Abstract
Changes in mesocorticolimbic dopamine (DA) neurons and their target cells can be induced throughout life and are important determinants of individual differences in susceptibility to psychopathology. The goal of my research is to gain insight into the nature of the cellular and molecular mechanism underlying the selective plasticity of mesocorticolimbic DA neurons. Here, I review work showing that the guidance cue netrin-1 is implicated in the organization, plasticity and function of mesocorticolimbic DA neurons in rodents. Developmental variations in netrin-1 receptor function result in selective reorganization of medial prefrontal DA circuitry during adolescence and in an adult phenotype protected against schizophrenia-like dopaminergic and behavioural abnormalities. Furthermore, in adulthood, expression of netrin-1 receptors is upregulated by repeated exposure to stimulant drugs of abuse in DA somatodendritic regions and is necessary for drug-induced behavioural plasticity. I propose that risk factors associated with DA-related adult psychiatric disorders alter netrin-1 function.
Introduction
The mesocorticolimbic dopamine (DA) circuitry has a continuous capacity to change. The work I describe here has been directed at deciphering the nature of the changes induced in these DA neurons and their connections by genetic abnormalities and by exposure to drugs or stressors at different times in life. I will outline my group’s findings to date and their implications for psychopathology.
Changes in mesocorticolimbic DA neurons and their target cells can be induced throughout life by exposure to external and internal events, such as perinatal complications, alterations in levels of gonadal hormones or repeated experience with drugs of abuse. Such changes are thought to be important determinants of individual differences in susceptibility to psychopathology.1–4 For instance, it is known that disruptions in the development of DA circuitry perinatally can contribute to the symptomatology of various psychiatric disorders, such as schizophrenia, that appear later in life.5,6 Even in adulthood, changes in the organization of DA circuitry brought about by exposure to stressors or stimulant drugs confer increased vulnerability to pathological drug-taking.7–9 What is missing to date is the specification of the molecular processes underlying such plasticity within both the developing and adult mesocorticolimbic DA systems.
Mesocortical and mesolimbic DA projections
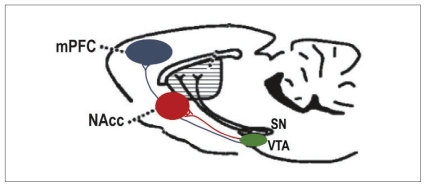
In adults, 2 primary projections of the mesocorticolimbic DA neurons arising in the ventral tegmental area (VTA) have been identified (Fig. 1). One group projects to the limbic forebrain, primarily to the ventral striatum (nucleus accumbens, mesolimbic), and the other one innervates the medial prefrontal cortex (mPFC; mesocortical). These 2 projections are anatomically distinct and physiologically differentiated. In rodents, it has been determined that mPFC but not nucleus accumbens–projecting DA neurons are innervated directly by cortical glutamatergic inputs,10 whereas nucleus accumbens–projecting DA cells receive direct glutamatergic innervation from brainstem neurons, including those located in laterodorsal pedunculopontine tegmental nuclei.11,12 Mesolimbic and mesocortical DA neurons also have different functional properties. Relative to nucleus accumbens DA projections, mPFC DA neurons have lower terminal density, a decreased number of DA transporters per terminal13,14 and lack somatodendritic DA D2 G-protein–coupled potassium channel (Girk2) autoreceptors.13 Furthermore, uptake of DA in the nucleus accumbens depends primarily on the DA transporter, whereas in the mPFC, DA uptake depends primarily on the norepinephrine (NE) transporter.15,16 Released synaptic DA in the mPFC but not in the nucleus accumbens is metabolized, in part, by catechol-O-methyltransferase (COMT17); COMT knockout mice exhibit increased DA levels in the mPFC but not in the nucleus accumbens.17,18 Such differences between the 2 projections and their targets may be key to understanding how each is differentially affected by events that induce plastic changes at different periods of development.
Fig. 1.
Mesocorticolimbic dopamine system in the rodent brain. The diagram shows the dopamine (DA) projections to the medial prefrontal cortex (mPFC) and ventral striatum (nucleus accumbens; NAcc) in the rodent brain viewed in the sagittal plane. Ventral tegmental area (VTA) DA neurons projecting to the mPFC are anatomically distinct from those projecting to the nucleus accumbens. SN = substantia nigra.
Reciprocal relations between mesolimbic and mesocortical DA systems
Evidence that these 2 projections can be differentially affected by a single manipulation comes from studies showing that exposure to pharmacologic agents or stressors produces divergent, even opposite, effects in mesolimbic and mesocortical DA neurons (e.g., see Castner and colleagues19,20 and Brake and colleagues21,22). This selective modulation appears to result, at least in part, from the fact that DA activity in the mPFC can lead to reduced DA activity in the nucleus accumbens. For example, selective lesions of the DA input to the mPFC result in increased stress- and drug-induced release of DA in the nucleus accumbens.23 Conversely, stimulation of DA receptors in the mPFC decreases DA release in the nucleus accumbens in response to these stimuli.24–26 Such an inverse functional relationship between the mPFC and nucleus accumbens DA systems suggests that alterations in the development, organization and/or rewiring of one of these DA systems could affect the normal functioning of the other. Indeed, increased expression of DA D2 receptors in the striatum has been shown to lead to reduced DA turnover in the mPFC.27 Furthermore, individuals with schizophrenia and stimulant drug addiction exhibit exaggerated striatal DA release in response to an amphetamine challenge, but appear to have blunted DA activity in the mPFC.28–36 (For possible mechanisms accounting for the inhibitory control of DA activity in mPFC over DA activity in the nucleus accumbens, see Carr and Sesack,10 Sesack and Pickel37 and Gao and colleagues.38)
Time course of mesolimbic and mesocortical DA development
Another important difference between the mesolimbic and mesocortical DA systems is the fact that they have distinct developmental timelines. In contrast to the DA innervation of the striatum, which reaches near adult density by postnatal day 20 in rats,39 the ingrowth of DA terminals into the mPFC is slow and continues until early adulthood. This slow, delayed maturation confers increased vulnerability for structural plasticity. In both rats and mice, mPFC DA innervation increases progressively beyond the weanling stage until early adulthood, undergoing a substantial change in DA fibre density and shape around puberty, with the highest density occurring in deeper layers V and VI.40,41 The late maturation of the mPFC DA innervation, which is also observed in non-human primates,42 is unique within the monoamine systems; both NE and serotonergic innervations of the mPFC reach adult density by the end of the first and third postnatal week, respectively, in rats.41,43,44 This slow course of the mPFC DA development is intriguing, considering that psychiatric symptoms often emerge postpubertally. A similar phenomenon has been observed in putative developmental animal models of schizophrenia.45–47 Taken together with the inverse functional relation described above, these findings have led to the hypothesis that factors associated with increased risk of developing DA-related psychiatric disorders in adulthood may target selectively molecular processes underlying mPFC DA development. The nature and precise time course of the development of the DA innervation of the prefrontal cortex in humans is not known. However, because the distribution pattern of DA axons is similar in the neocortex of the adult monkey and in the homologous regions of the human prefrontal cortex, it has been suggested that mesocortical DA axons undergo similar spatiotemporal modifications in both species (see Rosenberg and Lewis42).
Role of netrins in the differential plasticity within the mesolimbic and mesocortical DA systems
In our attempt to identify molecular processes responsible for the differential plasticity of VTA mPFC and nucleus accumbens DA neurons, my group has concentrated on a potential role for the developmental protein netrin-1. Netrin-1 is a guidance cue highly expressed by both DA neurons and their postsynaptic targets48,49 and, as such, is likely to be implicated in the very selective, precise and orchestrated organization of mesocorticolimbic DA connectivity. We proposed that netrin-1 signalling could be altered by genetic and environmental factors associated with vulnerability to develop DA-related psychopathologies. Furthermore, because netrin-1 and its receptors continue to be expressed in the adult brain, we also anticipated their role in the refined and enduring plasticity of adult DA neurons induced by repeated exposure to drugs of abuse.
Netrin-1 and its receptors DCC and UNC5 homologues
The most characterized member of the netrin family of guidance cues, netrin-1, is an approximately 65-kDa secreted protein evolutionarily related to the extracellular matrix protein laminin. Netrin-1 is made up of 3 domains (VI, V and C) and an amino terminal signal peptide. It participates in the developmental organization of neural networks as a bifunctional cue, either attracting or repelling extending axons and dendrites.50–53 Initially, netrin-1 was identified and characterized as an attractant of spinal cord commissural axons, but it was soon demonstrated to also function as a repellent of certain motor axons.50,51 These opposing responses to netrin-1 are now known to depend on the activation of different receptors or receptor complexes.
There are 2 families of netrin-1 receptors, the deleted in colorectal cancer (DCC) and the UNC5 homologues (UNC5H), with 4 homologues identified to date (UNC5H-D). Both DCC and UNC5H proteins are transmembrane immunoglobulin superfamily members. Whereas DCC receptors account for the attractant responses to netrin-1, UNC5H receptors alone, or as DCC-UNC5H receptor complexes, mediate netrin-1–induced chemorepulsion.54,55 Consistent with their function, DCC and UNC5H recruit downstream proteins that regulate cytoskeletal reorganization. The Rho family of small GTPases are key players in linking netrin-1 receptor signalling to cytoskeleton dynamics. The DCC-induced activation of 2 Rho family members, Cdc42 and Rac1, promotes neurite extension and mediates the attractive function of netrin-1.56,57 In contrast, chemorepulsion appears to be brought about by activation of RhoA.58–60 In addition, second messenger pathways dependent on Ca2+ influx, Src-family kinases, the serine-threonine kinase Pak1, the Wiskott–Aldrich syndrome–related protein family (NWASP) and the Arp2/3 actin-binding complex, also influence netrin-1 receptor signalling (for a more comprehensive review on signalling pathways implicated in DCC- and UNC5H-netrin-1 signalling see Wang and Poo,61 Bradford and colleagues62 and Rajasekharan and Kennedy63).
The attractive and repulsive actions of netrin-1 can be switched by regulating the availability of DCC and UNC5H receptors at the cell surface.64–66 Thus, the selectivity with which netrin-1 organizes neuronal connectivity is determined by the ratio of DCC:UNC5H receptors expressed by particular neurons at a particular time. Subtle alterations in this ratio at different developmental periods should result in discrete remodelling of specific neuronal circuits and in turn in significant changes in the function of these systems in adulthood.
Although netrin-1 is a diffusible protein, it can function as both a long- and short-range cue. Secreted netrin-1 can act as a chemotropic cue during development and guide growing axons that are far away from their appropriate targets through a gradient.67 However, netrin-1 can also have an effect on cellular processes found within the surrounding area of the cells that produce it. For instance, local expression of netrin-1 in the mouse optic disc is not required for long-range axonal pathfinding of retinal ganglion cells, but for allowing these axons to exit the optic disc into the optic nerve.68 Furthermore, as a target-derived short-range cue, netrin-1 also plays a critical role in the wiring events that take place once the axons have reached such a target. These events include axon arborization and synapse formation.69–71 Studies conducted in Caenorhabditis elegans show that netrin participates in synapse formation by regulating the assembly of proteins at the presynaptic site. This role is similar to its axonal guidance function: whereas netrin–DCC signalling recruits presynaptic proteins in axons, netrin–UNC5 signalling excludes presynaptic proteins from dendrites.70,71
The expression of netrin-1 and its receptors in the central nervous system persists into adulthood.72,73 Moreover, most embryonic and adult netrin-1 appears to be attached to the extracellular matrix and to the cell membrane, suggesting that it mainly functions as a short-range cue.50 One could therefore predict that not only during development,74 but also in adulthood, alterations in netrin-1 receptor expression within selective neuronal populations result in reorganization of already established synaptic networks.
Although my group’s studies focus on the role of netrin-1 signalling through DCC and UNC5H, there are other transmembrane proteins that bind netrin-1. Down syndrome cell adhesion molecule (DSCAM) has been identified as a novel netrin-1 receptor involved in signalling axon guidance.75 Neogenin, a second member of the DCC family in the vertebrate, is another netrin-1 receptor that appears to be involved in neurogenesis in the developing and adult brain.76 Expression of these netrin-1 receptors by DA neurons remains to be determined. Similarly, it is important to clarify that the role of netrin-1 is not restricted to neuronal connectivity, but also involves cell adhesion, cell migration, cell death and angiogenesis processes (for reviews see Bradford and colleagues,62 Baker and colleagues,77 Mehlen and Guenubeaud78 and Castets and Mehlen79).
DCC receptor signalling contributes to the development of mesocorticolimbic DA circuitry
Expression of netrin-1, DCC and UNC5H by DA neurons: peripubertal shift
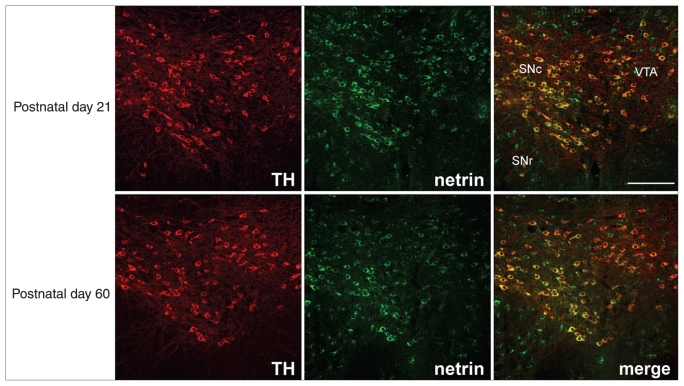
Since the late 1990s, neuroanatomic studies have demonstrated that although mRNA expression of netrin-1 spans across several brain regions in both the developing and adult rodent brain, its highest levels of expression are observed within midbrain DA cell body regions.72 Similarly, robust expression of dcc was reported in the developing and adult rodent VTA and substantia nigra pars compacta.72 Consistent with these findings, my group has recently shown that netrin-1 protein is highly expressed by the mPFC, nucleus accumbens and VTA neurons, including robust expression by DA cells (Fig. 2). Importantly, the pattern and intensity of expression of netrin-1 within these mesocorticolimbic DA regions remains indistinguishable from prepubertal age (postnatal day 21 ± 1 in mice; postnatal day 21 in rats) to adulthood (postnatal day 75 ± 15 in mice; postnatal day 90 in rats).48,49
Fig. 2.
Netrin-1 protein expression in the ventral tegmental area (VTA) of the mouse, before (postnatal day 21) and after puberty (postnatal day 60). Micrographs of coronal midbrain hemisections of wild-type BL6 male mice on postnatal days 21 and 60 showing immunofluorescence for netrin-1 (green) and tyrosine hydroxylase (TH; red). In all pictures, dorsal is on top, lateral on the left and medial on the right. Most tyrosine hydroxylase–positive neurons coexpress netrin-1 (merged); however, not all netrin-1–positive cells express tyrosine hydroxylase. The pattern of expression is similar at both ages. These findings are consistent with previous reports showing that the region of highest netrin-1 mRNA expression in the central nervous system is the midbrain dopamine somatodendritic region72 and with netrin-1 mRNA expression shown in the Allen Atlas (www.brain-map.org). In our experiments, we used a chicken antimouse netrin-1 antibody (1:7000; Novus Biological). We have tested the specificity of this antibody using Western blot. Lysates from whole rat brain were processed next to rat liver, which does not express netrin-1.80 A band of about 75 kDa was observed in the adult rat brain, consistent with the molecular weight of rat netrin-1. This band was not detected in the liver. In addition, we found that preadsorption of the netrin-1 antibody with a 10-fold excess of recombinant mouse netrin-1 (R&D Systems) before immunolabelling abolishes or dramatically reduces netrin-1 labelling (C. Manitt and C. Flores, Montréal, Que.: unpublished observations, 2011). Scale bar: 250 μm. SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata. Reproduced from Manitt et al.48
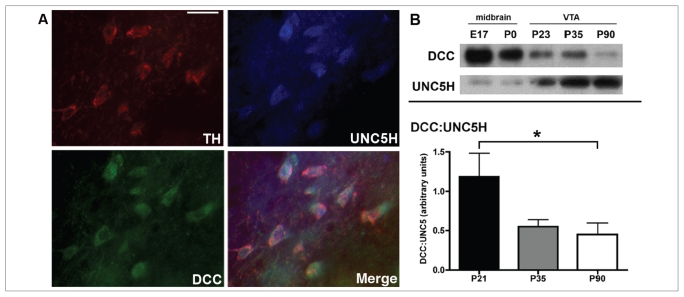
We have also characterized the spatiotemporal expression of DCC and UNC5H proteins in the VTA of both mice and rats from embryonic life to adulthood.48 In contrast to netrin-1, the expression of these receptors varies significantly across the lifespan. There are high levels of DCC expression in the embryonic VTA, with most DA neurons expressing DCC at all embryonic stages. In contrast, there is only scarce UNC5H expression at this time, and there are no tyrosine hydroxylase/UNC5H-positive neurons in the VTA. Whereas DCC continues to be expressed by DA neurons in the VTA at birth, UNC5H expression remains weak and does not colabel with tyrosine hydroxylase. At postnatal day 21, DCC is still expressed by DA neurons and, although the number of UNC5H-positive neurons increases relative to the earlier developmental stages, there is no UNC5H expression in DA cells. Deleted in colorectal cancer continues to be expressed in tyrosine hydroxylase–positive neurons during peripubertal (postnatal day 33 ± 2 in mice; postnatal day 33 in rats) and adult periods, consistent with previous reports.81–83 Remarkably, there is a dramatic emergence of UNC5H expression by DA neurons in the peripubertal period, which persists until adulthood.48,82,83
Importantly, from the peripubertal period onward, single VTA DA neurons coexpressed both UNC5H and DCC receptors (Fig. 3A48). These findings strongly suggest that netrin-1 plays a role in the development and adult function of mesocorticolimbic DA circuitries. The fact that single DA neurons express both DCC and UNC5H receptors is also consistent with the idea that subtle changes in their expression induced by events occurring at particular critical periods could lead to altered organization of DA connectivity. The peripubertal emergence of UNC5H expression by VTA DA neurons indeed represents a drastic shift in the DCC:UNC5H ratio in this region, leading to a predominance of UNC5H effects that endure into adulthood (Fig. 3B). This shift is likely to be associated with a change in the response of DA neurons to netrin-1 and may have a significant impact on their functional organization.
Fig. 3.
Expression of deleted in colorectal cancer (DCC) and UNC5 homologue (UNC5H) receptors in the ventral tegmental area (VTA). (A) Micrographs of coronal midbrain hemisections from adult male rats showing coexpression of DCC (green) and UNC5H (blue) by single VTA tyrosine hydroxylase (TH)–positive neurons (red). Scale bar: 25 μm. (B) Peripubertal shift in the levels of DCC and UNC5H receptor expression in the VTA. (Top panel) Western blot showing DCC and UNC5H protein expression in tissue lysates from rat midbrain at embryonic day 17 (E 17) and at birth (postnatal day [P] 0), and in VTA lysates from male rats on postnatal days 23, 35 and 90. It can be noticed that whereas the level of expression of DCC decreases with age, UNC5H receptor expression increases. (Bottom panel) Western blot for DCC and UNC5H immunoreactivity was performed on lysates from the VTA of wild-type male BL6 mice on postnatal days 21, 35 and 90 (n = 6–9 per age). The graph shows quantitative analysis of mean (and standard error of the mean) DCC and UNC5H expression levels in the VTA (expressed as a DCC:UNC5H ratio) for each sample. A shift toward UNC5H predominance occurs between the peripubertal period and adulthood. A 1-way analysis of variance performed on these data revealed significant differences between postnatal days 21 and 90. Reproduced from Manitt et al.48
The dcc +/− mice are protected against amphetamine-induced behavioural alterations
To determine whether developmental alterations in netrin-1 receptor expression affect the organization and function of mesocortical and/or mesolimbic DA circuitries, my group has now completed several studies using dcc heterozygous (+/−) mice, which develop with reduced levels of DCC protein.83–86 In contrast to dcc knockout homozygous mice, which die a few hours after birth, dcc +/− mice survive to adulthood and do not exhibit obvious phenotypic differences from their wild-type counterparts.87 We hypothesized that a DA phenotype might emerge in response to challenges that trigger DA neuron function, including exposure to stimulant drugs. It is noteworthy that a haploinsufficiency model is in fact quite relevant to the study of biologic phenomena. Variations rather than absence of protein levels are more likely to occur throughout the lifespan of animals, including humans. In fact, DCC heterozygosity in the human population has recently been identified.88 It is important to clarify that dcc haploinsufficiency in mice does not result in compensatory changes in UNC5H protein expression at least in the VTA, mPFC and nucleus accumbens.83
We have tested adult male dcc +/− and wild-type mice for a number of behaviours that reflect the state of DA functioning and that are altered in schizophrenia and in adult laboratory animals subjected to perinatal manipulations associated with increased risk for this disorder.
Locomotor activity
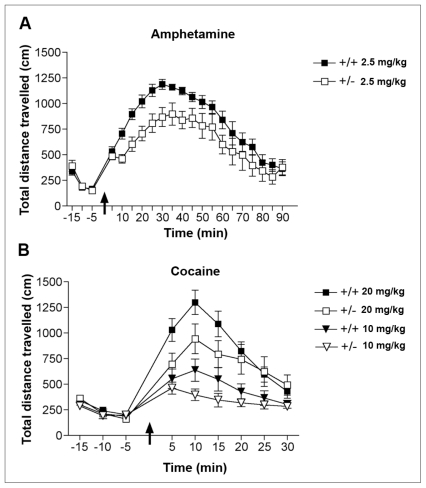
My group has found no genotypic differences between dcc +/− and wild-type mice in baseline locomotor activity or following an intraperitoneal injection of saline. However, dcc +/− mice show significantly reduced locomotor activation in response to a single injection of 2.5 mg/kg of the indirect DA agonist amphetamine compared with their wild-type littermates (Fig. 4A). This effect is quite robust and does not depend on sex, amphetamine dose or genetic background: it is observed in males and females, following 1.5, 2.5 or 4 mg/kg of amphetamine, and in mice of a pure BL6 strain or of a 129Sv/BL6 cross.83,84 Furthermore, adult dcc +/− mice show reduced locomotor responses to single injections of cocaine (Fig. 4B).
Fig. 4.
Blunted behavioural responses to stimulant drugs in adult dcc heterozygous (+/−) mice. (A) Mean (and standard error of the mean) locomotor activity before and after an injection of d-amphetamine sulfate (2.5 mg/kg, intraperitoneally; arrow) in adult male BL6 dcc +/− and wild-type (+/+) mice (n = 8 per group). Repeated-measures analysis of variance revealed a significant main effect of genotype. Reproduced with permission from Grant et al.83 (B) Mean (and standard error of the mean) locomotor activity before and after an injection of either 10 or 20 mg/kg, intraperitoneally, of cocaine hydrochloride (arrow) in adult male BL6 dcc +/− (n = 10 per group) and +/+ mice (low dose, n = 10; high dose, n = 12). A mixed-design, 3-way analysis of variance conducted on the first 25 minutes after cocaine injections revealed a significant main effect of genotype (F1,38 = 4.40, p = 0.042), but no significant genotype by dose interaction (p = 0.39).
Sensorimotor gating
My group has measured sensorimotor efficacy in adult dcc +/− and wild-type mice using prepulse inhibition of the acoustic startle response. Sensorimotor gating is impaired in schizophrenia,89 depends on proper mesocorticolimbic DA function90 and is disrupted by exposure to stimulant drugs, including amphetamine.91 Again, we found no differences in response to baseline prepulse inhibition between genotypes, consistent with the idea that the dcc +/− phenotype only becomes evident upon a challenge. Thus, when animals are given an intraperitoneal injection of 3.2 mg/kg of amphetamine, wild-type but not dcc +/− mice show amphetamine-induced deficits in prepulse inhibition. The dcc +/− mice remain resistant to amphetamine-induced prepulse inhibition impairment even after doubling the dose.83
Reward
My group has also tested whether adult dcc +/− mice show differential sensitivity to the rewarding effects of amphetamine using conditioned place preference. In this procedure, animals learn to associate the effects of a drug with a particular environment. Subsequent preference for the drug-paired environment is taken as an index of the rewarding properties of the drug. We have found that, in the postconditioning test, wild-type mice spend a significantly greater amount of time in the compartment previously paired with 2.2 mg/kg of amphetamine than in the one paired with saline. However, dcc +/− mice do not exhibit preference for either compartment. When a higher dose of amphetamine (4.4 mg/kg) was used, both groups exhibited significant preference for the amphetamine-associated compartment.83 These findings indicate diminished sensitivity to the incentive properties of amphetamine in adulthood in dcc +/− mice. Exaggerated behavioural response to amphetamine is observed in patients with schizophrenia and following repeated exposure to drugs of abuse and is a hallmark of animal models of these disorders. Together, these behavioural results show that dcc haploinsuffiency protects against this trait.
Adult dcc +/− mice exhibit enhanced mPFC activity, but reduced DA release in the nucleus accumbens
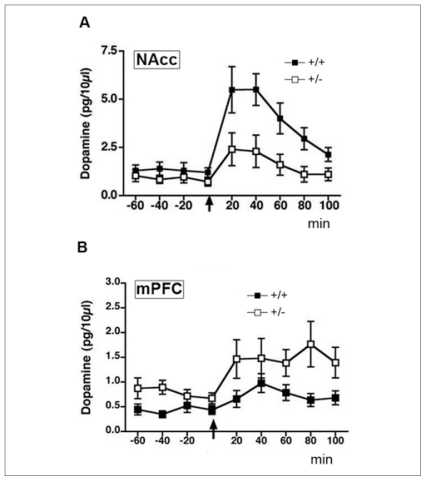
Amphetamine-induced locomotion, conditioning place preference and deficits in prepulse inhibition depend to a large extent on the ability of the drug to induce DA release in the nucleus accumbens.92,93 Thus, my group predicted that the behavioural phenotype observed in adult dcc +/− mice would be associated with altered amphetamine-induced DA release in this region. Results from in vivo microdialysis experiments on freely moving adult dcc +/− and wild-type mice revealed that this is the case. Consistent with the absence of a baseline behavioural phenotype, baseline extracellular concentrations of DA are not different between genotypes. The dcc +/− mice do, however, show considerably lower baseline extracellular concentrations of the DA metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanilic acid (HVA), indicating decreased DA activity within this region. Importantly, dcc +/− mice exhibit significantly blunted DA release in the nucleus accumbens when challenged with 2.5 mg/kg of amphetamine — less than half of the increase observed in wild-type mice (Fig. 5A83). Previous work I did resulted in consistent findings from drug-naive adult mice when I used high-performance liquid chromatography analysis of whole tissue punches taken from the nucleus accumbens.84
Fig. 5.
In vivo microdialysis measurements of mean (and standard error of the mean) baseline and amphetamine-induced dopamine (DA) concentrations in the nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC) of freely moving adult dcc heterozygous (+/−) and wild-type (+/+) mice. (A) Dopamine release in the nucleus accumbens (both core and shell divisions) of adult male dcc +/− (n = 10) and +/+ (n = 7) mice both before and after an injection of 2.5 mg/kg, intraperitoneally, of d-amphetamine sulfate (arrow). Two-way repeated-measures analysis of variance revealed that amphetamine-induced DA release is significantly reduced in dcc +/− mice (main effect of genotype: F1,15 = 6.04, p = 0.027). There were no significant differences between genotypes in baseline DA concentrations. (B) Dopamine release in the mPFC (pregenual mPFC, including cingulate 1, prelimbic and infralimbic subregions) of adult male dcc +/− and +/+ mice before and after an injection of 2.5 mg/kg, intraperitoneally, of d-amphetamine sulfate (arrow). Two-way repeated-measures analysis of variance revealed that in comparison to +/+ mice, dcc +/− exhibit increased extracellular concentrations of DA at baseline (main effect of genotype: F1,17 = 4.10, p = 0.05) and following treatment with amphetamine (main effect of genotype: F1,16 = 4.90, p = 0.041; n = 8 per group). Reproduced with permission from Grant et al.83
As mentioned in the introduction, DA activity in the mPFC can have inhibitory control over DA activity in the nucleus accumbens. My group, therefore, hypothesized that the reduced nucleus accumbens DA activity observed in dcc +/− mice at baseline and after the amphetamine challenge could result from mPFC DA hyperactivity. Indeed, we found that extracellular concentrations of DA, DOPAC and HVA at baseline are significantly elevated in the mPFC of adult dcc +/− mice and, furthermore, that the increase in DA in the mPFC induced by an injection of 2.5 mg/kg of amphetamine is significantly greater in dcc +/− than in wild-type mice (Fig. 5B83). Dopamine and DA metabolite levels measured from whole tissue punches of mPFC of drug-naive mice revealed an approximately 200% increase in both DA and DOPAC and an approximately 50% increase in HVA.84
These findings show that a reduction in DCC receptors during development results in greater mPFC DA activity, but reduced DA activity in the nucleus accumbens in the adult, confirming the original hypothesis that changes in netrin-1 signalling result in selective, even opposite, effects on mesolimbic versus mesocortical DA neurons. Furthermore, we suggest that the mPFC hyperactivity observed may account for the blunted amphetamine effects on DA release in the nucleus accumbens and on behaviour observed in adult dcc +/− mice. It is important to mention that these changes appear to be specific to the DA system because there are no genotypic differences in extracellular concentrations of NE in the mPFC at baseline or following amphetamine exposure.83
Reorganization of mPFC DA synaptic circuitry in adult dcc +/− mice
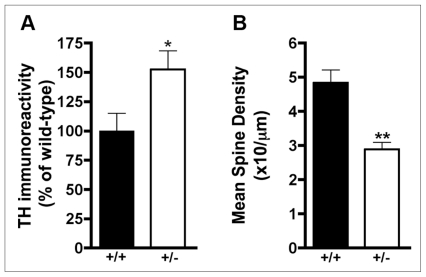
To investigate possible causes of the coexistence of hyper-and hypo-DA activity in the mPFC and nucleus accumbens, respectively, in dcc +/− mice, I conducted a series of molecular and neuroanatomic studies in drug-naive animals. Using Western immunoblotting we observed a significant increase in baseline levels of tyrosine hydroxylase immunoreactivity in the mPFC of adult dcc +/− mice compared with wild-type controls, without a corresponding increase in expression of the NE-converting enzyme, dopamine-β-hydroxylase (Fig. 6A84). There were no differences in tyrosine hydroxylase levels between genotypes in the nucleus accumbens or the dorsal striatum. To determine whether adult dcc +/− mice exhibit changes in number and/or distribution of DA presynaptic terminals in the mPFC, we conducted stereologic analysis of tyrosine hydroxylase–positive varicosities in this region. We found that the volume encompassed by tyrosine hydroxylase–positive varicosities in the pregenual mPFC was significantly greater in adult dcc +/− than in wild-type mice.49 We anticipate that the increase in the number of DA terminals in the mPFC of adult dcc +/− mice may result from axonal sprouting, especially because these mice have reduced (about 20%) tyrosine hydroxylase–positive neurons in the VTA.84 Because DA immunoreactive varicosities represent sites at which DA synthesis, packaging, release and reuptake most often occur,94 these results suggest that dcc +/− mice have an increased number of DA synapses within the mPFC. The number and/or distribution of DA presynaptic terminals in the nucleus accumbens does not differ between adult dcc +/− and wild-type mice (C. Manitt and C. Flores, Montréal, Que.: unpublished observations, 2011).
Fig. 6.
Presynaptic and postsynaptic changes in medial prefrontal cortex (mPFC) dopamine (DA) circuitry of adult dcc heterozygous (+/−) mice. (A) Western blot for mean tyrosine hydroxylase (TH) immunoreactivity (and standard error of the mean) was performed on lysates from tissue punches taken from the pregenual mPFC (including cingulate 1, prelimbic and infralimbic subregions) of adult male dcc +/− and wild-type (+/+) mice. Student t test analysis revealed increases in tyrosine hydroxylase immunoreactivity in dcc +/− mice (t6 = 2.39, p = 0.05; n = 4 per group). Reproduced with permission from Flores et al.84 (B) Mean (and standard error of the mean) basilar dendritic spine density in Golgi-Cox–stained pregenual mPFC layer V pyramidal neurons of adult male dcc +/− and +/+ mice. Student t test analysis revealed a significant reduction in spine density in dcc +/− mice (t10 = 6.477, p < 0.001; n = 6 per group). Reproduced with permission from Grant et al.83
It is intriguing that studies conducted in postmortem brains of patients with schizophrenia reported opposite findings. In comparison to healthy controls, the total length of tyrosine hydroxylase–immunoreactive axons is reduced in layer VI of the dorsomedial prefrontal cortex of patients with schizophrenia — an effect that does not seem to result from chronic exposure to antipsychotic medication.95
Adult dcc +/− mice show blunted amphetamine-induced locomotion, resistance to amphetamine-induced deficits in prepulse inhibition and reduced sensitivity to amphetamine-induced conditioning place preference. These mice also show blunted amphetamine-induced DA release in the nucleus accumbens, but greater DA activity in the mPFC. This latter finding is associated with increased DA innervation within this region. All these traits are opposite to those observed in developmental animal models of schizophrenia, in the disorder itself and to those exhibited following repeated exposure to stimulant drugs of abuse. We propose that reduced DCC receptor activity during development leads to selective reorganization of mesocortical DA circuitry. My group’s working hypothesis is that this reorganization results in enhanced DA function within the mPFC, which in turn reduces responsiveness of DA neurons terminating in the nucleus accumbens, protecting against the development of DA-related behavioural abnormalities.
Structural changes in postsynaptic layer V pyramidal neurons of dcc +/− mice
Consistent with the idea that reduced DCC leads to changes in mPFC DA circuitry specifically, dcc +/− mice also exhibit structural alterations in layer V mPFC pyramidal neurons that are suggestive of reorganization in synaptic connectivity. In comparison to wild-type mice, layer V, but not layer III, pyramidal neurons of dcc +/− mice show significantly reduced density of dendritic spines (Fig. 6B83). In contrast, there are no differences in dendritic spine density in the nucleus accumbens medium spiny neurons between genotypes. Moreover, pyramidal neurons in the mPFC layer V of dcc +/− mice show differential organization and complexity of their basilar dendritic arbors; they have fewer and shorter dendritic arbors than wild-type controls.49 The mPFC layer V is the region that receives the densest DA innervation,96,97 expresses in the rat the highest levels of mRNA of DA D1 and D2 receptors98 and sends projections to the nucleus accumbens.99
Peripuberty as a critical period for the effects of reduced DCC on mesocorticolimbic DA function
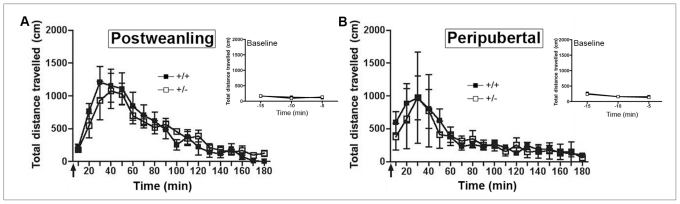
If the protective phenotype observed in adult dcc +/− mice results from alterations in mPFC DA circuitry, specifically, then it should become evident only on maturation of this system. To address this question, my group has examined whether postweanling (postnatal day 21 ± 1) and peripubertal (postnatal day 33 ± 2) male and female dcc +/− mice, which express reduced DCC levels throughout the brain, exhibit the behavioural, neurochemical and neuroanatomic alterations displayed by their adult counterparts. Our findings are striking: in contrast to adult dcc +/− mice, neither post-weanling nor peripubertal dcc +/− mice show blunted amphetamine-induced locomotor responses, elevated baseline concentrations of DA and DA metabolites in the mPFC, increased tyrosine hydroxylase protein levels in mPFC or reduced numbers of VTA DA neurons (e.g., Fig. 785). Significantly, the alterations in synaptic connectivity in mPFC DA circuitry observed in adult dcc +/− mice are not evident prepubertally.49 These results demonstrate clearly that the protective phenotype observed in adult dcc +/− mice only manifests after puberty, pointing to peripuberty as a critical period for the netrin-1–DA interaction.
Fig. 7.
Absence of the behavioural phenotype in prepubertal dcc heterozygous (+/−) mice. (A) Mean (and standard error of the mean) locomotor activity in postweanling (postnatal day 21 ± 1) male +/− and wild-type mice (+/+) in response to an injection of 2.5 mg/kg, intraperitoneally, of d-amphetamine sulfate (arrow). Baseline locomotor activity data obtained during a 15-minute period before the amphetamine injection are presented in the inset graph (n = 8 per group). (B) Mean (and standard error of the mean) locomotor activity in peripubertal (postnatal day 33 ± 2) male +/− and +/+ mice in response to an injection of 2.5 mg/kg, intraperitoneally, of d-amphetamine sulfate (arrow). Baseline locomotor activity data obtained during a 15-minute period before the amphetamine injection are presented in the inset graph (+/+, n = 10; +/−, n = 5). Reproduced with permission from Grant et al.85
The delayed emergence of structural changes in layer V mPFC pyramidal neurons of dcc +/− mice suggests that prior changes in synaptic formation and/or maintenance within this circuitry does not account for the adult phenotype. However, transient overexpression of DA D2 receptors in the striatum has been shown to produce reduced mPFC DA activity, selectively.27 The mesocortical dcc +/− phenotype could, therefore, result from prepubertal alterations in DA function in striatal regions. Our results to date indicate no differences in baseline DA activity in the nucleus accumbens or dorsal striatum.85 We are currently assessing for differences between genotypes in striatal density of D1 and D2 receptors using autoradiography.
The peripubertal period (adolescence) is thought to be a critical one for the establishment of individual differences in DA function and DA-related behaviours in adulthood. Intriguingly, this period coincides with the occurrence of dramatic maturational changes in the mesocortical DA system.41,100–105 Our findings suggest that netrin-1 signalling during adolescence may contribute to the organization of mPFC DA synaptic connectivity and, further, that risk factors associated with DA-related adult psychiatric disorders may target netrin-1 signalling mechanisms during development. Indeed, we have shown that ventral hippocampal lesions given to neonatal rats, a putative neurodevelopmental model of schizophrenia,46 produce dynamic, enduring and opposite changes in DCC expression in the mPFC and nucleus accumbens. Remarkably, for both regions, a “switch” in the direction of the effect occurs peripubertally. Alterations in mPFC, but not nucleus accumbens, DCC expression last into adulthood.106
Possible mechanisms mediating the effects of reduced DCC on the functional organization of the mesocortical DA circuitry
The cellular and molecular mechanisms by which netrin-1– DCC signalling may regulate the development of mesocortical DA circuitry are currently unknown. Based on my group’s results to date, we propose that 1 or more of the following processes is implicated.
Axonal branching
The postpubertal increases in tyrosine hydroxylase immunoreactivity and in the number of tyrosine hydroxylase–positive varicosities in the mPFC in dcc +/− mice suggest that reduced DCC leads to exaggerated axonal branching of mPFC DA fibres during puberty. This hypothesis is supported by the reduction in the total number of VTA DA neurons displayed by adult, but not prepubertal dcc +/− mice.84 Terminal arbors of individual DA axons have been shown to undergo extensive sprouting after partial loss of DA neurons in the substantia nigra.107 Furthermore, netrin-1 has been shown to promote axonal branching independently of axonal growth.108–110 This effect of netrin-1 seems to be more pronounced at specific developmental periods,109,111 is activity-dependent112,113 and can involve DCC-mediated signalling.69 Collateral branch formation, rather than extension of the primary growth cone, appears to be the main mechanism underlying cortical innervation.114 Thus, reduced DCC during development may lead to changes in DA wiring events that take place once axons from VTA DA neurons have reached the mPFC.
UNC5H-mediated cellular processes
The spatiotemporal regulation of the expression of guidance cue receptors is critical for axonal guidance, target selection/recognition and synaptogenesis. The emergence of UNC5H expression by DA neurons during the prepubertal period is, therefore, likely to be associated with critical events in the development of mPFC DA circuitry. Such events may include modifications in the number, shape and/or distribution of DA terminals. Because dcc +/− mice develop with reduced DCC levels, it is possible that UNC5H expression by DA neurons emerges earlier and that, as a consequence, the critical modifications in the development of mPFC DA circuitry occur earlier and/or are exaggerated in these mice. Initial examination of UNC5H/tyrosine hydroxylase immunofluorescent neurons and of the ratios of DCC:UNC5H in the VTA of postweanling, peripubertal and adult dcc +/− mice suggest that this is the case (C. Labelle-Dumais, A. Grant and C. Flores, Montréal, Que.: unpublished observations, 2009). In addition, my group is currently conducting experiments to determine whether the expression of UNC5H by VTA DA neurons is restricted to a specific subpopulation of projecting cells (e.g., mesocortical DA neurons).
Axon pruning
Netrin-1 signalling during the peripubertal period may also participate in the normal pruning of excitatory and inhibitory influences that occurs across species in the mPFC at that time.115–118 Netrin-1 signalling is involved in synaptogenesis, and guidance cues have been demonstrated recently to play a role in axon pruning.119 It is possible that the changes in synaptic circuitry within the mPFC observed in dcc +/− mice results from a reduction in the normal synaptic pruning that takes place peripubertally. Notably, excessive synaptic pruning in the prefrontal cortex has been proposed to occur in schizophrenia.120 Finally, developmental changes in the expression and/or activity of proteins involved in the regulation of DA synthesis, metabolism and clearance could also be involved.
Role of DCC receptor signalling in DA-related behavioural plasticity in adulthood
Sensitization to the effects of stimulant drugs
When animals, including humans, are repeatedly exposed to stimulant drugs, such as amphetamine, they develop increased sensitivity to their locomotor-activating and rewarding effects. This phenomenon, known as behavioural sensitization, is associated with sensitized drug-induced DA release in the nucleus accumbens.121–123 Cellular and molecular mechanisms underlying sensitization may be important to our understanding of the processes involved in the development of drug addiction and of stimulant drug–induced psychosis. Furthermore, the processes underlying sensitization within the midbrain DA systems may explain, at least in part, the high commorbidity between schizophrenia and drug abuse.
Sensitization develops gradually, persists over time and involves reorganization of mesocorticolimbic DA circuitry. Drug-induced neuronal modifications suggestive of alterations in patterns of synaptic connectivity have been identified in the VTA, nucleus accumbens and mPFC.124–127 The changes in dendritic structure in VTA neurons are particularly intriguing because the VTA is a critical site for the initiation of sensitization; administration of amphetamine directly into the VTA is sufficient to produce sensitized DA release in the nucleus accumbens and behavioural response to a subsequent systemic injection of this drug.122 Blockade of glutamate receptors directly in the VTA during stimulant drug pretreatment prevents the development of sensitization.128 Importantly, the reorganization of VTA neuronal circuitry by stimulant drugs of abuse is associated with actual functional changes in synaptic plasticity within this region.8
Upregulation of DCC expression in the VTA by amphetamine exposure during adulthood
Netrin-1 and its receptors continue to be highly expressed in mesocorticolimbic DA regions, including VTA DA neurons, of adult rodents. My group has hypothesized that the orchestrated functional reorganization of VTA DA circuitry by stimulant drugs may be achieved via selective spatiotemporal regulation of netrin-1 receptor expression. To test this hypothesis, we have conducted a series of studies using the amphetamine sensitization model in adult rats and mice, including dcc +/− mice. The amphetamine treatment regimen we have used in adult rats (3 mg/kg, 4 injections, once every day) and mice (4 mg/kg, 5 injections, once every other day) produces robust behavioural sensitization. That is, when 7 days after the pretreatment phase we challenge both amphetamine- and saline-pretreated animals with a low dose of amphetamine (1.5 and 2 mg/kg to rats and mice, respectively), drug-pretreated animals exhibit significantly greater amphetamine-induced locomotion than saline-pretreated animals.
We have found consistently that exposure to amphetamine results in upregulation (about double) of DCC expression in the VTA. Significantly, amphetamine regulates netrin-1 receptor expression in the VTA, selectively; we do not observe changes in the nucleus accumbens or mPFC. Furthermore, this upregulation results from the amphetamine pretreatment and not from the amphetamine challenge given on the sensitization test; amphetamine-pretreated animals given a saline injection on the test day exhibit increased DCC expression in the VTA. Finally, amphetamine-induced sensitization and upregulation of VTA DCC are prevented by cotreatment of an N-methyl-d-aspartate antagonist during the pretreatment phase.82,86 These findings suggest that the upregulation of VTA DCC expression by repeated amphetamine exposure may be implicated in the development of sensitization.
DCC signalling in the VTA is critical for amphetamine sensitization in adulthood
My group has now demonstrated that DCC function in the VTA during amphetamine pretreatment is required for the development of a sensitized response to amphetamine. Bilateral intra-VTA microinfusions of a DCC function–blocking antibody given 30 minutes before each amphetamine pretreatment injection prevent the development of sensitization in adult rats. This effect is evident 7 days after drug pretreatment and persists for at least 14 days. It is important to specify that on the test days for sensitization, animals do not receive VTA microinfusions of the antibody. Moreover, in parallel experiments, we have shown that the DCC function–blocking antibody can be visualized in the VTA 30 minutes, but not 7 days, after its microinfusion.86 The DCC antibody we used in these experiments has been previously shown to block DCC-mediated netrin-1 effects in vivo.69 Together, these results suggest that glutamate-mediated DCC receptor upregulation in the VTA is essential for the development of sensitization, most likely via effects on DA neurons and their local circuitry in the VTA.
Lack of sensitization in adult dcc +/− mice
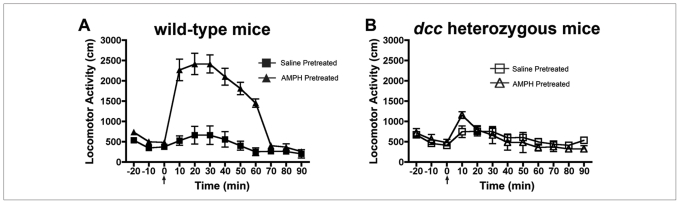
Consistent with a role for DCC receptor–signalling in the VTA in amphetamine-induced plasticity that my group has observed in adult rats, previous work I did has also shown that in contrast to wild-type mice, adult dcc +/− mice fail to develop sensitization when treated repeatedly with amphetamine (Fig. 8). Furthermore, the upregulation of DCC expression in the VTA seen in wild-type mice after repeated amphetamine is not observed in dcc +/− mice (Fig. 984,86).
Fig. 8.
Adult dcc heterozygous (+/−) mice do not develop sensitization when treated repeatedly with amphetamine. (A) Mean (and standard error of the mean) locomotor activity in response to 2 mg/kg, intraperitoneally, of d-amphetamine sulfate during the test for sensitization in wild-type (+/+) mice. This test was conducted 1 week after the last day of pretreatment with saline or amphetamine (4 mg/kg of amphetamine, 5 injections, once every other day; n = 7 per group). A 2-way mixed analysis of variance revealed that +/+ mice pretreated with amphetamine showed significantly greater locomotor activity in response to the amphetamine injection on the test day than +/+ mice exposed to this drug for the first time (i.e., saline pretreated; main effect of drug pretreatment: F1,12 = 15.2, p = 0.002). (B) Mean (and standard error of the mean) locomotor activity in response to 2 mg/kg, intraperitoneally, of d-amphetamine sulfate during the test for sensitization in dcc +/− mice. This test was conducted 1 week after the last day of pretreatment with saline (n = 4) or amphetamine (4 mg/kg of amphetamine, 5 injections, once every other day; n = 5). A 2-way mixed-measures analysis of variance revealed no significant difference in response to the amphetamine injection on the test day between amphetamine- and saline-pretreated dcc +/− mice (main effect of drug pretreatment: F1,7 = 0.07, p = 0.80). Reproduced with permission from Yetnikoff et al.86
Fig. 9.
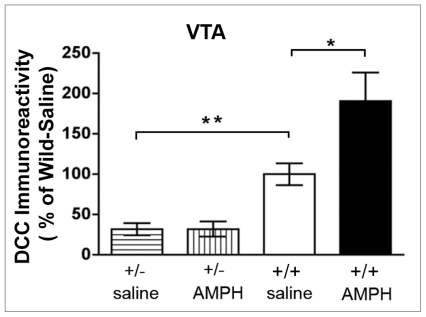
Effect of repeated amphetamine pretreatment on DCC receptor expression in the ventral tegmental area of adult dcc heterozygous (+/−) and wild-type (+/+) mice. Results from Western blot analysis of mean DCC immunoreactivity (and standard error of the mean) in the ventral tegmental area (VTA) of adult male dcc +/− and +/+ mice that received a pretreatment with saline or with d-amphetamine sulfate (AMPH; 4 mg/kg of amphetamine, 5 injections, once every other day). These mice were tested for sensitization 1 week after the last day of the pretreatment phase by injecting them with 2 mg/kg of amphetamine intraperitoneally (see Fig. 8); their brains were processed for Western blot immediately after this test (n = 7 per group). Repeated administration of amphetamine induced significant increases in DCC receptor expression in the VTA of +/+ mice, but did not alter DCC expression in dcc +/− mice. As expected, dcc +/− exhibited significantly less (about half) DCC expression than saline-pretreated wild-type controls (significant genotype × drug pretreatment interaction: F 1,15 = 4.79, p = 0.044; post hoc Student t tests: significant difference between amphetamine- and saline-pretreated +/+ groups, p < 0.05, and significant difference between saline-pretreated dcc +/− and saline-pretreated +/+ mice, p < 0.001). Reproduced with permission from Yetnikoff et al.86
Adult wild-type mice given repeated amphetamine show increased expression of the protein spinophilin in the VTA, whereas dcc +/− mice do not.86 Spinophilin is a protein associated exclusively with dendritic spines,129 and it is known that DCC–netrin-1 signalling is implicated in synaptic tagging.70,71 Thus, the role of DCC in sensitization appears to be related to drug-induced changes in synaptic connectivity within the VTA.
Exposure to amphetamine during the juvenile period reverses the protective phenotype of dcc +/− mice
Recent results are showing that the phenotype resilient against amphetamine-induced sensitization observed in dcc +/− mice in adulthood is not observed when these mice are treated repeatedly with amphetamine during the juvenile period. These findings have important clinical relevance because they suggest that in the human population, individuals with DCC heterozygosity88 have reduced risk of long-lasting DA and behavioural abnormalities induced by drugs of abuse. However, our findings also indicate that these individuals lose this “protection” if they are treated with stimulant drugs during childhood.130
Conclusion
Changes in the functional organization of mesolimbic and mesocortical DA circuitries at different periods of development appear to confer differential vulnerability to psychopathology. In my group’s search for molecular processes underlying the selective plasticity that these 2 DA projections and their targets undergo, we have found that netrin-1 signalling is critically implicated. We have demonstrated that netrin-1 is highly expressed by mesocorticolimbic DA neurons and their target regions, both before puberty and during adulthood. This pattern of expression appears to be maintained from early life to adulthood. Deleted in colorectal cancer and UNC5H receptors are also expressed by DA neurons. However, their pattern of expression is regulated developmentally. Whereas DCC receptors are expressed by VTA DA neurons throughout life, UNC5H expression by DA cells emerges only peripubertally. Importantly, single DA neurons coexpress DCC and UNC5H receptors in the adult VTA.
We have shown that DCC-mediated netrin-1 signalling plays a critical role in the development of mesocorticolimbic DA circuitries. Adult mice that develop with reduced levels of DCC protein expression, in comparison to wild-type controls, exhibit significantly reduced sensitivity to the effects of amphetamine on locomotion and reward, and are resistant against amphetamine-induced deficits in sensorimotor gating. These blunted behavioural responses are associated with diminished amphetamine-induced DA release in the nucleus accumbens, but with DA hyperactivity in the mPFC. These behavioural and DA phenotypes, which are opposite to those seen in schizophrenia or in developmental models of this disorder, are not observed in juvenile or peripubertal dcc +/− mice. Furthermore, we have identified that the increased DA activity in the mPFC observed in adult dcc +/− mice results from peripubertal alterations in the organization of DA synaptic connectivity within this region, including increased sprouting of DA axons and reduced dendritic arbor and spine density in layer V mPFC pyramidal neurons. The effects of DCC on the organization of mPFC DA circuitry appear to be selective to this region because we have not observed effects of reduced DCC on nucleus accumbens DA synaptic connectivity.
Our experiments conducted in adult rats and mice show that DCC-mediated netrin-1 signalling is implicated in the enduring DA and behavioural plasticity induced by repeated exposure to amphetamine in adulthood. Repeated amphetamine exposure leads to upregulation of DCC in the VTA selectively. Blockade of DCC receptor function in the VTA during repeated amphetamine treatment prevents sensitized behavioural response to a subsequent drug injection. Moreover, adult dcc +/− mice fail to develop sensitization when treated repeatedly with amphetamine and do not show drug-induced upregulation of DCC in the VTA. This protective phenotype against amphetamine-induced sensitization is not observed when dcc +/− mice are treated with amphetamine as juveniles.
It is important to clarify that we are aware that, in addition to the mPFC and nucleus accumbens DA systems, netrin-1 is likely to be implicated in the development, plasticity and function of DA neurons projecting to other targets and of other neurotransmitter systems. The effects of changes in netrin-1 signalling on other brain systems may contribute to the dcc phenotype we have observed in the dcc +/− mice. The fact that there is high expression of netrin-1, DCC and UNC5H by DA neurons in the substantia nigra pars compacta of adult rats and mice48 strongly suggest that netrin-1 signalling plays a role in the development, function and/or plasticity of the nigrostriatal DA system. Future studies will investigate this possibility. Furthermore, the combined, coordinated actions of netrin-1 and protein members of other families of guidance cues are likely to bring about the selective organization, function and plasticity of the mesocortical and mesolimbic DA systems. Indeed, members of the semaphorins, ephrins and slits families of guidance cues have been shown to participate in the development of mesocorticolimbic DA neurons (see Pasterkamp and colleagues,131 Cooper and colleagues,132 Dugan and colleagues133 and Lin and colleagues134).
Identifying developmental proteins that participate in the differential organization and function between mPFC and nucleus accumbens DA afferents can offer an important avenue for understanding, preventing and treating psychiatric disorders characterized by opposite dysfunction of these 2 systems. Our results indicate that netrin-1 signalling is a key factor. We are now in the process of assessing, in humans, whether genetic alterations in NTN1, DCC and/or UNC5H associate with increased risk or resilience to psychopathology, primarily schizophrenia and drug abuse.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (MOP-74709), the Natural Sciences and Engineering Research Council of Canada and salary awards from the Fonds de la recherche en santé du Québec. The work was accomplished as a result of the efforts of current and former lab members, primarily 2 current doctorate students, Alanna Grant and Leora Yetnikoff, and both a former and a current postdoctoral fellow, Cassandre Labelle-Dumais and Colleen Manitt, respectively. I thank Jane Stewart not only for her comments and suggestions on this manuscript, but also for all her support and guidance throughout my career. I also thank my collaborators Andreas Arvanitogiannis and Bryan Kolb, who have made essential contributions to the work reviewed here.
Footnotes
Competing Interests: None declared.
References
- 1.Le Moal M. Drug abuse: vulnerability and transition to addiction. Pharmacopsychiatry. 2009;42(Suppl 1):S42–55. doi: 10.1055/s-0029-1216355. [DOI] [PubMed] [Google Scholar]
- 2.Cabib S, Orsini C, Le Moal M, et al. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–5. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- 3.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 2009;206:587–602. doi: 10.1007/s00213-009-1504-9. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 6.Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(Suppl 1):i171–81. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–39. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisler S, Derst C, Veh RW, et al. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–74. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammel S, Hetzel A, Häckel O, et al. Unique properties of meso-prefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Sesack SR, Hawrylak VA, Matus C, et al. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morón JA, Brockington A, Wise RA, et al. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–95. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanda G, Pontieri FE, Frau R, et al. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–85. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 17.Käenmäki M, Tammimäki A, Myöhänen T, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114:1745–55. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- 18.Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–6. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castner SA, al-Tikriti MS, Baldwin RM, et al. Behavioral changes and [123I]IBZM equilibrium SPECT measurement of amphetamine-induced dopamine release in rhesus monkeys exposed to sub-chronic amphetamine. Neuropsychopharmacology. 2000;22:4–13. doi: 10.1016/S0893-133X(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 20.Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57:743–51. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Brake WG, Sullivan RM, Gratton A. Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J Neurosci. 2000;20:5538–43. doi: 10.1523/JNEUROSCI.20-14-05538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brake WG, Noel MB, Boksa P, et al. Influence of perinatal factors on the nucleus accumbens dopamine response to repeated stress during adulthood: an electrochemical study in the rat. Neuroscience. 1997;77:1067–76. doi: 10.1016/s0306-4522(96)00543-x. [DOI] [PubMed] [Google Scholar]
- 23.Ventura R, Alcaro A, Cabib S, et al. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- 24.Vezina P, Blanc G, Glowinski J, et al. Opposed behavioural outputs of increased dopamine transmission in prefrontocortical and subcortical areas: a role for the cortical D-1 dopamine receptor. Eur J Neurosci. 1991;3:1001–7. doi: 10.1111/j.1460-9568.1991.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 25.Banks KE, Gratton A. Possible involvement of medial prefrontal cortex in amphetamine-induced sensitization of mesolimbic dopamine function. Eur J Pharmacol. 1995;282:157–67. doi: 10.1016/0014-2999(95)00306-6. [DOI] [PubMed] [Google Scholar]
- 26.Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- 27.Kellendonk C, Simpson EH, Polan HJ, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–15. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Abi-Dargham A, van de Giessen E, Slifstein M, et al. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65:1091–3. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Abi-Dargham A. Probing cortical dopamine function in schizophrenia: What can D1 receptors tell us? World Psychiatry. 2003;2:166–71. [PMC free article] [PubMed] [Google Scholar]
- 30.Brozoski TJ, Brown RM, Rosvold HE, et al. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–32. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–71. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 32.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 33.Salo R, Ursu S, Buonocore MH, et al. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–9. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans AH, Pavese N, Lawrence AD, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–8. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 37.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 38.Gao M, Liu CL, Yang S, et al. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci. 2007;27:5414–21. doi: 10.1523/JNEUROSCI.5347-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voorn P, Kalsbeek A, Jorritsma-Byham B, et al. The pre- and post-natal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25:857–87. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 40.Kalsbeek A, Voorn P, Buijs RM, et al. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 41.Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 43.Lidov HG, Grzanna R, Molliver ME. The serotonin innervation of the cerebral cortex in the rat — an immunohistochemical analysis. Neuroscience. 1980;5:207–27. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- 44.Levitt P, Moore RY. Development of the noradrenergic innervation of neocortex. Brain Res. 1979;162:243–59. doi: 10.1016/0006-8993(79)90287-7. [DOI] [PubMed] [Google Scholar]
- 45.Boksa P. Animal models of obstetric complications in relation to schizophrenia. Brain Res Brain Res Rev. 2004;45:1–17. doi: 10.1016/j.brainresrev.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–97. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Manitt C, Labelle-Dumais C, Eng C, et al. Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS ONE. 2010;5:e11463. doi: 10.1371/journal.pone.0011463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manitt C, Mimee A, Eng C, et al. 2010 Neuroscience Meeting Planner. San Diego (CA): Society for Neuroscience; 2010. Mice heterozygous for the netrin-1 receptor DCC exhibit alterations in mesocortical dopamine synaptic connectivity. Program no. 471.1/FF18. [Google Scholar]
- 50.Manitt C, Kennedy TE. Where the rubber meets the road: netrin expression and function in developing and adult nervous systems. Prog Brain Res. 2002;137:425–42. doi: 10.1016/s0079-6123(02)37034-1. [DOI] [PubMed] [Google Scholar]
- 51.Barallobre MJ, Pascual M, Del Río JA, et al. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Suli A, Mortimer N, Shepherd I, et al. Netrin/DCC signaling controls contralateral dendrites of octavolateralis efferent neurons. J Neurosci. 2006;26:13328–37. doi: 10.1523/JNEUROSCI.2858-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furrer MP, Kim S, Wolf B, et al. Robo and Frazzled/DCC mediate dendritic guidance at the CNS midline. Nat Neurosci. 2003;6:223–30. doi: 10.1038/nn1017. [DOI] [PubMed] [Google Scholar]
- 54.Hong K, Hinck L, Nishiyama M, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–41. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 55.Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–17. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 56.Shekarabi M, Moore SW, Tritsch NX, et al. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J Neurosci. 2005;25:3132–41. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Saint-Cyr-Proulx E, Aktories K, et al. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J Biol Chem. 2002;277:15207–14. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]
- 58.Rajasekharan S, Bin JM, Antel JP, et al. A central role for RhoA during oligodendroglial maturation in the switch from netrin-1-mediated chemorepulsion to process elaboration. J Neurochem. 2010;113:1589–97. doi: 10.1111/j.1471-4159.2010.06717.x. [DOI] [PubMed] [Google Scholar]
- 59.Hata K, Kaibuchi K, Inagaki S, et al. Unc5B associates with LARG to mediate the action of repulsive guidance molecule. J Cell Biol. 2009;184:737–50. doi: 10.1083/jcb.200807029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore SW, Correia JP, Lai Wing Sun K, et al. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development. 2008;135:2855–64. doi: 10.1242/dev.024133. [DOI] [PubMed] [Google Scholar]
- 61.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 62.Bradford D, Cole SJ, Cooper HM. Netrin-1: diversity in development. Int J Biochem Cell Biol. 2009;41:487–93. doi: 10.1016/j.biocel.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Rajasekharan S, Kennedy TE. The netrin protein family. Genome Biol. 2009;10:239. doi: 10.1186/gb-2009-10-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muramatsu R, et al. The ratio of ‘deleted in colorectal cancer’ to ‘uncoordinated-5A’ netrin-1 receptors on the growth cone regulates mossy fibre directionality. Brain. 2010;133:60–75. doi: 10.1093/brain/awp266. [DOI] [PubMed] [Google Scholar]
- 65.Bouchard JF, Moore SW, Tritsch NX, et al. Protein kinase A activation promotes plasma membrane insertion of DCC from an intra-cellular pool: a novel mechanism regulating commissural axon extension. J Neurosci. 2004;24:3040–50. doi: 10.1523/JNEUROSCI.4934-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams ME, Wu SC, McKenna WL, et al. Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J Neurosci. 2003;23:11279–88. doi: 10.1523/JNEUROSCI.23-36-11279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kennedy TE, Wang H, Marshall W, et al. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–74. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deiner MS, Kennedy TE, Fazeli A, et al. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–89. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 69.Manitt C, Nikolakopoulou AM, Almario DR, et al. Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J Neurosci. 2009;29:11065–77. doi: 10.1523/JNEUROSCI.0947-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–6. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–73. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Livesey FJ, Hunt SP. Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol Cell Neurosci. 1997;8:417–29. doi: 10.1006/mcne.1997.0598. [DOI] [PubMed] [Google Scholar]
- 73.Volenec A, Zetterstrom TS, Flanigan TP. 6-OHDA denervation substantially decreases DCC mRNA levels in rat substantia nigra compacta. Neuroreport. 1998;9:3553–6. doi: 10.1097/00001756-199811160-00002. [DOI] [PubMed] [Google Scholar]
- 74.Harter PN, Bunz B, Dietz K, et al. Spatio-temporal deleted in colorectal cancer (DCC) and netrin-1 expression in human foetal brain development. Neuropathol Appl Neurobiol. 2010;36:623–35. doi: 10.1111/j.1365-2990.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- 75.Ly A, Nikolaev A, Suresh G, et al. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–54. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bradford D, Faull RL, Curtis MA, et al. Characterization of the netrin/RGMa receptor neogenin in neurogenic regions of the mouse and human adult forebrain. J Comp Neurol. 2010;518:3237–53. doi: 10.1002/cne.22397. [DOI] [PubMed] [Google Scholar]
- 77.Baker KA, Moore SW, Jarjour AA, et al. When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr Opin Neurobiol. 2006;16:529–34. doi: 10.1016/j.conb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Mehlen P, Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol. 2010;22:46–54. doi: 10.1097/CCO.0b013e328333dcd1. [DOI] [PubMed] [Google Scholar]
- 79.Castets M, Mehlen P. Netrin-1 role in angiogenesis: To be or not to be a pro-angiogenic factor? Cell Cycle. 2010:9. doi: 10.4161/cc.9.8.11197. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Kennedy TE, Serafini T, de la Torre JR, et al. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 81.Osborne PB, Halliday GM, Cooper HM, et al. Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience. 2005;131:671–81. doi: 10.1016/j.neuroscience.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 82.Yetnikoff L, Labelle-Dumais C, Flores C. Regulation of netrin-1 receptors by amphetamine in the adult brain. Neuroscience. 2007;150:764–73. doi: 10.1016/j.neuroscience.2007.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grant A, Hoops D, Labele-Dumais C, et al. Netrin-1 receptor-deficient mice show enhanced mesocortical dopamine transmission and blunted behavioural responses to amphetamine. Eur J Neurosci. 2007;26:3215–28. doi: 10.1111/j.1460-9568.2007.05888.x. [DOI] [PubMed] [Google Scholar]
- 84.Flores C, Manitt C, Rodaros D, et al. Netrin receptor deficient mice exhibit functional reorganization of dopaminergic systems and do not sensitize to amphetamine. Mol Psychiatry. 2005;10:606–12. doi: 10.1038/sj.mp.4001607. [DOI] [PubMed] [Google Scholar]
- 85.Grant A, Speed Z, Labelle-Dumais C, et al. Post-pubertal emergence of a dopamine phenotype in netrin-1 receptor-deficient mice. Eur J Neurosci. 2009;30:1318–28. doi: 10.1111/j.1460-9568.2009.06919.x. [DOI] [PubMed] [Google Scholar]
- 86.Yetnikoff L, Eng C, Benning S, et al. Netrin-1 receptor in the ventral tegmental area is required for sensitization to amphetamine. Eur J Neurosci. 2010;31:1292–302. doi: 10.1111/j.1460-9568.2010.07163.x. [DOI] [PubMed] [Google Scholar]
- 87.Fazeli A, Dickinson SL, Hermiston ML, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 88.Srour M, Rivière JB, Pham JM, et al. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- 89.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 90.Swerdlow NR, Martinez ZA, Hanlon FM, et al. Toward understanding the biology of a complex phenotype: rat strain and sub-strain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J Neurosci. 2000;20:4325–36. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tenn CC, Kapur S, Fletcher PJ. Sensitization to amphetamine, but not phencyclidine, disrupts prepulse inhibition and latent inhibition. Psychopharmacology (Berl) 2005;180:366–76. doi: 10.1007/s00213-005-2253-z. [DOI] [PubMed] [Google Scholar]
- 92.Swerdlow NR, Mansbach RS, Geyer MA, et al. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology (Berl) 1990;100:413–6. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- 93.Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benes FM, Vincent SL, Molloy R, et al. Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse. 1996;23:237–45. doi: 10.1002/(SICI)1098-2396(199608)23:4<237::AID-SYN1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 95.Akil M, Pierri JN, Whitehead RE, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–9. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 96.Fallon J, Loughlin S. Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia. In: Jones E, Peters A, editors. Cerebral cortex. Vol. 6. New York (NY): Plenum; 1987. pp. 41–127. [Google Scholar]
- 97.Van Eden CG, Hoorneman EM, Buijs RM, et al. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–62. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- 98.Vincent SL, Khan Y, Benes FM. Cellular colocalization of dopamine D1 and D2 receptors in rat medial prefrontal cortex. Synapse. 1995;19:112–20. doi: 10.1002/syn.890190207. [DOI] [PubMed] [Google Scholar]
- 99.Carr DB, O’Donnell P, Card JP, et al. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci. 1999;19:11049–60. doi: 10.1523/JNEUROSCI.19-24-11049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 101.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 102.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 103.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–98. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 105.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 106.Flores C, Bhardwaj SK, Labelle-Dumais C, et al. Altered netrin-1 receptor expression in dopamine terminal regions following neonatal ventral hippocampal lesions in the rat. Synapse. 2009;63:54–60. doi: 10.1002/syn.20584. [DOI] [PubMed] [Google Scholar]
- 107.Finkelstein DI, Stanic D, Parish CL, et al. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 108.Lim YS, Mallapur S, Kao G, et al. Netrin UNC-6 and the regulation of branching and extension of motoneuron axons from the ventral nerve cord of Caenorhabditis elegans. J Neurosci. 1999;19:7048–56. doi: 10.1523/JNEUROSCI.19-16-07048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dent EW, Barnes AM, Tang F, et al. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–12. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hao JC, Adler CE, Mebane L, et al. The tripartite motif protein MADD-2 functions with the receptor UNC-40 (DCC) in netrin-mediated axon attraction and branching. Dev Cell. 2010;18:950–60. doi: 10.1016/j.devcel.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halloran MC, Kalil K. Selective neurite outgrowth of cultured cortical neurons on specific regions of brain cryostat sections. J Comp Neurol. 1996;371:72–84. doi: 10.1002/(SICI)1096-9861(19960715)371:1<72::AID-CNE4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 112.Tang F, Kalil K. Netrin-1 induces axon branching in developing cortical neurons by frequency-dependent calcium signaling pathways. J Neurosci. 2005;25:6702–15. doi: 10.1523/JNEUROSCI.0871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J Neurosci. 2008;28:143–53. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14:2161–77. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 116.Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–96. [PubMed] [Google Scholar]
- 117.Gonzalez-Burgos G, Kroener S, Zaitsev AV, et al. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–37. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- 118.Andersen SL, Thompson AT, Rutstein M, et al. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–9. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 119.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–76. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence. J Psychiatr Res. 1982–1983;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 121.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 122.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alcantara AA, Lim HY, Floyd CE, et al. Cocaine- and morphine-induced synaptic plasticity in the nucleus accumbens. Synapse. 2011;65:309–20. doi: 10.1002/syn.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mueller D, Chapman CA, Stewart J. Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor. Neuroscience. 2006;137:727–35. doi: 10.1016/j.neuroscience.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 126.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sarti F, Borgland SL, Kharazia VN, et al. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci. 2007;26:749–56. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- 128.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 129.Ouimet CC, Katona I, Allen P, et al. Cellular and subcellular distribution of spinophilin, a PP1 regulatory protein that bundles F-actin in dendritic spines. J Comp Neurol. 2004;479:374–88. doi: 10.1002/cne.20313. [DOI] [PubMed] [Google Scholar]
- 130.Yetnikoff L, Arvanitogiannis A, Flores C. 2010 Neuroscience Meeting Planner. San Diego (CA): Society for Neuroscience Abstracts; 2010. The adult phenotype of netrin-1 receptor deficient mice is reversed by prepubertal administration of repeated amphetamine. Program no. 367.9/GG12. [Google Scholar]
- 131.Pasterkamp RJ, Kolk SM, Hellemons JA, et al. Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration. BMC Dev Biol. 2007;7:98. doi: 10.1186/1471-213X-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cooper MA, Kobayashi K, Zhou R. Ephrin-A5 regulates the formation of the ascending midbrain dopaminergic pathways. Dev Neurobiol. 2009;69:36–46. doi: 10.1002/dneu.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dugan JP, Stratton A, Riley HP, et al. Midbrain dopaminergic axons are guided longitudinally through the diencephalon by Slit/Robo signals. Mol Cell Neurosci. 2011;46:347–56. doi: 10.1016/j.mcn.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin L, Rao Y, Isacson O. Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Mol Cell Neurosci. 2005;28:547–55. doi: 10.1016/j.mcn.2004.11.009. [DOI] [PubMed] [Google Scholar]