decreased airway hypersensitivity, pulmonary mucus production and Th2-associated cytokine production as well as increased viral clearance.
■ Notch ligand Delta-like 4 expression on DCs is important for the regulation of Th2-associated responses.
Role of DCs in RSV vaccine development
■ Development of an inactivated or subunit RSV vaccine may require Toll-like receptor agonist supplementation to induce both sufficient DC maturation and a Th1-skewed response to prevent disease exacerbation upon natural infection.
■ RSV disease pathology is mediated by conventional DC responses, thus future vaccines should induce plasmacytoid DC responses that limit RSV infection and help generate adequate CD8 T-cell responses.
Conclusion & future perspective
■ We review data that demonstrates that DCs infected with RSV in vitro mature but are inhibited in their ability to stimulate naive T cells. The exact mechanisms remain to be clarified, as well as the role of RSV-induced DC inhibition of naive T-cell activation in vivo.
■ The data reviewed here indicates that DC composition is very important in the generation of a productive and protective immune response against RSV, which should be considered in the development of future RSV vaccines.
Acknowledgments
This work is supported by the NIH Grant AI 063520 (to SM Varga).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■ of considerable interest
- 1.Heilman CA. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J. Infect. Dis. 1990;161(3):402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 1991;88(3):1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Experi. Med. 1997;186(3):421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore ML, Newcomb DC, Parekh VV, et al. STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J. Immunol. 2009;183(3):2016–2026. doi: 10.4049/jimmunol.0803167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer JE, Johnson JE, Kuli-Zade RK, et al. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J. Virol. 1997;71(11):8672–8677. doi: 10.1128/jvi.71.11.8672-8677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tekkanat KK, Maassab H, Berlin AA, et al. Role of interleukin-12 and stat-4 in the regulation of airway inflammation and hyperreactivity in respiratory syncytial virus infection. Am. J. Pathol. 2001;159(2):631–638. doi: 10.1016/S0002-9440(10)61734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durbin JE, Johnson TR, Durbin RK, et al. The role of IFN in respiratory syncytial virus pathogenesis. J. Immunol. 2002;168(6):2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 12.Geurtsvankessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal. Immunol. 2008;1(6):442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 13.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31(3):412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 2010;376(9743):835–843. doi: 10.1016/S0140-6736(10)61226-3. [DOI] [PubMed] [Google Scholar]
- 15.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-γ in naive T cells. Immunology. 2003;109(1):49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16■.De Graaff PM, De Jong EC, Van Capel TM, et al. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J. Immunol. 2005;175(9):5904–5911. doi: 10.4049/jimmunol.175.9.5904. [Examines dendritic cell (DC) maturation after direct respiratory syncytial virus (RSV) infection. This paper also demonstrates that RSV-infected DCs produce a soluble factor that inhibits naive T-cell proliferation.] [DOI] [PubMed] [Google Scholar]
- 17.Guerrero-Plata A, Casola A, Suarez G, et al. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2006;34(3):320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobson L, Everard ML. Persistent of respiratory syncytial virus in human dendritic cells and influence of nitric oxide. Clin. Exp. Immunol. 2008;151(2):359–366. doi: 10.1111/j.1365-2249.2007.03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones A, Morton I, Hobson L, Evans GS, Everard ML. Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus. Clin. Exp. Immunol. 2006;143(3):513–522. doi: 10.1111/j.1365-2249.2005.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Nouen C, Munir S, Losq S, et al. Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology. 2009;385(1):169–182. doi: 10.1016/j.virol.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munir S, Le Nouen C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J. Virol. 2008;82(17):8780–8796. doi: 10.1128/JVI.00630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothoeft T, Fischer K, Zawatzki S, Schulz V, Schauer U, Korner Rettberg C. Differential response of human naive and memory/effector T cells to dendritic cells infected by respiratory syncytial virus. Clin. Exp. Immunol. 2007;150(2):263–273. doi: 10.1111/j.1365-2249.2007.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23■.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J. Immunol. 2006;177(9):6263–6270. doi: 10.4049/jimmunol.177.9.6263. [Demonstrates that plasmacytoid DCs (pDCs) promote viral clearance and regulate pulmonary immunopathology following RSV infection.] [DOI] [PubMed] [Google Scholar]
- 24.Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One. 2011;6(1):E16458. doi: 10.1371/journal.pone.0016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25■.Guerrero-Plata A, Kolli D, Hong C, Casola A, Garofalo RP. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J. Immunol. 2009;182(5):3072–3083. doi: 10.4049/jimmunol.0802262. [Examines the recruitment of conventional DCs and pDCs in the lung and lung-draining LNs following RSV infection as well as their cytokine profile.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26■■.Gonzalez PA, Prado CE, Leiva ED, et al. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc. Natl Acad. Sci. USA. 2008;105(39):14999–15004. doi: 10.1073/pnas.0802555105. [Demonstrates that RSV-infected DCs do not form an appropriate immune synapse with CD4 T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudd BD, Luker GD, Luker KE, Peebles RS, Lukacs NW. Type I interferon regulates respiratory virus infected dendritic cell maturation and cytokine production. Viral Immunol. 2007;20(4):531–540. doi: 10.1089/vim.2007.0057. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, Schlender J, Guenthner-Biller M, et al. Replication-dependent potent IFN-a induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 2004;173(10):5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 29.Schlender J, Hornung V, Finke S, et al. Inhibition of Toll-like receptor 7- and 9-mediated α/β interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 2005;79(9):5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J. Virol. 2009;83(20):10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31■■.Chi B, Dickensheets HL, Spann KM, et al. α and λ interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol. 2006;80(10):5032–5040. doi: 10.1128/JVI.80.10.5032-5040.2006. [Demonstrates that RSV-infected DCs secrete a soluble factor that inhibits the proliferation of CD4 T cells. This paper also shows that blocking a combination of cytokine receptors restores naive T-cell proliferation and activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez PA, Carreno LJ, Figueroa CA, Kalergis AM. Modulation of immunological synapse by membrane-bound and soluble ligands. Cytokine Growth Factor Rev. 2007;18(1–2):19–31. doi: 10.1016/j.cytogfr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Beyer M, Bartz H, Horner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J. Allergy Clin. Immunol. 2004;113(1):127–133. doi: 10.1016/j.jaci.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 34■■.Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, Van Bleek GM. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 2009;83(14):7235–7243. doi: 10.1128/JVI.00452-09. [Examines the migration of CD11c+ cDCs and CD103+ cDCs into the lung-draining lymph nodes following RSV infection. This paper also demonstrates that migrating cDCs from the lung are efficient at stimulating naive T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35■■.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 2006;203(5):1153–1159. doi: 10.1084/jem.20052359. [Demonstrates that pDCs regulate Th2-associated responses that results in increased pulmonary immunopathology following RSV infection. This report also demonstrates that pDCs mediate viral clearance within the lung in an IFN-α-dependent manner.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soukup JM, Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin. Immunol. 2003;107(3):178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 37.Reis E, Sousa C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006;6(6):476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 38.De Heer HJ, Hammad H, Soullie T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 2004;200(1):89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39■■.Gill MA, Palucka AK, Barton T, et al. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J. Infect. Dis. 2005;191(7):1105–1115. doi: 10.1086/428589. [Demonstrates that cDCs and pDCs are recruited to the nasal mucosa of RSV-infected children.] [DOI] [PubMed] [Google Scholar]
- 40.Gill MA, Long K, Kwon T, et al. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J. Infect. Dis. 2008;198(11):1667–1676. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruno L, Seidl T, Lanzavecchia A. Mouse pre-immunocytes as non-proliferating multipotent precursors of macrophages, interferon-producing cells, CD8 α+ and CD8 α- dendritic cells. Eur. J. Immunol. 2001;31(11):3403–3412. doi: 10.1002/1521-4141(200111)31:11<3403::aid-immu3403>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Peters N, Laza-Stanca V, Nawroly N, Johnston SL, Schwarze J. Local CD11c+ MHC class II- precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J. Immunol. 2006;177(4):2536–2542. doi: 10.4049/jimmunol.177.4.2536. [DOI] [PubMed] [Google Scholar]
- 43.Reizis B. Regulation of plasmacytoid dendritic cell development. Curr. Opin. Immunol. 2010;22(2):206–211. doi: 10.1016/j.coi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 2008;3(3):E1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osterholzer JJ, Ames T, Polak T, et al. CCR2 and CCR6, but not endothelial selectins, mediate the accumulation of immature dendritic cells within the lungs of mice in response to particulate antigen. J. Immunol. 2005;175(2):874–883. doi: 10.4049/jimmunol.175.2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46■■.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur. J. Immunol. 2010;40(4):1042–1052. doi: 10.1002/eji.200939778. [Demonstrates that cDCs promote Th2-associated responses following RSV infection that correlate with pulmonary immunopathology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silver E, Yin-Declue H, Schechtman KB, Grayson MH, Bacharier LB, Castro M. Lower levels of plasmacytoid dendritic cells in peripheral blood are associated with a diagnosis of asthma 6 yr after severe respiratory syncytial virus bronchiolitis. Pediatr. Allergy Immunol. 2009;20(5):471–476. doi: 10.1111/j.1399-3038.2008.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117(4):515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 49.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands δ1 and δ4. J. Immunol. 2008;180(3):1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudd BD, Schaller MA, Smit JJ, et al. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 2007;178(9):5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 51■■.Schaller MA, Neupane R, Rudd BD, et al. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J. Exp. Med. 2007;204(12):2925–2934. doi: 10.1084/jem.20070661. [Demonstrates that DCs regulate Th2-associated responses following RSV infection through the notch ligand dll4, resulting in decreased pulmonary immunopathology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferbas JJ, Toso JF, Logar AJ, Navratil JS, Rinaldo CR., Jr CD4+ blood dendritic cells are potent producers of IFN-α in response to in vitro HIV-1 infection. J. Immunol. 1994;152(9):4649–4662. [PubMed] [Google Scholar]
- 53.Biron CA. Role of early cytokines, including α and β interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin. Immunol. 1998;10(5):383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 54.Bossert B, Marozin S, Conzelmann KK. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 2003;77(16):8661–8668. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of α, β, and λ interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J. Virol. 2004;78(8):4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15(1):34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57■■.Johnson TR, Rao S, Seder RA, Chen M, Graham BS. TLR9 agonist, but not TLR7/8, functions as an adjuvant to diminish FI-RSV vaccine-enhanced disease, while either agonist used as therapy during primary RSV infection increases disease severity. Vaccine. 2009;27(23):3045–3052. doi: 10.1016/j.vaccine.2009.03.026. [Demonstrates that vaccination with ultraviolet-inactivated RSV results in decreased DC maturation, resulting in the production of non-neutralizing antibodies that form harmful immune complexes upon natural RSV infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur. J. Immunol. 1996;26(9):2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 59.Mckee AS, Munks MW, Macleod MK, et al. Alum induces innate immune responses through macrophage and masT cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 2009;183(7):4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 1994;68(8):5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 1996;70(5):2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24(23):5027–5035. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 63.Schickli JH, Dubovsky F, Tang RS. Challenges in developing a pediatric RSV vaccine. Hum. Vaccin. 2009;5(9):582–591. doi: 10.4161/hv.9131. [DOI] [PubMed] [Google Scholar]
- 64.Wright PF, Karron RA, Belshe RB, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25(42):7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Power UF, Nguyen TN, Rietveld E, et al. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J. Infect. Dis. 2001;184(11):1456–1460. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- 66.Wathen MW, Kakuk TJ, Brideau RJ, Hausknecht EC, Cole SL, Zaya RM. Vaccination of cotton rats with a chimeric FG glycoprotein of human respiratory syncytial virus induces minimal pulmonary pathology on challenge. J. Infect. Dis. 1991;163(3):477–482. doi: 10.1093/infdis/163.3.477. [DOI] [PubMed] [Google Scholar]
- 67.Falsey AR, Walsh EE, Capellan J, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines – nonadjuvanted vaccine or vaccine adjuvanted with alum – given concomitantly with influenza vaccine to high-risk elderly individuals. J. Infect. Dis. 2008;198(9):1317–1326. doi: 10.1086/592168. [DOI] [PubMed] [Google Scholar]
- 68.Tokita D, Sumpter TL, Raimondi G, et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J. Hepatol. 2008;49(6):1008–1018. doi: 10.1016/j.jhep.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castilow EM, Varga SM. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 2008;3(5):445–454. doi: 10.2217/17460794.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kruijsen D, Schijf MA, Lukens MV, et al. Local innate and adaptive immune responses regulate inflammatory cell influx into the lungs after vaccination with formalin inactivated RSV. Vaccine. 2011;29(15):2730–2741. doi: 10.1016/j.vaccine.2011.01.087. [DOI] [PubMed] [Google Scholar]