Abstract
Introduction
TGF-β1 works through Smad dependent mechanisms and contributes to the pathogenesis of Peyronie’s Disease (PD). Pentoxyfylline (PTX) antagonizes the effects of TGF-β1 and has been utilized for the management of PD although the mechanisms of action are not entirely clear.
Aim
We studied cell-signaling pathways through which TGF-β1 and PTX mediate collagen metabolism, elastin expression, and elastogenesis in tunica albuginea derived fibroblasts (TADF).
Methods
TADF from men with and without PD were cultured and treated with TGF-β1 and PTX as monotherapy at differing concentrations and time points. Combination treatment (TGF-β1 followed by PTX and vice versa) was also investigated.
Main Outcome Measures
RT-PCR and Western blotting were utilized to assess differences in elastin metabolism and cellular signaling between groups.
Results
TGF-β1 increased mRNA and protein expression of elastin in a time and dose dependent fashion. PTX did not interfere with TGF-β1 mediated upregulation of elastin mRNA and protein in TADF. However, pre-treatment of TADF with PTX was associated with decreased activity of the Smad2 pathway and enhanced phosphorylation of the inhibitory Smad6 in PTX pre-treated TADF.
Conclusion
Expression of elastin mRNA and protein is upregulated in TADF by TGF-β1. PTX has no effect on elastin and collagen production but has been shown to attenuate both collagen fiber deposition and elastogenesis in TADF. This change in fiber deposition is likely mediated via the TGF/Smad pathway.
Keywords: TGF-β1, Fibroblast, Pentoxyfylline, Elastin, Collagen, Peyronie’s Disease
1. INTRODUCTION
TGF-β1 is a potent modulator of the extracellular matrix in a variety of human fibrotic diseases.1 It is known to be intimately related to the Peyronie’s disease (PD) phenotype, and treatment with TGF-β1 has been shown to induce a PD-like condition in animal models2, 3. Much of this effect has been attributed to TGF-β1 induced enhancement of collagen production in fibroblasts4, 5. TGF-β1 has also been demonstrated to increase elastin mRNA levels in both dermal and lung fibroblasts6, 7.
TGF-β1exerts the majority of its’ downstream effects via action on Smad proteins, a family of TGF receptor substrates that have the capacity to act as transcription factors in the cell nucleus.1 Smad proteins frequently interact with one another during the course of activation; specifically, Smad2 and Smad 3 typically interact together, whereas Smad1 and Smad5 also share activity. While the majority of Smad proteins have stimulatory effects on gene transcription the inhibitory Smads (Smad6 and Smad7) inhibit the activity of other Smad proteins.8, 9 The Smad proteins have been shown to play very important roles in the modulation of collagen I production and thereby a critical role in extracellular fibrotic conditions, such as PD.1
Pentoxyfylline (PTX), a nonselective phosphodiesterase inhibitor with anti-inflammatory properties in vivo and in vitro, has been shown in both in vitro and in vivo (rat) experiments to induce regression of collagen and TGF-β1 induced plaque10. In a previous study, it was demonstrated that TGF-β1 enhances collagen and elastin production in tunica albuginea derived fibroblast cells (TADF) and that this effect is attenuated by pre-treatment with PTX.1 The current study is an assessment of the impact of TGF-β1 with or without PTX on elastin metabolism in tunica albuginea derived fibroblasts (TADF) at the mRNA, protein, and cellular signaling level.
2. MATERIALS AND METHODS
2.1. Tissue harvesting and cell culture
Our Institutional Committee on Human Research approved all procedures regarding the collection and use of human tissues. Fibrotic tunica plaques (PT) were harvested from 12 patients with chronic (>12 months duration) Peyronie’s disease who were undergoing surgery for correction of penile curvature. Normal tunica (NT) was harvested from 6 patients who were undergoing penile prosthesis placement. All cavernosal tissue was stripped from the biopsy specimens so as to ensure a pure culture of tunica-derived tissues.
TADF were procured as previously described11. Briefly, the tunica tissues were washed 3 times in sterile phosphate-buffered saline (PBS) and cut into 2–3 mm3 segments. The segments were placed evenly onto a 100-mm cell culture dish (Falcon-Becton Dickinson Labware, Franklin Lakes, NJ). Ten minutes later 10 ml of Dulbecco’s Modified Eagle Medium (DMEM) containing penicillin (100 units/ml), streptomycin (100 ug/ml), and 10% FBS was pipetted into the dish. The dish was kept undisturbed in a humidified 37°C incubator with 5% CO2. Five days later, tissue segments that had detached from the dish were removed, and the culture medium was replaced. This process was repeated after another five days of culture. When small islands of cells were noticeable, wells were treated with trypsin and transferred to a fresh dish. Expansion of each cell strain was continued with change of medium every 3 days and passages approximately every 10 days. All cells used in the following experiments were from passages 4 through 10. All experiments were repeated in triplicate on TADF from each subject (i.e. 12 PT and 6 NT) and all data are presented as the average of three independent experiments.
2.2. Cell treatment with TGF and PTX
Cells were cultured as detailed above. The cells were then kept in a humidified 37°C incubator with 5% CO2 until they manifested 80% confluence. After 80% confluence was attained, the cells were incubated in serum free DMEM overnight and subsequently treated with TGF-β1 and/or PTX in DMEM with 0.1% BSA as detailed below.
For the dose response study, TGF-β1 at concentrations of 0, 0.01, 0.1, 1, 10 and 100 ng/ml was used to treat the TADF treatment for 24hr. For the time response study, 1ng/ml TGF-β1 was used to treat TADF for 0hr, 4hr, 16hr, 24hr and 2 weeks. To evaluate the interaction between PTX and TGF-β1 in vitro, five groups were studied: 1) Control group: TADF treated with 0.1% BSA medium only; 2) TGF-β1 group: TADF treated with 1ng/ml TGF-β1 for 24 hours; 3) PTX group: TADF treated with 100 uM PTX for 24 hours; 4) TGF-β1 and PTX group: TADF treated with 1ng/ml TGF-β1 for 6 hours followed by 100uM PTX for another 24 hours 5) PTX and TGF-β1 group: TADF treated with PTX at 0, 0.01, 10, and 100 uM for 6hr followed by 1ng/ml TGF-β1 for another 24 hours.
2.3. RNA preparation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
RNA from TADF cells was isolated by the Tri-Reagent RNA extraction method (Molecular Research Center, Cincinnati, OH). Despite the manufacturer’s claim, RNA prepared by this method is often contaminated by trace amounts of DNA, as assessed by polymerase chain reaction (PCR) with b-actin primers (data not shown). To remove the contaminating DNA, each RNA sample (20–50 ug) was treated with 10 units of RNase-free DNase I (Roche Molecular Biochemicals, Pleasanton, CA) at 37 C for 30 min. The RNA was then purified by phenol/chloroform extraction and ethanol precipitation. Quantity and purity of RNAs were measured by spectrophotometry with UV adsorption at wavelengths 260 and 280. RNA integrity was visualized by the sharpness of the 28s and 18s ribosomal RNA bands in agarose gels. Reverse transcription-polymerase chain reaction (RT-PCR) was performed in an RT step and a PCR step, as described previously. Briefly, 2.5 μg of each RNA were reverse transcribed in a reaction volume of 20 μl. This RT product was then diluted 5 fold with TE buffer (10mM Tris–HCl, pH 8, 1mM EDTA). One μl of each dilution was used in a 10 μl PCR to identify the optimal input within the linear amplification range. PCR was performed in DNA Engine thermocycler (MJ Research, Watertown, MA) under calculated temperature control. The primer pairs for RT-PCR analysis of Elastin (ELN), fibulin 5 (aka DANCE, important for elastogenesis), lysyl oxidase-like 1 (LOXL-1, also important for elastogenesis), and β-actin genes are listed in Table 1. The cycling program was set for 35 cycles of 94°C, 10 s; 55 °C, 10 s; 72°C, 10 s, followed by one cycle of 72 °C, 5 min. The PCR products were electrophoresed in 1.5% agarose gels in the presence of ethidium bromide, visualized by UV fluorescence, and recorded by the ChemiImager 4000 System (Alpha Innotech, San Leandro, CA). Quantification of the PCR products was determined by the densitometry program of the ChemiImager 4000 System.
Table 1.
Oligonucleotide primers
| Gene | Primers | Size of PCR product (bp) |
|---|---|---|
| ELN | Forward :TGGAGGCAAACCTCTTAAGCCAGT | |
| Reverse : GCACCGAACTTTGCTGCTGCTTTA | 630 | |
| β-actin | Forward: TCTACAATGAGCTGCGTGTG | |
| Reverse: AATGTCACGCACGATTTCCC | 368 |
2.4. Protein isolation and Western blot analysis
The cellular protein samples from the 24 hour time point were prepared by homogenization of cells in a lysis buffer containing 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% sodium docecyl sulfate, aprotinin (10 μg/ml), leupeptin (10 μg/ml), and PBS. Cell lysates containing 20 μg of protein were electrophoresed in sodium docecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (Millipore Corp, Bedford, MA, USA). The membrane was stained with Ponceau S to verify the integrity of the transferred proteins and to monitor the unbiased transfer of all protein samples. Detection of target proteins on the membranes were performed with an electrochemiluminescence kit (Amersham Life Sciences Inc, Arlington Heights, IL, USA) with the use of primary antibodies for pSmad1/5, pSmad2, Smad1/5, Smad2, Smad4, Smad6, pMAPK, MAPK, pAkt, Akt, Elastin, Fibulin 5, and β-actin (listed in table 2). After the hybridization of secondary antibodies, the resulting images were analyzed with ChemiImager 4000 (Alpha Innotech Corporation, San Leandro, CA, USA) to determine the integrated density value of each protein band.
Table 2.
Antibodies used in this study
| Target protein | Supplier | Catalog no. | Concentration |
|---|---|---|---|
| p-SMAD1/5 (Ser463/465) | Cell Signaling | 9516 | 1:1000 |
| SMAD5 | Cell Signaling | 9517 | 1:1000 |
| p-SMAD2 (Ser465/467) | Cell Signaling | 3108 | 1:1000 |
| SMAD2 | Cell Signaling | 3122 | 1:1000 |
| SMAD4 | Cell Signaling | 9515 | 1:1000 |
| SMAD6 | Cell Signaling | 9519 | 1:1000 |
| p-p38(Thr180/182) | Cell Signaling | 9216s | 1:1000 |
| p38 | Cell Signaling | 9212 | 1:1000 |
| pAkt(Ser473) | Cell Signaling | 9271s | 1:1000 |
| Akt | Cell Signaling | 9272 | 1:1000 |
| ELN | Abcam | ab21610 | 1:10000 |
| DANCE | Santa Cruz Biotech | sc30170 | 1:500 |
| β-actin | sigma | A5441 | 1:3000 |
2.5. Statistics
Statistical analysis was performed according to the Primer of Biostatistics, 3rd edition (Glantz SA, McGraw-Hill, Inc, New York, NY, USA). Data are expressed as means ± standard deviation. Analysis of variance (ANOVA) on data from all groups was used to determine the p value. If the p value was less than 0.05, multiple comparisons of data from paired groups were performed with Bonferroni correction. All calculations were performed using Prism GraphPad v 4.0 (GraphPad Software, La Jolla, USA). Statistical significance was set at p < 0.05.
3. RESULTs
3.1. TGF -β1 stimulates the synthesis of elastin in a time- and dose-dependent manner in TADF cells
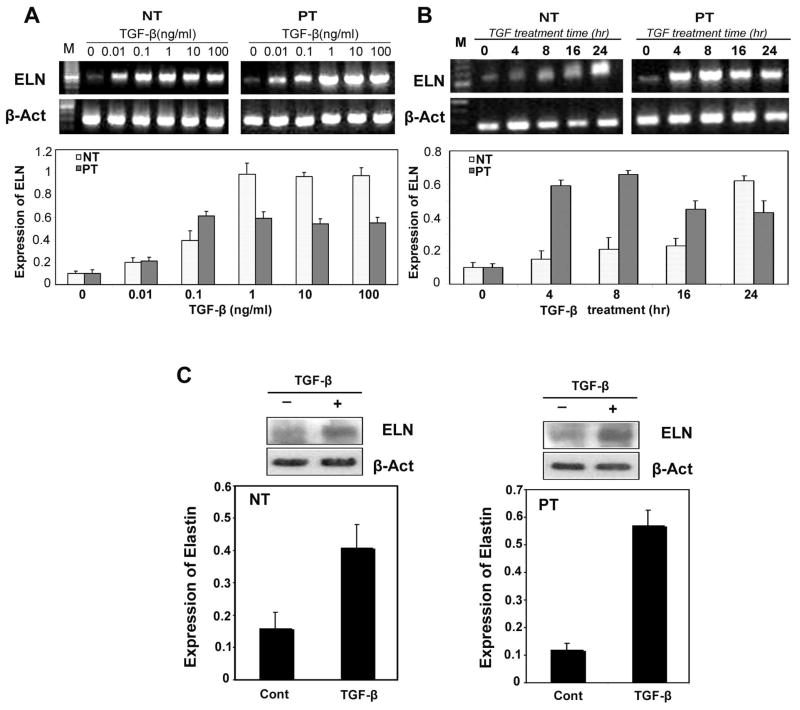
TGF-β1 increased elastin mRNA expression in cultured TADF in a dose-dependent fashion. Maximal induction was observed at concentrations ≥0.01 ng/ml TGF-β1 for both PT and NT TADF although the magnitude of increase in NT was significantly less than PT (Fig. 1A). Steady-state elastin mRNA levels were significantly increased in TGF-β1 treated TADF relative to untreated TADF. Interestingly, NT TADF did not significantly increase their elastin mRNA production until 24 hours after TGF-β1 treatment, implying a slower response in NT cells relative to PT cells (Fig 1B). The level of DANCE (Fibulin 5) and LOXL1 mRNA did not change in response to TGF-β1 treatment. Enhanced production of elastin protein in both NT and PT TADF cells treated with TGF-β1 was confirmed by Western blot (Fig 1C).
Fig 1. TGF-β1 stimulates the synthesis of elastin in TADF cells.
A. rtPCR for elastin mRNA: TADF cells were treated with TGF-β1 for 24 hr at different dosages ( 0, 0.01, 0.1,1, 10 and 100ng/ml). TGF-β1 increased elastin mRNA in a dose-dependent fashion, and maximal induction was observed at concentrations ≥0.01 ng/ml TGF-β1 (P<0.05). B. rtPCR for elastin mRNA: TADF cells were treated with 1ng/ml TGF-β1 for 0hr, 4 hr, 8 hr, 16 hr and 24hr. Elastin mRNA levels were increased significantly after 4h~8h and peaked at 8~16 h treatment with TGF-β in PT cells. The NT cells responded more slowly than PT cells but manifested a significant elastin mRNA response at the 24 hour time point. C. Western Blot for elastin: In NT (left panel) and PT cells (right panel), Elastin protein level was significantly increased with 1ng/ml TGF-β1 in both groups (P<0.05). (NT=6, PT=12)
3.2. TGF -β1 activates the dual Smad pathway in TADF cells
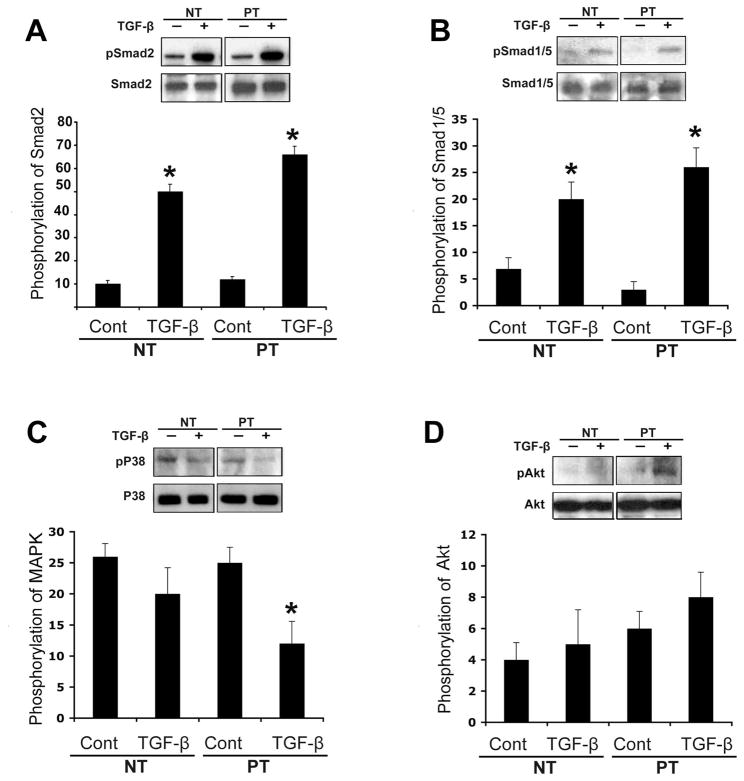
Several cellular pathways related to TGF-β1 were investigated in PT and NT TADF. TGF-β1 treatment of TADF was shown to enhance the phosphorylation of Smad 2 at Ser 473, and the phosphorylation of Smad1/5 at Ser 463/465 in both PT and NT TADF (Figure 2). The pAkt/Akt pathway was not significantly involved in this effect. Interestingly, the p38 pathway was inhibited in NT cells to a slight but insignificant extent by TGF-β1; however, TGF-β1 produced a significant (P<0.05) inhibition of p38 in PT cells. (Fig 2)
Fig 2. TGF-β1 up-regulates the dual Smad pathway in TADF.
A. TGF-β1 significantly activated Smad2 by phosphorylation of Ser 473 in TADF cells ( *P<0.05). B. TGF-β1 also significantly activated Smad1/5 by phosphorylation of Ser 463/465 (*P<0.05). C. TGF-β1 decreased the phosphorylation state of p38 in PT cells (P<0.05) but the difference did not attain statistical significance in NT cells( P>0.05). D. TGF-β1 did not significantly affect the phosphorylation status of Akt in either NT or PT cells (P>0.05). (nNT=6, nPT=12)
3.3. PTX attenuates activation of TGF -β1/Smad1(5) pathway by increased Smad6 in TADF cells
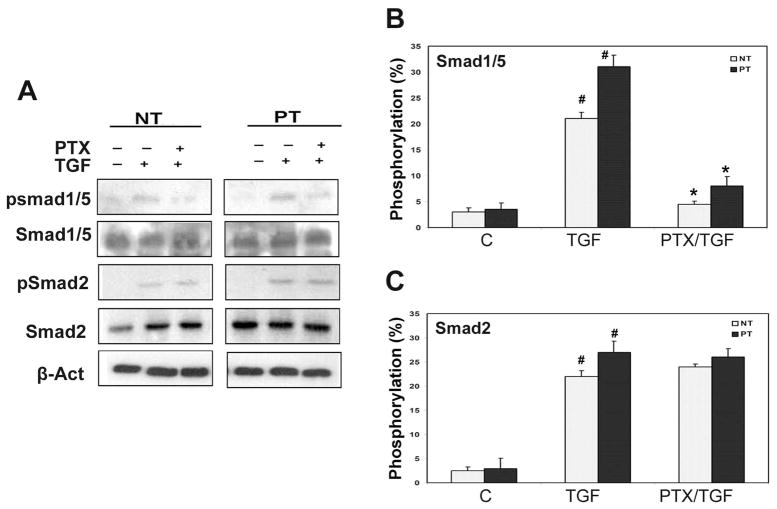
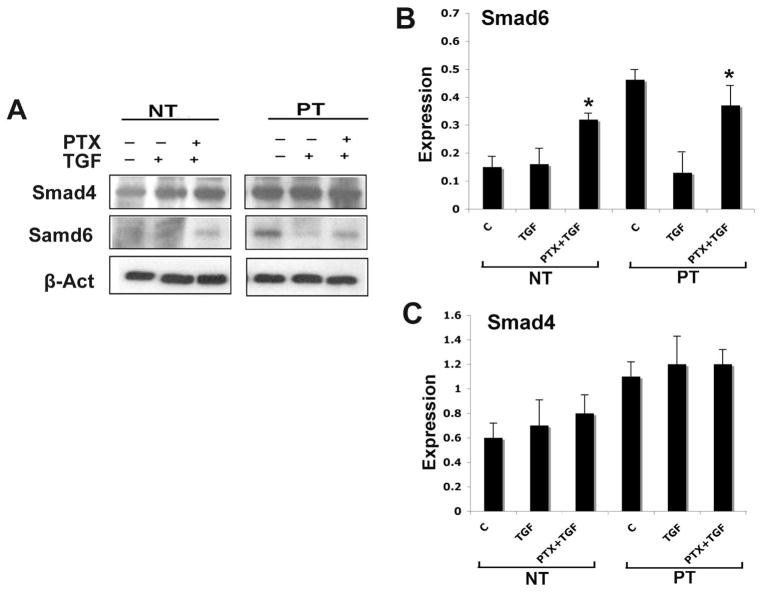
Pretreatment with PTX significantly decreased Smad1/5 phosphorylation in response to TGF-β1; a larger increase was noted in PT cells relative to NT cells. The phosphorylation of Smad 2 was not affected at 24 hours (Fig 3B&C). Baseline production of the Smad6 was higher in PT cells relative to NT cells; TGF-β1 treatment reduced Smad6 expression to levels similar to what was observed in NT cells (Fig 4A&B). PTX pretreatment increased the expression of I-Smad 6 at 24 hours in both NT and PT cells (Fig 4A&B). The expression of co-Smad 4 was maintained at the same level in response to both TGF-β1 and PTX/TGF-β1 treatments (Fig 4A&C).
Fig 3. PTX decreases phosphorylation of the Smad1(5)/Smad6 pathway.
TADF cells were treated with vehicle, TGF-β1 (1ng/ml), or the combination of TGF-β1 and PTX (TGF-β1 followed by PTX, or PTX followed by TGF-β1) for 24 hr. A. Western blot of pSmad1/5, Samd1/5, pSmad2, and Smad2, in TADF cells. All TGF-β1 treatments increased phosphorylation of both Smad 1/5 and Smad2 ( # P<0.01). B. Phosphorylation level of Smad1/5 in TADF. Pretreatment with PTX significantly decreased the phosphorylation level of Smad1/5 (*P<0.01). C. Phosphorylation level of Smad2 in TADF Pretreatment with PTX did not affect the phosphorylation level of Smad2 (#p>0.05).
Fig 4. PTX increases expression of Smad6.
TADF cells were treated with vehicle, TGF-β1 (1ng/ml), or the combination of TGF-β1 and PTX (TGF-β1 followed by PTX, or PTX followed by TGF-β1) for 24 hr. A. Western blot of Smad4 and Smad6 in TADF cells. B. Expression of Smad6 in TADF. TGF-β1 treatment decreased expression of Smad6 in PT cells but not other types. Pretreatment with PTX in TADF cells increased Smad6 expression significantly in both NT and PT compared to TGF-β1 only (* P<0.05). The magnitude of PTX-mediated increase in Smad6 in the PT cells was greater compared to what was observed in NT cells but the difference was not statistically significant. (nNT=6, nPT=12)). C. Expression of Smad4 in TADF Expression of Smad4 was not affected by TGF-β1 or PTX.
4. DISSCUSSION
The TGF-β super-family binds to two receptors, TβRI and TβRII, to regulate downstream molecules and subsequent gene expression. After the TGF-β1 ligand binds to a receptor, signal transduction occurs predominantly by members of the Smad family. The activation of Smad proteins is achieved through the phosphorylation of specific receptor-regulated Smads (R-Smads) by an activated receptor. This leads to formation of “Smad complexes” which accumulate in the nucleus and regulate target genes.8, 9
There are two dominant branches for TGF-β super family signaling pathways: a TGF-β/Nodal/Activin branch that signals through activation of Smad2/3, and a BMP/GDF branch that signals through Smad1, 5 and 8. It has been reported that TGF-β activates both Smad2/3 and Smad1/5 in endothelial cells and fibroblasts12. When stimulated with TGF-β1, fibroblasts from patients with PD show rapid nuclear translocation of Smad2/3, as soon as 15 min after stimulation.13 Inhibition of the TGF-β1 type I receptor was recently shown to induce regression of PD plaques and reduced expression of phospho-Smad 2/3.14 These data suggest that the TGF/Smad 2/3 pathway plays a major role in the induction of the Peyronie’s phenotype.
Elastin is present in penile tissues, including the tunica albuginea.15, 16 It is known that elastin metabolism regulation is mainly achieved by modulation of mRNA transcription and stabilization17. The translated precursor elastin monomers (tropoelastin) from mRNA are secreted primarily from fibroblasts and smooth muscle cells into the extracellular matrix (ECM). In the ECM, tropoelastin is assembled into elastin fibers by action of lysyl oxidase (LOX), lysyl oxidase-like proteins 1 to 4, and fibulin 518, 19.
The effects of TGF-β1 in stimulating collagen expression are well established.1 It has also been demonstrated that TGF-β1 stimulates the expression of elastin in lung and skin fibroblasts6. These effects are thought to be mediated in large part via phosphorylation of Smad2 and Smad320 and stabilization of elastin mRNA17.
Since TGF-β1 is known to activate Smad 2/3 in tunica fibroblasts and this effect has been linked to elastin mRNA production, we hypothesized that TGF-β/Smad modulates elastin metabolism in tunica albuginea fibroblasts and that this effect may be related to the PD phenotype. It was previously determined that TGF-β1 induces elastin and collagen protein upregulation in a time- and dose-dependent manner in TADF cells.1 IN the current study, it was demonstrated that these effects may be mediated by activation of both the Smad2/3 and Smad1/5 pathways in both NT and PT cells, as well as inhibition of the p38 pathway in PT cells. Although it has been reported that the MAPK21 and Akt pathways6 are involved in downstream TGF-β activated pathways in other fibroblasts, our current results implies cell specificity of TGF-β1 signaling in TADF cells.
Interestingly, I-Smad6 expression in untreated control PT cells was higher than what was observed in NT cells; treatment with TGF-β1 led to a dramatic decline in I-Smad6 expression in PT cells but not NT cells. The reason for the difference in these cells types is not entirely clear but it is implied that Smad6 may be expressed at a higher level in PT cells, possibly as a mechanism intended to regulate the inflammatory process of PD in vivo.
The TGF-β1 response of TADF derived from men with clinical PD was more rapid and robust with respect to elastin mRNA and protein production, collagen production, and p38 inactivation when compared to cells from normal men. It is of particular interest that p38 was down-regulated by TGF-β1 in PT cells from model system, as the p38 pathway is most typically activated by TGF-β1.22 The reason for this seemingly paradoxical reaction is at this time unclear but may signify that p38 signaling is disrupted in clinical PD or at least in this model system. The consequences of p38 activation by TGF-β1 in various in vitro systems are at this time unclear and may not always be of obvious functional significance.22 In any event, our observations imply that there are important cellular differences in response to TGF-β1 exist between men with and without PD; cells from normal tunica tissues have the same capacity to respond to TGF-β1 but this response is not as robust as what is observed in plaque tissue derived cells.
PTX, a non-specific phosphodiesterase inhibitor, has been used in a variety of clinical inflammatory and fibrotic conditions23–26. PTX inhibits TGF-mediated collagen expression through modulation of the ERK1/2 and p38(HOG) pathways24–27. We hypothesized that PTX might also play a role in elastin and collagen metabolism in TADF. Pre-treatment with PTX did significantly increase the activity of I-Smad6 in TADF; I-Smad6 is in turn known to down-regulate phosphorylation/activation of R-Smad 1/5, the “side-branch” of the TGF pathway8. The effects of PTX in enhancing Smad 6 activity were more pronounced in PT cells relative to NT cells but this difference did not attain statistical significance. As PTX pre-treatment has been associated with a decline in TGF-β1 mediated elastogenesis and collagen deposition in TADF cells, 1 we conclude that Smad 1/5 plays a stimulatory role in elastogenesis and collagen deposition in TADF and this effect is inhibited by PTX via an I-Smad6 dependent mechanism.
Interestingly, the prior report by Kucich et al. in lung derived fibroblasts reported that elastin mRNA stabilization (with or without TGF-β1 treatment) was suppressed by Smad7 (a known inhibitor of Smad2 activity) treatment but not by Smad6 treatment17. As there was no suppression of Smad2 phosphorylation in our study, Smad7 may not play a large role in elastin metabolism in TADF. Ergo, there may be tissue specific differences in Smad regulation of elastin metabolism between various human fibroblasts.
Limitations of this study include the in vitro study design; the behavior of cultured monolayers of fibroblasts in vitro may not be representative of what occurs in the more complex in vivo environment. The precise mechanisms by which PTX mediates Smad metabolism and produces the observed effect remain to be elucidated. Our assessment of intracellular signaling activity at the 24 hour time point may have missed important alterations that occur before or after that time point; it is known that TGF-β1 may have delayed autocrine effects on a number of signaling pathways. Future experiments should be performed to clarify the time course of Smad activation in this model system. Additional investigations in this model system using various inhibitors of intracellular signaling will further clarify the precise signaling relationships at work in TADF cells.
5. CONCLUSIONS
Production of collagen and elastin in TADF cells is stimulated by TGF-β1; this effect is associated with increased activation of the dual TGF-β/Smad pathways. Pre-treatment of TADF with PTX attenuates the TGF-β1 mediated increase in elastogenesis, possibly by down-regulation of Smad1/5 via an iSmad6 dependent mechanism. Since there were no PTX mediated changes in expression/activity of the proteins essential to assembly of elastin fibers in the ECM, the precise mechanisms by which PTX attenuates TGF-β1 mediated elastogenesis remain unclear. Further studies are required to elucidate the molecular mechanisms underlying these observations.
Acknowledgments
XXX
References
- 1.Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev. 2006;5:563–9. doi: 10.1016/j.autrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.El-Sakka AI, Hassoba HM, Chui RM, Bhatnagar RS, Dahiya R, Lue TF. An animal model of Peyronie's-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol. 1997;158:2284–90. doi: 10.1016/s0022-5347(01)68236-3. [DOI] [PubMed] [Google Scholar]
- 3.El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997;158:1391–4. [PubMed] [Google Scholar]
- 4.Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995;153:826–34. [PubMed] [Google Scholar]
- 5.Cutroneo KR. TGF-beta-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen. 2007;15 (Suppl 1):S54–60. doi: 10.1111/j.1524-475X.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuang PP, Zhang XH, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-beta in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2007;292:L944–52. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- 7.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–40. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 8.Ishida W, Hamamoto T, Kusanagi K, et al. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275:6075–9. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- 9.Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- 10.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Lin GT, Wang Z, Liu BC, Lue TF, Lin CS. Identification of potential biomarkers of Peyronie's disease. Asian J Androl. 2005;7:237–43. doi: 10.1038/aja.2005.45. [DOI] [PubMed] [Google Scholar]
- 12.Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28:6889–902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haag SM, Hauck EW, Szardening-Kirchner C, et al. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie's disease. Eur Urol. 2007;51:255–61. doi: 10.1016/j.eururo.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JK, Piao S, Shin HY, et al. IN-1130, a novel transforming growth factor-beta type I receptor kinase (activin receptor-like kinase 5) inhibitor, promotes regression of fibrotic plaque and corrects penile curvature in a rat model of Peyronie's disease. J Sex Med. 2009;6:1284–96. doi: 10.1111/j.1743-6109.2009.01216.x. [DOI] [PubMed] [Google Scholar]
- 15.Bossart MI, Spjut HJ, Scott FB. Ultrastructural analysis of human penile corpus cavernosum. Its significance in tumescence and detumescence. Urology. 1980;15:448–56. doi: 10.1016/0090-4295(80)90003-5. [DOI] [PubMed] [Google Scholar]
- 16.Akkus E, Carrier S, Baba K, et al. Structural alterations in the tunica albuginea of the penis: impact of Peyronie's disease, ageing and impotence. Br J Urol. 1997;79:47–53. doi: 10.1046/j.1464-410x.1997.26511.x. [DOI] [PubMed] [Google Scholar]
- 17.Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-beta stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-delta, and p38. Am J Respir Cell Mol Biol. 2002;26:183–8. doi: 10.1165/ajrcmb.26.2.4666. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 19.Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. Embo J. 2007;26:3283–95. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 21.Fu Y, O'Connor LM, Shepherd TG, Nachtigal MW. The p38 MAPK inhibitor, PD169316, inhibits transforming growth factor beta-induced Smad signaling in human ovarian cancer cells. Biochem Biophys Res Commun. 2003;310:391–7. doi: 10.1016/j.bbrc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Han J, Ito Y, Bringas P, Jr, Deng C, Chai Y. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell. 2008;15:322–9. doi: 10.1016/j.devcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deree J, Martins J, de Campos T, et al. Pentoxifylline attenuates lung injury and modulates transcription factor activity in hemorrhagic shock. J Surg Res. 2007;143:99–108. doi: 10.1016/j.jss.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 24.Hung KY, Huang JW, Chiang CK, Tsai TJ. Preservation of peritoneal morphology and function by pentoxifylline in a rat model of peritoneal dialysis: molecular studies. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn369. [DOI] [PubMed] [Google Scholar]
- 25.Lin SL, Chen RH, Chen YM, et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol. 2005;16:2702–13. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 26.Ng YY, Chen YM, Tsai TJ, Lan XR, Yang WC, Lan HY. Pentoxifylline inhibits transforming growth factor-beta signaling and renal fibrosis in experimental crescentic glomerulonephritis in rats. Am J Nephrol. 2009;29:43–53. doi: 10.1159/000150600. [DOI] [PubMed] [Google Scholar]
- 27.Chen YM, Wu KD, Tsai TJ, Hsieh BS. Pentoxifylline inhibits PDGF-induced proliferation of and TGF-beta-stimulated collagen synthesis by vascular smooth muscle cells. J Mol Cell Cardiol. 1999;31:773–83. doi: 10.1006/jmcc.1998.0910. [DOI] [PubMed] [Google Scholar]