Abstract
The present study investigated the impact of sleep deprivation on several aspects of affective functioning in healthy participants selected from three different developmental periods: early adolescence (ages 10–13), midadolescence (ages 13–16), and adulthood (ages 30–60). Participants completed an affective functioning battery under conditions of sleep deprivation (a maximum of 6.5 hours total sleep time on the first night followed by a maximum of 2 hours total sleep time on the second night) and rest (approximately 7–8 hours total sleep time each night for two consecutive nights). Less positive affect was observed in the sleep-deprived, compared to rested, condition. This effect held for 9 of the 12 positive affect items on the PANAS-C. Participants also reported a greater increase in anxiety during a catastrophizing task and rated the likelihood of potential catastrophes as higher when sleep deprived, relative to when rested. Early adolescents appraised their main worry as more threatening when sleep deprived, relative to when rested. These results support and extend previous research underscoring the adverse affective consequences of sleep deprivation.
Keywords: sleep deprivation, affect, anxiety, adolescents
It is well established that sleep deprivation adversely impacts mood (Pilcher & Huffcutt, 1996). Healthy participants who slept only 5 hours per night for 1 week experienced a progressive worsening in mood (Dinges et al., 1997). Moreover, a study by Yoo et al. (2007) reported a 60% increase in amygdala activity in response to an emotional stimulus when it was viewed under conditions of sleep deprivation compared to when rested. This large increase in amygdala reactivity was associated with decreased activity in the medial–prefrontal cortex, a region known to exert top-down control on the limbic area (including the amygdala) and functioning to regulate or modulate emotional responses as appropriate for the context. These results are consistent with other neuroimaging research demonstrating that sleep deprivation influences cognitive control or frontal executive functions in general (Drummond et al., 1999; Muzur, Pace-Schott, & Hobson, 2002). Adequate sleep, in contrast, is proposed to contribute to improved affect regulation through enhanced prefrontal cortex (PFC) functioning (Silk et al., 2007). The present study sought to extend this research in three ways.
First, previous research has tended to examine affect broadly, with little attention to the impact on specific discrete emotions. In the former domain, Dinges et al. (1997) and Drake et al. (2001) used the Profile of Mood States (POMS; McNair, Lorr, & Druppleman, 1971). The POMS indices affect via six broad scales (tension-anxiety, depression-dejection, anger-hostility, fatigue, confusion, and vigor). A total mood disturbance score is derived by summing the scores across all six factors, with vigor weighted negatively. Dinges and colleagues (1997) found that sleep deprivation resulted in increased fatigue, confusion, tension-anxiety, and total mood disturbance and decreased vigor. Drake and colleagues (2001) demonstrated increases in fatigue and confusion and decreases in vigor. Zohar, Tzischinsky, Epstein, and Lavie (2005) furthered this broad affect research by assessing positive in addition to negative affect. Using composite scores of four positive and four negative affect items, the findings indicated that sleep loss both intensified negative affect and diminished positive affect in the context of daily events. Franzen, Siegle, and Buysse (2008) also reported that sleep deprivation decreased positive affect. However, these authors reported that sleep deprivation did not impact negative affect based on the Positive Affect and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). They found that negative affect did increase when it was assessed with a visual analog scale (Monk, 1989). Haack and Mullington (2005) examined positive and negative factor-derived variables including optimism-sociability, tiredness-fatigue, and anger-aggression in the context of partial sleep deprivation and observed decreased optimism-sociability. Following the precedent of these studies, the present study examined both positive and negative affect broadly. In addition, given the lack of research on the impact of sleep deprivation on individual emotions, we sought to expand previous research by embracing a discrete emotions perspective (e.g., Izard, 1977). The impact of sleep deprivation on specific emotions was investigated (e.g., joyful, proud, ashamed, afraid) to determine the degree to which particular emotions drive the affective changes of sleep deprivation.
Second, building upon the discrete emotions approach, this study sought to examine one particular affective state in greater depth: anxiety. Anxiety is a clinically relevant cognitive-affective state that helps humans plan for, and adapt to, the future (Barlow, 2002). Anxiety is functional in that it mobilizes the individual for fight or flight in the presence of appraised danger (Power & Dalgleish, 1997). However, anxiety has a number of negative consequences. For example, anxiety has adverse effects on cognitive performance (see Eysenck, 1992, for a review). Theoretically these effects should be exacerbated under conditions of sleep deprivation given that (a) a sleep-deprived state appears to diminish medial-PFC activity in the context of emotional challenges (Yoo et al., 2007; Killgore, Balkin, & Wesensten, 2006); and (b) the medial-PFC is an important brain region activated in anxiety states for exerting inhibition of the amygdala (e.g., Davidson, 2002; Phelps, Delgado, Nearing, & LeDoux, 2004). An investigation of anxiety and sleep is also important because of the association between sleep disturbance and anxiety disorders (Harvey, Hairston, Gruber, & Gershon, 2008; Papadimitriou & Linkowski, 2005). For example, persistent insomnia is associated with an increased risk of developing an anxiety disorder (Breslau, Roth, Rosenthal, & Andreski, 1996) and sleep disturbance predicts the subsequent development of Posttraumatic Stress Disorder (Koren, Arnon, Lavie, & Klein, 2002). To the best of our knowledge only one study has examined sleep deprivation and anxiety. Consistent with the hypothesis that sleep deprivation will intensify anxiety, Sagaspe and colleagues (2006) found that healthy participants (n = 12) exhibited an increase in self-reported anxiety following a night of sleep deprivation. In the present study, we sought to extend this literature by examining a number of contributors to anxious states including: worry (Dalgleish & Power, 1999), which is considered a “central psychological process” implicated in anxiety (Power & Dalgleish, 1997, p. 220); catastrophizing, which involves automatic internal “what if” questions similar to Beck’s automatic thinking in depression (Ingram & Kendall, 1987; Startup & Davey, 2001); and appraisal of threat (Power & Dalgleish, 1997), such as inflated perceptions of the likelihood of various threats (Butler & Mathews, 1987).
Third, the affective consequences of sleep deprivation in adolescence, and particularly across development in adolescence, have not been extensively examined. The dearth of experimental research is a critical gap. Adolescence is a period during which individuals already face affective challenges with limited resources. For example, the PFC, shown to be important in affective responses and regulation (Davidson, 2002; Ochsner et al., 2004), does not reach full maturity until after adolescence (Giedd, 2004). In fact, during the adolescent years the relative size of the PFC decreases and it is “prominently remodeled” (Spear, 2000, p. 112). Given that the PFC has been shown in adults to be adversely impacted when exposed to emotional stimuli after sleep deprivation, sleep deprivation in adolescence could particularly compromise functioning. In addition, biological sleep need appears to increase in adolescence (Carskadon, 2002). Despite this evidence of the importance of sleep in adolescence, there is concern about an “epidemic of sleep deprivation” in this age group (e.g., Hansen, Janssen, Schiff, Zee, & Dubocovich, 2005). A multitude of factors contribute including a circadian rhythm shift toward a delayed phase (Jenni, Achermann, & Carskadon, 2005) as well as psychosocial influences such as more evening activities (particularly involving electronic media; Van den Bulck, 2003), greater social opportunities, increased academic responsibilities, and more extracurricular activities (Carskadon, Wolfson, Acebo, Tzischinsky, & Seifer, 1998). Given this combination of sleep deprivation and potentially increased vulnerability to sleep loss, the current study sought to evaluate the affective consequences of sleep deprivation in adolescents using an experimental paradigm. Early adolescents were a focus in order to capture the initial developmental transition to adolescence. Moreover, the design accounted for gender differences in adolescence by including boys 1 year older than girls, given that boys begin puberty later than girls (Gordon & Laufer, 2005).
In the present study, adolescents and adults completed an affective functioning battery under conditions of sleep deprivation and rest (order counterbalanced). The first aim was to examine the effects of sleep deprivation on positive and negative affect. Based on previous research, the first hypothesis tested was that participants would experience less positive affect and more negative affect in the sleep-deprived, compared to rested, condition (Dinges et al., 1997; Franzen et al., 2008; Pilcher & Huffcutt, 1996; Zohar et al., 2005). Relatedly, we explored the impact of sleep deprivation on discrete positive (interested, excited, happy, strong, energetic, calm, cheerful, active, proud, joyful, delighted, lively) and negative (sad, frightened, ashamed, upset, nervous, guilty, scared, miserable, jittery, afraid, lonely, mad, disgusted, blue, gloomy) emotions. Given the absence of previous research addressing this level of specificity, this aspect of the study was included on an exploratory basis. The second aim was to conduct an in-depth examination of one particular negative affective state—namely, anxiety. Based on the association between sleep deprivation and anxiety observed in previous research (Sagaspe et al., 2006), we predicted that participants would demonstrate heightened anxiety when sleep deprived as compared to when rested. Five contributors to anxious states were examined: number of worries generated, rating of the most threatening worry, length of catastrophizing sequence generated, anxiety ratings, and likelihood ratings of potential catastrophes. The final aim was to extend the literature by examining the aforementioned potential impact of sleep deprivation on affective functioning during adolescence. Given the ongoing development of the prefrontal cortex during adolescence and its importance for affect regulation (Davidson, 2002; Giedd, 2004), we hypothesized that adolescents would display increased affective reactivity, relative to adults.
Methods
Participants
Participants were included if they (a) did not meet Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) criteria for any past or current Axis I disorder; (b) did not meet criteria for any past or current sleep disorder; (c) did not have any medical conditions; and (d) had no history of head trauma. Participants were 20 early adolescents (girls ages 10–12, boys ages 11–13), 24 midadolescents (girls ages 13–15, boys ages 14–16), and 20 adults (ages 30–60). Two different adolescent age groups (i.e., early and mid) were selected in order to examine the differential impact of sleep deprivation on affect across development. Specific age ranges were based on the mean ages from samples used in previous research examining early and mid-late pubertal development (e.g., Jenni et al., 2005; Silk et al., 2009). Furthermore, the age inclusion criterion for the girls was set at one year earlier on the basis that girls begin puberty 1–2 years earlier than boys (Gordon & Laufer, 2005). The rationale for excluding young adults from the adult group was threefold: 1) it ensured clear differentiation from the adolescent groups by avoiding the delayed phase that can persist into early adulthood (Richardson & Malin, 1996); 2) it avoided the dramatic temporary sleep pattern shifts of college-student populations during which individuals may adopt a chronically sleep-deprived lifestyle (Lack, 1986); and 3) it ensured neurological differences from the adolescent group, given that the prefrontal cortex does not reach adult dimensions until the early 20s (Giedd, 2004). Adults over 60 were excluded because of age-related changes in the sleep-wake cycle such as sleep maintenance difficulties, reduced sleep duration, and lowered arousal threshold (e.g., Campbell & Murphy, 2007; Dement, Miles, & Carskadon, 1982).
Measures
Structured clinical interview for DSM–IV (SCID)
The SCID is a semistructured interview designed to assess DSM–IV diagnostic criteria for Axis I disorders (Spitzer, Williams, Gibbon, & First, 1996). Adult participants’ diagnoses were assessed using the SCID. It has shown good reliability for the majority of disorders (Skre, Onstad, Torgersen, & Kringlen, 1991; Williams et al., 1992).
Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime version (K-SADS-PL)
The K-SADS-PL (Kaufman, Birmaher, Brent, Rao, et al., 1997), a semistructured interview that assesses diagnostic criteria of a broad spectrum of childhood disorders, was administered to all potential adolescent participants and one parent of each participant. The K-SADS-PL has been shown to generate reliable and valid child psychiatric diagnoses (Kaufman et al., 1997).
Duke Structured Interview for Sleep Disorder (DSISD)
The DSISD is a semistructured interview that assesses research diagnostic criteria for sleep disorders (Edinger et al., 2004). The DSISD was used to assess sleep disorders in all participants.
Self-Rating Scale for Pubertal Development
The Self-Rating Scale for Pubertal Development is a self-report measure of pubertal status adapted by Carskadon and Acebo (1993) from an earlier interview-based puberty rating instrument (Petersen, Crockett, Richards, & Boxer, 1988). Both early and midadolescent participants completed the scale, which includes items rating physical development that are combined to classify the adolescents’ pubertal development into a categorical maturation score: (1) prepubertal, (2) beginning pubertal, (3) midpubertal, (4) advanced pubertal, and (5) postpubertal. The measure has established validity and reliability (Carskadon & Acebo, 1993).
Sleep diary
The sleep diary is a self-report measure completed upon waking in which participants estimate a number of sleep variables including total sleep time (TST). The sleep diary has been shown to be a reliable estimate (Morin & Espie, 2003) and is considered the gold standard subjective measure of sleep (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006).
Actigraphy
Actigraphs are wrist watch-like devices that provide an estimate of the sleep/wake cycle via movement using a sensor, a processor and memory. The correlation between actigraphy- and polysomnography-defined sleep estimates ranges from .88 –.97 in adult normal sleepers (Cole, Kripke, Gruen, Mullaney, & Gillin, 1992; Jean-Louis et al., 1997; Sadeh, Alster, Urbach, & Lavie, 1989). Actigraphy has also been shown to provide a good estimate of TST in healthy adolescents (Acebo & Carskadon, 1993; Johnson et al., 2007).
Stanford Sleepiness Scale (SSS)
The SSS is a one-item measure of subjective sleepiness (Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973). Response options range from 1 “feeling active, vital, alert, and wide awake” to 7 “no longer fighting sleep, sleep onset soon; having dream-like thoughts.” The SSS has been widely used in sleep-deprivation research (e.g., Babkoff, Caspy, & Mikulincer, 1991).
Child Version of the PANAS (PANAS-C)
The PANAS-C is a child version of the PANAS. It resembles the adult scale in form, content, and instructions. The measure lists 12 positive and 15 negative descriptors (e.g., happy, upset). Participants are instructed to “complete the items based on how you are feeling right now” using a 5-point Likert scale (1 “very slightly or not at all” to 5 “extremely”; Laurent et al., 1999). The PANAS-C has been shown to have strong psychometric properties and good convergent and discriminant validity (Laurent et al., 1999). Note that the PANAS-C was administered to all participants (including adults) to allow for comparability across all participants.
Procedure
Study procedures were approved by the Institutional Review Board of the University of California, Berkeley. Participants were compensated for their time and effort. The grade level of the wording of all instructions used at the laboratory visits was checked using Microsoft Word, 2007 to ensure comprehension by all participants. The grade level ranged from 0.5 to 6.0, with the majority of instructions at the third-grade level.
In the first session, all adult participants provided written informed consent. Adolescent participants provided assent and their parents provided consent. Doctoral student interviewers with previous experience conducting structured clinical interviews administered the SCID or KSADS and the DSISD. When parent and child reports were discrepant, any Axis I psychopathology or sleep disorders endorsed by either parent or child rendered the participant ineligible for further participation.
Eligible participants were asked to keep sleep diaries throughout the study and a portion (66%) of participants also wore actigraphs (Mini Mitter Actiwatch, model AWLP).1 For data analysis, actigraphy was always used when available on the basis that actigraphy provides accurate estimates of sleep in both adolescents and adults (Johnson et al., 2007; Morgenthaler et al., 2007). When actigraphy was not available, sleep diary was used; the two can be used interchangeably to assess sleep start, sleep end, and assumed sleep times (Werner, Molinari, Guyer, & Jenni, 2008). An average of 5 nights of sleep was analyzed as an indicator of habitual sleep. After this habitual sleep period participants completed one of the two conditions: the sleep-deprived or the rested condition. Approximately 1 week later the other condition was completed.
Sleep-deprived condition
The sleep-deprivation manipulation occurred over the course of two nights, with the majority of the sleep loss occurring on the second, in-laboratory night. On the first night, participants spent the night at home, but were asked to aim for a maximum sleep time of approximately 6.5 hours. Compliance was checked using participants’ actigraphy or sleep diary. This information was available for 80% percent of participants.2
Participants then visited the laboratory on the second night at 10:00 p.m. at which time they completed a baseline SSS rating. They were then continuously monitored throughout the night by trained laboratory staff and were permitted to read, watch movies, play board games, and interact with the laboratory staff in order to ensure wakefulness. Snacks such as fruit, crackers, yogurt, and cheese were available. No caffeine or other stimulants were allowed. To simulate real world conditions, exposure to light was not controlled (Dinges et al., 1997). Between 03:00 a.m. and 5:00 a.m. participants were given a 2-hr sleep opportunity. At 8:00 a.m. participants again completed the SSS. Between 08:00 a.m. and 10:00 a.m., the affective functioning battery was administered.
The rationale for the two-night sleep-deprivation protocol was threefold. First, to the best of our knowledge this is the first experimental study of sleep deprivation in adolescents. Hence, the ethical and safety ramifications of total sleep deprivation were unclear. Second, we were concerned that after one night of total sleep deprivation the adolescent participants would fail to remain awake during the morning affective functioning battery. We consequently provided a 2-hr sleep opportunity on the second sleep-deprivation night to discharge the homeostat. Finally, partial sleep deprivation may be more analogous to the type of real-world sleep deprivation experienced by individuals.
Rested condition
Participants were asked to extend their habitual sleep time to 8.5 hours at home for two consecutive nights. Compliance was checked using actigraphy or sleep diary. These data were available for 83% of nights.2 At 10:00 a.m. the morning following the second night, participants visited the laboratory and completed the SSS and then the affective functioning battery. A few participants in each group completed the rested condition at 8:00 a.m. due to scheduling constraints (n = 3 in the early adolescent group, n = 3 in the midadolescent group, and n = 4 in the adult group). There were no differences between groups in the number of participants at 8:00 a.m.
Affective Functioning Battery
The data for the present study were collected as part of a larger research project designed to examine the effects of sleep deprivation on affective functioning. The affective functioning battery consisted of a number of cognitive-affective tasks (e.g., the balloon analog risk task and the future thinking task) separated by neutral film clips. The conceptually related tasks are presented in this paper. This is the first article published on this study and future publications will not overlap with the tasks and analyses reported here.
Positive and negative affect
Participants rated their baseline positive and negative affect (based on their feelings “right now”) using the PANAS-C. The PANAS-C was administered as participants’ first task, immediately prior to the worry generation and most threatening worry tasks.
Worry generation and most threatening worry
Participants were given a page with seven marked lines and were asked to briefly list all the topics about which they were worried. Next, following Davey and Levy (1998), participants were asked to rate how seriously threatening the worry was or how scared they were that the worry would come true on a scale of 0 “not at all scared or threatened that the worry will come true” to 100 “almost certain that the worry will come true/the worry is extremely threatening.” The experimenter then confirmed with the participant that the worry ranked as most threatening was the worry about which he or she was most concerned and about which he or she spent the most time thinking. Following this confirmation, the most threatening worry was then used for the catastrophizing task.
Catastrophizing task
A practice trial based on an area of interest (e.g., soccer) was administered to ensure that participants understood the procedure. The catastrophizing interview then began. The interviewer asked the participant “What is it that worries you about X?” where X was the participant’s most threatening worry. Where Y denotes the participant’s answer to the latter question, the participant was then asked “What is it that worries you about Y?” Where Z denotes the answer the participant provided for this question, the interviewer then asked “What is it that worries you about Z?” This process continued until the participant could think of no more responses or until the participant reported a similar answer three consecutive times. The total number of catastrophic steps was recorded (Vasey & Borkovec, 1992).
Participants rated their anxiety immediately before and after completing the catastrophizing procedure (Harvey & Greenall, 2003) on a one-item scale (0 “not at all anxious” to 100 “extremely anxious”). In addition, after the catastrophizing interview participants were asked to rate the likelihood that each catastrophe that they reported during the interview would actually occur (0 “not at all likely” to 100 “extremely likely”; Vasey & Borkovec, 1992). This rating is referred to as the catastrophe likelihood rating (Harvey & Greenall, 2003).
Results
Participant Characteristics
There were no significant differences across groups in gender or race/ethnicity (see Table 1). The groups differed on household income, with the adult group having lower household income than the families of the adolescent groups. There were expected group differences in age, education level, and pubertal status. All adolescent participants were at least in the early pubertal stage. There were no group differences in participants’ habitual TST (see Table 2).
Table 1.
Participant Characteristics
| Demographic variable | Early adolescents (n = 20) |
Midadolescents (n = 24) |
Adults (n = 20) |
p |
|---|---|---|---|---|
| Mean age (SD) | 11.50 (0.83) | 14.29 (0.86) | 41.20 (9.97) | .001 |
| Race/ethnicity | ns | |||
| African American | 2 | 2 | 4 | |
| Asian American | 2 | 9 | 5 | |
| Caucasian | 14 | 10 | 10 | |
| Hispanic | 2 | 1 | 1 | |
| Native American | 0 | 0 | 0 | |
| Other/biracial | 0 | 2 | 0 | |
| Gender | ns | |||
| Male | 10 | 15 | 10 | |
| Female | 10 | 9 | 10 | |
| Mean years education (SD) | 6.32 (1.16) | 9.17 (0.98) | 16.40 (2.19) | .001 |
| Peterson pubertal status | .01 | |||
| Prepubertal | 0 | 0 | N/A | |
| Early-pubertal | 4 | 1 | N/A | |
| Mid-pubertal | 11 | 8 | N/A | |
| Late-pubertal | 3 | 13 | N/A | |
| Postpubertal | 0 | 2 | N/A | |
| Employment status | N/A | |||
| Full time | N/A | N/A | 9 | |
| Part time | N/A | N/A | 10 | |
| Unemployed | N/A | N/A | 1 | |
| Annual household income | .01 | |||
| Less than $50, 000 | 2 | 4 | 9 | |
| Greater than $50,000 | 17 | 18 | 8 |
Note. SD = Standard deviation.
Table 2.
Manipulation Checks
| Early adolescents |
Midadolescents | Adults | |
|---|---|---|---|
| Habitual sleep (based on five days of monitoring) | |||
| TST | 7.82 (1.25) | 7.37 (1.72) | 6.79 (0.81) |
| Sleep-deprived condition | |||
| First night TST | 6.31 (1.44) | 6.26 (1.60) | 5.73 (1.15) |
| Second night maximum TST | 2.00 (0.00) | 2.00 (0.00) | 2.00 (0.00) |
| TST over first and second nights | 8.31 (1.44) | 8.26 (1.60) | 7.73 (1.15) |
| Two nights of mean habitual TST | 15.64 (2.50) | 14.74 (3.44) | 13.58 (1.62) |
| Sleep debt accrued during sleep-deprived condition | 7.33 | 6.48 | 5.85 |
| SSS 10:00 p.m. | 2.42 (1.35) | 2.38 (0.88) | 2.44 (1.03) |
| SSS 8:00 a.m. | 3.68 (1.45) | 3.46 (1.41) | 4.25 (1.34) |
| Rested condition | |||
| Average TST of first and second nights | 8.14 (1.02) | 7.80 (0.91) | 7.18 (0.85) |
| Total TST over first and second nights | 16.28 (2.03) | 15.60 (1.83) | 14.37 (1.70) |
| SSS 8:00 a.m. or 10:00 a.m. | 2.29 (0.92) | 1.92 (0.88) | 1.54 (0.52) |
Note. Mean values presented with standard deviations in parentheses. SSS = Stanford Sleepiness Scale; TST = total sleep time. All times are quoted in hours.
Manipulation Checks
Sleep-deprivation manipulation check
Recall that participants were asked to aim for a maximum sleep time of 6.5 hours on the first night of the sleep-deprivation condition. As indicated in Table 2, mean first night TST was less than 6.5 hours (76% below 6.5 hours, 6% between 6.5–7 hours, 8% between 7 and 7.5 hours, and 8% over 7.5 hours).3 A one-way analysis of variance (ANOVA) indicated there were no differences between groups. Given that the second night maximum TST was 2 hours, over the two nights participants accrued a substantial sleep debt, relative to habitual TST (see Table 2). The three age groups did not differ in the sleep debt accrued.
A repeated-measures ANOVA was conducted on SSS ratings in the sleep-deprived condition with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Time (10:00 p.m., 8:00 a.m.) as the within-subject factor (see Table 2). There was no significant main effect of Age Group, F(2, 56) < 1, ns, but there was a significant main effect of Time, F(1, 56) = 40.13, p < .001, with participants reporting more sleepiness at 8 a.m. relative to 10 p.m. The Age Group × Time interaction was not significant, F(2, 56) < 1, ns. In sum, the TST and SSS data suggest that the sleep-deprivation manipulation delivered was successful.
Rested manipulation check
Recall that participants were asked to extend their habitual sleep to 8.5 hours for two nights. A one-way ANOVA on average TST of the first and second nights indicated there was an Age Group difference in average TST, F(2, 44) = 3.93, p < .05. Tukey follow-up tests indicated a significant difference such that adults obtained less sleep compared to the early adolescents, p < .05. No other comparisons were significant. An inspection of the mean values in Table 2 indicates that participants did not comply with the 8.5 hour TST instructions. Paired t tests examined the difference between average TST of the first and second nights and habitual sleep in each group. Adults had significantly more sleep on the rested manipulation nights compared to their habitual sleep, t(13) = 2.94, p < .05. This result remained after applying the Bonferroni correction of p < .017. The same comparisons were not significant in either adolescent age group. In sum, given these results we shifted our original conceptualization from an extended sleep condition to a habitually rested condition.
Comparison of sleep-deprived and rested SSS scores
A repeated-measures ANOVA was conducted on morning sleepiness ratings with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor (see Table 2). There was no significant main effect of Age Group, F(2, 51) < 1, ns, but there was a significant main effect of Sleep Condition, F(1, 51) = 95.70, p < .001, with participants reporting more sleepiness on the sleep-deprived, compared to rested, morning. There was also an Age Group × Sleep Condition interaction F(2, 51) = 3.48, p < .05. Follow-up tests indicated that in the rested condition, early adolescents were more sleepy than adults, t(31) = 2.46, p < .05. This same comparison was not significant in the sleep-deprived condition. The SSS data show that the sleep-deprivation manipulation was effective in increasing sleepiness.
Affective Functioning Battery
Positive affect
A two-way repeated-measures ANOVA was conducted on the PANAS-C composite positive affect with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There was a main effect of Sleep Condition, F(1, 55) = 34.11, 4, p < .001, such that participants reported less positive affect when sleep deprived, compared to rested. There was no main effect of Age Group, F(2, 55) < 1, ns, and there was no Sleep Condition × Age Group interaction, F(2, 55) < 1, ns. Next, a two-way repeated-measures ANOVA was conducted on the PANAS-C positive affect individual items with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) and Affect Item (interested, excited, happy, strong, energetic, calm, cheerful, active, proud, joyful, delighted, lively) as the within-subject factors. Again, there was a main effect of Sleep Condition, F(1, 55) = 34.11, , p < .001, such that participants reported less positive affect when sleep deprived, compared to rested, and no main effect of Age Group, F(2, 55) < 1, ns. The only interaction to reach significance was the Sleep Condition × Affect Item interaction, F(11, 605) = 3.31, p < .001, . Follow-up paired t tests were conducted on the individual affect items between the sleep-deprived and rested conditions. There were significant differences on all affect items except calm, such that participants rated each item lower in the sleep-deprived, compared to rested, condition (see Table 3). After applying the Bonferroni correction of p < .004, all items remained significant except joyful and lively.
Table 3.
Positive and Negative Affect
| Sleep deprived |
Rested | p | |
|---|---|---|---|
| Positive affect | |||
| Composite | 21.76 (8.61) | 30.14 (1.55) | .001 |
| Interested | 2.11 (1.09) | 2.85 (1.10) | .001 |
| Excited | 1.66 (0.98) | 2.38 (1.16) | .001 |
| Happy | 1.97 (0.94) | 2.82 (1.09) | .001 |
| Strong | 1.61 (0.75) | 2.56 (1.22) | .001 |
| Energetic | 1.65 (0.98) | 2.58 (1.05) | .001 |
| Calm | 3.00 (1.20) | 3.21 (1.03) | ns |
| Cheerful | 1.81 (0.99) | 2.35 (1.10) | .001 |
| Active | 1.56 (0.82) | 2.58 (1.02) | .001 |
| Proud | 1.56 (0.82) | 1.98 (1.15) | .001 |
| Joyful | 1.81 (1.53) | 2.26 (1.13) | .05 |
| Delighted | 1.53 (0.75) | 2.00 (1.07) | .001 |
| Lively | 1.61 (0.84) | 2.89 (4.09) | .05 |
| Negative affect | |||
| Composite | 16.91 (3.00) | 16.76 (2.67) | ns |
| Sad | 1.21 (0.52) | 1.15 (0.40) | ns |
| Frightened | 1.08 (0.33) | 1.03 (0.18) | ns |
| Ashamed | 1.02 (0.13) | 1.02 (0.13) | ns |
| Upset | 1.13 (0.38) | 1.13 (0.34) | ns |
| Nervous | 1.16 (0.41) | 1.32 (.059) | .05 |
| Guilty | 1.02 (0.13) | 1.08 (0.33) | ns |
| Scared | 1.02 (0.13) | 1.11 (0.37) | ns |
| Miserable | 1.23 (0.64) | 1.05 (0.22) | .05 |
| Jittery | 1.27 (0.58) | 1.35 (0.63) | ns |
| Afraid | 1.05 (0.22) | 1.11 (0.37) | ns |
| Lonely | 1.15 (0.41) | 1.10 (0.30) | ns |
| Mad | 1.03 (0.18) | 1.06 (0.25) | ns |
| Disgusted | 1.08 (0.33) | 1.05 (0.22) | ns |
| Blue | 1.21 (0.52) | 1.11 (0.37) | ns |
| Gloomy | 1.26 (0.60) | 1.31 (0.71) | ns |
Note. Mean values presented with standard deviations in parentheses.
Negative affect
A two-way repeated-measures ANOVA was conducted on the PANAS-C composite negative affect with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There was no main effect of Sleep Condition, F(1, 55) < 1, ns, or Age Group F(2, 55) < 1, ns, or a Sleep Condition × Age Group interaction, F(2, 55) = 1.35, ns. Next, a two-way repeated-measures ANOVA was conducted on the PANAS-C negative affect individual items with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) and Affect Item (sad, frightened, ashamed, upset, nervous, guilty, scared, miserable, jittery, afraid, lonely, mad, disgusted, blue, gloomy) as the within-subject factors. Mean values are presented in Table 3. Again, there was no main effect of Sleep Condition, F(1, 55) < 1, ns, or Age Group F(2, 55) < 1, ns. The only interaction to reach significance was the Sleep Condition × Affect Item interaction, F(14, 770) = 1.90, , p < .05. Follow-up paired t tests indicated that participants were more nervous in the rested, compared to sleep-deprived, condition, and more miserable in the sleep-deprived, compared to rested, condition. After applying the Bonferroni correction of p < .003, neither of the follow-up tests was significant.
Worry generation and most threatening worry
Mean values for these tasks are presented in Table 4. A two-way repeated-measures ANOVA was conducted on number of worries with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There was no main effect of Sleep Condition, F(1, 59) < 1, ns. There was a main effect of Age Group, F(2, 59) = 7.83, , p < .05. Post hoc tests indicated that in the rested condition, adults generated more worries than midadolescents, t(42) = 2.10, p < .05 and early adolescents, t(38) = 3.87, p < .001, and midadolescents generated more worries than early adolescents, t(42) = 1.95, p < .05. In the sleep-deprived condition, adults generated more worries than early adolescents, t(36) = 2.39, p < .01. There was no Age Group × Sleep Condition interaction, F(2, 59) < 1, ns.
Table 4.
Worry Generation, Most Threatening Worry, and Catastrophizing Task
| Early adolescents | Midadolescents | Adults | |
|---|---|---|---|
| Worry generation and most threatening worry | |||
| Number of worries | |||
| Sleep deprived | 2.16 (1.21) | 2.83 (1.27) | 3.42 (1.46) |
| Rested | 2.00 (1.67) | 3.04 (1.51) | 3.84 (1.26) |
| Most threatening worry | |||
| Sleep deprived | 64.00 (34.29) | 56.36 (23.26) | 54.12 (26.29) |
| Rested | 34.87 (26.22) | 55.00 (29.36) | 54.47 (23.70) |
| Catastrophizing task | |||
| Number of catastrophic steps | |||
| Sleep deprived | 7.58 (4.55) | 7.70 (3.28) | 7.56 (3.62) |
| Rested | 8.74 (5.69) | 8.61 (4.86) | 8.06 (3.83) |
| Pre-post anxiety difference score1 | |||
| Sleep deprived | 1.22 (2.05) | 0.96 (1.12) | 1.50 (2.68) |
| Rested | 0.33 (0.91) | 0.21 (1.32) | 1.00 (1.97) |
| Catastrophe likelihood rating | |||
| Sleep deprived | 53.34 (25.12) | 43.14 (24.08) | 56.59 (24.98) |
| Rested | 46.67 (24.02) | 40.52 (26.42) | 42.08 (27.02) |
Note. Mean values are presented with standard deviations in parentheses.
The Pre-post anxiety difference score was calculated by subtracting the pretask anxiety rating from the posttask anxiety rating.
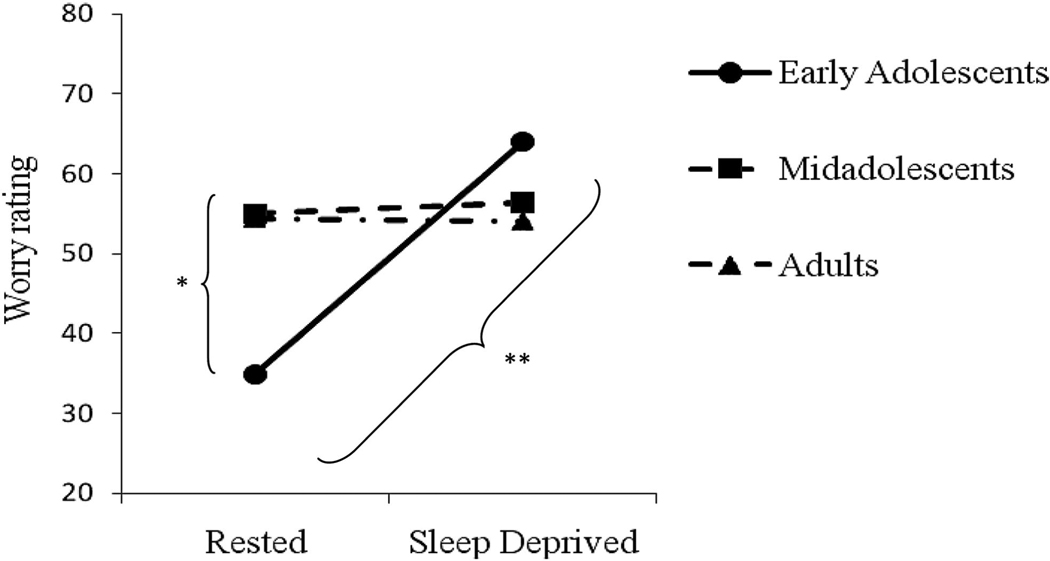
A two-way repeated-measures ANOVA was conducted on the rating of the most threatening worry with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There was an Age Group × Sleep Condition interaction, F(2, 51) = 4.47, , p < 05 (presented in Figure 1). Follow-up tests indicated that the early adolescents appraised the most threatening worry as significantly more threatening when sleep deprived compared to rested, t(14) = 3.44, p < .01. Additionally, in the rested condition, adults appraised the most threatening worry as significantly more threatening than early adolescents, t(33) = 2.34, p < .05. No other follow-up tests were significant. There was no main effect of Age Group, F(2, 51) < 1, ns. There was a main effect of Sleep Condition, F(1, 51) = 4.36, , p < .05, such that the most threatening worry was appraised as more threatening when sleep deprived, compared to when rested, though this appears to be a function of the two-way interaction.
Figure 1.
Rating of the most threatening worry across the rested and sleep-deprived conditions. Rating scale ranges from 0–100. Statistically significant differences are indicated with asterisks: * p < .05. ** p < .01.
Catastrophizing task
The mean values for this task are presented in Table 4. A two-way repeated-measures ANOVA was conducted on the number of catastrophic steps in the catastrophizing sequence with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There were no main effects of Sleep Condition, F(1, 57) = 1.51, ns, or Age Group, F(2, 57) < 1, ns. There was no Age Group × Sleep Condition interaction, F(2, 57) < 1, ns.
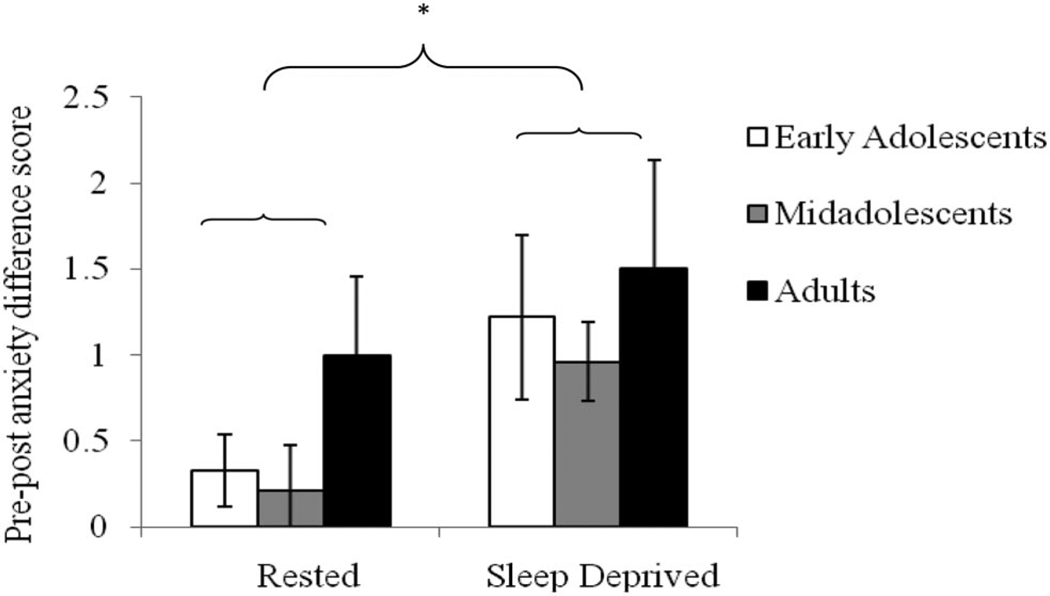
To examine the effect of catastrophizing on anxiety, we calculated the difference between the pretask and posttask anxiety ratings (“pre-post anxiety difference score”). A two-way repeated measures ANOVA was conducted on the pre-post anxiety difference score with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There was a main effect of Sleep Condition, F(1, 67) = 6.17, , p < .05, such that participants experienced a greater increase in anxiety during the task in the sleep-deprived, compared to rested, condition (presented in Figure 2). There was no main effect of Age Group, F(2, 57) = 1.31, ns, nor an Age Group × Sleep Condition interaction F(2, 57) < 1, ns.
Figure 2.
Pre-post anxiety difference score across the rested and sleep-deprived conditions. The statistically significant difference is indicated with an asterisk: * p < .05.
A two-way repeated measures ANOVA was then conducted on the catastrophe likelihood rating with Age Group (early adolescent, midadolescent, adult) as the between-subjects factor and Sleep Condition (sleep-deprived, rested) as the within-subject factor. There was a main effect of Sleep Condition, F(1, 49) = 3.79, , p < .05, with participants rating the average likelihood of each catastrophe coming true as higher in the sleep-deprived, compared to rested, condition. There was no main effect of Age Group, F(2, 49) < 1, ns, nor an Age Group × Sleep Condition interaction, F(2, 49) < 1, ns.
Controlling for affect differences between conditions
To address the possible concern that the results might be accounted for by differences in positive and negative affect at the beginning of the affective functioning battery, we reran the analyses controlling for the difference between the rested and sleep-deprived composite positive affect and the difference between the rested and sleep-deprived composite negative affect. The pre-post anxiety difference score was excluded from these analyses because the original analysis already accounted for the pretask anxiety rating. The results did not change except for the catastrophe likelihood rating. For the latter, the main effect for Sleep Condition did not remain when positive affect was covaried.
Discussion
The overall aim of this study was to investigate the impact of sleep deprivation on affect in a sample of adolescents and adults. We compared participants under two conditions: sleep deprivation and rest.
Our first prediction was that participants would experience less positive and more negative affect in the sleep-deprived, compared to rested, condition. Taking positive affect first, consistent with our hypothesis, participants reported less positive affect in the sleep-deprived condition relative to the rested condition. This result replicates previous research (Franzen et al., 2008; Zohar et al., 2005) and extends it by showing that this pattern of findings held for an array of discrete positive emotions including interested, excited, happy, strong, energetic, cheerful, active, proud, and delighted. These findings suggest that the affective changes following sleep deprivation reported in previous studies were not likely artificially driven by one or two particular emotions or sleep-related affective items. On the contrary, the results across these discrete positive emotions lend additional credence to the broad affective findings of previous research.
Contrary to our hypothesis, self-reported negative affect as measured by the PANAS-C did not increase following sleep deprivation. While this finding is consistent with Franzen et al. (2008), it is in contrast to other previous research reporting that sleep deprivation increases negative mood (e.g., Dinges et al., 1997). A number of possibilities may account for this difference. First, our study and the study by Franzen et al. (2008) used the PANAS, while Dinges et al. (1997) used the POMS. The POMS may indicate greater mood disturbance in part because some of its scales overlap with sleepiness, such as fatigue and vigor. Moreover, the apparent floor effect of responses to the PANAS-C negative affect items across conditions (see Table 3) may suggest that the PANAS does not assess the type of negative affect experienced when sleep deprived. In support of this proposal, Franzen et al. (2008) observed increased negative affect on a visual analog scale but not on the PANAS, and in this study we found increased anxiety but not increased negative affect according to the PANAS-C. Second, the study by Zohar et al. (2005) raises the possibility that context may be an important factor. Specifically, Zohar et al. (2005) showed that sleep loss increased negative affect following a goal-thwarting event. Taken together, future research should incorporate multiple measures of affect and consider the context.
Our second prediction was that participants would demonstrate heightened anxiety under conditions of sleep deprivation. Partial support for this hypothesis was evident. While participants did not generate more worries or longer catastrophizing sequences when sleep deprived relative to when rested, they did report more anxiety as a result of catastrophizing and they rated the likelihood of the catastrophes coming true as higher when sleep deprived, as compared to when rested. We note that the latter result did not hold when we controlled for positive affect. However, there were no significant correlations between positive affect and catastrophe likelihood rating in either condition, suggesting that the changed result when positive affect was covaried may have been due to a loss of statistical power. The anxiety results are consistent with a prior study suggesting an association between sleep deprivation and anxiety (Sagaspe et al., 2005). These data are also broadly consistent with previous research indicating that in the context of affective stimuli sleep-deprived individuals experience heightened amygdala activity and diminished medial-PFC activity (Yoo et al., 2007). It is interesting that the two outcome measures that did not reflect increases in anxiety in the sleep-deprived, compared to rested, condition were the tasks that required generating worries or catastrophes. Given the known adverse consequences of sleep deprivation on generative cognitive tasks (Van Dongen, Maislin, Mullington, & Dinges, 2003), perhaps the cognitive effort required for these tasks prevented demonstrable anxiety increases in the sleep-deprived condition. It is also possible that the relative cognitive difficulty of these tasks, compared to the simpler tasks of appraising threat, rating anxiety, and rating the likelihood of catastrophes, led to increased, compensatory PFC activation (e.g., Lungu, Binenstock, Pline, Yeaton, & Carey, 2007; Drummond et al., 2000) and consequent better regulation of anxiety.
An important goal of this study was to extend the literature on the effects of sleep deprivation in adolescence. We found that adolescents experienced less positive affect when sleep deprived, compared to when rested (as did the adults). These findings are particularly noteworthy given that good sleep has been proposed to be a mechanism by which positive affect protects against depression in adolescents (Dahl, 1996; Forbes & Dahl, 2005). Moreover, we observed that adolescents reported more anxiety as a result of catastrophizing and rated the likelihood of the catastrophes as higher when sleep deprived, compared to when rested (as did the adults). Together, these findings raise the potential importance of sufficient sleep for regulation of anxiety in both adolescents and adults. Two age group differences emerged. First, the early adolescent group appraised their most threatening worry as significantly more threatening when sleep deprived, compared to when rested, whereas this finding was not observed in the midadolescent and adult groups. This finding is consistent with evidence that younger adolescents exhibit poorer affective decision-making—a process reliant on PFC maturation—compared to older adolescents (Hooper, Luciana, Conklin, & Yarger, 2004). It is possible that the early adolescents in the present study experienced increased threat appraisal under conditions of sleep deprivation, compared to rest, given the earlier stage of the development of the PFC in this age group, relative to midadolescents and adults (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Giedd, 2004). We also note that when rested, the early adolescent group appraised their most threatening worry as less threatening than the adult and midadolescent groups. While there is dearth of research on worry over the course of adolescence (Leen-Feldner, Feldner, Tull, Roemer, & Zvolensky, 2006), this result is consistent preliminary evidence that worry-relevant processes increase throughout adolescence (e.g., Vasey, Crnic, & Carter, 1994; Hampel & Petermann, 2005). Indeed, considering both this initial evidence and the lower rested threat appraisal level in the present study, it is notable that the early adolescents exceeded the threat appraisal levels of midadolescents and adults when sleep deprived. Second, the three age groups differed on the number of worries generated, with adults generating the most worries and early adolescents the least. This result likely stems from developmental differences in cognitive fluency (particularly writing fluency; e.g., Woodcock, McGrew, & Mather, 2001).
Several limitations are important to consider. First, the relatively small sample size may have limited the statistical power of the study to detect differences, particularly between the three age groups. Second, not all participants complied with the instructions (i.e., a maximum of 6.5 hours TST on the first sleep-deprivation night and 8.5 hours TST on each of the two rested nights). However, all participants accrued substantial sleep deprivation on the second (laboratory) sleep-deprivation night and the average TST on the rested nights exceeded habitual TST. Future studies should consider conducting the sleep manipulations entirely in the laboratory to ensure full compliance. In addition, 8.5 hours TST may be unrealistic for some adult participants based on sleep need (e.g., Wehr, 1991). Third, not all participants wore actigraphs, introducing variability between diary and actigraphy data into the manipulation checks. We note, however, that there was good correlation between the two measures. Fourth, the design did not control for circadian influences and some evidence suggests that such effects may be important to consider in studies of positive affect (Murray, Allen, & Trinder, 2002; Hasler, Mehl, Bootzin, & Vazire, 2008). Hence, future research in this area is needed to control for potential circadian influences, though for adolescent samples there may be greater ecological validity in the absence of circadian controls given that many adolescents are required to function at biologically inappropriate times on a daily basis (Carskadon, Acebo, & Jenni, 2004). Finally, our sample size was insufficient to examine the impact of pubertal status. Given data suggesting that pubertal status may be a more critical factor than age in terms of affective reactivity (Silk et al., 2009), this is an important topic for future research.
In conclusion, the findings of the current study suggest that sleep deprivation reduces positive affect across an array of positive emotions. Furthermore, sleep deprivation increases anxiety, whether measured directly through self-report or indirectly through likelihood ratings of catastrophes. Early adolescents appear to be particularly vulnerable to increased threat appraisal under conditions of sleep deprivation, potentially as a result of the ongoing development of the PFC. Our results align with the body of evidence indicating adverse affective consequences of sleep deprivation. The findings extend the current literature by beginning to elucidate these effects in three new domains: specific positive emotions, anxiety, and adolescents.
Acknowledgments
This project was supported by National Institute of Mental Health Grant No. R24 MH067346 awarded to R.E.D.
Footnotes
There was good correlation between actigraphy and diary (r = .52, p < .01)
Some participants did not comply with keeping a sleep diary and wearing an actigraph during the at-home portions of the study.
Participants who exceeded 6.5 hours of TST on the first night of sleep deprivation were not excluded because the majority of the sleep deprivation occurred on the second (laboratory) night.
The partial eta squared effect sizes are reported with the results of the major dependent variables. Small effect sizes are less than .06, moderate effect sizes range from .06 –.14, and large effect sizes are greater than .14 (Cohen, 1973).
Contributor Information
Lisa S. Talbot, Department of Psychology, University of California, Berkeley
Eleanor L. McGlinchey, Department of Psychology, University of California, Berkeley
Katherine A. Kaplan, Department of Psychology, University of California, Berkeley
Ronald E. Dahl, Department of Psychiatry, University of Pittsburgh
Allison G. Harvey, Department of Psychology, University of California, Berkeley
References
- Acebo C, Carskadon MA. An evaluation of children’s self reported sleep measures. Sleep Research. 1993;22:53. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Babkoff H, Caspy T, Mikulincer M. Subjective sleepiness ratings: The effects of sleep deprivation, circadian rhythmicity and cognitive performance. Sleep. 1991;14:534–539. doi: 10.1093/sleep/14.6.534. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. 2nd ed. New York: Guilford Press Publications; 2002. [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G, Mathews A. Anticipatory anxiety and risk perception. Cognitive Therapy and Research. 1987;11:551–565. [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Campbell SC, Murphy PJ. The nature of spontaneous sleep across adulthood. Journal of Sleep Research. 2007;16:24–32. doi: 10.1111/j.1365-2869.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Adolescent sleep patterns biological, social, and psychological influences. Cambridge, U.K.: Cambridge University Press; 2002. [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Annals of the New York Academy of Sciences. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep: Journal of Sleep Research and Sleep Medicine. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in communication science. Human Communication Research. 1973;28:473–490. [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8:3–27. [Google Scholar]
- Dalgleish T, Power M, editors. Handbook of cognition and emotion. Chichester, West Sussex, U.K.: Wiley; 1999. [Google Scholar]
- Davey GCL, Levy S. Catastrophic worrying: Personal inadequacy and a perseverative iterative style as features of the catastrophizing process. Journal of Abnormal Psychology. 1998;107:576–586. doi: 10.1037//0021-843x.107.4.576. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Dement WC, Miles LE, Carskadon MA. “White paper” on sleep and aging. Journal of American Geriatrics Society. 1982;30:25–50. doi: 10.1111/j.1532-5415.1982.tb03700.x. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–267. [PubMed] [Google Scholar]
- Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. NeuroReport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Anxiety: The cognitive perspective. Hove, U.K.: Erlbaum; 1992. [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Laufer MR. Physiology of puberty. In: Emans SJH, Goldstein DP, Laufer MR, editors. Pediatric and adolescent gynecology. 5th ed. Philadelphia: Lippincott, Williams, & Wilkins; 2005. pp. 120–155. [Google Scholar]
- Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Hampel P, Petermann F. Age and gender effects on coping in children and adolescents. Journal of Youth and Adolescence. 2005;34:73–83. [Google Scholar]
- Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Greenall E. Catastrophic worry in primary insomnia. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:11–23. doi: 10.1016/s0005-7916(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Hairston IS, Gruber J, Gershon A. Anxiety and sleep. In: Antony MA, Stein MB, editors. The handbook of anxiety and anxiety disorders. New York: Oxford University Press; 2008. [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviours associated with positive affect. Journal of Research in Personality. 2008;42:1537–1546. [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Kendall PC. The cognitive side of anxiety. Cognitive Therapy and Research. 1987;11:523–536. [Google Scholar]
- Izard CE. Human emotions. New York: Plenum Press; 1977. [Google Scholar]
- Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: II. A novel approach to scoring and interpreting sleep-wake activity. Perceptual and Motor Skills. 1997;85:219–226. doi: 10.2466/pms.1997.85.1.219. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, Redline S. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: A comparison of three data modes. Sleep. 2007;30:899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 hours of sleep deprivation. Journal of Sleep Research. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: A 1-Year prospective study of injured survivors of motor vehicle accidents. American Journal of Psychiatry. 2002;159:855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- Lack LC. Delayed sleep and sleep loss in university students. Journal of American College Health. 1986;35:105–110. doi: 10.1080/07448481.1986.9938970. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE, Jr, Rudolph KD, Potter KI, Lambert S, Gathright T. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. [Google Scholar]
- Leen-Feldner EW, Feldner MT, Tull MT, Roemer L, Zvolensky MJ. An examination of worry in relation to anxious responding to voluntary hyperventilation among adolescents. Behavior Research & Therapy. 2006;44:1803–1809. doi: 10.1016/j.brat.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Lungu OV, Binenstock MM, Pline MA, Yeaton JR, Carey JR. Neural changes in control activation of a continuous task. The Journal of Neuroscience. 2007;27:3010–3016. doi: 10.1523/JNEUROSCI.5051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Druppleman LF. EITS manual for the profile of mood states. San Diego: Educational and Industrial Test Services; 1971. [Google Scholar]
- Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Research. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:900–905. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Press Publishers; 2003. [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: Investigation of a circadian component in positive affect. Chronobiology International. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends in Cognitive Sciences. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Mackey S. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. International Review of Psychiatry. 2005;17:229–236. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Power M, Dalgleish T. Cognition and emotion: From order to disorder. Hove, U.K.: Psychology Press; 1997. [Google Scholar]
- Richardson GS, Malin HV. Circadian rhythm sleep disorders: Pathophysiology and treatment. Journal of Clinical Neurophysiology. 1996;13:17–31. doi: 10.1097/00004691-199601000-00003. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Alster J, Urbach D, Lavie P. Actigraphically based automatic bedtime sleep-wake scoring: Validity and clinical applications. Journal of Ambulatory Monitoring. 1989;2:209–216. [Google Scholar]
- Sagaspe P, Sanchez-Ortuno M, Charles A, Taillard J, Valtat C, Bioulac B, Philip P. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain and Cognition. 2006;60:76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21:7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Vanderbilt-Adriance E, Shaw DS, Forbes EE, Whalen DA, Ryan ND, Dahl RE. Resilience among children and adolescents at risk for depression: Mediation and moderation across social and neurobiological contexts. Development and Psychopathology. 2007;19:841–865. doi: 10.1017/S0954579407000417. [DOI] [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the structured clinical interview for DSM–III–R axis I (SCID-I) Acta Psychiatrica Scandinavica. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First M. Structured clinical interview for DSM–IV (SCID) Washington, DC: American Psychiatric Association; 1996. [Google Scholar]
- Startup HM, Davey GCL. Mood as input and catatrophic worrying. Journal of Abnormal Psychology. 2001;110:83–96. doi: 10.1037//0021-843x.110.1.83. [DOI] [PubMed] [Google Scholar]
- Van den Bulck J. Text messaging as a cause of sleep interruption in adolescents, evidence from a cross-sectional study. Journal of Sleep Research. 2003;12:263. doi: 10.1046/j.1365-2869.2003.00362.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Borkovec TD. A catastrophizing assessment of worrisome thoughts. Cognitive Therapy and Research. 1992;16:505–520. [Google Scholar]
- Vasey MW, Crnic KA, Carter WG. Worry in childhood: Developmental perspective. Cognitive Therapy and Research. 1994;18:529–549. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect. The PANAS scales. Journal of Personality and Social Psychology. 1988:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod) Journal of Clinical Endocrinology and Metabolism. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Archives of Pediatric Adolescent Medicine. 2008;162:350–358. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Wittchen H. The Structured Clinical Interview for DSM–III–R (SCID): Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Yoo S, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep-a prefrontal amygdala disconnect. Current Biology. 2007;17:877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epsten R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: A cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]