Abstract
Purpose
To investigate the distribution and activity of phosphodiesterase 5 (PDE5) in the urethra.
Materials and Methods
Rat tissues were examined for expression of PDE5 and alpha-smooth muscle actin (SMA). Urethral PDE5 activity was examined by tissue bath in the presence of sildenafil.
Results
Anti-SMA antibody stained all known smooth muscles in all tested tissues. It also revealed the presence of a small amount of smooth muscle fibers in the levator ani muscle. Anti-PDE5 antibody stained the smooth muscles in the penis and bladder but not the striated leg muscle. However, it stained predominantly the striated muscle in the urethra and levator ani muscle. In the urethra, the amount of PDE5 in the striated muscle was 6 times as high as in the smooth muscle. Within the urethral striated muscle, PDE5 expression was localized to the Z-band striations. Intermingling of the smooth and striated muscles were clearly visible on both the inner and outer rims of the circularly arranged striated muscle layer. Relaxation of pre-contracted urethral tissues by sodium nitroprusside (SNP) was enhanced by sildenafil, indicating the presence of PDE5 activity, which was primarily located in the striated muscle as judged by PDE5 staining.
Conclusions
PDE5, despite its presumed smooth muscle specificity, was predominantly expressed in the striated muscle of the urethra and the levator ani muscle. These results are consistent with earlier studies in which these striated muscles were found to be developmentally related to smooth muscle. They also suggest that these striated muscles are possibly regulated by PDE5.
INTRODUCTION
The urethral sphincter in several species consists of circular and longitudinal smooth muscle layers that are surrounded by striated muscle fibers 1. In continent women the urethral striated muscle, which predominates over the middle and distal thirds of the organ 2, was found to contribute a third of urethral closure pressure 3. Similar urethral distribution of the striated musculature and neuroanatomy has been demonstrated in the rat, supporting the usefulness of the rat as an experimental model for studying female urinary incontinence 4.
Contraction of both the smooth and striated muscle types is thought to be important in maintaining urinary continence, while relaxation of both muscle types is essential for effective voiding 5. In vitro studies have demonstrated that nitric oxide (NO) is the main neurotransmitter involved in mediating relaxation of the urethral smooth muscle 6. The identification of nitrergic nerve fibers in the human striated urethral sphincter also suggests that NO may also play a role in the control of the urethral striated muscle 7, 8.
After its release from nitrergic nerve fibers, NO diffuses into smooth muscle cells and activates guanyl cyclase, which then catalyzes the conversion of GTP to cGMP. Elevated levels of cGMP activate protein kinase G, which then phosphorylates gap junctions, potassium channels, and calcium channels, leading to smooth muscle relaxation. Thus, the smooth muscle relaxation effect of NO is mediated by cGMP, and the breakdown of which is necessary for smooth muscle contraction. In most types of smooth muscle the breakdown of cGMP is principally carried out by phosphodiesterase 5 (PDE5) 9.
While the importance of PDE5 in regulating smooth muscle tone is well documented, issues regarding the expression and/or function of PDE5 in striated muscles have been subjects of much debate 10. From recent studies it is increasingly evident that PDE5 is indeed expressed and functional in cardiomyocytes 11, 12, but whether PDE5 is expressed in skeletal myocytes remains unknown. In the urethra, PDE5 mRNA and protein have been detected by RT-PCR 13 and by immunofluorescence staining 14, respectively, but neither of these two studies provided data concerning the striated muscle. As such, we wished to examine the PDE5 expression pattern in the urethra, particularly in concern with the striated muscle. We also wished to examine PDE5 expression in the levator ani muscle because of its importance in maintenaning urinary continence 15.
MATERIALS AND METHODS
Animals
Three-month-old male and female Sprague-Dawley rats weighing 250–300 g were obtained from Charles River Laboratories (Wilmington, MA, USA). All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at our institution.
Immunofluorescence staining and image analysis
The penis of one male rat and the bladder, urethra with attached levator ani muscle, and leg muscle of 12 female rats were harvested and fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 h followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetic USA, Torrance, CA) and stored at −70 °C until use. Fixed frozen tissue specimens were cut at 10 microns, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min. For immunostaining, the slides were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated at room temperature with anti-PDE5 (kindly provided by Dr. Visweswariah of Indian Institute of Science, India) and anti-α-SMA antibody (Abcam Inc., Cambridge, MA) for 1.5 h. Control tissue sections were similarly prepared except no primary antibody was added. After rinses with PBS, the sections were incubated with FITC-conjugated goat anti-rabbit or Texas red-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). After rinses with PBS, the slides were incubated with 4′,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Sigma-Aldrich, St. Louis, MO). The immunostained tissues were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments Inc., Melville, NY). PDE5 expression, which was represented by the green color in the photographs, was quantified by counting the number pixels.
In vitro functional study
Sildenafil (Pfizer Inc., New York, NY) was employed in tissue bath experiments to probe for the presence PDE5 activities in the urethra. Female rats (n=13) were anesthetized with intraperitoneal injection of pentobarbital (200 mg/kg). A midline abdominal incision was made to expose the urinary bladder, which was then isolated together with the proximal part of the urethra (3 to 4 mm in length). The urethra was then separated from the bladder and mounted in a vertical tissue bath (Myobath Tissue Bath System II, World Precision Instrument, Sarasota, FL), each 10 ml-chamber of which was filled with Krebs’ solution (NaCl, 86.87 mM; KCl, 5.16 mM; MgSO4, 1.22 mM; NaHCO3, 25.56 mM; CaCl2, 1.33 mM and dextrose, 1.01 mM, pH 7.6) that was maintained at 37°C and continuously aerated with 95% O2 and 5% CO2. Through an L- shaped metal wire, the ring-shaped urethral segment was connected to a force-displacement transducer (FORT 25, World Precision Instrument), applied with a resting tension of 6 mN, and allowed to equilibrate for 60 min. The urethra specimens were then treated with sildenafil (0.1 μM) (n=7) or its solvent dimethyl sulfoxide (DMSO, Sigma-Aldrich) (n=6). Thirty min later, phenylephrine (PE, 10 μM, Sigma-Aldrich, St. Louis, MO) was added to contract the urethra. As the contraction of each of the specimens began to reach a plateau, cumulative amounts of sodium nitroprusside (SNP, 1 nM to 100 μM, Sigma-Aldrich) were added to relax the urethra. The isometric tension was recorded with the data acquisition system (Lab TRAX-4, World Precision Instrument). The concentration of SNP producing half the relaxation of PE-induced tension was calculated and expressed as the −log EC50.
Statistical Analysis
Data were analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA) and shown as the mean ± standard error of the mean (SEM) with n indicating the number of samples. Student’s unpaired t-test was used to evaluate the results. Differences with p < 0.05 were considered significant.
RESULTS
Immunolocalization of PDE5 expression
We used the cavernous smooth muscle and the leg skeletal muscle as positive and negative controls, respectively. The results show that PDE5 and SMA were co-localized in the cavernous smooth muscle while no such colocalization was found in the leg muscle (Fig. 1). In both tissues some PDE5 immunoreactivities are visible in areas where there were no muscle cells, suggesting the presence of platelets. In the leg muscle, SMA was also identified with blood vessels. In the bladder, PDE5 was expectedly co-localized with SMA. However, in the urethral sphincter and the levator ani muscle, this smooth muscle specificity of PDE5 was lost. Specifically, in these two tissues, the locations of PDE5 and SMA immunoreactivities were nearly mutually exclusive, that is, PDE5 stained predominantly the striated muscle while SMA the smooth muscle.
Figure 1.
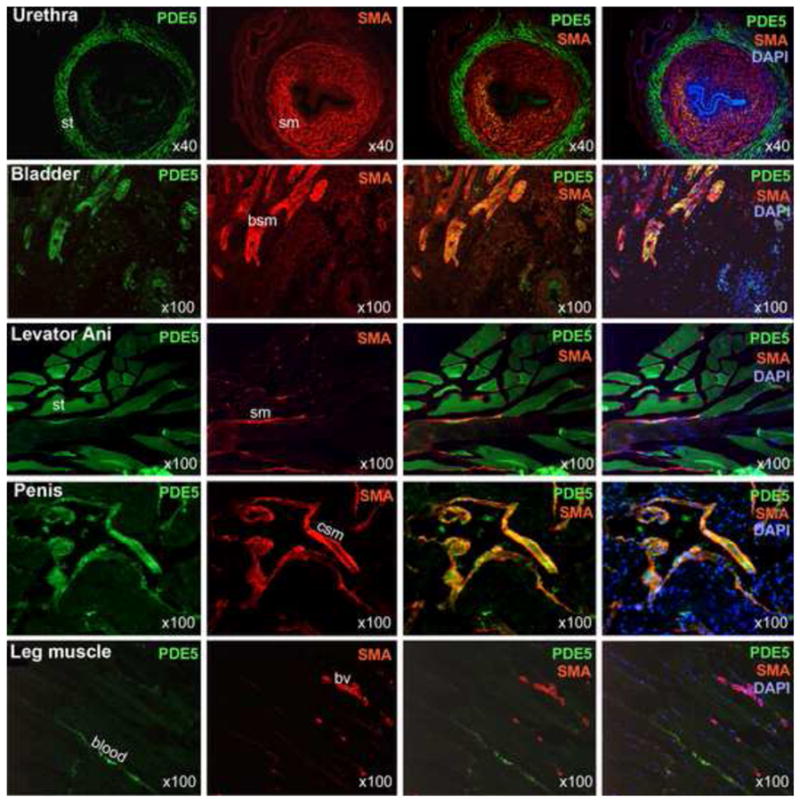
Localization of PDE5 expression. SMA staining (red) was used to identify smooth muscles (sm) in the indicated tissues. The specificity of the anti-SMA was confirmed by the staining of all of the known smooth muscles including the bladder smooth muscle (bsm), the cavernous smooth muscle (csm), and the vascular smooth muscle of blood vessels (bv). The presence of smooth muscle in the levator ani smooth muscle specimens has been reported previously (see Discussion). PDE5 staining (green) of smooth muscles is clearly visible in the bladder and penis but is less in the urethra and leg blood vessels. PDE5 staining of the striated muscle (st) is prominent in the urethra and levator ani muscle. DAPI staining (blue) serves to locate cellular nuclei. Optical magnification is indicated on the right lower corner of each picture.
Differential distribution of PDE5 and smooth muscle actin in the urethra
Both the longitudinal and circular smooth muscle layers were stained prominently by SMA but minimally by PDE5 (Fig. 2). On the other hand, the striated muscle was stained strongly by PDE5 but not by SMA. However, on the outskirts of the striated muscle there were two structures that were stained by SMA but not by PDE5. While one structure is the blood vessels, the other, which runs parallel to the circular striated muscle, is apparently another layer of smooth muscle. Noteworthy is that even the smooth muscle in the blood vessels was not stained by the supposedly smooth muscle-specific PDE5.
Figure 2.
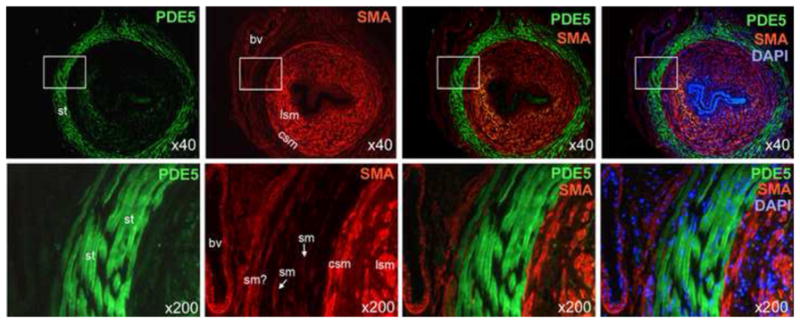
Differential distribution of PDE5 and smooth muscle actin in the urethra. SMA staining (red) mainly occurred in the longitudinal smooth muscle (lsm) and the circular smooth muscle (csm). Lesser SMA staining was found with blood vessels (bv) and presumed smooth muscle (sm?) located outside of the striated muscle (st) layer. PDE5 staining (green) was found mainly in the striated muscle. Note that the two blood vessels are also devoid of PDE5 staining. DAPI staining (blue) serves to locate cellular nuclei. Optical magnification is indicated on the right lower corner of each picture. Boxed areas in the 40x pictures are shown in the 200x pictures.
Differential expression of PDE5 in the urethral smooth and striated muscles
By counting the pixel numbers of PDE5 (green)-stained areas within the striated and smooth muscle compartments (Fig. 3A), we obtained results that PDE5 expression in the striated muscle was approximately 6 times as high as in the smooth muscle (Fig. 3B).
Figure 3.
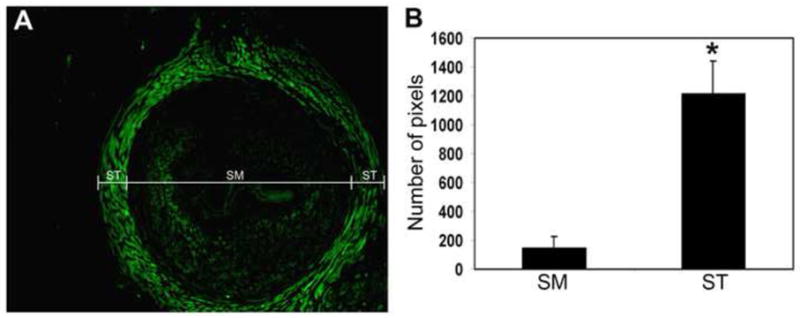
Differential expression of PDE5 in the urethral smooth and striated muscles. The pixel numbers of the PDE5 (green)-stained areas within the striated muscle (ST) and the smooth muscle (SM) compartments (A) of each urethra sample were counted. The results are compiled and shown in B. The number of pixels shown on the y-axis is the average number of 12 urethra samples from 12 different female rats. * indicates p<0.01.
Localization of PDE5 expression to the Z-band striations
High-power imaging reveals that PDE5 expression was localized to the Z-bands in the urethral striated muscle (Fig. 4A).
Figure 4.
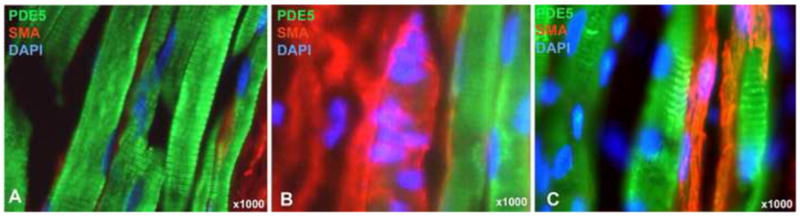
Detailed structures of the urethral musculature. At 1000x magnification, PDE5 staining (green) revealed the characteristic striations of striated muscles, as demonstrated clearly in A. The same picture (A) also shows the SMA (red)-stained fibers are sandwiched between the much larger striated fibers. Pictures B and C show the intermingling striated and smooth muscle fibers on the outer and inner rims, respectively, of the circular striated muscle layer. DAPI staining (blue) serves to locate cellular nuclei. Note that the intermingled fiber centrally located in B can also be seen at the 200x magnification in Fig. 2 (labeled by “sm?”). In this case, the absence of DAPI staining allows better visualization of the green color within the red color.
Identification of the smooth-striated transitions in the urethral sphincter
In Fig. 2 the presence of smooth muscle within the “striated zone” is clearly visible. In Fig. 4B & C, co-localization of PDE5 and SMA is visible in both the outer and inner rims of the striated zone.
Identification of PDE5 activity
As shown in Fig. 5, SNP-induced relaxation of urethral preparations was indeed enhanced by sildenafil. The −log EC50 values for SNP were 5.69 ± 0.25 with DMSO and 6.73 ± 0.29 with sildenafil (P = 0.0355).
Figure 5.
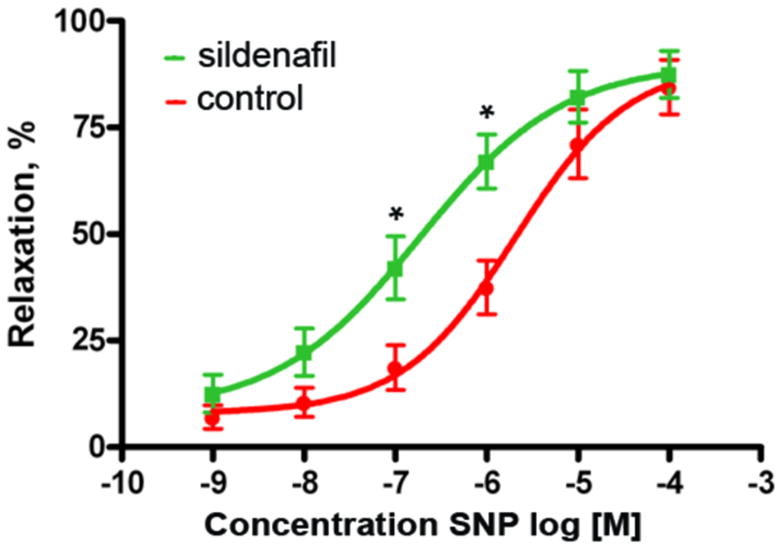
Identification of PDE5 activity. Urethral samples shaped as rings were used in tissue bath experiments. They were treated with sildenafil (n=7) or its solvent (n=6, as controls), contracted with phenylephrine, and then relaxed with sodium nitroprusside (SNP) at the indicated concentrations. * indicates p<0.05.
DISCUSSION
The importance of PDE5 in regulating smooth muscle tone is well documented. But whether it is expressed in striated muscles, particular the cardiac muscle, has been a contentious issue 10. Recent studies however have presented clear evidence that PDE5 not only is expressed but also plays important functions in cardiomyocytes 11, 12. Explanations for why earlier studies failed to detect PDE5 expression in the heart may include the subcellular localization of PDE5 in particulate fractions, specifically the myocyte Z-bands 11.
In the present study, we show that PDE5 was expectedly undetectable in the leg muscle but was abundantly expressed in the striated muscle of the urethra and the levator ani muscle. Although generally considered similar to other skeletal muscles, these two muscles have in fact been shown to be developmentally related to the smooth muscle 16, 17. Specifically in regard to the levator ani muscle, it has been shown that, when exposed to stress, the muscle type could transform from skeletal into smooth 17. Furthermore, the levator ani muscle is not purely skeletal but rather composed of both skeletal and smooth muscles 17, and this is also clearly shown in the present study (Fig. 1).
Unlike limb skeletal muscles, which arise embryonically from the somites, the urethral striated and smooth muscles derive from the splanchnic mesoderm 16. Thus, despite being “striated”, the urethral striated muscle shares the same embryonic origin with urethral smooth muscles and expresses a “smooth muscle-specific” protein, as we demonstrated in this study with the identification of PDE5 expression. However, it is still surprising that in this study we also found that PDE5 was expressed much more abundantly in the striated than in the smooth muscle of the urethra. This would imply that the PDE5 signaling pathway, or even together the upstream NO/cGMP pathway, plays a more significant role in the striated than in the smooth muscle. Although we did not separate the striated from the smooth muscle components in the tissue bath experiments, we did show that sildenafil, a selective PDE5 inhibitor, was able to enhance the relaxation effect of SNP. Since PDE5 was predominantly expressed in the striated muscle of the urethra, we believe that the observed effect of sildenafil was exerted on the urethral striated muscle. Thus, our data provide an explanation for the unexpected observation made by Wibberley et al 18 - specifically possible PDE5 activities in the urethral striated muscle. In addition, due to PDE5’s predominant expression in the urethral striated muscle and the exclusive expression of SMA in the urethral smooth muscle, double staining for these two proteins allowed us to clearly visualize the smooth-striated transition zones (Fig. 4). Although histological data concerning the smooth-striated transition have been reported previously 2, our photographs provide far greater details.
As to why the previous study did not mention PDE5 expression in the urethral striated muscle 14, one possibility is that the tissue preparations in that study contained only the smooth muscle as described in its materials and methods section. While it is also possible that there may be species differences, this is a less likely explanation as several studies have demonstrated the anatomical and functional similarities between human and rat urethras 4. Therefore, we believe that the discovery of PDE5 expression in the urethral striated muscle is clinically relevant and should encourage future studies on the functions of PDE5 as well as other cGMP signaling molecules in the urethra. Likewise, the discovery of PDE5 expression in the levator ani muscle could lead to a better understanding of pelvic floor disorders.
CONCLUSIONS
PDE5, which has long been considered smooth muscle specific, is predominantly expressed in the striated muscle of the urethra and the levator ani muscle. These discoveries point to the need to redirect our focuses on how urinary continence is regulated by cGMP signaling.
Acknowledgments
This work was supported by grants from Mr. Arthur Rock, the Rock Foundation, and the National Institutes of Health (DK64538 and DK069655). We would like to thank Dr. Visweswariah of Indian Institute of Science for providing the anti-PDE5 antibody.
Key of Definitions for Abbreviations (only include abbreviations used 3 times or more in manuscript)
- PDE5
phosphodiesterase 5
- SMA
alpha-smooth muscle actin
- SNP
sodium nitroprusside
- NO
nitric oxide
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- SEM
standard error of the mean
References
- 1.Elbadawi A. Comparative neuromorphology in animals. In: Torrens M, Morrison JFB, editors. The Physiology of the lower urinary tract. London ; New York: Springer-Verlag; 1987. pp. 23–52. [Google Scholar]
- 2.Colleselli K, Stenzl A, Eder R, Strasser H, Poisel S, Bartsch G. The female urethral sphincter: a morphological and topographical study. J Urol. 1998;160:49. doi: 10.1016/s0022-5347(01)63025-8. [DOI] [PubMed] [Google Scholar]
- 3.Rud T, Andersson KE, Asmussen M, Hunting A, Ulmsten U. Factors maintaining the intraurethral pressure in women. Invest Urol. 1980;17:343. [PubMed] [Google Scholar]
- 4.Kim RJ, Kerns JM, Liu S, Nagel T, Zaszczurynski P, Lin DL, et al. Striated muscle and nerve fascicle distribution in the female rat urethral sphincter. Anat Rec (Hoboken) 2007;290:145. doi: 10.1002/ar.20420. [DOI] [PubMed] [Google Scholar]
- 5.Brading AF. The physiology of the mammalian urinary outflow tract. Exp Physiol. 1999;84:215. doi: 10.1111/j.1469-445x.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 6.Andersson KE, Persson K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J Urol. 1994;12:274. doi: 10.1007/BF00191207. [DOI] [PubMed] [Google Scholar]
- 7.Ho KM, McMurray G, Brading AF, Noble JG, Ny L, Andersson KE. Nitric oxide synthase in the heterogeneous population of intramural striated muscle fibres of the human membranous urethral sphincter. J Urol. 1998;159:1091. [PubMed] [Google Scholar]
- 8.Ho KM, Ny L, McMurray G, Andersson KE, Brading AF, Noble JG. Co-localization of carbon monoxide and nitric oxide synthesizing enzymes in the human urethral sphincter. J Urol. 1999;161:1968. [PubMed] [Google Scholar]
- 9.Lin CS, Xin ZC, Lin G, Lue TF. Phosphodiesterases as therapeutic targets. Urology. 2003;61:685. doi: 10.1016/s0090-4295(02)02439-1. [DOI] [PubMed] [Google Scholar]
- 10.Lin CS, Lin G, Xin ZC, Lue TF. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006;12:3439. doi: 10.2174/138161206778343064. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CS, Lin G, Lue TF. Isolation of two isoforms of phosphodiesterase 5 from rat penis. Int J Impot Res. 2003;15:129. doi: 10.1038/sj.ijir.3900983. [DOI] [PubMed] [Google Scholar]
- 14.Werkstrom V, Svensson A, Andersson KE, Hedlund P. Phosphodiesterase 5 in the female pig and human urethra: morphological and functional aspects. BJU Int. 2006;98:414. doi: 10.1111/j.1464-410X.2006.06217.x. [DOI] [PubMed] [Google Scholar]
- 15.Wallner C, Dabhoiwala NF, DeRuiter MC, Lamers WH. The anatomical components of urinary continence. Eur Urol. 2009;55:932. doi: 10.1016/j.eururo.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Borirakchanyavat S, Baskin LS, Kogan BA, Cunha GR. Smooth and striated muscle development in the intrinsic urethral sphincter. J Urol. 1997;158:1119. doi: 10.1097/00005392-199709000-00109. [DOI] [PubMed] [Google Scholar]
- 17.Shafik A, Asaad S, Doss S. The histomorphologic structure of the levator ani muscle and its functional significance. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:116. doi: 10.1007/s001920200026. [DOI] [PubMed] [Google Scholar]
- 18.Wibberley A, Nunn PA, Naylor AM, Ramage AG. An investigation of the effects of zaprinast, a PDE inhibitor, on the nitrergic control of the urethra in anaesthetized female rats. Br J Pharmacol. 2002;136:399. doi: 10.1038/sj.bjp.0704735. [DOI] [PMC free article] [PubMed] [Google Scholar]


