Abstract
Background
Autologous bone marrow mononuclear cell (BMMC) therapy has shown promise for improving cardiac function post myocardial infarction. However, the efficiency of such therapy for diabetic patients remains unknown.
Methods
BMMCs were harvested from type II diabetic male BKS.Cg-m+/+Leprdb/J mice or C57BLKS/J (non-diabetic control) mice and were isolated using Ficoll-based separation as seen in clinical trials. Cell characterization was performed by flow cytometry. Cell viability was determined by apoptosis and proliferation assays. Female BKS.Cg-m+/+Leprdb/J mice underwent left anterior descending (LAD) artery ligation and were randomized into 3 groups receiving 2.5×106 diabetic BMMCs (n=8), 2.5×106 control BMMCs (n=8), or PBS (n=6), respectively. Echocardiography and invasive hemodynamic measurements were used to assess cardiac function at week 5. Post-mortem cell survival was quantified by Taqman RT-PCR for male Sry gene.
Results
BKS.Cg-m+/+Leprdb/J BMMCs showed a significantly lower mononuclear fraction (using flow cytometry) and a significantly lower proliferation rate compared with C57BLKS/J BMMCs. Echocardiography and invasive hemodynamic measurements showed significant in vivo improvement in fractional shorting (40.1±1.2% vs. 30.3±1.9%; P=0.001) and cardiac output (4,166±393 vs. 2,246±462 μl/min; P=0.016) for mice treated with control BMMCs injection compared to those treated with diabetic BMMCs, respectively. This difference could not be attributed to difference in cell engraftment as Taqman RT-PCR showed no significant difference in cell survival in infarcted hearts between the two groups.
Conclusions
Diabetic BMMCs are significantly impaired in their ability to improve cardiac function following myocardial infarction as compared to control BMMCs. The findings here could have significant clinical implication regarding autologous BMMC therapy in diabetic patients.
BACKGROUND
Cardiovascular disease remains the number one cause of morbidity and mortality in the Western world (1). Myocardial infarction (MI) is, by nature, an irreversible injury (2). In recent years, numerous animal studies on cell therapy as potential treatment for MI have been conducted. In particular, bone marrow mononuclear cells (BMMCs) show promising results (3–6). In fact, translation of such therapy to clinical trails has been made, although these studies have produced mixed outcomes (7–9).
Approximately 17 million patients are diagnosed with diabetes mellitus (DM) in the US and the number of patients is still rising (10). As a consequence, patients often develop diabetic vasculopathy, which is a systemic disease characterized by severe impairment of the development of collateral vessels (11, 12). Therefore, diabetic patients are two to four times more likely to develop cardiovascular disease (13). Different mechanisms have been suggested to explain this disorder, including endothelial dysfunction (14), down regulation of VEGF by hyperglycemias (15), and dysfunction in endothelial progenitor cells (EPCs) (16–19) that impairs ischemia-induced angiogenesis (19).
Previous studies have shown that in animal models of hind-limb ischemia, autologous BMMCs from diabetic animals have impaired capacity to improve ischemia-induced angiogenesis. Despite inconsistent results in animal studies (20–22), translation to the clinic has been made (23, 24). However, the efficiency of autologous BMMC transplantation for treatment of myocardial infarction in patients with DM remains unknown. In this study, we investigated the therapeutic efficacy of autologous BMMC transplantation for treatment of acute myocardial infarction in a mouse model of type II DM.
METHODS
Animals
10 to 12 weeks old male BKS.Cg-m+/+Leprdb/J (db/db; Jackson Lab, Bar Harbor, ME) mice (diabetes mellitus type 2) or male C57BLKS/J mice (healthy litter mate control; Jackson Lab, Bar Harbor, ME) were used as BMMC donors. In the db/db mice elevations of plasma insulin begin at 10 to 14 days and elevations of blood sugar at four weeks. These homozygous mutant mice are polyphagic, polydipsic, and polyuric. Delayed wound healing, increased metabolic efficiency, peripheral neuropathy and myocardial disease are seen in C57BLKS-Leprdb homozygotes. Blood glucose levels were measured with FreeStyle glucose meters and FreeStyle test strips (Abbott Laboratories, Abbott Park, IL) before surgery or harvesting BMMCs. In db/db mice, diabetes was confirmed when the blood glucose level after 4 hours of fasting was > 240 mg/dl. Female BKS.Cg-m+/+Leprdb/J mice were used as recipients for all in vivo experiments. Diabetes was confirmed as described above. Animal care was provided in accordance with the Stanford University School of Medicine guidelines and policies for the use of laboratory animals.
Preparation of BMMCs
Bone marrow cells were harvested from the long bones of 10- to 12-week male BKS.Cg-m+/+Leprdb/J mice (db/db BMMCs) or age matched C57BLKS/J mice (control BMMCs) by flushing with PBS using a 25-gauge needle. After passing through a 70 μm strainer, the isolate was centrifuged at 1200 rpm for 5 minutes, washed, and resuspended into 5 ml of saline. To acquire the mononuclear fraction, the bone marrow isolate was centrifuged for 35 minutes at 1900 rpm using a 14 ml tube with 5mL Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) gradient and 5 cell/saline suspension. BMMCs were washed again and counted using a Hemacytometer (Fischer Scientific, Pittsburgh, PA). BMMCs were prepared freshly for application.
Flow cytometry analysis
The BMMCs’ identity was investigated by fluorescent-activated cell sorting (FACS) with antibodies recognizing Sca-1 (progenitor cells), Mac-1 (macrophages), GR-1 (granulocytes), CD45 (hematopoietic cells), CD34 (EPCs), and CD133 (EPCs; Becton, Dickinson and Company. San Jose, CA). BMMCs (1 × 106) were incubated in 2% fetal bovine serum/PBS at 4°C for 30 minutes with 1 μl of monoclonal fluorescein isothiocyanate (FITC)-conjugated antibodies and processed through a FACSCalibur system with CellQuest software (Becton, Dickinson and Company, San Jose, CA) according to the manufacturer’s protocol. For each antibody, the experiment was repeated independently three times.
Apoptosis assay
Quantification of apoptotic cells was performed using an Annexin-V-FITC Apoptosis Detection Kit (CD106 (Becton, Dickinson and Company, San Jose, CA) according to the manufacturer’s instructions. 1×106 BMMCs cells from both db/db and control mice were plated in 2 ml of medium (IDIM with 10% FBS and 1% P/S-L-G, all from Invitrogen Corporation (Gibco), Carlsbad, CA) into a 6-well plate. They were then incubated at 37°C under normoxia conditions (78% CO2/21% O2) and hypoxia conditions (95% CO2/5% O2), using Nillups-Rothenberg Modular Incubator Chamber (Del Mar, CA). Afterwards, cells were collected, washed, and resuspended in 500 μl of binding buffer, and 5 μl of Annexin-V-FITC with 10 μl of propidium iodide (PI) was added and incubated at room temperature for 10 minutes. Analyses were performed with FACSCalibur and CellQuest software (Becton, Dickinson and Company, San Jose, Ca) according to manufacturer’s protocol. The cells in the FITC-positive and PI-negative fraction were regarded as apoptotic cells. This experiment was repeated three times.
Cell proliferation assay
Proliferation was determined by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 1×105 BMMCs cells from both db/db (n=2) and control (n=2) mice were plated in 100 μl medium (IDIM with 10% FBS en 1% P/S-L-G (all from Invitrogen, Carlsbad, CA) into a 96-well plate and were incubated under normoxia and hypoxia conditions as described above for 32 hours. 20 μl of MTT was added to each well, and the plate was further incubated for another 4 hours in same conditions. Absorbance was determined with a multi-well absorbance reader (Genios, Tecan Systems Inc., San Jose, CA) at 490 nm with included manufacture software (Magellon v6.2). Experiments were carried out as quadruplicates. Absorbance values were expressed as percentages.
Surgical model for myocardial infarction
10- to 12-week old female diabetic db/db mice were randomized into three groups receiving either 2.5×106 db/db BMMCs (n=8, db/db group), 2.5×106 control BMMCs (n=8, control group), or phosphate buffered saline (n=6, PBS group). Animals were intubated with a 20-gauge angiocath (Ethicon Endo-Surgery, Inc., Cincinnati, OH) and placed under general anesthesia with 2–3% mixture of isoflurane and 100% O2 using a rodent ventilator (Harvard Apparatus, Holliston, MA). Myocardial infarction (MI) was created by ligation of the left anterior descending (LAD) artery with 8-0 ethilon suture through a left anterolateral thoracotomy as described before (25). Two doses were injected into the infarcted area via the lateral and medial infarct zones using a Hamilton syringe with a 29-gauge needle. A total volume of 50 μl was injected containing 2.5×106 cells or PBS alone, depending on group randomization. Surgery was performed by a single experienced micro-surgeon (G.H.).
Echocardiographic determination of left ventricular contractility
Echocardiography was performed at postoperative days 7, 14, and 35. Animals received continuous inhaled anesthetic (1.5–2% isoflurane) for the duration of the imaging session. Two-dimensional transversal-targeted M-mode traces were obtained at the level of the papillary muscles using a 14.7-MHz transducer on a Sequoia C512 Echocardiography system (Siemens, Malvern PA). Analysis of the M-mode images was performed using DicomWorks 1.3.5 (http://dicom.online.fr) analysis software. Left ventricular end-diastolic diameter (EDD) and end-systolic diameter (ESD) were measured by a blinded investigator (R.J.S.) and used to calculate fractional shortening (FS) by the following formula: FS = (EDD−ESD)/EDD as described (26).
Measurements of hemodynamics with pressure-volume loops
Invasive, steady-state hemodynamics measurements were conducted by closed-chest pressure-volume loop analysis prior to sacrifice at week 5. The animal was placed under general anesthesia as described above. After midline neck incision, a 1.4-F conductance catheter (Millar Instruments, Houston TX) was advanced retrogradely through the right carotid artery into the left ventricle. Surgery was performed by a single blinded micro-surgeon (G.H.) with over 6 years of experience with this model. The measurements of segmental conductance were recorded, allowing extrapolation of the left ventricular volume, which was coupled with pressure. These data were analyzed by a blinded investigator (K.E.A.v.d.B.) using PVAN 3.4 Software (Millar Instruments, Houston, TX) and Chart/Scope Software (AD Instruments, Colorado Springs, CO).
TaqMan PCR for male specific Sry gene
At week 5, animals were sacrificed and hearts were explanted, minced, homogenized in 2 ml DNAzol (Invitrogen, Carlsbad, CA) and 500 ng DNA was processed for Taqman PCR using primers (TaqMan Gene Expression Assays, Alied Biosystems, Foster City, CA) specific for the Sry locus. RT-PCR reactions were conducted in iCylcer IQ Real-Time Detection System (Bio-Rad, Hercules, CA). Detection levels were compared to a standard curve to assess the number of viable cell per sample. Each sample was carried out in sextuplicate and the average was used for the analysis.
Statistical analysis
Unless otherwise stated, data are presented as mean ± SEM. Comparisons between groups were done by independent sample t-tests or analysis of variance (ANOVA) least significant difference post hoc tests, where appropriate. Differences were considered significant for P-values <0.05. Statistical analysis was performed using SPSS statistical software for Windows.
RESULTS
Functional characterization of db/db BMMCs vs. control BMMCs in vitro
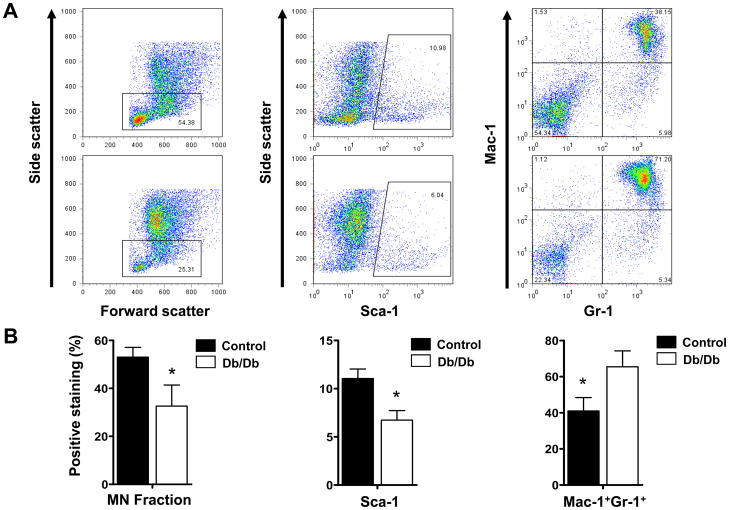
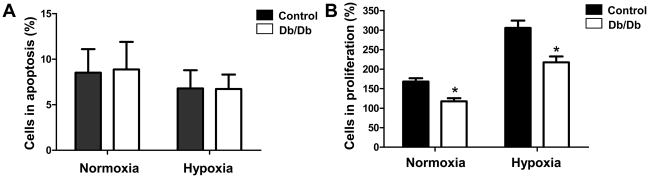
To understand the characteristics of BMMCs isolated from the db/db and the wild-type control mice, we first analyzed their surface markers. After bone marrow purification using Ficoll gradient, the mononuclear fraction in the db/db group was significantly lower (32.64± 8.75%) than that in the control group (53.06± 4.03%) (Fig 1a–b, left panels). This resulted in a significantly lower percentage of Sca-1+ progenitor cells in the db/db group (6.73±0.99%) compared to the control group (11.05±0.09%) (Fig 1a–b, middle panels). In the db/db group, there is a significantly higher percentage of Mac-1+Gr-1+ granulocytes (65.43± 8.79%) than in the control group (41.03± 7.39%) (Fig 1a–b, right panels). We found that the total number of CD45negCD34+ or CD45negCD133+ endothelial progenitor cells was low in both db/db and control mice (0.6 – 1.2% of total BMMCs), and did not find significant difference between the two groups. Apoptosis of BMMCs was measured after 24 hours of incubation in either normoxic conditions or hypoxic conditions (Fig 2a). In normoxic conditions, we found no difference (P=0.916) between control BMMCs (8.51±2.58%) and db/db BMMCs (8.87±1.99%). According to Ohnishi et al., hypoxia induces mesenchymal stem cells to upregulate genes encoding for cell survival (27). Therefore, we also examined whether BMMCs would show a similar robustness during hypoxia and if there would be a difference between db/db BMMCs and the control BMMCs. Both groups showed slight decrease in apoptosis compared to a normoxic environment, but again there was no statistical difference (P=0.985) between the control BMMCs (6.79±3.03%), and db/db BMMCs (6.73±1.59%) Previous reports have demonstrated impaired proliferation of EPCs in the setting of hyperglycemia (18, 19). Therefore, we investigated the proliferation potency of db/db vs. control BMMCs in vitro. After 36 hours in culture, db/db BMMCs showed a significantly impaired proliferation rate as compared to control BMMCs (Fig 2b) under both normoxic conditions (117.79±7.69% vs. 168.13±8.67%; P<0.05) and hypoxic conditions (217.38±15.26% vs. 305.87±18.50%; P<0.05).
Figure 1.
Characterization of bone marrow mononuclear cells. (A) The identity of BMMC subpopulation was investigated by fluorescent-activated cell sorting (FACS) with antibodies recognizing Sca-1, Mac-1, GR-1, CD45, CD34, and CD133. (B) After Ficoll gradient (an isolation procedure as seen in clinical trials), the db/db group has significantly lower mononuclear (MN) cell fraction and Sca-1 population but higher Mac-11+/Gr-1+ fraction. This indicates that the db/db BMMC injections used have a significantly lower fraction of MN cells than the control BMMC injections. Moreover, within the db/db group there is a higher contamination of granulocytes. No significant differences in CD45negCD34+ or CD45negCD133+ were found (data not shown). Graphs represent 3 independent experiments. Abbreviations: BMMCs, bone marrow mononuclear cells.
Figure 2.
In vitro cell viability and proliferation assays. (A) Apoptosis measured after 24 hours of incubation in either normoxic conditions or hypoxic conditions. BMMCs from control or db/db donors showed the same trend in normoxia. (B) For proliferation capacity, BMMCs were cultured for 36 hours under normoxic and hypoxic conditions and cell quantitation was performed with the MTT assay. After 36 hours, db/db BMMCs showed a significantly impaired proliferation rate as compared to control BMMCs under both normoxic conditions and hypoxic conditions. Data are expressed as percentage relative to 100% at t=0 (*P <0.05). Abbreviations: MTT assay, (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Functional effects of BMMC transplantation into db/db mice following MI
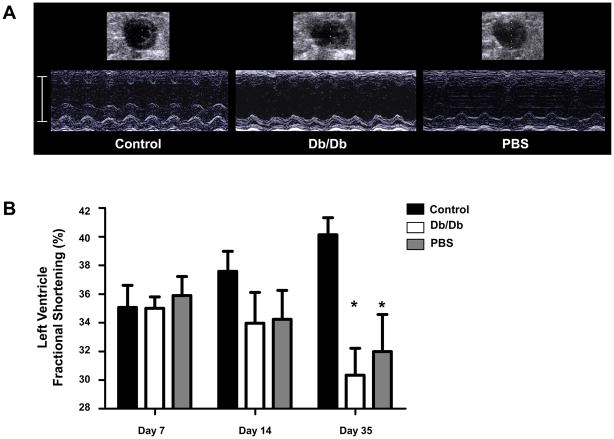
Several animal studies have shown the therapeutic efficiency of BMMC transplantation after myocardial infarction (3–5, 28). However, less well known is the efficacy of BMMC transplantation in the setting of DM. Therefore, we investigated the therapeutic efficacy of autologous BMMCs transplantation for treatment of acute myocardial infarction in a mouse diabetic model. Echocardiographic measurements of cardiac performance were conducted at days 7, 14, and 35 after cell transplantation (Fig 3a). At day 35, left ventricular fractional shortening (LVFS) for animals treated with control BMMCs was significantly higher (40.1±1.2%) than that for animals treated with db/db BMMCs (30.3±1.9%, P=0.001) and PBS control (32.0±2.6%, P=0.01). LVFS between db/db BMMCs and PBS treated groups was not significantly different (Fig 3b).
Figure 3.
Left ventricular function following BMMC therapy. (A) Representative M-mode images of heart at 5 weeks after LAD ligation with administration of control BMMCs (left), db/db BMMCs (middle), or PBS control (right). (B) Quantification of left ventricular fractional shortening (LVFS) demonstrated a significant improvement at 5 weeks post-MI in animals receiving control BMMCs compared to db/db BMMCs or PBS (*P < 0.05). Abbreviations: M-mode, motion mode; PBS, phosphate-buffered saline; LAD, left anterior descending.
Validation of non-invasive measurements of left ventricular dimensions
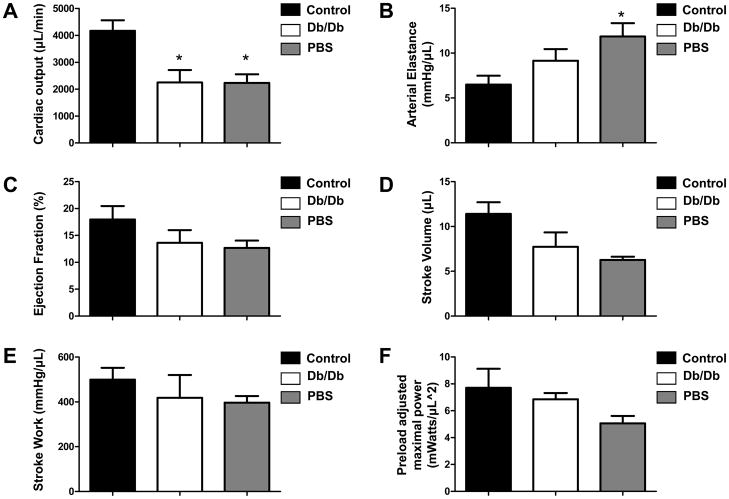
To confirm echocardiographic findings of improved cardiac contractility, we next performed invasive hemodynamic measurements (Fig 4). As expected, the cardiac output was significantly better in animals treated with control BMMCs (4166±393 μl/min) compared with animals treated with db/db BMMCs (2246±462 μl/min; P=0.016) and PBS control (2232±320 μl/min; P=0.019). Moreover, the arterial elastance was significantly decreased in control BMMC-treated animals (6.49±0.98 mmHg/μl) compared with PBS (11.85±1.47 mmHg/μl; P=0.04). No significant changes in arterial elastance were detected vis-à-vis db/db BMMC-treated group. This trend persisted for the other hemodynamic measurements performed, though they did not achieve statistical significant difference (Fig 4 and Supplemental Table 1).
Figure 4.
Invasive hemodynamic measurements using pressure-volume loops. (A) At day 35, cardiac output was significantly increased in hearts receiving control BMMCs compared to hearts receiving db/db BMMCs or PBS (*P<0.05). (B) Moreover, arterial elastance was significantly decreased in hearts receiving control BMMCs compared with hearts receiving PBS (*P<0.05). (C–F) At day 35, there was a trend toward improved functional recovery in animals receiving control BMMCs compared with animals receiving db/db BMMCs or PBS. This trend did not achieve statistical significance compared with db/db BMMCs.
Quantification of BMMC survival
In our protocol, transplanted BMMCs were derived from male mice and transplanted into female recipients, which facilitates quantification of male cells in the explanted female hearts by tracking the Sry locus found on the Y chromosome. Earlier findings by Müller-Ehmsen et al. (29) have confirmed the validity and robustness of this quantification technique. In both groups, cell survival was <1% of total injected cell number at day 35 (12,912±7238 in the db/db group vs. 15,174±2428 in the control group; P=0.81). This poor long-term survival rate is consistent with findings from previous studies (6, 30). Taken together, these data show that there is no difference in cell survival at day 35 between control BMMCs and db/db BMMCs (Fig 5).
Figure 5.
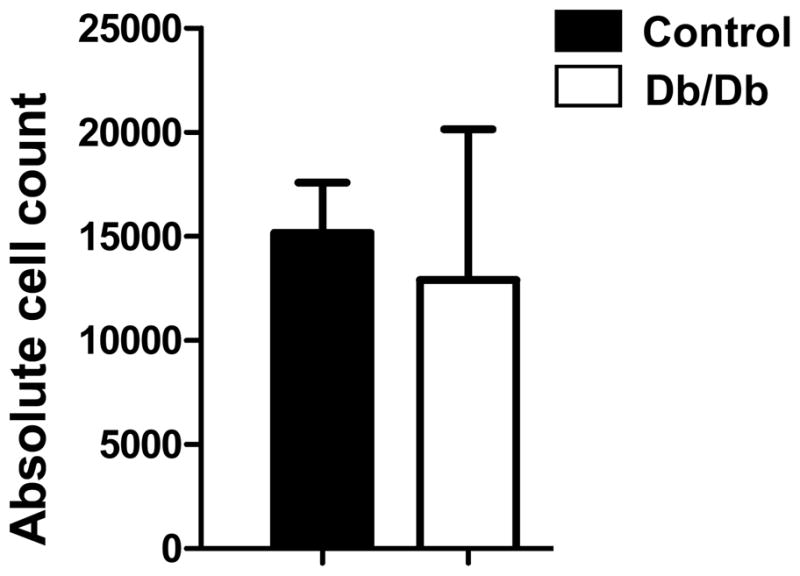
Real-time polymerase chain reaction quantification of surviving male transplanted BMMCs within female heart. Ex vivo Taqman analysis of hearts undergoing LAD ligation following injection of db/db BMMCs versus control BMMCs revealed no significant difference in survival of transplanted cells. In both hearts cell, survival at day 35 after surgery was <1% (n=3 in control and n=5 in db/db group).
DISCUSSION
Bone-marrow derived cell therapy in the setting of diabetes has shown impaired therapeutic efficacy in endothelialization, ischemic hind-limb, or wound-healing models (17–19, 22). We now present additional evidence that in the setting of myocardial infarction, transplantation of diabetic BMMCs has severe limitations in its therapeutic efficacy. Our data can be summarized as follows: (a) autologous diabetic (db/db) BMMCs transplantation does not lead to a significant preservation of cardiac function after myocardial infarction compared to healthy (control) BMMC injection (as shown by echocardiography and pressure volume loops); (b) this lack of preservation could be as a result of a significantly lower mononuclear fraction of the db/db BMMCs injection (as shown by flow cytometry analysis) and/or as a result of significant lower proliferation rate of the db/db BMMCs (as shown by the proliferation assay); and (c) this lack of preservation does not, however, seem to be related to a lower survival rate of db/db cells as there were no differences in either in vitro apoptosis assay or ex vivo TaqMan Sry quantitation between the two groups. Clinical trials such as ASTAMI, TOPCARE-CHD and REPAIR-AMI (7–9) contain large subpopulations of patients suffering diabetes (10 to 29 % of BMMC treated group). Therefore, this animal model gives us a clear view of cell transplantation in diabetic mice after MI and may shed additional insights into these outcomes.
One limitation of our study is that we used murine cells rather than human cells. Although we could have transplanted human cells into mice, our aim was to mimic the clinical environment by creating an autologous transplantation model instead of a xenotranplantation model, which would have likely further influenced cell survival. In contrast to previous studies (18, 31), we did not select BMMC sub-populations in order to promote a higher rate of therapeutic angiogenesis. Although the most efficient BMMC sub-population able to induce beneficial effects for angiogenesis is not yet known, some evidence indicates that circulating EPCs could increase functional activity to improve neovascularization after ischemia (31, 32). Unfortunately, animal (17, 33) and human (18) studies have shown that EPCs from diabetic donors have a disabled function for endothelial regeneration.
In our study, we did not find an absolute lower number of EPCs in the db/db mice as compared to healthy controls, however an impaired function of these cells may have attributed to the poor cardiac functional recovery of mice transplanted with db/db BMMCs. Another explanation for the dysfunction of the transplanted db/db BMMCs could be that the lower mononuclear fraction results in a significantly lower percentage of Sca-1+ progenitor cells (as shown in Fig 1a). In addition, in db/db mice, there is a significantly higher percentage of contaminating Mac-1+Gr-1+ granulocytes following BMMC isolation. Co-transplantation of these inflammatory cells into the infarcted myocardium may interfere with the therapeutic potential of progenitor cells. Van den Akker et al. reported similar granulocyte contamination of mononuclear cell fractions after Ficoll-based separation in septic patients (34), due to the induced stress response. It is possible that db/db animals have a form of chronic stress response due to their diseased diabetic state; however, further investigation is needed to support this hypothesis. One could also argue that the isolation of the db/db BMMCs was not optimal before transplantation and if we could further purify the progenitor cells, the cardiac function could be significantly improved. Nevertheless, for our study, we adopted a similar protocol (Ficoll-based separation) that was used in previous clinical trials (7–9). The results of this study suggest that diabetic patients enrolled in BMMN cell trials were transplanted with lower amounts of progenitor cells compared to non-diabetic patients because of lower yields and higher granulocytes contamination.
The poor proliferation capacity could also contribute to the dysfunction of the transplanted diabetic BMMCs. In our study, we have demonstrated that diabetic BMMCs have a significantly lower proliferation rate under both normal oxygen and hypoxic circumstances. It is unclear whether these db/db BMMCs have an absolute lower proliferation capacity or for some other reasons just proliferate less robustly compared to healthy BMMCs. One way to address this issue in the future may be to image BMMCs tagged with molecular beacons that would allow non-invasive monitoring as shown previously (28). Nevertheless, the significant difference in cell proliferation after 36 hours in vitro indicates that the diabetic BMMCs have an inferior capacity to expand compared to the healthy BMMCs.
In summary, this study demonstrated that db/db BMMCs have no favorable effect on cardiac function after infarction compared to control BMMCs. Therefore, our findings question the effectiveness of the autologous BMMC therapy as a treatment for MI in patients with co-existing diabetic disease. However promising cell therapy may be, this data should be an impetus for further research on the mechanism of action of diabetic BMMCs.
Supplementary Material
Acknowledgments
GRANT SUPPORT
This work was supported in part by grants from Burroughs Wellcome Foundation (J.C.W.), HL085899-02 (J.C.W.), the American Heart Association Medical Student Research Award (J.A.G.), the Netherlands Heart Foundation (J.A.G.), and grants from the University of Leiden (J.A.G.)
Abbreviations
- MI
Myocardial Infarction
- BMMCs
Bone Marrow Mononuclear Cells
- PBS
Phosphate-Buffered Saline
- RT-PCR
Real-Time Polymerase Chain Reaction
- DM
Diabetes Mellitus
- EPC
Endothelial Progenitor Cell
- Db/db
BKS.Cg-m+/+Leprdb/J
- Control
C57BLKS/J
- FITC
Fluorescein Isothiocyanate
- PI
Propidium Iodide
- FACS
Fluorescent-Activated Cell Sorting
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- LAD
Left Anterior Descending
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 4.Yoon YS, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–38. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 6.van der Bogt KE, Sheikh AY, Schrepfer S, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–9. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 8.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 10.Economic costs of diabetes in the U S. 2007 Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin LE. Glucose, VEGF-A, and diabetic complications. Am J Pathol. 2001;158:1181–4. doi: 10.1016/S0002-9440(10)64066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feener EP, King GL. Endothelial dysfunction in diabetes mellitus: role in cardiovascular disease. Heart Fail Monit. 2001;1:74–82. [PubMed] [Google Scholar]
- 13.Lewis EJ, Xu X. Abnormal glomerular permeability characteristics in diabetic nephropathy: implications for the therapeutic use of low-molecular weight heparin. Diabetes Care. 2008;31 (Suppl 2):S202–7. doi: 10.2337/dc08-s251. [DOI] [PubMed] [Google Scholar]
- 14.Abaci A, Oguzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–42. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 15.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–63. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubbon RM, Rajwani A, Wheatcroft SB. The impact of insulin resistance on endothelial function, progenitor cells and repair. Diab Vasc Dis Res. 2007;4:103–11. doi: 10.3132/dvdr.2007.027. [DOI] [PubMed] [Google Scholar]
- 17.Ii M, Takenaka H, Asai J, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 18.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 19.Capla JM, Grogan RH, Callaghan MJ, et al. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2007;119:59–70. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 20.Sica V, Williams-Ignarro S, de Nigris F, et al. Autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the diabetic mouse hindlimb. Cell Cycle. 2006;5:2903–8. doi: 10.4161/cc.5.24.3568. [DOI] [PubMed] [Google Scholar]
- 21.Hirata K, Li TS, Nishida M, et al. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol. 2003;284:H66–70. doi: 10.1152/ajpheart.00547.2002. [DOI] [PubMed] [Google Scholar]
- 22.Li TS, Furutani A, Takahashi M, et al. Impaired potency of bone marrow mononuclear cells for inducing therapeutic angiogenesis in obese diabetic rats. Am J Physiol Heart Circ Physiol. 2006;290:H1362–9. doi: 10.1152/ajpheart.00766.2005. [DOI] [PubMed] [Google Scholar]
- 23.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 24.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–60. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 25.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 26.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–39. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166–77. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 28.van der Bogt KESA, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh AY, Lin SA, Cao F, et al. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–84. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 32.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 33.Awad O, Jiao C, Ma N, Dunnwald M, Schatteman GC. Obese diabetic mouse environment differentially affects primitive and monocytic endothelial cell progenitors. Stem Cells. 2005;23:575–83. doi: 10.1634/stemcells.2004-0185. [DOI] [PubMed] [Google Scholar]
- 34.van den Akker EL, Baan CC, van den Berg B, et al. Ficoll-separated mononuclear cells from sepsis patients are contaminated with granulocytes. Intensive Care Med. 2008;34:912–6. doi: 10.1007/s00134-007-0989-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.