Abstract
Erectile Dysfunction (ED) is the most common male sexual dysfunction presented for treatment, and the most thoroughly studied sexual dysfunction in men. In the late 20th century, important discoveries were made regarding both the physiologic processes of penile erection and the pathophysiology of ED. These discoveries led to the commercial introduction of the phosphodiesterase type 5 inhibitors (PDE5I), a class of medications which now accounts for the largest segment of the ED market. While these drugs are highly efficacious for many men, a relatively large subset of ED patients who do not respond to PDE5I has been identified. Recognition of this subset of the ED population and the ageing of the population has driven researchers to investigate novel treatment targets for ED. Increased research efforts have resulted in the development of several orally available compounds that combine high efficacy with low rates of adverse events. In this review we report on various compounds that regulate penile erection both centrally (Clavulanic acid, Dopamine and Melanocortin receptor agonists) and peripherally (novel PDE5I, soluble and particulate Guanylil Cyclase activators, Rho-kinase inhibitors and Maxi-K channel openers), and discuss the preclinical and clinical evidence supporting the development of these emerging drugs for ED.
Keywords: Erectile Dysfunction, Therapy, phosphodiesterase 5 inhibitor, dopamine, maxi-K channel, melanocortin receptor, Rho-kinase, guanylil cyclase
1. Background
Erectile Dysfunction (ED) was defined by the NIH Consensus Development Panel on Impotence as the persistent inability to attain and maintain an erection sufficient for sexual intercourse.[1] It is the most thoroughly studied sexual dysfunction in men and the most common sexual complaint of men presenting to their healthcare providers.[2] Notwithstanding variations in definitions and methodology, various large scale studies (both cross-sectional and longitudinal) substantiate the global presence of this disease, with an estimated overall prevalence rate that ranges between 10% and 20% worldwide.[3] There is a strong correlation between age and ED, with prevalence increasing steadily from 6.5% in men aged 20–39 years to 77.5% in those 75 years and older.[4] ED is associated with various comorbidities including psychological factors, cardiovascular diseases, diabetes mellitus, metabolic syndrome and smoking. Iatrogenic causes of ED include cavernous nerve injury during radical pelvic surgery and the use of various medications, particularly thiazides, some beta-blockers, hormonal blockade, and antidepressants.[5]
Historically, treatment of ED was limited to surgical options and intracavernosal or intraurethral administration of vasoactive drugs. In the 1980–90s, research on the mechanisms of corpus cavernosum smooth muscle relaxation led to the discovery of nitric oxide (NO) as the most important peripheral neurotransmitter in erectile function, which in turn resulted in the development and introduction of phosphodiesterase-5 inhibitors (PDE5I).[6–9]
2. Medical need
Despite the huge success of PDE5I, the demand for alternative pharmacotherapeutic options for ED continues to rise as a result of the increased proportion of elderly in the population, and increasing recognition that a approximately one-third of ED patients do not respond to PDE5I.[10] Research continues on compounds that may benefit those men that do not experience satisfactory results from oral PDE5I. This review article aims to provide a comprehensive overview of novel compounds that are currently under investigation in both preclinical and clinical settings for the treatment of ED.
3. Existing treatments
3.1 Selective phosphodiesterase 5 inhibitors
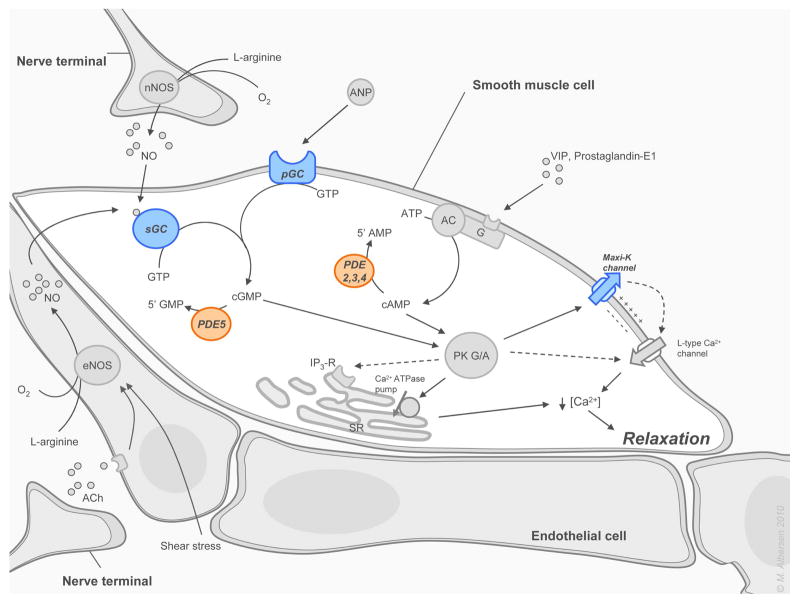
PDE5I are non-hydrolysable analogs of 3′,5′-cyclic-guanosine monophosphate (cGMP) and exert their beneficial effects on smooth muscle relaxation by competitively binding to the catalytic site of PDE5, the enzyme responsible for breakdown of cGMP to 5′-guanosine monophosphate (GMP). By slowing the degradation of cGMP by PDE5, these drugs produce an intracellular accumulation of cGMP in smooth muscle cells in the corpus cavernosum and in the walls of the supplying arteries (figure 1). This results in relaxation of the smooth muscle, increased arterial blood flow, and penile tumescence.[11]
Figure 1. Molecular mechanisms of penile smooth muscle relaxation.
Pathways involved in the relaxation of the cavernous smooth muscle cells are explained in detail in section 3.1 on the physiology of penile erection. Abbreviations are found in the same section and in the abbreviation index. Tartgets for emerging stimulatory acting drugs are depicted in italic and blue. Targets for emerging inhibitory acting drugs are depicted in italic and red.
Guidelines on the treatment of ED generally recommend PDE5I as the initial and reference treatment for ED.[12,13] These recommendations are based on numerous trials which establish both the efficacy and the safety profile of the PDE5I currently available in the United States: sildenafil, tadalafil and vardenafil. [14,15] Overall efficacy rates are 60–70% with on-demand treatment regimens.[10] Of the patients that initially do not respond to PDE5I, between 30–50% may be converted to responders by counseling the patient and his partner. Some patients who fail to achieve an erection when taking PDE5I on-demand, benefit from a daily dosing regimen.[16,17] Furthermore, in the male suffering from ED in the context of late onset hypogonadism, addition of Testosterone suppletion might enhance PDE5I-therapy.[18]
As the efficacy of PDE5I depends on the integrity of the NO pathway in producing cGMP, it is evident that patients in whom this pathway is disturbed or defective will benefit far less than the general population from PDE5I. Disease states that diminish NO availability include denervation of the erectile tissue following radical prostatectomy; severe diabetes with neuropathy and endothelial dysfunction; metabolic syndrome; and downregulation of nitric oxide synthase (NOS) expression as may be seen in atherosclerosis, ageing and hypogonadism.[19]
The safety profile of the currently available PDE5I is excellent, based on post-marketing data and further demonstrated by the recent FDA approvals for daily use of PDE5I. Sildenafil was approved in daily high dosage for the treatment of pulmonary hypertension, and tadalafil at low-dose (up to 5 mg daily) for ED.[2,20] In post-marketing pharmacological surveillance, no increase in myocardial infarction rates in patients who received these agents has occurred compared to expected rates in age-matched populations.[14,20] In spite of these data, there are certain heart-related precautions in the use of PDE5I. PDE5I are relatively contraindicated in patients with unstable angina pectoris, recent myocardial infarction, certain arrhythmias, and poorly controlled hypertension. Furthermore, patients who are treated with nitrates or nitrate-donors should not take PDE5I, and use of PDE5I with α-blockers may result in postural hypotension.[5] The most common adverse events from PDE5I are attributable to specific inhibition of PDE5 and vasodilatation in tissues other than the penis, and include headache, facial and ocular hyperemia, nasal congestion, myalgia, and back pain. Adverse events account for about 25% of cases in which PDE5I are discontinued, with the most common reason for discontinuation of PDE5I being lack of efficacy.[15] There have been rare reports of serious adverse events such as seizures, nonarteritic ischemic optic neuritis and acute hearing loss. While the role of PDE5I in causing these disorders remains a controversial issue, the majority of reports on the topic have been rather anecdotal.[15,20,21] Other adverse events can be attributed to cross-reactivity with other PDE-isoforms. Vision disturbances, which are believed to result from cross-reactivity of PDE5I against PDE6 (an isoform of PDE that is abundantly present in the cones of the retina) have been reported with PDE5I use. Tadalafil has been shown to cross-react with PDE11 to some extent, although no consequences of this cross-reactivity are currently known. None of the available PDE5I has shown clinically significant cross-reactivity with PDE isoforms other than PDE6.[17]
3.2 Apomorphine SL
Apomorphine is a centrally acting non-selective dopamine agonist with more potent D2-like effects, which acts by binding to dopaminergic receptors of hypothalamic neurons. Apomorphine has lower efficacy and satisfaction rates compared to PDE5I, and is most effective in patients with mild to moderate ED.[13] It is rapidly absorbed due to the sublingual route of administration and erections are achieved in 20 minutes in more than two-thirds of the patients.[5] The most common adverse events are due to nonspecific binding to other subtypes of the dopamine receptor and include nausea, headache and dizziness. Apomorphine is not contraindicated in patients taking nitrates and may therefore represent first-line oral treatment in this class of patients.[13,22,23] Currently, intranasal administration of apomorphine (VR-004) is under investigation.[24] In a phase II trial, it was safe and well-tolerated without serious treatment-related adverse events. The most common adverse event was headache. The development of the drug for the indication of male sexual dysfunction has been suspended however until a development partner is found.[25]
3.3 Yohimbine
Yohimbine is a natural peripherally and centrally acting alpha-blocker. There is little evidence on its efficacy in the treatment of ED and it is therefore not currently recommended in most guidelines on the management of ED.[13]
3.4 Intracavernous and intra-urethral administration of vasoactive substances
Penile injection of vasoactive substances has been utilized since the 1980s as a treatment for ED and provides a good safety profile, and a rapid of onset of action. The most commonly utilized substance, and currently the only one with US FDA approval as a treatment of ED, is prostaglandin E1 (PGE1). PGE1 binds to specific G-protein-coupled receptors (GPCR) in the membrane of smooth muscle cells, and activates adenylyl cyclase (AC). AC facilitates cleavage of ATP to cAMP, a second messenger with downstream effects analogous to cGMP in the establishment of smooth muscle relaxation. This effect is independent of the NO pathway and is therefore particularly useful as a treatment for ED after radical prostatectomy. Overall satisfaction rates with intracavernous PGE1 therapy are almost 80%.[26] Other drugs which are commonly used for intracavernous injection therapy are phentolamine and papaverine. Vasoactive Intestinal Peptide (VIP) is available for intracavernous injection therapy in some countries although its efficacy is low and it is typically only used as a component in combination therapy. These substances can be injected alone or in combination (so called bimix or trimix). PGE1 is also available for intraurethral administration (medicated urethral system for erection, MUSE).
The major disadvantages of injection therapy include the risk of priapism, variable degrees of pain with injection in 50% of patients, and the risk of penile fibrosis after long-term use. Patients using injection therapy should undergo a thorough training program prior to initiation of therapy. MUSE may have some side-effects in common with intracavernous injection of PGE1, and may also be associated with hypotension, syncope, urethral burning or pain in the patient, and vaginal irritation in the partner.[5] While these therapies provide a valid alternative for patients who do not respond to oral therapy, the self-injection or urethral administration is a major drawback for many patients compared to oral therapy.
4. Scientific rationale: physiology of penile erection
Upon sexual stimulation, the hypothalamus is exposed to input of various substances, of which dopamine appears to be the primary erectogenic neurotransmitter and serotonin the principle erection inhibiting neurotransmitter.[27] The specific dopamine receptor subtype responsible for erectogenic effects is unclear. Although it was formerly believed that the effects were mediated in large part by D2 receptors, recent evidence points toward the D4 receptor subtype as a mediator for the pro-erectogenic effects of dopamine.[28] Dopamine-containing nerve endings impinge on oxytocinergic cell bodies in the paraventricular nucleus which project, in turn, to extra-hypothalamic brain areas such as the hippocampus, the ventral medulla and the spinal cord. Oxytocin has been localized in pathways descending from the hypothalamus to the brain stem and spinal sacral autonomic centers controlling the erectile tissues of the penis.[29]
In addition to central pathways, an erectogenic stimulus may originate in the sacral spinal erection center in response to direct tactile stimulation of the penis, exciting sensory neurons which synapse in the sacral erection center located in the S2–S4 region. Exiting through the sacral neural foramina, efferent neurons from the sacral erection center pass anterior and lateral to the rectum as the nervi erigentes to reach the pelvic plexus. In this location, preganglionic fibers relay in ganglia, and postganglionic non-adrenergic, non-cholinergic (NANC) fibers pass in the cavernous nerves to the corpora cavernosa. These nerves run alongside the posterolateral side of the prostatic capsule and perforate the urogenital diaphragm to enter the cavernous bodies at the level of the crura.[30] This anatomic localization makes these nerves particularly susceptible to damage during radical pelvic surgery.[31]
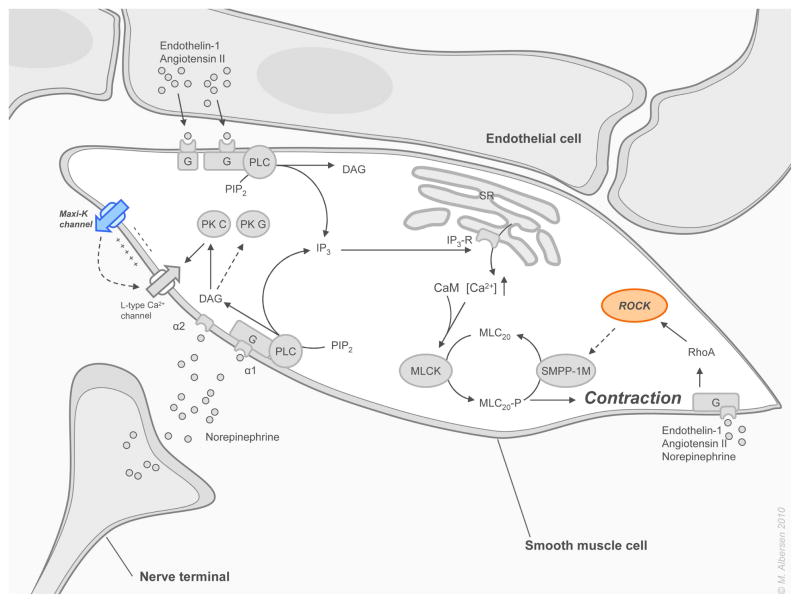
NO is released from NANC nerve terminals in the corpus cavernosum in response to a neural stimulus and also from the endothelium in response to 1) the release of acetylcholine (Ach) by parasympathetic endothelial nerve endings, and 2) by the shear stress elicited by increased blood flow in the corporeal sinusoids. NO is synthesized in the nerve terminals and in the endothelium by action of the tissue specific enzyme nitric oxide synthase (NOS), which catalyzes the production of NO and citrulline from oxygen and L-arginine. NO passively diffuses into cavernous smooth muscle cells where it binds to the heme moiety of soluble guanylate cyclase (sGC) and thereby activates this enzyme, which catalyses the breakdown of guanosine triphosphate (GTP) into cGMP. In turn, cGMP incites a cascade of protein-kinase G (PKG) mediated intracellular events. Although being the most important pathway leading to smooth muscle relaxation, it is supported by other pathways with cAMP as a second messenger, which in turn activates protein-kinase A (PKA). PKG and PKA activate a series of cellular events via phosphorylation of various targets in the smooth muscle cell. These events lead to inhibition of the noradrenergic pathway; opening of Maxi-K channels; and activation of the Ca2+ ATP-ase transporter in the membrane of the sarcoplasmic reticulum (SR). The principle end result of these processes appears to be a reduction in intracellular calcium content. (figure 1). When the cytoplasmic calcium concentration falls below 500 nmol, calcium dissociates from calmodulin (CaM), which in turn dissociates from myosin light chain kinase (MLCK), thus inactivating it. With its kinase inactivated and its phosphate moiety removed by smooth muscle myosin phosphatase (SMPP-1M), the myosin light-chain (MLC) becomes inactive and can no longer bind the myosin head to actin. This leads to relaxation of the cavernous smooth muscle cell (figure 2). With relaxation of the smooth muscle, vasodilation and enhancement of blood flow into the cavernosal sinusoids occurs, leading to penile erection. Blood becomes trapped in the corporal bodies by compression of subtunical venules against the tunica albuginea (full erection phase) and contraction of the voluntary ischiocavernosus muscle (rigid erection phase). [30–32]
Figure 2. Molecular mechanisms of penile smooth muscle contraction.
Pathways involved in the maintenance and establishment of contraction of the cavernous smooth muscle cells are explained in detail in section 3.1 on the physiology of penile erection. Abbreviations are found in the same section and in the abbreviation index. Tartgets for emerging stimulatory acting drugs are depicted in italic and blue. Targets for emerging inhibitory acting drugs are depicted in italic and red.
These pro-erectogenic parasympathetic mechanisms are counterbalanced by sympathetic input from nerve fibers that also run in the cavernous nerves. The sympathetic nervous system maintains the penis in a flaccid state by tonic release of norepinephrine from the nerve terminals which stimulates the α1-adrenergic GPCR on the cavernosal smooth muscle cells. This activates a cascade of events initiated by phospholipase C (PLC), which splits phosphatidylinositol bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 activates its receptor (IP3-R) on the SR, inducing release of calcium from internal stores to the cytoplasm, while DAG activates protein-kinase C (PKC), thereby promoting conduction of Ca2+ into the cell by L-type calcium channels in the cell membrane. Ca2+ binds to CaM, and this complex activates MLCK. This results in contraction of the smooth muscle cell and maintenance of penile flaccidity (figure 2). Supporting pathways in the maintenance of cavernosal flaccidity are the inhibition of PKG by DAG, and the activation of rho-kinase (ROCK) by the ras homolog gene family member A (RhoA) which affects the smooth muscle tone by altering calcium-sensitivity. Inhibitory phosphorylation of SMPP-1M by ROCK leads to sensitization of myofilaments to intracellular basal calcium and thus to contraction (vide infra).[30,32,33]
5. Current research goals
5.2.1 Central regulation of erection
Recently, increased attention has been given to the central regulation of the erectile state of the penis. Dopamine and melanocortin-receptors have been identified as important mediators in the generation of pro-erectile impulses originating in the CNS.[27,28,34] For both receptors, various subtypes have been identified and research has focused on means to produce pro-erectile effects without instigating adverse events by using agents with subtype selective activities at receptors in the CNS.
For centrally acting compounds to be able to enhance erectile function, an intact neuronal transmission is required, as well as functioning erectile tissue in the penis. Hence, drugs that act centrally to enhance pro-erectile impulses may be of lesser value in patients with severe organic ED and tissue defects in the cavernous arteries and corporal bodies. It has been established that apomorphine is most effective in mild-to-moderate organic ED and psychogenic ED, and is less effective in patients suffering from severe organic ED.[10,24] However, preclinical studies have shown synergistic effects when centrally acting drugs are combined with drugs acting peripherally in the corpus cavernosum. Therefore, central agents may be useful for potentiation of peripherally acting drugs as combination therapy for severe ED, with monotherapy using central agents a valid option in mild and/or psychogenic ED.[35] Many centrally acting compounds may have beneficial effects on both male and female sexual dysfunction.[36]
5.2.2 Stimulation of smooth muscle relaxation
A complex interplay between the mechanisms described above (4) determines the erectile state of the penis. Complex cross-talk mechanisms within the autonomic nervous system pathways ensure silencing of the sympathetic pathway when pro-erectogenic stimuli activate the parasympathetic pathway, and vice versa.[32] Modulation of pathways leading to smooth muscle relaxation and inhibition of the pathways maintaining smooth muscle contraction have been the focus of intense research in the past two decades. Methods for direct activation of the cGMP and cAMP pathways have been studied as a means to bypass the need for endogenous NO production. This research was based on the finding that certain diseases abolish endogenous NO production (vide supra). While effective supplementation of exogenous NO has proven difficult, various compounds directly targeting and activating downstream effectors in the NO-cGMP cascade are promising therapeutic options.[2] Various substances that do not depend on NO secretion for modulation of endothelial function have been developed for the treatment of hypertension and other cardiovascular diseases. These compounds are now under investigation for their potential as treatments of ED; examples include ROCK-inhibitors and direct activators of sGC. While some of these compounds are in phase II and phase III trials for cardiovascular diseases, research focusing on utilization of these drugs for ED is more preliminary at the current time.
5.2.3 Inhibition of smooth muscle contraction
While a vast body of ED research focuses on the potentiation of smooth muscle relaxation, certain disease states such as diabetes and atherosclerosis are known to amplify the adrenergic pathway leading to smooth muscle contraction, in large part by activation of RhoA and ROCK. A variety of compounds designed to inhibit this enzyme have been developed; these ROCK-inhibitors have shown promise as potential treatments for ED.[37–41] Furthermore, research efforts have focused on directly influencing the polarization state of the smooth muscle cells by targeting ion channels in the cell membrane. Inducing hyperpolarization and relaxation of the smooth muscle cell by activating certain ion channels (such as calcium-regulated “Maxi-K” potassium channels) has been a focus of intense research with promising results in both pharmacotherapy and gene-therapy.[42–44]
6. Competitive environment
6.1 Centrally acting compounds
6.1.1 Clavulanic acid (Zoraxel™)
Zoraxel (RX-10100) is a tablet containing clavulanic acid that is being developed as an orally administered treatment for ED. Clavulanic acid has been shown to inhibit glutamate carboxypeptidase II in the brain, thereby reducing the release of glutamate, and putatively modulating serotonin and dopamine release. Clavulanic acid has a stimulatory effect on three aspects of male sexuality: libido, erection and ejaculation. It might therefore hold promise for hypoactive sexual desire disorder and for the treatment of certain symptoms of hypogonadism. Indeed, clavulanic acid was shown to stimulate mounting behavior and decrease ejaculatory latency time in rats.[45] A 6 month US double-blind, placebo-controlled, dose-ranging Phase IIa trial in 40 male patients with ED has been completed. Preliminary results showed a dose-dependent effect on the IIEF baseline score. RX-10100 demonstrated improved erectile function and significant improvement in the quality of life measures. It was safe and well tolerated with no serious adverse events. A Phase IIb trial is planned.[25]
6.1.2 Dopamine receptor agonists (ABT-724 and ABT-670)
ABT-742 is a novel compound that is currently under preclinical investigation for its potential to selectively inhibit the D4 receptor. In a rodent study, the drug was administered subcutaneously and intracavernous pressure (ICP) changes during cavernous nerve electrostimulation were measured. Mean peak ICPs of 139.6, 134.1 and 92 cmH2O were observed after treatment with ABT-742 at doses of 0.0025 μmol/kg, 0.025 μmol/kg, and 0 μmol/kg (control) respectively. There was no direct effect on cavernous smooth muscle contractility in-vitro. The compound facilitated erectile responses when injected into the cerebral ventricles, but not when injected intrathecally at the spinal level, suggesting that ABT-724’s pro-erectile effects are mediated by supraspinal dopaminergic mechanisms.[28] The selectivity of this compound for the D4 receptor was established in-vitro and in vivo in a conscious ferret model; ferrets treated with the compound did not develop stereotypical behaviors that correlate with the sensation of nausea after administration of doses several-fold higher than the effective dose. The effects of ABT-724 in combination with the PDE5I sildenafil have been investigated in rats. In the presence of sildenafil, a 10-fold increase in erectile events was observed. These data suggest that the combination of a centrally acting and a peripherally acting agent might be beneficial for the treatment of patients suffering from ED.[28] ABT-670 is a more recent development and is a D4-selective agonist with superior oral bioavailability compared to ABT-724. Limited in-vivo assays show promising efficacy for this compound.[46] Company information states that ABT-724 is currently enrolled in phase II, and ABT-670 is investigated in phase I trials. However, information on the results of these studies has not been published.[25]
6.1.3 Melanocortin receptor agonists (Melanotan II and Bremelanotide)
Melanotan II (MT2) was derived from Melanotan I, which was originally developed as a skin melanin-pigmentation enhancing therapeutic for use in humans. The beneficial effects of MT2 on sexual function in both male and female subjects were discovered by accident. In a phase 1 clinical trial to determine the efficacy of MT2 in enhancing skin pigmentation in human males, it was noted that the peptide invariably induced an erection in nearly all male participants.[34]
Bremelanotide (PT-141) is a metabolite of MT2 which was developed later in response to the discovery that MT2 was able to induce erections in men. Bremelanotide appears to act on melanocortin receptor- 3 (MCR-3) and MCR-4 receptors in the CNS, where it enhances sexual arousal and penile erections through the release of dopamine.[47] The central actions of this drug also makes it suitable for the treatment of certain female sexual dysfuntions.[36] The CNS site of action of these compounds was demonstrated in preclinical experimental models in which MT2 did not relax rabbit corpus cavernosum strips, and direct intracavernosal injection of MT2 failed to alter ICP in rats.[48,49] It has been shown in a double-blind, placebo-controlled study that the subcutaneous administration of MT2 to men with organic and psychogenic ED induced penile erections in the absence of visual or tactile sexual stimulation. [50]
From a clinical perspective, the potential utility of MT2 is limited by its route of administration (subcutaneous) and onset of action (>90 min).[51] Bremelanotide is available as an atomizer for intranasal administration, and response to the peptide is rather immediate, initiating a penile response in a matter of minutes. Both phase I and phase II trials have been completed for bremelanotide. In a 12 week phase IIb trial recruiting 726 non-diabetic men with ED, IIEF EF domain scores where significantly higher in subjects that received bremelanotide 5 mg relative to those that received placebo.[52] Bremelanotide also has efficacy in producing erections in men with diabetes and those who do not respond to sildenafil.[27,53]
When used for therapy in ED, both MT2 and bremelanotide are unlikely to enhance skin pigmentation or cause satiety under normal use as these effects typically require prolonged dosing.[34] The most common adverse events in phase I and phase II trials were nausea, flushing, and a stretch-and-yawn response. However, the US FDA had significant concerns regarding blood pressure increases that were seen in phase I and II trials; plans for a phase III trial have been delayed because of these concerns.[25]
6.2 Peripherally acting compounds
6.2.1 Compounds stimulating smooth muscle relaxation
6.2.1.1 Novel PDE5I
The development of compounds that are superselective and highly potent inhibitors of PDE5 continues. Over 400 substances are currently under investigation for their inhibitory capacity of PDE5.[54] For comprehensiveness of this review, we choose to discuss only those compounds that have at least completed phase II testing.
6.2.1.1.1 Udenafil
Udenafil has been launched under the name Zydena™ in South Korea and Russia but is not yet available in Western countries. An ongoing US Phase III trial in 1,120 ED patients is underway. A single multicenter double blind randomized placebo-controlled phase III trial confirmed that udenafil is effective in a population of Korean men with ED of varying etiology and severity. There was a mean increase of 7.52 and 9.93 in IIEF erectile function (EF) domain score in the group receiving 100 and 200 mg of the compound, respectively, versus a 0.2 point increase in the placebo group.[55] Udenafil’s pharmacokinetic profile includes a Tmax of 0.8–1.3 hr. and a T½ of 7.3–12.1 hr, which would confer unique clinical properties of both relatively rapid onset and long duration of action.[25,55] Udenafil is claimed to have a low cross-reactivity with the other PDE isoforms. The most common adverse events reported are facial flushing, nasal congestion, ocular hyperemia and headache. Only 2 out of 110 subjects in the udenafil group withdrew from the study because of adverse events.[25,55]
6.2.1.1.2 Mirodenafil
Mirodenafil is a novel PDE5I with low cross-reactivity against other PDE isoforms that has been tested in South Korea. A Phase III multicenter, randomized, double-blind study was conducted with 223 subjects who were randomized to placebo or mirodenafil at fixed doses of 50 or 100 mg for 12 weeks on an “on-demand” basis. Only half of the subjects were actually naïve to PDE5I. This study was performed in a population of Korean men with ED of varied spectrum etiology and severity, including psychogenic ED. There was a mean 7.6 and 11.6 point increase in IIEF erectile function domain score in the group receiving 50 and 100 mg. of the compound, respectively, versus a 3.4 point increase in the placebo group.[56] Adverse events consisted of facial flushing, headache, nausea, and ocular hyperemia. Most adverse events were mild, and resolved spontaneously by the end of treatment. Visual disturbances did not occur. Four subjects terminated the study because of adverse events.[56] With a Tmax of 1.5 hr. and a T½ of 2.4 hr. it has no clear pharmacokinetic advantage over the currently available PDE5I but the short-half life may be useful for subjects who experience bothersome side effects.[57]
6.2.1.1.3 Lodenfil Carbonate
Lodenfil Carbonate is a PDE5I currently under development in Brazil and is a dimer consisting of two Lodenafil molecules attached to a carbonate bridge. After ingestion the bridge is broken resulting in the delivery of two molecules of the active compound. Phase II and III trials have been recently completed. The recently conducted phase III-trial was performed in Brazil in 350 men and showed good efficacy of the drugs with a mean increase in the IIEF EF domain score of 0.9, 5.0, and 7.2 in subjects receiving placebo, 40 mg. and 80 mg. in an on-demand fashion for 4 weeks. Tmax was 1.2 hr. and T½ was 2.36 hr. Adverse effects were relatively common, occurring in 28.7% of those treated with placebo, 40.9% of those treated with Lodenfil Carbonate 40 mg, and 49.5% of those treated with Lodenfil Carbonate 80 mg. The incidence of headache, flushing, visual disorders and dizziness was significantly higher in the Lodenafil Carbonate group than the placebo group. [58,59]
6.2.1.1.4 Avanafil
Avanafil (TA-1790) is a fast-acting, selective PDE5I inhibitor currently under development. In preclinical studies, co-administration of avanafil and nitrates caused less of a decrease in blood pressure than sildenafil and nitrates. In vitro, avanafil showed greater inhibition of PDE5 than sildenafil. In a US randomized, double-blind, placebo-controlled Phase III trial (REVIVE) in 646 men with ED, avanafil 50, 100 and 200mg demonstrated a rapid onset of full effect at 30 min. post-administration, with no restrictions on food or alcohol consumption. Subjective efficacy was reported by 29%, 67%, 69% and 72% of subjects within 15 min. of treatment with placebo, avanafil 50, 100 and 200 mg, respectively. The most commonly reported adverse events included headache, flushing and nasal congestion. The Tmax was 35 min. and the T½ was 60–90 min, making it not only a fast acting, but also a rapidly metabolized compound. Phase II trials in South Korea are completed. Phase III trials in patients with diabetes-induced ED and ED following radical prostatectomy are ongoing.[25]
6.2.1.1.5 SLx-2101
SLx-2101 is converted after ingestion to its metabolite, SLx-2081, which continues to be active. It is currently under development in the US for the treatment of ED, endothelial dysfunction and congestive heart failure. It is also under investigation (phase IIb) as a treatment for hypertension and might therefore be a good choice for the treatment of ED in hypertensive patients. A Phase IIa trial to assess the safety, tolerability and efficacy of different single doses in ED patients known to respond to PDE5I was completed. Interim results confirmed activity 36–48 hr. after a single oral dose of SLx-2101 10mg. The drug had good safety, tolerability, pharmacokinetic and pharmacodynamic profiles.[11,25] In a double-blind, randomized Phase I trial in 40 healthy male volunteers, six subjects each received SLx-2101 at 5, 10, 20, 40 or 80 mg and ten subjects received placebo. There was clinically meaningful erectile activity as documented by RigiScan plethysmography in these healthy adult males with or without visual sexual stimulation following single oral doses of SLx-2101 across the dose range of this study. In addition, there was evidence of improvement in endothelial function, based on RH-PAT index assessments, following single oral doses of SLx-2101 across the dose range at 9.5 hours and 24 hours post-dose compared with baseline. SLx-2101 was safe and generally well tolerated at all doses in this study. Headache was the most frequently reported adverse event following administration of SLx-2101 or placebo. No back pain, myalgia nor painful, prolonged erections were reported.[11,25]
6.2.1.1.6 Herbal phosphodiesterase inhibitors (Icariin)
Numerous herbal substances are under investigation for their proposed PDE-inhibitory properties.[60] Icariin, a flavonol glycoside and major constituent of the plant commonly known as “horny goat weed”, is one such compound. In China, horny goat weed, or Epimedii herba has been used for centuries to enhance sexual drive and performance. Recently, it was discovered that this enhancement is due to an inhibitory effect of both PDE4 and PDE5 by icariin.[61,62] The substance has been shown to enhance erectile function in an in-vivo rat model.[63] In a recent study by our group, icariin was able to improve erectile function in-vivo, prevent fibrotic alterations in the corpus cavernosum and was shown to enhance neuroregeneration after cavernous nerve injury in rats when administered daily by oral gavage.[64] In another study, the beneficial effects of icariin were potentiated when it was given as part of a herbal combination preparation, Etana, which also contained Epimedium grandiflorum, a substance known to promote the release of NO in the erectile tissue.[65]
6.2.1.2 sGC activators (YC-1, Bay-2272, A-350619)
Since PDE5I are NO-dependent, they have shown unsatisfactory results in various subpopulations of patients who lack endogenous NO supply. Classical NO donors (i.e. organic nitrates) have shown poor efficacy in treating ED and carry the risk of side effects secondary to nonspecific interaction of NO with other biological molecules.[19] Treatment pathways that do not require endogenous NO production are therefore being explored as potential treatment targets for ED. One class of molecules under consideration are activators of sGC.[66] The first molecule of this class that was studied for the treatment of ED is YC-1, a compound originally identified as an inhibitor of platelet aggregation.[67] In in-vivo experiments in rats, peak ICP after electrical stimulation of the cavernous nerves was significantly higher in animals treated with YC-1 than in animals treated with vehicle.[68] Administration of NOS-inhibitor L-NAME did not impair the erectile response after treatment with YC-1, indicating that the mechanism of action this drug is not NO-dependent.[68,69] However, the compound has been shown to enhance the sensitivity of sGC to NO. In corpus cavernosum strips, relaxation of smooth muscle has been shown to be mediated by an increase in cGMP, stressing the role of sGC activation by YC-1.[69,70]
YC-1 also inhibits PDE and stimulates the synthesis of NO at therapeutic concentrations.[19] This property has been identified in other compounds such as NCX-911 (vide infra).[71] Two newer compounds (BAY41-2272 and A-350619) have been shown to be more potent activators of sGC than YC-1. These compounds are heme-dependent sGC-stimulators as they share a crucial dependency on the presence of the reduced prosthetic heme moiety in the sGC enzyme and strong synergistic enzyme activation when administred with NO. Both compounds have been investigated in erectile tissue, and have been shown to relax rabbit cavernosal tissue strips subjected to phenylephrine induced contraction.[19,72–74] Furthermore, both compounds have been shown to produce penile erection in animal models. Synergistic effects of sGC activators and organic nitrate donors were observed, suggesting enhanced activity during sexual arousal.[19,73] Further studies have shown that BAY41-2272 enhances nitrergic relaxation responses in the anococcygeus muscle of streptozotocin diabetic rats, a property not observed after treatment with sildenafil.[75] These suggests that sGC activation may be more efficacious than PDE5I in the treatment of diabetes-induced ED and in ED related to cavernous nerve injury during radical pelvic surgery. The newer sGC activators are currently in phase I and phase II for other indications. To our knowledge their efficacy and safety profiles have not yet been evaluated in men with ED.
Another group of compounds in this class is the heme-independent sGC activators. The activation of sGC by these compounds was even stronger after oxidation or removal of the prosthetic heme group of sGC, indicating a previously unknown mechanism of enzyme activation. This class of drugs has shown great potential since these drugs target a modified, oxidized state of sGC that is present in both animal models and in human disease states.[76] While holding great promise for the treatment of ED, these compounds have yet to be evaluated for this indication.[74]
6.2.1.3 pGC activators (atrial natriuretic peptide and uroguanylin)
Another pathway that is potentially useful as a pharmacological target for the treatment of ED is the particulate guanylate cyclase (pGC).[32,77] This membrane-bound enzyme is the target for endogenous natriuretic peptides such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide, and guanylin peptides such as uroguanylin (UGN). Three subtypes (A, B and C) have been identified with different affinities for the various peptides. Both ANP and UGN have been studied in vitro for their human corpus-cavernosum (HCC) relaxant capacities. Both were able to enhance relaxation of HCC strips versus vehicle in organ-bath experiments. This relaxant capacity of UGN (this experiment was not conducted with ANP) was mediated in large part by a rise in intracellular cGMP and was not influenced by the addition of L-NAME, a NOS inhibitor, nor by ODQ, a sGC specific inhibitor.[78] These results may hold promise for the future treatment of ED in patients who lack endogenous NO supply.
6.2.1.4 Nitric oxide-releasing PDE5I (NCX-911 – Sildenafil Nitrate)
NCX-911 is a recently developed compound that has been shown to possess three erection-potentiating capacities. NCX-911 and sildenafil citrate have a similar selectivity and potency with respect to inhibiting PDE5. However unlike sildenafil citrate, NCX-911 has been shown to release NO spontaneously, and by directly activating sGC and inhibiting PDE5 it causes an increase in the intracellular cGMP concentration without the need for endogenous NO supply.[71,79] NCX-911 has a more potent relaxant effect on precontracted HCC strips relative to sildenafil citrate. [80,81] In in-vivo studies in anesthetized rats, NCX-911 had more potent proerectile effects than sildenafil citrate when the cavernous nerve was stimulated submaximally, resembling a disease state with suboptimal neural input.[79] NCX-911 was similar to BAY 41-2722, but was more potent than sildenafil, in establishing relaxation of anococcygeus muscle strips of streptozocin-induced diabetic rats in vitro.[75] Furthermore, the effectiveness of this compound in the cavernous tissue of hypercholesterolemic rats has been proved in-vitro.[79] These results combined suggest that NCX-911, like other NO-independent compounds, is a potential candidate for treatment of conventionally difficult-to-treat ED in patients with comorbidities like diabetes, hyperlipidemia, and ED patients after radical prostatectomy.
6.2.2 Compounds inhibiting smooth muscle contraction
6.2.2.1 Rho-kinase inhibitors (Y-27632, Fasudil, SAR-407889)
A class of molecules that inhibit ROCK has become a focus of research in the treatment of ED.[39–41,82] The RhoA/ROCK pathway plays an important role in maintaining the flaccid state of the penis. Binding of norepinephrine, angiotensin II or endothelin-1 activates GPCRs, resulting in release of a dissociation inhibitor of RhoA, which then in turn activates ROCK. ROCK phosphorylates and inactivates SMPP-1M, allowing MLC to stay phosphorylated and consequently actin-contracted. Inhibition of ROCK thus provides a mechanism of smooth muscle relaxation that is independent of NO (figure 2).[32] In diabetes, the RhoA/ROCK pathway is hyper-activated, and suppresses eNOS activity.[37,83] Therefore, it has been speculated that the development of ED in diabetes is at least partially mediated by ROCK. In atherosclerotic male rats, elevated cavernosal ROCK activity, decreased total expression of eNOS, and impaired erectile function were observed.[84]
Inhibition of ROCK by a selective ROCK-inhibitor, Y-27632, was shown to induce relaxation of rat corpus cavernosum strips in-vitro, and caused an increase in ICP in an in vivo rat model. The inhibition of ROCK elicited an erectile response by a process that was not mediated by NO or cGMP.[82] Fasudil (HA-1077), an orally available ROCK-inhibitor, was used to investigate the role of chronic ROCK-inhibition in the prevention of both pelvic atherosclerosis and resulting vasculogenic ED in rats, with promising results. The treatment with fasudil partly but significantly ameliorated the development of pelvic atherosclerosis and plasma level of von Willebrand factor, in addition to normalizing erectile function, cavernosal Rho kinase activity, and total eNOS expression.[84] Both Y-27632 and Fasudil have been investigated in-vitro in the corpus cavernosum of diabetic mice. Diabetic mouse corpus cavernosum exhibited relaxation similar to what was observed in tissue from non-diabetic mice in response to the Rho-kinase inhibitors.[85] Therefore, the use of these agents may provide a therapeutic benefit in diabetic erectile dysfunction.
While Y-27632 is still in a preclinical phase, Fasudil has been launched for other indications. SAR-407899 is another ROCK-inhibitor under development that has been shown to be 8-fold more active than fasudil in the treatment of hypertension.[86] It was studied for the treatment of ED in a randomized, double-blind Phase II trial in 20 patients with erectile dysfunction. The results of this phase II trial have, to our knowledge, not been published at the time of writing of this review.
6.2.2.2 Maxi-K channel openers (NS1619, BMS-223131)
Most drugs currently under investigation for the treatment of ED target second messenger pathways that modulate intracellular calcium concentration leading to smooth muscle cell relaxation or contraction. Calcium content may also be modulated by interference in the trans-membrane conductance of other ions. An ion-channel that has drawn special attention in achieving smooth muscle cell hyperpolarization is the maxi-K channel, also known as BK channel, which conducts potassium ions through the cell membrane.[87] These channels are activated (opened) by changes in membrane electrical potential and/or by increases in concentrations of intracellular Ca2+. Opening of maxi-K channels results in cell membrane hyperpolarization and a decrease in cell excitability.[44,87,88] Confirming the role of Maxi-K channels in the establishment of an erection, knock-out mice lacking this channel are unable to get an erection. Gene therapy with the maxi-K gene has been shown to restore erectile function in atherosclerotic monkeys.[43,89] NS1619 is a putative selective Maxi-K channel opener. Its effectiveness has been confirmed in in-vivo experiments in rats where the compound was able to produce a physiologically relevant increase, compared to untreated controls, in the ICP upon submaximal current stimulation of the cavernous nerve.[87] Furthermore, 3-Thio-quinolinones are under investigation and have shown relaxation of corporal tissue strips in vitro.[90] BMS-223131, an orally available maxi-K channel opener is being evaluated in phase 2a study, although the results have not yet been reported.[87]
7. Conclusions
The worldwide market value of ED was $3.2 billion in 2006, with the US market being responsible for two-thirds of global sales. Given the ageing of the general population and direct-to-consumer advertising projecting the desire of men of all ages to maintain an active sexual lifestyle, a market growth rate of 4.9% is expected for the period 2006–2012.[91,92] Due to increasing demand and prospective market growth, pharmaceutical companies are competing to develop more potent and better tolerated drugs. In this article we reviewed the current ED market and important or promising targets for pharmacological therapy for ED. A wide variety of molecular targets have been identified and special attention has been given to compounds that bypass the need for endogenous NO production, one of the principle limitations of currently available oral ED medications.
8. Expert opinion
By influencing targets in different pathways leading to the development of penile erection, the various compounds that are currently in development will undoubtedly cause a major change in treatment strategies of ED. Currently available ED guidelines recommend treatment algorithms that are virtually identical for every patient, regardless of the underlying pathology causing the ED. We expect future strategies to allow treatment protocols tailored to the specific needs of each individual patient, taking into consideration the efficacy of erectile performance enhancement and the potential for adverse events. This tailored approach may include combination of various emerging drugs to enhance efficacy in difficult-to-treat patients. Individual tailoring of treatment to the specific needs of the patient does require an extensive knowledge of the etiology of ED. Careful characterization and study of these novel compounds by the physician and pharmacologist treating the increasing population of patients suffering from ED will be essential. We hope this review helps not only the sexual medicine specialist or urologist, but also the general practicioner and pharmacologist in the understanding of emerging drugs for ED.
Table 1.
Compounds currently in clinical development phases.
| Compound | Company | Stage of development | Mechanism of action | |
|---|---|---|---|---|
| RX 10100; Zoraxel; clavulanic acid | Rexahn Pharmaceuticals | Phase IIa | Enhancement of Dopamine release in CNS | |
| PT-141; Bremelanotide | Palatin Technologies | Phase IIb Phase III delayed |
Melanocortin-receptor agonist | |
| Udenafil; Zydena | Dong-A pharmaceuticals, Warner Chilcott (US) | Phase III (US), launched (S-Korea) | PDE5I | t½: 12 hr Tmax: 1–1.3 hr |
| SK3530; Mirodenafil | SK chemical | Launched (S-korea) | PDE5I | t½: 2.4 hr Tmax: 1.5 hr |
| Lodenafil Carbonate | Cristalia productos Quimicos Pharmaceuticos |
Phase III | PDE5I | t½: 2.36 hr Tmax: 1.2 hr |
| Avanafil | Vivus (US), Mitsubitshi Tanabe Pharma |
Phase IIa | PDE5I | t½: 1.25 hr Tmax: 0.58 hr |
| SLX-2101 | Surface Logix | Phase IIa | PDE5I | t½: 9–14 hr Tmax:1 hr |
| Icariin | Available in herbal preparations | PDE4I, PDE5I | ||
| HA-1077; Fasudil | Asahi Kasei Pharma | Launched for other indications. | ROCK inhibition | |
| SAR-407889 | Sanofi-Aventis | Phase IIa | ROCK inhibition | |
| BMS-223131 | Bristol-Myers Squibb | Phase IIa | Maxi-K channel opener | |
Acknowledgments
The authors would like to thank Miss Misty Bond, commissioning editor of this journal, for her excellent help in obtaining information from the pharmaprojects database.
Alphabetical index of important abbreviations occuring in figures and/or text
- AC
adenylyl cyclase
- Ach
acetylcholine
- AMP
5′-adenosine monophosphate
- ANP
atrial natriuretic peptide
- ATP
adenosine-5′-triphosphate
- cAMP
3′,5′-cyclic-adenosine monophosphate
- cGMP
3′,5′-cyclic-guanosine monophosphate
- DAG
diacylglycerol
- ED
erectile dysfunction
- G
G-protein
- GMP
5′-guanosine monophosphate
- GPCR
G-protein coupled receptor
- GTP
guanosine-5′-triphosphate
- HCC
human corpus cavernosum
- IP3
inositol triphosphate
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- NANC
non-adrenergic, non-cholinergic
- NO
nitric oxide
- NOS
nitric oxide synthase
- eNOS
endothelial NOS
- nNOS
neuronal NOS
- PDE
phosphodiesterase
- PDE5I
phosphodiesterase 5-inhibitor
- PGE1
prostaglandin E1
- PIP2
Phosphatidylinositol bisphosphate
- PKA
PKC, PKG, protein kinase A,C,G
- PLC
phospolipase C
- RhoA
Ras homolog gene family, member A
- ROCK
rho-kinase
- sGC
pGC, soluble and particulate guanylyl cyclase
- SMPP-1M
smooth muscle myosin phosphatase
- SR
sacroplasmic reticulum
- UGN
uroguanylin
- VIP
vasoactive intestinal peptide
References
- 1.NIH consensus conference. impotence. NIH consensus development panel on impotence. JAMA. 1993 Jul 7;270(1):83–90. [PubMed] [Google Scholar]
- 2.Uckert S, Mayer ME, Stief CG, Jonas U. The future of the oral pharmacotherapy of male erectile dysfunction: Things to come. Expert Opin Emerg Drugs. 2007 May;12(2):219–28. doi: 10.1517/14728214.12.2.219. [DOI] [PubMed] [Google Scholar]
- 3.Derogatis LR, Burnett AL. The epidemiology of sexual dysfunctions. J Sex Med. 2008 Feb;5(2):289–300. doi: 10.1111/j.1743-6109.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 4.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ Urologic Diseases in America Project. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006 Jan 23;166(2):207–12. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 5.Albersen M, Shindel AW, Lue TF. Reviews in clinical gerontology. Cambridge University press; Sexual dysfunction in the older man. [DOI] [Google Scholar]
- 6.Martinez-Pineiro L, Trigo-Rocha F, Hsu GL, von Heyden B, Lue TF, Tanagho EA. Cyclic guanosine monophosphate mediates penile erection in the rat. Eur Urol. 1993;24(4):492–9. doi: 10.1159/000474357. [DOI] [PubMed] [Google Scholar]
- 7.Trigo-Rocha F, Aronson WJ, Hohenfellner M, Ignarro LJ, Rajfer J, Lue TF. Nitric oxide and cGMP: Mediators of pelvic nerve-stimulated erection in dogs. Am J Physiol. 1993 Feb;264(2 Pt 2):H419–22. doi: 10.1152/ajpheart.1993.264.2.H419. [DOI] [PubMed] [Google Scholar]
- 8.Trigo-Rocha F, Hsu GL, Donatucci CF, Lue TF. The role of cyclic adenosine monophosphate, cyclic guanosine monophosphate, endothelium and nonadrenergic, noncholinergic neurotransmission in canine penile erection. J Urol. 1993 Apr;149(4):872–7. doi: 10.1016/s0022-5347(17)36250-x. [DOI] [PubMed] [Google Scholar]
- 9.Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996 Jun;8(2):47–52. [PubMed] [Google Scholar]
- 10.Hatzimouratidis K, Hatzichristou DG. A comparative review of the options for treatment of erectile dysfunction: Which treatment for which patient? Drugs. 2005;65(12):1621–50. doi: 10.2165/00003495-200565120-00003. [DOI] [PubMed] [Google Scholar]
- 11**.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, McVary K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010 Jan;7(1 Pt 2):524–40. doi: 10.1111/j.1743-6109.2009.01627.x. This paper summarizes the major findings of the 3rd international consultation on sexual medicine and provides a comprehensive and up-to-date review of currently available pharmacotherapy for ED. [DOI] [PubMed] [Google Scholar]
- 12.Hackett G, Kell P, Ralph D, Dean J, Price D, Speakman M, et al. British society for sexual medicine guidelines on the management of erectile dysfunction. J Sex Med. 2008 Aug;5(8):1841–65. doi: 10.1111/j.1743-6109.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 13.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. Eur Urol. 2010 Feb 20; doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano F, Jackson G, Montorsi F, Martin-Morales A, Raillard P. Safety of sildenafil citrate: Review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010 Jan;64(2):240–55. doi: 10.1111/j.1742-1241.2009.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzimouratidis K, Hatzichristou D. Phosphodiesterase type 5 inhibitors: The day after. Eur Urol. 2007 Jan;51(1):75, 88. doi: 10.1016/j.eururo.2006.07.020. discussion 89. [DOI] [PubMed] [Google Scholar]
- 16.Bella AJ, Deyoung LX, Al-Numi M, Brock GB. Daily administration of phosphodiesterase type 5 inhibitors for urological and nonurological indications. Eur Urol. 2007 Oct;52(4):990–1005. doi: 10.1016/j.eururo.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 17.Shindel AW. 2009 update on phosphodiesterase type 5 inhibitor therapy part 1: Recent studies on routine dosing for penile rehabilitation, lower urinary tract symptoms, and other indications (CME) J Sex Med. 2009 Jul;6(7):1794, 808. doi: 10.1111/j.1743-6109.2009.01347.x. quiz 1793, 1809–10. [DOI] [PubMed] [Google Scholar]
- 18.Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004 Aug;172(2):658–63. doi: 10.1097/01.ju.0000132389.97804.d7. [DOI] [PubMed] [Google Scholar]
- 19.Cellek S, Rees RW, Kalsi J. A rho-kinase inhibitor, soluble guanylate cyclase activator and nitric oxide-releasing PDE5 inhibitor: Novel approaches to erectile dysfunction. Expert Opin Investig Drugs. 2002 Nov;11(11):1563–73. doi: 10.1517/13543784.11.11.1563. [DOI] [PubMed] [Google Scholar]
- 20.Shindel AW. 2009 update on phosphodiesterase type 5 inhibitor therapy part 2: Updates on optimal utilization for sexual concerns and rare toxicities in this class. J Sex Med. 2009 Sep 6;9(2352):64. doi: 10.1111/j.1743-6109.2009.01447.x. quiz 2365–6. [DOI] [PubMed] [Google Scholar]
- 21.Bella AJ, Brant WO, Lue TF, Brock GB. Non-arteritic anterior ischemic optic neuropathy (NAION) and phosphodiesterase type-5 inhibitors. Can J Urol. 2006 Oct;13(5):3233–8. [PubMed] [Google Scholar]
- 22.Heaton JP, Altwein JE. The role of apomorphine SL in the treatment of male erectile dysfunction. BJU Int. 2001 Oct;88( Suppl 3):36–8. doi: 10.1046/j.1464-4096.2001.00121.x. [DOI] [PubMed] [Google Scholar]
- 23.Heaton JP. Central neuropharmacological agents and mechanisms in erectile dysfunction: The role of dopamine. Neurosci Biobehav Rev. 2000 Jul;24(5):561–9. doi: 10.1016/s0149-7634(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 24.Riley A, Main M, Morgan F. Inhalation device allows novel administration of apomorphine in men with erectile dysfunction-efficacy and safety findings. J Sex Med. 2009 Oct 20; doi: 10.1111/j.1743-6109.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 25.Pharmaprojects - copyright to citeline drug intelligence (an informa business).
- 26.Alexandre B, Lemaire A, Desvaux P, Amar E. Intracavernous injections of prostaglandin E1 for erectile dysfunction: Patient satisfaction and quality of sex life on long-term treatment. J Sex Med. 2007 Mar;4(2):426–31. doi: 10.1111/j.1743-6109.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 27*.Hellstrom WJ. Clinical applications of centrally acting agents in male sexual dysfunction. Int J Impot Res. 2008 Jul;20(Suppl 1):S17–23. doi: 10.1038/ijir.2008.18. This review by Wayne Hellstrom provides the reader with an overview of erectile pathways originating in the CNS and potential and current therapeutic targets in the CNS. [DOI] [PubMed] [Google Scholar]
- 28.Brioni JD, Moreland RB, Cowart M, Hsieh GC, Stewart AO, Hedlund P, et al. Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6758–63. doi: 10.1073/pnas.0308292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskerville TA, Douglas AJ. Interactions between dopamine and oxytocin in the control of sexual behaviour. Prog Brain Res. 2008;170:277–90. doi: 10.1016/S0079-6123(08)00423-8. [DOI] [PubMed] [Google Scholar]
- 30.Lue TF. Erectile dysfunction. N Engl J Med. 2000 Jun 15;342(24):1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 31.Albersen M, Joniau S, Claes H, Van Poppel H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv Urol. 2008:594868. doi: 10.1155/2008/594868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Lin CS, Xin ZC, Wang Z, Lin G, Lue TF. Molecular yin and yang of erectile function and dysfunction. Asian J Androl. 2008 May;10(3):433–40. doi: 10.1111/j.1745-7262.2008.00396.x. Well written review on the molecular pathways influencing the erectile state of the penis and the interaction between those various pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008 Jan–Feb;20(1):17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- 34.Hadley ME, Dorr RT. Melanocortin peptide therapeutics: Historical milestones, clinical studies and commercialization. Peptides. 2006 Apr;27(4):921–30. doi: 10.1016/j.peptides.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Hedlund P. Centrally acting drugs for erectile dysfunction: Do they have a future? current sexual health reports. 2007;4:71–76. [Google Scholar]
- 36.Perelman MA. Clinical application of CNS-acting agents in FSD. J Sex Med. 2007 Nov;4( Suppl 4):280–90. doi: 10.1111/j.1743-6109.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 37**.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: A mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):9121–6. doi: 10.1073/pnas.0400520101. Influential basic science report on pathophysiological processes causing ED in diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010 Jan;7(1 Pt 2):445–75. doi: 10.1111/j.1743-6109.2009.01624.x. This paper summarizes the major findings of the 3rd international consultation on sexual medicine and provides a comprehensive and up-to-date review of contemporary concepts in ED. [DOI] [PubMed] [Google Scholar]
- 39.Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, et al. Antagonism of rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001 Jan;7(1):119–22. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 40.Chitaley K, Webb RC, Mills TM. Rho-kinase as a potential target for the treatment of erectile dysfunction. Drug News Perspect. 2001 Dec;14(10):601–6. [PubMed] [Google Scholar]
- 41.Mills TM, Lewis RW, Wingard CJ, Chitaley K, Webb RC. Inhibition of tonic contraction--a novel way to approach erectile dysfunction. J Androl. 2002 Sep–Oct;23(5):S5–9. [PubMed] [Google Scholar]
- 42.Christ GJ, Wang HZ, Venkateswarlu K, Zhao W, Day NS. Ion channels and gap junctions: Their role in erectile physiology, dysfunction, and future therapy. Mol Urol. 1999;3(2):61–73. [PubMed] [Google Scholar]
- 43**.Christ GJ, Andersson KE, Williams K, Zhao W, D’Agostino R, Jr, Kaplan J, et al. Smooth-muscle-specific gene transfer with the human maxi-K channel improves erectile function and enhances sexual behavior in atherosclerotic cynomolgus monkeys. Eur Urol. 2008 Dec 25; doi: 10.1016/j.eururo.2008.12.016. Although out of the scope of this review, this excellent basic research papers clarifies the role of maxi-K channels in erectile physiology. [DOI] [PubMed] [Google Scholar]
- 44.Spektor M, Rodriguez R, Rosenbaum RS, Wang HZ, Melman A, Christ GJ. Potassium channels and human corporeal smooth muscle cell tone: Further evidence of the physiological relevance of the maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J Urol. 2002 Jun;167(6):2628–35. [PubMed] [Google Scholar]
- 45.Chan JS, Kim DJ, Ahn CH, Oosting RS, Olivier B. Clavulanic acid stimulates sexual behaviour in male rats. Eur J Pharmacol. 2009 May 1;609(1–3):69–73. doi: 10.1016/j.ejphar.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Patel MV, Kolasa T, Mortell K, Matulenko MA, Hakeem AA, Rohde JJ, et al. Discovery of 3-methyl-N-(1-oxy-3′,4′,5′,6′-tetrahydro-2′H-[2,4′-bipyridine]-1′-ylmethyl)benzam ide (ABT-670), an orally bioavailable dopamine D4 agonist for the treatment of erectile dysfunction. J Med Chem. 2006 Dec 14;49(25):7450–65. doi: 10.1021/jm060662k. [DOI] [PubMed] [Google Scholar]
- 47*.King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H. Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem. 2007;7(11):1098–106. This article provides a comprehensive overview in current knowledge of the role of these receptors in the treatment of ED. [PMC free article] [PubMed] [Google Scholar]
- 48.Wessells H, Blevins JE, Vanderah TW. Melanocortinergic control of penile erection. Peptides. 2005 Oct;26(10):1972–7. doi: 10.1016/j.peptides.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wessells H, Hruby VJ, Hackett J, Han G, Balse-Srinivasan P, Vanderah TW. MT-II induces penile erection via brain and spinal mechanisms. Ann N Y Acad Sci. 2003 Jun;994:90–5. doi: 10.1111/j.1749-6632.2003.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 50.Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, et al. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: Double-blind, placebo controlled crossover study. J Urol. 1998 Aug;160(2):389–93. [PubMed] [Google Scholar]
- 51.Martin WJ, MacIntyre DE. Melanocortin receptors and erectile function. Eur Urol. 2004 Jun;45(6):706–13. doi: 10.1016/j.eururo.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Kaminetsky J, zinner N, gittleman M, auerbach S, fischkoff S. phase IIb study of bremelanotide in the treatment of ED in non-diabetic males. proceedings of the american urology association annual meeting; 19–24 may 2007; anaheim, USA. [Google Scholar]
- 53.Safarinejad MR, Hosseini SY. Salvage of sildenafil failures with bremelanotide: A randomized, double-blind, placebo controlled study. J Urol. 2008 Mar;179(3):1066–71. doi: 10.1016/j.juro.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 54.Eros D, Szantai-Kis C, Kiss R, Keri G, Hegymegi-Barakonyi B, Kovesdi I, et al. Structure -activity relationships of PDE5 inhibitors. Curr Med Chem. 2008;15(16):1570–85. doi: 10.2174/092986708784911524. [DOI] [PubMed] [Google Scholar]
- 55.Kim BH, Lim HS, Chung JY, Kim JR, Lim KS, Sohn DR, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young korean subjects. Br J Clin Pharmacol. 2008 Jun;65(6):848–54. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paick JS, Ahn TY, Choi HK, Chung WS, Kim JJ, Kim SC, et al. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med. 2008 Nov;5(11):2672–80. doi: 10.1111/j.1743-6109.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim BH, Yi S, Kim J, Lim KS, Kim KP, Lee B, et al. Influence of alcohol on the hemodynamic effects and pharmacokinetic properties of mirodenafil: A single-dose, randomized-sequence, open-label, crossover study in healthy male volunteers in korea. Clin Ther. 2009 Jun;31(6):1234–43. doi: 10.1016/j.clinthera.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Glina S, Toscano I, Gomatzky C, de Goes PM, Junior AN, Claro JF, et al. Efficacy and tolerability of lodenafil carbonate for oral therapy in erectile dysfunction: A phase II clinical trial. J Sex Med. 2009 Feb;6(2):553–7. doi: 10.1111/j.1743-6109.2008.01079.x. [DOI] [PubMed] [Google Scholar]
- 59.Glina S, Fonseca GN, Bertero EB, Damiao R, Rocha LC, Jardim CRF, et al. Efficacy and tolerability of lodenafil carbonate for oral therapy of erectile dysfunction: A phase III clinical trial. J sex med. doi: 10.1111/j.1743-6109.2010.01711.x. in press. [DOI] [PubMed] [Google Scholar]
- 60.Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M. A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine. 2010 Feb;49(2):123–9. doi: 10.1016/j.cyto.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Ning H, Xin ZC, Lin G, Banie L, Lue TF, Lin CS. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology. 2006 Dec;68(6):1350–4. doi: 10.1016/j.urology.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 62.Xin ZC, Kim EK, Lin CS, Liu WJ, Tian L, Yuan YM, et al. Effects of icariin on cGMP-specific PDE5 and cAMP-specific PDE4 activities. Asian J Androl. 2003 Mar;5(1):15–8. [PubMed] [Google Scholar]
- 63.Liu WJ, Xin ZC, Xin H, Yuan YM, Tian L, Guo YL. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J Androl. 2005 Dec;7(4):381–8. doi: 10.1111/j.1745-7262.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 64.Shindel AW, Xin ZC, Lin G, Fandel TM, Huang YC, Banie L, et al. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (epimedium spp.) in vitro and in vivo. J Sex Med. 2010 Feb 5; doi: 10.1111/j.1743-6109.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qinna N, Taha H, Matalka KZ, Badwan AA. A new herbal combination, etana, for enhancing erectile function: An efficacy and safety study in animals. Int J Impot Res. 2009 Sep–Oct;21(5):315–20. doi: 10.1038/ijir.2009.18. [DOI] [PubMed] [Google Scholar]
- 66.Brioni JD, Nakane M, Hsieh GC, Moreland RB, Kolasa T, Sullivan JP. Activators of soluble guanylate cyclase for the treatment of male erectile dysfunction. Int J Impot Res. 2002 Feb;14(1):8–14. doi: 10.1038/sj.ijir.3900801. [DOI] [PubMed] [Google Scholar]
- 67.Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994 Dec 15;84(12):4226–33. [PubMed] [Google Scholar]
- 68.Mizusawa H, Hedlund P, Brioni JD, Sullivan JP, Andersson KE. Nitric oxide independent activation of guanylate cyclase by YC-1 causes erectile responses in the rat. J Urol. 2002 May;167(5):2276–81. [PubMed] [Google Scholar]
- 69.Hsieh GC, O’Neill AB, Moreland RB, Sullivan JP, Brioni JD. YC-1 potentiates the nitric oxide/cyclic GMP pathway in corpus cavernosum and facilitates penile erection in rats. Eur J Pharmacol. 2003 Jan 1;458(1–2):183–9. doi: 10.1016/s0014-2999(02)02730-9. [DOI] [PubMed] [Google Scholar]
- 70.Nakane M, Hsieh G, Miller LN, Chang R, Terranova MA, Moreland RB, et al. Activation of soluble guanylate cyclase causes relaxation of corpus cavernosum tissue: Synergism of nitric oxide and YC-1. Int J Impot Res. 2002 Apr;14(2):121–7. doi: 10.1038/sj.ijir.3900843. [DOI] [PubMed] [Google Scholar]
- 71.Kalsi JS, Kell PD, Cellek S, Ralph DJ. NCX-911, a novel nitric oxide-releasing PDE5 inhibitor relaxes rabbit corpus cavernosum in the absence of endogenous nitric oxide. Int J Impot Res. 2004 Apr;16(2):195–200. doi: 10.1038/sj.ijir.3901157. [DOI] [PubMed] [Google Scholar]
- 72.Kalsi JS, Rees RW, Hobbs AJ, Royle M, Kell PD, Ralph DJ, et al. BAY41-2272, a novel nitric oxide independent soluble guanylate cyclase activator, relaxes human and rabbit corpus cavernosum in vitro. J Urol. 2003 Feb;169(2):761–6. doi: 10.1097/01.ju.0000043880.58140.69. [DOI] [PubMed] [Google Scholar]
- 73.Miller LN, Nakane M, Hsieh GC, Chang R, Kolasa T, Moreland RB, et al. A-350619: A novel activator of soluble guanylyl cyclase. Life Sci. 2003 Jan 17;72(9):1015–25. doi: 10.1016/s0024-3205(02)02361-5. [DOI] [PubMed] [Google Scholar]
- 74.Gur S, Kadowitz PJ, Hellstrom WJ. Exploring the potential of no-independent stimulators and activators of soluble guanylate cyclase for the medical treatment of erectile dysfunction. Curr Pharm Des. 2010 Mar 4; doi: 10.2174/138161210791164162. [DOI] [PubMed] [Google Scholar]
- 75*.Kalsi JS, Ralph DJ, Madge DJ, Kell PD, Cellek S. A comparative study of sildenafil, NCX-911 and BAY41-2272 on the anococcygeus muscle of diabetic rats. Int J Impot Res. 2004 Dec;16(6):479–85. doi: 10.1038/sj.ijir.3901224. Impressive basic scientific work that stresses the potential of these compounds in the treatment of diabtes-related disorders. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol. 2009;191(191):309–39. doi: 10.1007/978-3-540-68964-5_14. [DOI] [PubMed] [Google Scholar]
- 77.Kun A, Kiraly I, Pataricza J, Marton Z, Krassoi I, Varro A, et al. C-type natriuretic peptide hyperpolarizes and relaxes human penile resistance arteries. J Sex Med. 2008 May;5(5):1114–25. doi: 10.1111/j.1743-6109.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 78.Sousa CM, Havt A, Santos CF, Arnaud-Batista FJ, Cunha KM, Cerqueira JB, et al. The relaxation induced by uroguanylin and the expression of natriuretic peptide receptors in human corpora cavernosa. J Sex Med. 2010 Jan 19; doi: 10.1111/j.1743-6109.2009.01672.x. [DOI] [PubMed] [Google Scholar]
- 79.Riffaud JP, Bernarbe J, Giuliano F, Jones R, Jeremy J. Pharmacological profile of sildenafil nitrate (NCX 911) in various models of penile erection. inflammation res. 2001;50(suppl 3):S84. [Google Scholar]
- 80.Kalsi JS, Ralph DJ, Thomas P, Bellringer J, Minhas S, Kell PD, et al. A nitric oxide-releasing PDE5 inhibitor relaxes human corpus cavernosum in the absence of endogenous nitric oxide. J Sex Med. 2005 Jan;2(1):53–7. doi: 10.1111/j.1743-6109.2005.20105.x. [DOI] [PubMed] [Google Scholar]
- 81.Seidler M, Uckert S, Waldkirch E, Stief CG, Oelke M, Tsikas D, et al. In vitro effects of a novel class of nitric oxide (NO) donating compounds on isolated human erectile tissue. Eur Urol. 2002 Nov;42(5):523–8. doi: 10.1016/s0302-2838(02)00397-4. [DOI] [PubMed] [Google Scholar]
- 82.Chitaley K, Webb RC, Mills TM. The ups and downs of rho-kinase and penile erection: Upstream regulators and downstream substrates of rho-kinase and their potential role in the erectile response. Int J Impot Res. 2003 Apr;15(2):105–9. doi: 10.1038/sj.ijir.3900964. [DOI] [PubMed] [Google Scholar]
- 83.Chang S, Hypolite JA, Changolkar A, Wein AJ, Chacko S, DiSanto ME. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via rho-kinase beta. Int J Impot Res. 2003 Feb;15(1):53–62. doi: 10.1038/sj.ijir.3900947. [DOI] [PubMed] [Google Scholar]
- 84.Park K, Kim SW, Rhu KS, Paick JS. Chronic administration of an oral rho kinase inhibitor prevents the development of vasculogenic erectile dysfunction in a rat model. J Sex Med. 2006 Nov;3(6):996–1003. doi: 10.1111/j.1743-6109.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 85.Buyukafsar K, Un I. Effects of the rho-kinase inhibitors, Y-27632 and fasudil, on the corpus cavernosum from diabetic mice. Eur J Pharmacol. 2003 Jul 11;472(3):235–8. doi: 10.1016/s0014-2999(03)01905-8. [DOI] [PubMed] [Google Scholar]
- 86.Lohn M, Plettenburg O, Ivashchenko Y, Kannt A, Hofmeister A, Kadereit D, et al. Pharmacological characterization of SAR407899, a novel rho-kinase inhibitor. Hypertension. 2009 Sep;54(3):676–83. doi: 10.1161/HYPERTENSIONAHA.109.134353. [DOI] [PubMed] [Google Scholar]
- 87*.Christ GJ. K channels as molecular targets for the treatment of erectile dysfunction. J Androl. 2002 Sep–Oct;23(5):S10–9. Comprehensive overview of the role of maxi-K channels in erectile physiology. [PubMed] [Google Scholar]
- 88.Lee SW. Physiological roles and properties of potassium channels in corporal smooth muscle. Drugs Today (Barc) 2000 Feb–Mar;36(2–3):147–54. doi: 10.1358/dot.2000.36.2-3.568788. [DOI] [PubMed] [Google Scholar]
- 89.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J Physiol. 2005 Sep 1;567(Pt 2):545–56. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boy KM, Guernon JM, Sit SY, Xie K, Hewawasam P, Boissard CG, et al. 3-thio-quinolinone maxi-K openers for the treatment of erectile dysfunction. Bioorg Med Chem Lett. 2004 Oct 18;14(20):5089–93. doi: 10.1016/j.bmcl.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 91.Lifestyle drugs market outlook. key indications, epidemiology, and emergent drugs. [last accessed 10 february 2010];new healthcare report - published december 2007. sample information available on www.globalbusinessinsights.com.
- 92. [last accessed 10 february 2010];Erectile dysfunction market assessment & analysis. 2007–2022 abstract. available on www.marketresearch.com. [homepage on the Internet]