Abstract
Described is the construction of a furanyl-cylcobutanone fragment suited for incorporation into a synthesis of the naturally occurring anti-cancer agent Providencin.
Keywords: Providencin, Cyclobutanone, Furan, Bromo Ketone
1. Introduction
The gorgonians encompass approximately 500 species of sea fans, sea plumes and sea whips found in oceans throughout the world.i These gorgonian octocorals have proven to be an abundant source of secondary metabolites that possess a diverse range of structural features and biological activities.ii In 2003 Rodriguez and co-workers isolated Providencin (1) from the Caribbean sea plume Pseudopterogorgia kallos.3 Providencin demonstrated anti-cancer activity against human breast (MCF7), lung (NCI-H460) and CNS (SF-268) cancer cell lines. The growth inhibition of treated cells compared to untreated cells was 57, 39, and 94% respectively.iii Unfortunately, the dearth of naturally occurring 1 has prevented further biological testing.
The structure and relative stereochemistry of 1 were determined through a combination of NMR spectroscopy and X-ray crystallographic analysis. These studies revealed 1 to be a highly oxygenated diterpene containing an unprecedented [12.2.0]hexadecane ring system. Some of the more intriguing structural features include the trans-fused cyclobutanol moiety and the tri-substituted furan.3 The unique structure and biological activity of 1 combine to make it an attractive target for total synthesis. Although several reports have appeared describing synthetic approaches to 1 a total synthesis has remained elusive.iv
2. Results and discussions
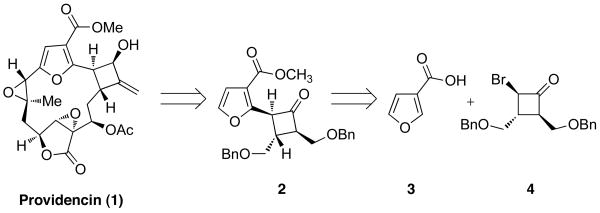
As illustrated retrosynthetically in Scheme 1, we envisioned incorporating a preassembled furanyl cyclobutane segment (2) that would in turn derive from the coupling of 3-furoic acid (3) with a suitably protected cyclobutane electrophile. In accord with this plan we chose to initially focus on the preparation of 4 wherein bromine would serve as the leaving group in the coupling event.
Scheme 1.
Retrosynthetic Analysis
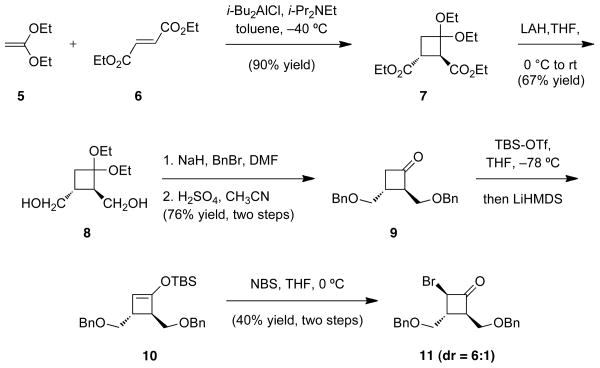
Synthesis of bromocyclobutanone 4 began with the [2+2] cycloaddition of diethyl ketene acetal (5) and diethyl furmarate (6) which, under the conditions developed by Brunner,v furnished the cycloadduct 7 in excellent yield (Scheme 2).vi Although our intention was to go forward with racemic material we did note a report from Bisacchi and co-workers demonstrating that 7 can be resolved via conversion to diastereomeric bis-amide derivatives followed by crystallization.vii Reduction of the racemic diester (7) using LAH furnished the known diol 8,7 which was converted to the corresponding bis-benzyl ether under standard conditions. Removal of the acetal provided cyclobutanone 9 and set the stage for incorporation of bromine. To this end, numerous approaches were explored and the most efficient conversion was observed by first converting 9 to the corresponding silyl enol ether 10. Subsequent treatment of 10 with NBS provided the bromocyclobutanone 11, which was isolated as a 6:1 mixture of diastereomers, with the major diastereomer having the illustrated trans-trans-relationship between the substituents.
Scheme 2.
Preparation of the bromocyclobutanone.
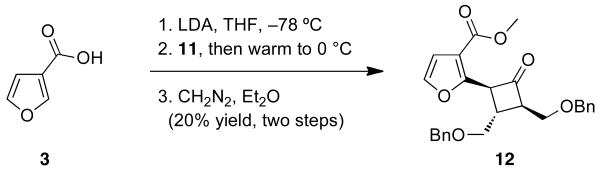
Having assembled the requisite bromocyclobutanone, we turned to its coupling with 3. In the event, 3-furoic acid was cooled to −78 °C and treated sequentially with 2 equivalents of LDA and then 11. After warming to 0 °C the reaction was quenched and the crude extract was treated with diazomethane. The latter facilitated handling and characterization of the desired product (12), which was isolated as a single diastereomer in 20% yield (Scheme 3). NMR studies, including 1D nOe and 2D ROESY experiments confirmed the illustrated trans-trans-relationship between substituents.
Scheme 3.
Initial coupling reaction.
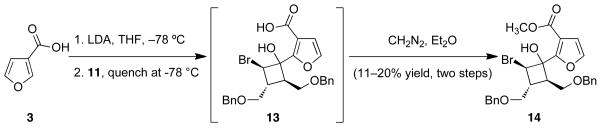
Gratified by the successful coupling, our efforts turned to improving the yield; thus, we began varying the reaction conditions. These studies revealed that quenching the coupling reaction at −78 °C prior to treatment with diazomethane results in the formation of tertiary alcohol 14 (Scheme 4). In addition to gaining mechanistic insight, we noted that both 14 and the intermediate acid (13) are quite labile and difficult to isolate. Moreover, subsequent studies demonstrated that exposure of 14 to base promotes formation of 12 via a 1,2-shift and displacement of bromine.viii
Scheme 4.
Low temperature quenching study.
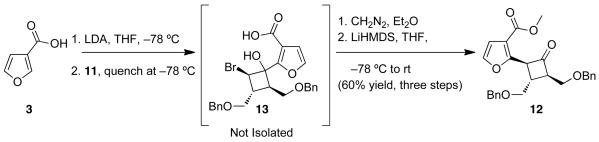
Further optimization studies revealed that LiHMDS is the base of choice for promoting the 1,2-shift. This latter observation coupled with the noted lability of the intermediate alcohols led to the development of optimized sequence wherein the dianion addition, esterification, and 1,2-shift were carried out without isolation of the intermediate alcohols 13 and 14 Scheme 5). Under these conditions the desired product 12 could be obtained in 60% yield as a single diastereomer. NMR studies, including 1D nOe and 2D ROESY experiments confirmed the illustrated trans-trans-relationship between substituents.
Scheme 5.
Optimized coupling sequence.
3. Conclusion
A convergent and efficient synthesis of a furanyl-cylcobutanone fragment (12) suited for incorporation into a synthesis of the naturally occurring anti-cancer agent Providencin (1) has been achieved. Efforts to complete the synthesis utilizing this key fragment are currently underway.
4. Experimentalix
Cyclobutanone 9
To a solution of diol 8 (4.8g, 23 mmol) in DMF (100 mL) was added 95% NaH dispersion in mineral oil (1.1 g, 46 mmol) at 0 °C. After 30 min, benzyl bromide (5.5 mL, 46 mmol) and NaI (10 mg) were added and the reaction mixture was allowed to warm to room temperature. After 48 hours the reaction was quenched at 0 °C by slow addition of saturated NH4Cl solution and diluted with ether. The biphasic solution was separated and the aqueous phase was extracted with ether. The combined organic phases were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The derived mixture of desired material and two monobenzylated products were separated by flash chromatography (gradient elution10 to 100% EtOAc:hexanes) and the mono protected products were re-subjected to the protection conditions to furnish the desired intermediate acetal (7.0 g, 80% combined yield). A portion of the latter acetal (4.5g, 12 mmol) was dissolved in CH3CN (120 mL) cooled to 0 °C and treated dropwise with H2SO4 (10 mL, 1 M aqueous). The reaction mixture was warmed to room temperature and after 30 min was diluted with EtOAc. The biphasic solution was separated and the organic phase washed sequentially with water, saturated NaHCO3 solution and brine, dried over Na2SO4, filtered and concentrated in vacuo. The crude product was purified by flash chromatography (9:1 hexanes:EtOAc) to yield 9 (3.7g, 95% yield) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 7.36-7.24 (m, 10H), 4.55 (s, 2H), 4.50 (s, 2H), 3.72 (dd, J = 10.0, 5.0 Hz, 1 H), 3.64 (d, J = 5.9 Hz, 2 H), 3.59 (dd, J = 9.9, 4.3 Hz, 1 H), 3.37-3.29 (m, 1 H), 3.03 (ddd, J = 17.6, 9.0, 2.4 Hz, 1 H), 2.87 (ddd, J = 17.6, 6.9, 3.0 Hz, 1 H), 2.78-2.70 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 208.81, 138.56, 138.50, 130.20, 128.87, 128.80, 128.15, 128.02, 127.92, 73.53, 73.50, 72.64, 67.35, 63.19, 48.55, 28.47; IR (thin film, NaCl) 2859 (m), 1781 (sh, s), 1723 (m), 1495 (w), 1453 (m), 1346 (w), 1254 (w), 1112 (m), 1027)w) cm-1 : HRMS (FAB) m/z found 327.16, calcd. for C20H25O4 [M+H3O]; 327.16.
Bromocyclobutanone 11
Cyclobutanone 9 (0.134 g, 0.43 mmol) was dissolved in THF (10 mL) and cooled to −78 °C and TBS-OTf (0.29 mL, 1.29 mmol) was added slowly. Then a 1M solution of LiHMDS in THF (2.2 mL, 2.15 mmol) was added rapidly down the side of the flask. After 1 h the reaction was carefully quenched with saturated NH4Cl solution. The aqueous layer was extracted with Et2O. The combined organics were washed with H2O and dried over MgSO4 and then concentrated in vacuo. The crude material was dissolved in THF (10 mL) and cooled to 0 °C. The resultant solution was treated with NBS (0.084 g, 0.47 mmol, added in one portion) and allowed to warm to room temperature and stir for 1 h. The reaction was quenched with H2O and the aqueous layer was extracted with Et2O. The combined organics were washed with brine, dried over MgSO4 and concentrated in vacuo. The crude material was flashed (10:1 hexanes:EtOAc) to furnish 11 (0.065 g, 40% yield, two steps). Rf = 0.28, 4:1 hexanes:EtOAc; 1H NMR (400 MHz, CDCl3) δ 7.39–7.29 (m, 10H), 4.99 (dd, J=7.7, 2.4 Hz, 1H), 4.64–4.56 (m, 2H), 4.56–4.50 (m, 2H), 3.83 (dt, J=9.9, 3.9 Hz, 1H), 3.78–3.70 (m, 2H), 3.65–3.56 (m, 2H), 2.87–2.81 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 199.3, 137.9, 137.8, 128.6, 128.5, 128.0, 127.8, 127.7, 127.6, 73.3, 73.2, 67.9, 66.2, 58.4, 48.3, 41.0 IR (NaCl thin film): 3030(w), 2857(w), 1792(s), 1113(m), 737(m), 697(m); HRMS (ESI–APCI) m/z calcd. for C20H25BrNO3 [M+NH4]+: 406.1012, found: 406.1011.
Furanyl Cyclobutanone 12
A solution of 3-furoic acid 3 (0.028 g, 0.249 mmol) in THF (3 mL) was cooled to −78 °C and treated with a 0.5M solution of LDA in (1 mL, 0.497 mmol). The reaction was stirred for 30 min at −78 °C and then a solution of bromocyclobutanone 11 (0.088 g, 0.226 mmol) in THF (1 mL) was added. When starting material was consumed as evidenced by TLC the reaction was quenched with saturated NH4Cl solution. The aqueous layer was extracted with Et2O and the combined organic phases were washed with H2O, dried over MgSO4 and concentrated in vacuo. The crude material was stirred in Et2O at room temperature and excess diazomethane was added. The reaction was filtered through MgSO4 and then concentrated in vacuo. The crude cyclobutanol (0.098 g, 0.19 mmol) was dissolved in THF (3 mL) and cooled to −78 °C. The resultant mixture was treated dropwise with a 1M solution of LiHMDS in THF (0.19 mL, 0.19 mmol). The reaction was warmed to room temperature and when complete by as evidenced byTLC analysis, it was diluted with EtOAc and washed with 1M HCl. The aqueous layer was extracted with EtOAc and the combined organic phases were washed with brine, dried over MgSO4 and concentrated in vacuo. The crude material was flashed in 4:1 – 2:1 hexanes:EtOAc to furnish cyclobutanone 12 (0.082 g, 60% yield, three steps). Rf = 0.17, 4:1 hexanes:EtOAc; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J=0.9 Hz, 1H), 7.38–7.17 (m, 10H), 6.83 (d, J=0.9 Hz, 1H), 4.55 (s, 2H), 4.40 (s, 2H), 3.90 (s, 3H), 3.86 (dd, J=19.0, 5.5 Hz, 1H), 3.59 (dd, J=16.3, 5.8 Hz, 1H), 3.54–3.49 (m, 2H), 3.03 (dd, J=14.1, 5.1 Hz, 1H), 2.23 (quint., J=5.9 Hz, 1H), 2.04–1.96 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 186.5, 163.3, 152.2, 144.2, 138.4, 138.3, 128.5, 128.4, 127.8, 127.6, 121.7, 113.6, 72.9, 72.8, 70.8, 66.7, 52.5, 30.6, 28.3, 26.7; IR (NaCl thin film): 3030(w), 2924(m), 2857(m), 1732(s), 1485(m), 1306(m), 1094(s), 738(m), 698(m); HRMS (ESI–APCI) m/z calcd. for C26H26NaO6 [M+Na]+: 457.1622, found: 457.1625
Alcohol 14
A solution of 3-furoic acid (3) (0.123 g, 1.1 mmol) in THF (10 mL) was cooled to −78 °C and treated with a 0.5M solution LDA in THF (4.4 mL, 2.2 mmol). The reaction was stirred for 30 min at −78 °C and then bromocyclobutanone 11 (0.383 g, 1 mmol) in THF (1 mL) was added at −78 °C. When the starting material was consumed as evidenced by TLC the reaction was quenched with saturated NH4Cl solution. The aqueous layer was extracted with Et2O. The combined organics were washed with H2O and dried over MgSO4 and concentrated in vacuo. The crude material was dissolved in Et2O, treated with excess diazomethane at room temperature, and concentrated in vacuo. The crude material was flashed in 20:1 – 10:1 – 4:1 hexanes:EtOAc to furnish 14 (0.101 g, 20% yield). Rf = 0.31, 4:1 hexanes:EtOAc; 1H NMR (400 MHz, CDCl3) δ 7.37–7.21 (m, 8H), 7.10–7.08 (m, 2H), 6.66 (d, J=1.9 Hz, 1H), 6.13 (d, J=0.7 Hz, 1H), 5.49 (d, J=8.9 Hz, 1H), 4.58 (s, 2H), 4.14 (dd, J=11.7, 8.7 Hz, 2H), 3.89 (d, J=6.8 Hz, 2H), 3.78 (s, 3H), 3.35–3.28 (m, 2H), 2.95 (q, J=4.8 Hz, 1H), 2.83–2.76 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 165.7, 160.2, 140.9, 138.2, 138.0, 128.5, 128.3, 128.0, 127.7, 127.5, 114.6, 111.5, 75.2, 73.5, 73.2, 72.3, 68.8, 52.3, 51.0, 49.0, 38.7; IR (NaCl thin film): 3301(w), 3030(w), 2950(w), 2857(m), 1725(m), 1693(s), 1312(s), 1209(s), 1073(s), 740(s), 698(s); HRMS (ESI–APCI) m/z calcd. for C26H28BrO6 [M+H]+: 515.1064, found: 515.105
Supplementary Material
Acknowledgments
Funding from the NIH (1 RO1 CA 93591) and NSF (CHE-1058292) are gratefully acknowledged. JLW thanks Amgen, Merck, Bristol-Myers Squibb for financial support. Dr. Chris Rithner, Don Heyse and Don Dick are acknowledged for their assistance with NMR analysis and instrumentation.
Footnotes
Dedicated to Professor Satoshi Omura on the Occasion of His Receiving the Tetrahedron Prize.
Supplementary data: Supplementary data (proton NMR, carbon NMR and IR spectra for 11, 12 and 14; ROESY spectra for 11 and 12) associated with this article can be found at doi: XXX.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- i.http://www.ceoe.udel.edu/kiosk/seafan.html
- ii.Roethle PA, Hernandez PT, Trauner D. Organic Letters. 2006;8:5901–5904. doi: 10.1021/ol062581o. [DOI] [PubMed] [Google Scholar]
- iii.Marrero J, Rodriguez AD, Baran P, Raptis RG. Organic Letters. 2003;5:2551–2554. doi: 10.1021/ol034833g. [DOI] [PubMed] [Google Scholar]
- iv.(a) Bray CD, Pattenden G. Tetrahedron Letters. 2006;47:3937–3939. [Google Scholar]; (b) White JD, Jana S. Organic Letters. 2009;11:1433–1436. doi: 10.1021/ol900152p. [DOI] [PubMed] [Google Scholar]; (c) Schweizer E, Gaich T, Brecker L, Mulzer J. Synthesis. 2007:3807–3814. [Google Scholar]; (d) Gaich T, Arion V, Mulzer J. Heterocycles. 2007;74:855–862. [Google Scholar]; (e) Gaich T, Weinstabl H, Mulzer J. Synlett. 2009:1357–1366. [Google Scholar]
- v.Brunner A. U.S. Patent 6 025 519. 2000
- vi.Bérubé A. Dissertation. Yale University; New Haven, CT: 2006. [Google Scholar]
- vii.Bisacchi GS, Braitman A, Cianci CW, Clark JM, Field AK, Hagen ME, Hockstein DR, Malley MF, Mitt T, Slusarchyk WA, Sundeen JE, Terry BJ, Tuomari AV, Weaver ER, Young MG, Zahler R. Journal of Medicinal Chemistry. 1991;34:1415–1421. doi: 10.1021/jm00108a026. [DOI] [PubMed] [Google Scholar]
- viii.Mechanistically, one can envision a 1,2-shift of the furan moiety; however, displacement of bromine to form an intermediate epoxide, followed by ring opening and 1,2-shift of hydride is also plausible. Epimerization of any intervening mixtures derived form either process could furnish the observed isomer. To provide some further insight into this issue, we attempted to assign the relative stereochemistry of 14 spectroscopically. Unfortunately these efforts were not fruitful. We thank a referee for discussions of mechanistic possibilities.
- ix.Unless otherwise stated, reactions were magnetically stirred in flame-dried glassware under an atmosphere of nitrogen. Triethylamine (Et3N) and methanol were dried over calcium hydride. Benzene, tetrahydrofuran, dichloromethane, toluene, and diethyl ether were dried using a solvent purification system manufactured by SG Water U.S.A., LLC. All other commercially available reagents were used as received.All reactions were monitored by thin-layer chromatography (TLC) using Silicycle glass-backed extra hard layer, 60 Å plates (indicator F-254, 250 μm). Column or flash chromatography was performed with the indicated solvents using Silicycle SiliaFlash® P60 (230-400 mesh) silica gel as the stationary phase. All melting points were obtained on a Gallenkamp capillary melting point apparatus and are uncorrected.Infrared spectra were obtained using a Midac M1200 FTIR or a Nicolet Avatar 320 FTIR. 1H and 13C NMR spectra were recorded on a Bruker AM-500, Bruker Avance DPX-500, Bruker Avance DPX-400, or Varian Inova 400 spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) relative to internal residual solvent peaks from indicated deuterated solvents.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.