Abstract
Introduction
Hyperlipidemia has been associated with erectile dysfunction (ED) via damage to the cavernous endothelium and nerves. Adipose tissue-derived stem cells (ADSC) have been shown to differentiate into endothelial cells and secrete vasculotrophic and neurotrophic factors.
Aim
To assess whether ADSC have therapeutic effects on hyperlipidemia-associated ED.
Methods
Twenty-eight male rats were induced to develop hyperlipidemia with a high fat diet (hyperlipidemic rats, HR). Ten additional male rats were fed a normal diet to serve as controls (normal rats, NR). Five months later, all rats were subjected to ADSC isolation from paragonadal fat. The cells were cultured for one week, labeled with 5-ethynyl-2′-deoxyuridine (EdU), and then injected autologously into the corpus cavernosum of 18 HR. The remaining 10 HR rats were injected with phosphate-buffered saline (PBS). At 3 and 14 days post-transplantation, 4 rats in the HR+ADSC group were sacrificed for tracking of the transplanted cells. At one month post-transplantation, all remaining rats were analyzed for serum biochemistry, erectile function and penile histology.
Main Outcome Measures
Erectile function was assessed by intracavernous pressure measurement during electrostimulation of the cavernous nerve. Cavernous nerves, endothelium, and smooth muscle were assessed by immunohistochemistry.
Results
Serum total cholesterol and low-density lipoprotein levels were significantly higher in HR than in NR. High-density lipoprotein level was significantly lower in HR than in NR. Mean intracavernous pressure/mean arterial pressure ratio was significantly lower in HR+PBS than in NR+PBS or HR+ADSC. Neuronal nitric oxide synthase (nNOS)-positive nerve fibers and endothelial cells were fewer in HR+PBS than in HR+ADSC. Smooth muscle content was significantly higher in both HR groups than in NR.
Conclusions
Hyperlipidemia is associated with abnormalities in both the nerves and endothelium. Treatment with ADSC ameliorates these adverse effects and holds promise as a potential new therapy for ED.
INTRODUCTION
Hyperlipidemia is a major risk factor for the development of erectile dysfunction (ED) [1] It has been demonstrated that there is a linear relationship between severity of hyperlipidemia and risk of ED; Wei reported that for every mmol/liter increase in total cholesterol there was an associated 32% increase in risk of ED, whereas there was a substantial decline in risk of ED for every mmol/liter increase in high density lipoprotein (HDL) [2] Hyperlipidemia may impair erectile function by affecting endothelial function as a common mechanism [3–5]. At the cellular level, endothelial dysfunction results in impaired bioavailability of nitric oxide (NO) [6]
Phosphodiesterase type-5 (PDE5) inhibitors are an important first line treatment for men with ED including those with coexisting hyperlipidemia [7] The use of PDE5 inhibitors has a proven record of safety in preclinical and human trials. However, PDE5 inhibitors do not produce adequate relief in all patients with hyperlipidemia. In a prospective, randomized, double-blind, placebo-controlled study, it has been reported that 10 mg challenge dose of vardenafil may often fail to achieve initial penetration (16%) or completion of intercourse (28%) for men with hyperlipidemia [8]. In addition, these medications have potential adverse effects including headache, flushing, dyspepsia, nasal congestion, abnormal vision, diarrhea, dizziness, skin rash, rhinitis, and back pain [9] Numerous alternative treatments are available for the management of ED but their efficacy and tolerability are variable; a means to treat the underlying disease process of ED would be preferable to currently available interventions.
Adipose-derived stem cells (ADSC) are multipotent progenitor cells isolated from the stromal vascular fraction of adipose tissue [10] In previous studies, we have demonstrated that ADSC can differentiate into neuron-like, endothelial and smooth muscle cells in vitro [11,12]. This capacity makes ADSC an attractive potential therapy for conditions known to be associated with disruption of endothelial tissues.
In this study, we investigate the efficacy of ADSC in the treatment of impaired penile hemodynamics and penile histological changes associated with hyperlipidemia in a rat model.
METHODS
Animal Groups and Experimental Design
Thirty eight (38) 3-month old male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA, USA). All animal care, treatments and procedures were approved by the Institutional Animal Care and Use Committee at our institution.
Ten rats fed a diet of standard rat chow served as negative controls. The remaining 28 rats (hyperlipidemic rats, HR) were fed a high-fat diet consisting of 2% cholesterol and 10% lard (Zeigler Brothers, Gardner, PA, USA) for 5 months. This has been shown in prior studies to induce a condition of hyperlipidemia and impaired penile hemodynamics [13].
At the five month time point, all rats underwent paragonadal fat harvest for procurement of ADSC. ADSC were cultured for one week and labeled with 5-ethynyl-2′-deoxyuridine (EdU). The 28 HR were divided into two groups: 1) injection of phosphate buffered saline (PBS) into the corpus cavernosum (HR+PBS, n=10), and 2) Injection of ADSC in PBS into the corpus cavernosum (HR+ADSC, n=18). Negative control animals underwent PBS injection into the corpus cavernosum (NR+PBS). At the 3 and 14 day time-points after ADSC transplantation, four rats were randomly selected from the HR+ADSC group and were euthanized with IP pentobarbital (200 mg/kg) and bilateral thoracotomy. The penis samples were harvested for EdU staining.
One month after injection the remaining rats underwent erectile function testing. Animals were then sacrificed, and serum samples and penile tissues were obtained for biochemical and immunohistochemical analysis.
Harvest and Processing of ADSC and EdU Labeling
Animals were anesthetized with isoflurane. A midline abdominal incision was made to expose the perigonadal fat pad. A specimen of para-testicular fat was harvested and placed in ice cold PBS. The animal was then closed in two layers with absorbable suture and anesthesia was weaned.
Fat tissue was rinsed with PBS in a 50 ml conical tube. Adipose tissue was removed from the upper layer to a fresh tube, and digested in 0.075% collagenase I (Sigma-Aldrich Cp., St Louis, MO, USA) for 1 hr at 37°C with shaking. The top lipid layer was removed and the remaining liquid portion was centrifuged at 220 × g for 10 min. The pellet was then treated with 160 mM NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and plated at a density of 1 × 106 cells in a 10-cm dish. The culture dish was placed in a 5% CO2 incubator for 3–5 days to allow the formation of ADSC colonies. For the purpose of cell tracking, ADSC were treated with 10 μM EdU (Invitrogen, Carlsbad, CA, USA) overnight. A total of 2 × 106 EdU-labeled ADSC were collected into a 2-ml conical tube containing 0.5 ml PBS; these ADSC were subsequently utilized for injection.
ADSC Injection
All animals were anesthetized with isoflurane and a 1.5 cm oblique incision was made in the lower abdominal midline. The skin was dissected from the anterior surface of the penis and the penis was retracted anterior with the foreskin left intact. Using blunt dissection the penile base and crura were exposed. The corpus cavernosum was then gently cannulated using a 25 gauge needle. Animals underwent injection of 0.5 ml PBS (NR+PBS and HR+PBS group), or 2 million autologous ADSC in 0.5 mL PBS (HR+ADSC) into the left corpus cavernosum. After treatment the wound was closed in one layer with absorbable suture.
Functional Evaluation
At the one month time point all rats were anesthetized with ketamine and midazolam (100 mg/kg and 5 mg/kg, respectively) by IP injection. A lower midline abdominal incision was made and the cavernous nerves isolated bilaterally. The penis was denuded of overlying skin and cannulated with a heparinized 25 gauge needle connected to a real time continuous pressure transducer. The cavernous nerves were then stimulated with a stainless steel bipolar hook electrode attached to a multi-jointed clamp; stimulation parameters were 50 second continuous trains at 20 Hz, 1.5 mAmp. Real time response of the erectile tissue was determined by change in intracavernous pressure (ICP); the maximum change in ICP was utilized for further analysis.
After functional testing, systemic blood pressure was measured via aortic cannulation. Mean arterial pressure (MAP) was calculated by the formula MAP = (2/3 diastolic blood pressure + 1/3 systolic blood pressure). After aortic puncture, serum samples were obtained, and then animals were sacrificed with bilateral thoracotomy. Penile tissues were obtained for immunohistochemistry.
Immunohistochemical Staining
After euthanasia, tissue samples were fixed in cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, PH 8.0, for 4 hours followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (American Master Tech Scientific, Inc, Lodi, CA, USA) and stored at −70°C until use. Sections were cut at 5 μm, mounted into charged slides and air dried for 5 min. representative slides were stained with Masson’s trichrome for connective tissue and smooth muscle histology.
For immunohistochemical examination, tissue sections were stained with Click-iT™ reaction cocktail (Invitrogen, Carlsbad, CA, USA) followed by Dilute Hoechst (Invitrogen, Carlsbad, CA, USA), mouse anti-neuronal nitric oxide synthase (nNOS, BD Transduction Laboratories, Franklin Lakes, NJ, USA), mouse anti- rat endothelial cell antigen-1 (RECA-1, Abcam Inc, Cambridge, MA, USA), mouse anti-α-smooth muscle actin (α-SMA, Sigma-Aldrich, St. Louis, MO, USA) and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL, Roche Diagnostics Corporation, Indianapolis, IN, USA) using standard techniques.
Image and Statistical Analysis
Image analysis was performed by computerized densitometry using Image-Pro Plus imaging software (Media Cybernetics, Silver Spring, MD, USA) coupled to a digital still camera (Nikon DXM1200) and ACT-1 software (Nikon Instruments Inc., Melville, NY, USA).
To quantify EdU staining, corpus cavernosum was analyzed at 40 magnification and expressed as the number of EdU-positive nuclei in the corpus cavernosum. For nNOS staining, dorsal penile nerve was examined at × 100 magnification and expressed as the number of nNOS-positive nerve fibers in the dorsal penile nerves [14]. For RECA-1 and α-SMA staining, bilateral fields at × 40 magnification pictures of the penis composed of one half of the corpus cavernosum was analyzed and expressed as the percentage of positive area versus total area of the corpus cavernosum. For Masson’s trichrome staining, bilateral fields at × 40 magnification pictures of the penis composed of one half of the corpus cavernosum but excluding the sinusoidal spaces were analyzed for smooth muscle (stained in red) and collagen (stained in blue). The data was expressed as the smooth muscle/collagen ratio [15]
Data was analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) and expressed as mean ± standard error of the mean for continuous variables. The continuous data was compared the groups using one-way analysis of variance. The Student-Neuman-Keuls test was used for post-hoc comparisons. Statistical significance was set at p < 0.05.
RESULTS
A lethal reaction to ketamine anesthesia at the time of functional testing occurred in 2 rats (1 each from HR+PBS and HR+ADSC groups). They were excluded from all subsequent analyses.
Body Weight and Serum Biochemistry
Total body weight was significantly higher in HR than in rats fed a normal diet (NR) (p < 0.05). Serum total cholesterol and LDL levels were significantly higher and HDL level was significantly lower in HR than in NR (p < 0.01). There were no significant differences in glucose, triglyceride or testosterone levels between the three groups (Table 1).
Table 1.
Physiological variable and serum biochemistry
| Groups | NR + PBS | HR + PBS | HR + ADSC | P value |
|---|---|---|---|---|
| Body weight (g) | 666 ± 53.9 * | 754 ± 68.4 | 739 ± 66.8 | 0.0095 |
| Total cholesterol (mg/dL) | 81.5 ± 14.20 * | 172.3 ± 53.33 | 142.2 ± 14.09 | 0.0008 |
| HDL (mg/dL) | 55.7 ± 8.69 * | 41.2 ± 5.61 | 44.6 ± 5.94 | 0.0064 |
| LDL (mg/dL) | 11.6 ± 4.24 * | 127.1 ± 52.97 | 98.5 ± 19.29 | < 0.0001 |
| Triglyceride (mg/dL) | 73.7 ± 67.67 | 57.9 ± 36.58 | 35.6 ± 28.04 | 0.3957 |
| Glucose (mg/dL) | 275 ± 85.0 | 217 ± 43.5 | 240 ± 26.9 | 0.2421 |
| Testosterone (pg/mL) | 1446 ± 735.1 | 943 ± 347.4 | 950 ± 347.1 | 0.1816 |
NR+PBS = rats fed a normal diet that received phosphate buffered saline (PBS), HR+PBS = rats fed a high-fat diet that received PBS, HR+ADSC = rats fed a high-fat diet that received adipose-derived stem cells (ADSC) injection into penis, HDL = high density lipoprotein, LDL = low density lipoprotein.
Six rats were randomly selected for biochemistry analysis from each group.
One-way analysis of variance (ANOVA) was used, and data presented as mean ± standard error of the mean.
Versus other groups p < 0.05.
Functional Study
Representative ICP measurement from electrostimulation of cavernous nerve in NR+PBS, HR+PBS and HR+ADSC rats are presented in figure 1. Mean ICP/MAP ratios were significantly lower in the HR+PBS (0.28 ± 0.268) compared to the NR+PBS group (0.84 ± 0.186) and HR+ADSC (0.59 ± 0.312) groups (Table 2). The NR+PBS group also had higher mean ICP/MAP ratio than the HR+ADSC group, but the difference did not reach statistical significance. MAP was not significantly different between the three groups.
Figure 1.
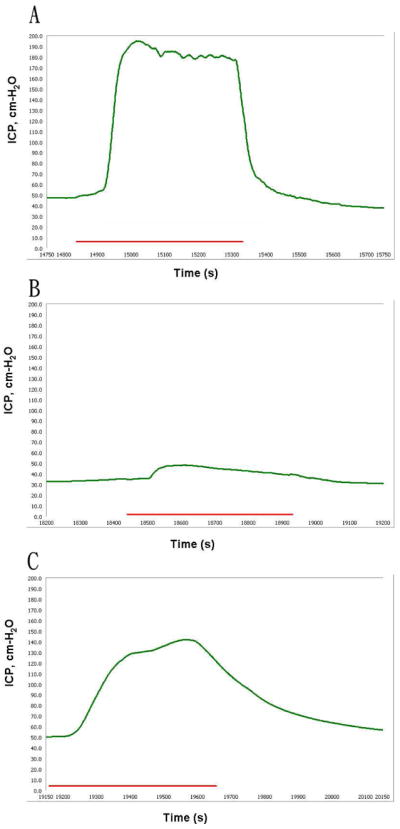
Measurement of erectile function.
Representative graphs are presented for rats fed a normal diet and received phosphate buffered saline (A), rats fed a high-fat diet and received PBS (B), and rats fed a high-fat diet and received ADSC (C). The green curve and red bar represent the intracavernous pressure (ICP) values in response to nerve stimulation and the duration of cavernous nerve stimulation (50 seconds), respectively.
Table 2.
Functional studies in the experimental animals
| Groups | NR + PBS | HR + PBS | HR + ADSC | P value |
|---|---|---|---|---|
| Peak ICP (cm-H2O) | 122 ± 24.6 * | 41 ± 37.2 | 84 ± 40.0 † | 0.0001 |
| MAP (cm-H2O) | 138 ± 41.6 | 145 ± 34.0 | 152 ± 45.6 | 0.7616 |
| ICP/MAP ratios | 0.84 ± 0.186 | 0.28 ± 0.268 * | 0.59 ± 0.312 | 0.0005 |
NR+PBS = rats fed a normal diet that received phosphate buffered saline (PBS), HR+PBS = rats fed a high-fat diet that received PBS, HR+ADSC = rats fed a high-fat diet that received adipose-derived stem cells (ADSC) injection into penis, ICP = intracavernous pressure, MAP = mean arterial blood pressure, ratio = peak ICP/MAP.
One each rat in the HR+PBS and HR+ADSC groups suddenly died after Ketamine injection; they were excluded.
One-way analysis of variance (ANOVA) was used, and data presented as mean ± standard error of the mean.
Versus other groups p < 0.05,
Significantly different versus HR + PBS.
Tracking Transplanted ADSC
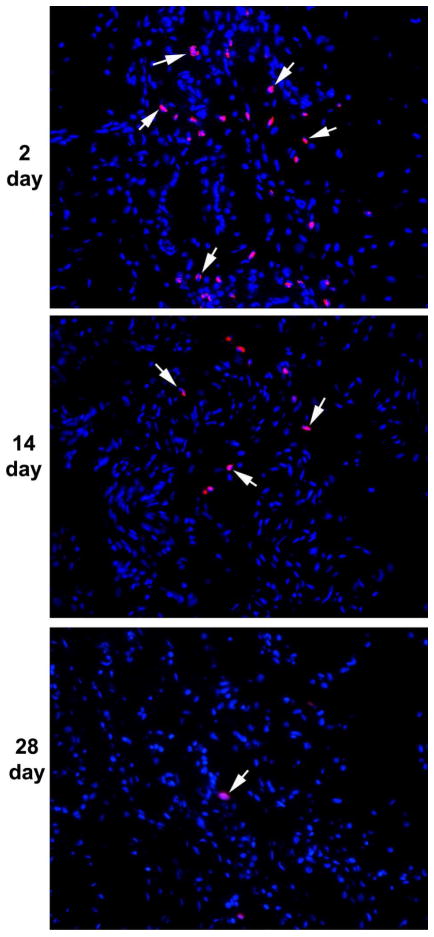
Autologous EdU-labeled ADSC were transplanted into corpus cavernosum (HR+ADSC group) and identified by Alexa-594 stain. The histological data indicated the presence of EdU-labeled cells in the corpus cavernosum. Many more EdU-labeled cells were identified in the 3 day time point (109 ± 60.0) relative to the 14 day time point (30 ± 13.9); EdU-positive nuclei were rare at the 1 month time point (2 ± 0.8, p = 0.0726). It is suggesting that ADSC either migrated or died rather than differentiating into somatic cells (Fig. 2).
Figure 2.
Tracking transplanted ADSC.
The corpus cavernosum was stained with Alexa-594 (red fluorescence) and DAPI (blue fluorescence) for the visualization of EdU-labeled cells (arrows) and cell nuclei, respectively. Note the decline of the number of EdU-labeled cells from 2 to 14 and to 28 days post-transplantation. Magnification is X 200.
nNOS-Positive Nerve Fibers in the Penile Dorsal Nerves
The HR+PBS group had lower nNOS-positive nerve fibers relative to NR+PBS (Fig. 3). nNOS-positive nerve fiber content in the HR+ADSC was significantly greater than what was observed in the NR+PBS and HR+PBS group (Table 3).
Figure 3.
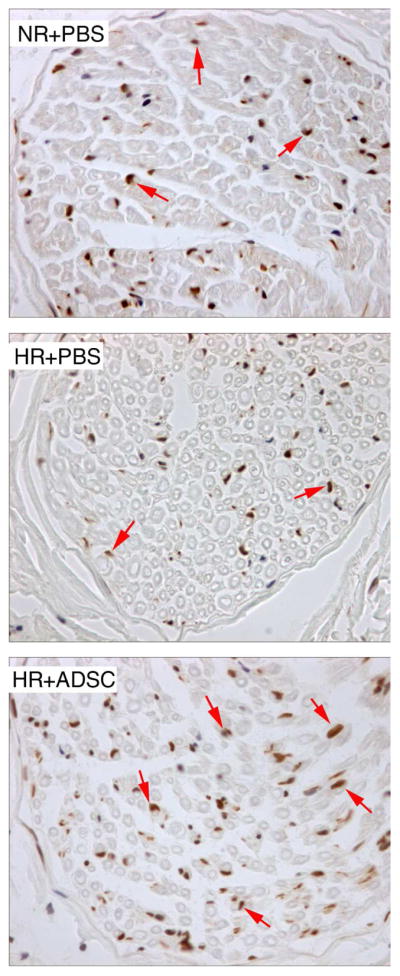
Analysis of neuronal nitric oxide synthase (nNOS)-positive nerve fibers in penile dorsal nerves.
Representative graphs are presented for rats fed a normal diet and received PBS (NR+PBS), rats fed a high-fat diet and received PBS (HR+PBS), and rats fed high-fat diet and received ADSC (HR+ADSC). nNOS positive fibers are stained brown (arrows). The number of nNOS-positive nerve fibers was higher in NR+PBS and HR+ADSC than in HR+PBS. Magnification is X 400.
Table 3.
nNOS, RECA-1, α-SMA and Masson’s Trichrome stain data in the experimental animals
| Groups | NR+PBS | HR+PBS | HR+ADSC | P value |
|---|---|---|---|---|
| nNOS (number) | 645 ± 92.4 | 489 ± 73.9 | 859 ± 79.5 † | 0.0169 |
| RECA-1 percentage (%) | 7.4 ± 0.82 | 4.1 ± 0.63 * | 7.4 ± 1.08 | 0.0131 |
| α-SMA percentage (%) | 10.0 ± 0.97 | 12.8 ± 0.76 | 13.7 ± 1.21 ‡ | 0.0490 |
| SM/collagen ratio | 0.12 ± 0.018 * | 0.17 ± 0.012 | 0.18 ± 0.011 | 0.0119 |
NR+PBS = rats fed a normal diet that received phosphate buffered saline (PBS), HR+PBS = rats fed a high-fat diet that received PBS, HR+ADSC = rats fed a high-fat diet that received adipose-derived stem cells (ADSC) injection into penis, nNOS = neuronal nitric oxide synthase, RECA-1 = rat endothelial cell antigen-1, α-SMA =α-smooth muscle cell actin, SM = smooth muscle.
One-way analysis of variance (ANOVA) for continuous variables; data is shown as mean ± standard error of the mean.
One each rat in the HR+PBS and HR+ADSC groups suddenly died after ketamine injection and were excluded.
Versus other groups p < 0.05,
Significantly different versus HR + PBS,
Significantly different versus NR+PBS.
Endothelial Integrity
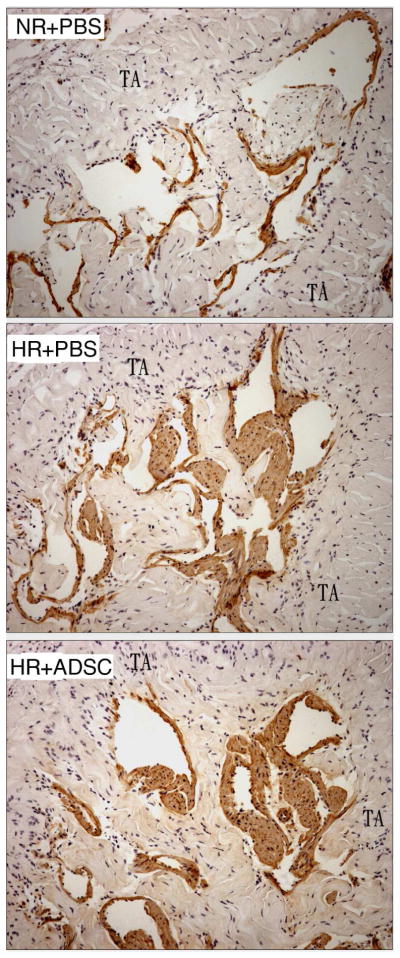
RECA-1 staining for endothelial tissue showed intense positivity in the corpus cavernosum from the NR+PBS and HR+ADSC groups; there was minimal RECA-1 positivity in the corpora of the HR+PBS group (Fig. 4). There was no significant difference in RECA-1 positivity between the NR+PBS and HR+ADSC groups (Table 3). There was also intense positivity for RECA-1 between the tunica albuginea and Buck’s fascia in the NR+PBS (133 ± 10.9) and HR+ADSC (112 ± 13.2) groups; there was minimal RECA-1 positivity in the HR+PBS group in this area (78 ± 9.5, p = 0.0058).
Figure 4.
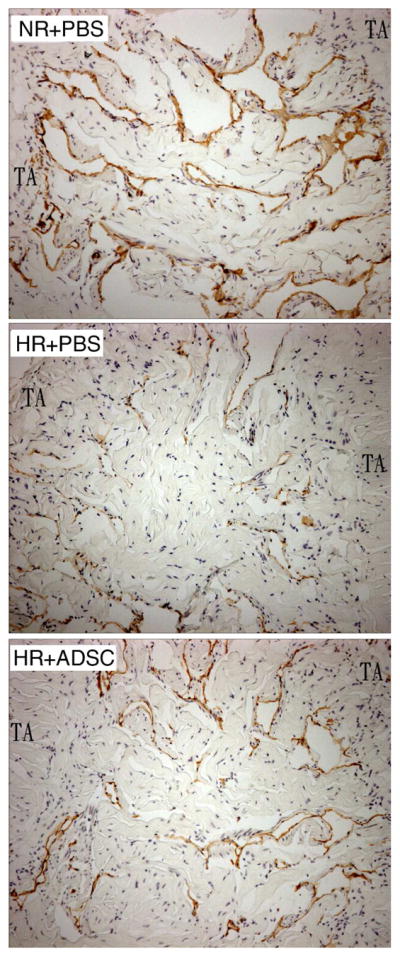
Analysis of endothelium content in the corpus cavernosum.
Representative graphs are presented for rats fed a normal diet and received PBS (NR+PBS), rats fed a high-fat diet and received PBS (HR+PBS), and rats fed high-fat diet and received ADSC (HR+ADSC). Endothelial cells are stained brown with the RECA-1 antibody. The number of RECA-1 positive cells was higher in NR+PBS and HR+ADSC than in HR+PBS. Magnification is X 100. TA = tunica albuginea.
Smooth Muscle Content
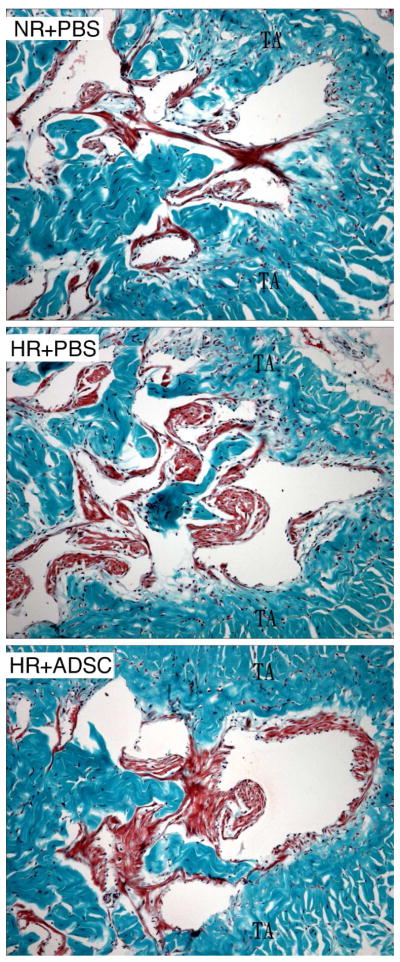
All HR groups had significantly higher smooth muscle content in the corpus cavernosum relative to the NR+PBS group (Fig. 5 & 6). Tissue sections from the HR+ADSC group had higher smooth muscle percentage than the HR+PBS group (Table 3). However, there was no significant difference between animals fed a high-fat diet.
Figure 5.
Analysis of smooth muscle content in the corpus cavernosum by trichrome staining.
Representative graphs are presented for rats fed a normal diet and received PBS (NR+PBS), rats fed a high-fat diet and received PBS (HR+PBS), and rats fed high-fat diet and received ADSC (HR+ADSC). Smooth muscle and connective tissue are stained red and blue, respectively. The smooth muscle content was higher in HR than in NR. Magnification is X 100. TA = tunica albuginea.
Figure 6.
Analysis of smooth muscle content in the corpus cavernosum by alpha-smooth muscle actin staining. Representative graphs are presented for rats fed a normal diet and received PBS (NR+PBS), rats fed a high-fat diet and received PBS (HR+PBS), and rats fed high-fat diet and received ADSC (HR+ADSC). Smooth muscle is stained brown. The smooth muscle content was higher in HR than in NR. Magnification is X 100. TA = tunica albuginea.
TUNEL Immunohistochemistry
There were no significant differences in TUNEL positivity between groups (data not shown).
DISCUSSION
There is a growing body of evidence that endothelial dysfunction plays a pivotal role in the development of ED in men with hyperlipidemia [16,17]. In the current study, it was determined that penile hemodynamics are substantially inferior in rats fed a high-fat diet for six months relative to those fed a normal diet. Markers for endothelial function and NOS activity were depressed in hyperlipidemic rats treated with saline alone. ADSC transplantation led to substantial improvement in penile hemodynamics in hyperlipidemic rats. It is implied that 1) hyperlipidemia in rats produces a phenotype similar to ED via endothelial dysfunction, and 2) ADSC may ameliorate hyperlipidemia related ED.
The precise mechanism(s) by which ADSC enhance erectile function remains to be elucidated. Histological data from our study suggests that ADSC treatment enhances neuronal and endothelial content of the penis. However, the scant staining for EdU in the corpus cavernosum at the 1 month time point implies that differentiation of stem cells plays a relatively minor role in the efficacy of this treatment, despite prior evidence that ADSC possess the capacity to develop into smooth muscle-like, endothelial-like, and neuronal-like cells in vitro.
As cellular differentiation does not appear to play a major role in the therapeutic effect of ADSC in this model system, it is logical to hypothesize that the paracrine release of cytokines and growth factors is responsible for the observed effects. It has been demonstrated that low dose basic Fibroblast Growth Factor (bFGF) increases nNOS expression in hyperlipidemic rabbit corporal tissue [18]. In a rat model system, treatment with Vascular Endothelial Growth Factor (VEGF) by corporal injection has been associated with greater expression of eNOS and iNOS [19]. ADSC have been shown to secrete both VEGF and bFGF; this action may underlie their efficacy in treatment of hyperlipidemia associated compromise of penile hemodynamics in this model system [20,21]. Further study of the specific cytokines and growth factors secreted by ADSC in vivo is required to clarify this issue.
An interesting finding of our study was that smooth muscle content was significantly higher in the HR than in the controls. This finding supports prior reports of increased smooth muscle content in hyperlipidemic rats [13,22]. LDL has been shown to act as a classic growth factor promoting vascular smooth muscle cell growth via a mitogenic signals normally elicited by classic growth factors [23] It is logical to conjecture that this effect may explain the observation of increased smooth muscle content in our model system. This increase in smooth muscle content may play a role in impaired penile hemodynamics by enhancement of the contractile properties of the corpora cavernosa. This paradigm, while somewhat contrary to the usual concept of smooth muscle content as an indicator of corporal health, merits further exploration.
The principal limitations of our study include the limited number of animals and a lack of molecular data on the underlying mechanisms of tissue effect. Questions also remain about the optimal dosing of ADSC and the long term durability of our results. These important questions will be the subject of additional research projects. Another critical question is the long-term safety of ADSC with respect to the possibility of tumor formation. Investigation of the long term fate of ADSC is required before human trials can be considered, although the lack of ADSC in our 1 month follow-up tissues sections implies that the potential of these cells to persist and undergo malignant transformation is limited.
CONCLUSIONS
Intracavernous injection of ADSC improved erectile function in rats that developed hyperlipidemia-associated ED. The underlying mechanism is likely cytokine and growth factor secretion although stem cell differentiation may also play a role. ADSC may have promise in the treatment of hyperlipidemia-related ED.
Acknowledgments
This work was supported by grants from the Arthur Rock Foundation and the National Institutes of Health (DK045370).
References
- 1.Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet. 1985;1:181–184. doi: 10.1016/s0140-6736(85)92023-9. [DOI] [PubMed] [Google Scholar]
- 2.Wei M, Macera CA, Davis DR, Hornung CA, Nankin HR, Blair SN. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol. 1994;140:930–937. doi: 10.1093/oxfordjournals.aje.a117181. [DOI] [PubMed] [Google Scholar]
- 3.Bocchio M, Desideri G, Scarpelli P, Necozione S, Properzi G, Spartera C, Francavilla F, Ferri C, Francavilla S. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004;171:1601–1604. doi: 10.1097/01.ju.0000116325.06572.85. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–184. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 5.Pegge NC, Twomey AM, Vaughton K, Gravenor MB, Ramsey MW, Price DE. The role of endothelial dysfunction in the pathophysiology of erectile dysfunction in diabetes and in determining response to treatment. Diabet Med. 2006;23:873–878. doi: 10.1111/j.1464-5491.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 6.Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006;45:268–276. doi: 10.1016/j.vph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Montague DK, Jarow JP, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, Milbank AJ, Nehra A, Sharlip ID. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230–239. doi: 10.1097/01.ju.0000164463.19239.19. [DOI] [PubMed] [Google Scholar]
- 8.Valiquette L, Montorsi F, Auerbach S. First-dose success with vardenafil in men with erectile dysfunction and associated comorbidities: RELY-I. Int J Clin Pract. 2006;60:1378–1385. doi: 10.1111/j.1742-1241.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92:9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- 10.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 12.Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gholami SS, Rogers R, Chang J, Ho HC, Grazziottin T, Lin CS, Lue TF. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol. 2003;169:1577–1581. doi: 10.1097/01.ju.0000055120.73261.76. [DOI] [PubMed] [Google Scholar]
- 14.Fandel TM, Bella AJ, Lin G, Tantiwongse K, Lin CS, Pohl J, Lue TF. Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2008;5:1866–1875. doi: 10.1111/j.1743-6109.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 15.Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, Rajfer J, Gonzalez-Cadavid N. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008;20:202–212. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 16.Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5:1066–1078. doi: 10.1111/j.1743-6109.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 17.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 18.Xie D, Pippen AM, Odronic SI, Annex BH, Donatucci CF. Intracavernosal basic fibroblast growth factor improves vasoreactivity in the hypercholesterolemic rabbit. J Sex Med. 2006;3:223–232. doi: 10.1111/j.1743-6109.2005.00174.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin CS, Ho HC, Chen KC, Lin G, Nunes L, Lue TF. Intracavernosal injection of vascular endothelial growth factor induces nitric oxide synthase isoforms. BJU Int. 2002;89:955–960. doi: 10.1046/j.1464-410x.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 21.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 22.Ryu JK, Shin HY, Song SU, Oh SM, Piao S, Han JY, Park KW, Suh JK. Downregulation of angiogenic factors and their downstream target molecules affects the deterioration of erectile function in a rat model of hypercholesterolemia. Urology. 2006;67:1329–1334. doi: 10.1016/j.urology.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Gouni-Berthold I, Sachinidis A. Does the coronary risk factor low density lipoprotein alter growth and signaling in vascular smooth muscle cells? Faseb J. 2002;16:1477–1487. doi: 10.1096/fj.02-0260rev. [DOI] [PubMed] [Google Scholar]