Abstract
Highly homologous B-cell receptors, characterized by non-random combinations of immunoglobulin heavy-chain variable (IGHV) genes and heavy-chain complementarity determining region-3 (HCDR3), are expressed in a recurrent fraction of patients affected by chronic lymphocytic leukemia (CLL). We investigated the IGHV status of 1131 productive IG rearrangements from a panel of 1126 CLL patients from a multicenter Italian study group, and correlated the presence and class of HCDR3 stereotyped subsets with the major cytogenetic alterations evaluated by FISH, molecular prognostic factors, and the time to first treatment (TTFT) of patients with early stage disease (Binet A). Stereotyped HCDR3 sequences were found in 357 cases (31.7%), 231 of which (64.7%) were unmutated. In addition to the previously described subsets, 31 new putative stereotypes subsets were identified. Significant associations between different stereotyped HCDR3 sequences and molecular prognostic factors, such as CD38 and ZAP-70 expression, IGHV mutational status and genomic abnormalities were found. In particular, deletion of 17p13 was significantly represented in stereotype subset #1. Notably, subset #1 was significantly correlated with a substantially reduced TTFT compared to other CLL groups showing unmutated IGHV, ZAP-70 or CD38 positivity and unfavorable cytogenetic lesions including del(17)(p13). Moreover, subset #2 was strongly associated with deletion of 13q14, subsets #8 and #10 with trisomy 12, whereas subset #4 was characterized by the prevalent absence of the common cytogenetic abnormalities. Our data from a large and representative panel of CLL patients indicate that particular stereotyped HCDR3 sequences are associated with specific cytogenetic lesions and a distinct clinical outcome.
Introduction
Chronic lymphocytic leukemia (CLL) is a common disorder characterized by the monoclonal accumulation of B lymphocytes with a distinct phenotype (CD5-positive, CD23-positive, CD22-negative and low level of surface Ig) and a highly variable clinical course [1]–[3]. A different clinical outcome has been associated with peculiar cellular and molecular markers and/or specific genomic alterations [4]–[6]. In particular, the mutational status of the immunoglobulin heavy-chain variable (IGHV) gene defines two disease subgroups; one subgroup is characterized by the absence of somatic mutation in CLL cells and has the worst clinical course and outcome, whereas the other, with somatic mutations in IGHV genes, has a more benign prognosis and outcome [7], [8]. A biased repertoire of IGHV-diversity (D)- joining (J) genes has been reported to characterize the B cell receptor (BCR) in CLL, with a different prevalence of certain genes in the mutated (M) or unmutated (UM) group, respectively [9]. Moreover, more than 20% of CLL patients exhibit closely homologous (“stereotyped”) heavy chain complementary-determining region 3 (HCDR3) sequences and approximately 1% of these also carry virtually identical IGHV amino-acid sequences [10]–[13]. These findings have suggested that clones sharing stereotyped BCRs may expand because of stimulation by a restricted set of epitopes and that antigenic driving may play an important role in the pathogenesis of the disease [6], [14]–[16].
Although recent data have suggested the existence of specific correlation between stereotyped subsets and common cytogenetic lesions [17], [18] or clinical outcome [10], [13], [19], [20], it remains to be defined whether the expression of distinct BCRs in CLL may be relevant to the molecular and cytogenetic profile and/or to the clinical outcome in at least a fraction of patients.
In the present study, we investigated the BCR repertoire in 1126 CLL patients recruited by a multicenter Italian study group. Based on previously reported criteria [10], [21] and canonical sequence alignment procedures, we searched for the known stereotyped subsets in three publicly available data sets [10], [22], [23], as well as for potential novel subsets by performing a pair-wise alignment in the proprietary dataset. The most represented stereotyped subsets were then investigated for their association with the common molecular and cytogenetic features as well as for their impact on clinical outcome of early stage patients (Binet A).
Methods
Patient samples
Written informed consent was obtained from all patients in accordance with the declaration of Helsinki and the study was approved by the local Ethics Review Committee (Comitato Etico Provinciale, Modena, Italy). All patients were diagnosed according to the National Cancer Institute Working Group criteria [24]. Our dataset counted a total of 1126 CLL patients with productive IGHV-D-J rearrangement included in retrospective (745 patients) and prospective (381 patients, O-CLL1-GISL protocol) multicenter Italian studies from all over the country. In all cases genomic, cytogenetic and molecular analyses were performed on highly purified peripheral mononuclear B-cells from blood samples collected within one year of diagnosis, provided that the patient remained untreated.
Molecular and FISH analyses
CLL IGHV gene usage and mutation were determined as previously described and the 98% homology cut-off value was used to discriminate the M or UM IGHV configuration [9]. ZAP-70 and CD38 expression were investigated by immunophenotypic analysis as previously described [25]–[27]. Specifically, a cut-off ≥20% or ≥30% positive cells was chosen to discriminate ZAP-70 or CD38 positive from negative patients. Cytogenetic abnormalities involving deletions at chromosomes 11q23, 13q14 and 17p13 and trisomy of chromosome 12 were investigated by fluorescence in situ hybridization (FISH) as previously described [28]. FISH analyses were performed in all of the patients for whom biological material was available, and no prior selection based on age or disease progression was applied. Time to First Treatment (TTFT) was defined as time from diagnosis to first line treatment (event) or to last follow-up (censored observation). Treatment was decided uniformly in all participating centers based on documented progressive and symptomatic disease according to NCI working guidelines [24]. TTFT information was available for 739 patients (661 staged as Binet A; 56 as Binet B and 22 as Binet C), median follow up was 30 months (range 1–316 months), and 237 (32.1%) patients had received treatment by the end of the follow up.
Identification of stereotyped subsets and statistical analysis
We assigned a stereotyped cluster label to our HCDR3 sequences by means of pair-wise alignment with known stereotyped sequences available from different public databases [10], [22], [23]. In concordance with previously proposed methods, we applied a primary filter excluding pairs of sequences whose length differed more than 3 amino acids and we considered as stereotyped those sequences sharing more than 60% identity on alignments showing less than 3 gaps [10], [29]. Such analysis was performed using a global alignment algorithm [30] with BLOSUM62 as the similarity matrix [31] under the BioStrings package for Bioconductor. The same approach was applied to discover new potential stereotyped clusters with pair-wise alignments of the sequences from the proprietary database. A supplemental “GX” number was assigned to novel putative subsets not previously included in the Murray et al and Bomben et al nomenclature system [22], [23]. All contingency analyses were performed by Fisher's Exact test. The competing effect of death on the relationship between TTFT and stereotyped BCRs was modeled by proportional hazards of competing risks. Correlation with TTFT was tested between the considered groups in Binet A patients using the crr function of cmprsk package in R software [32]. A P value <0.05 was considered significant for all statistical calculations. All data were statistically analyzed using conventional procedures available in R (www.r-project.org).
Results
A total of 1126 CLL patients were investigated for productive IGHV-D-J rearranged sequences; 5 patients carried a double in frame productive rearrangement. Based on the 98% homology criteria, 405/1126 patients (36%) were classified as UM (Table 1 and Figure S1). IGHV, IGHD and IGHJ gene type and distribution are reported in Figure S2A–C [10], [22], [23]. ZAP-70 and CD38 expression were positive in 367/1011 (36.3%) and 306/1051 (29.1%%) of cases, respectively. Interphase FISH was performed on 704 patients and at least one abnormality was found in 466/704 (66.2%) of cases. Based on proposed hierarchical classification [4]–[6], del(13)(q14) was found as the sole abnormality in 287/704 (40.7%); trisomy 12 was found in 98/704 (13.9%) patients and associated with del(13)(q14) in 9 cases; del(11)(q23) was found in 46/704 (6.5%) patients and associated with del(13)(q14) and trisomy 12 in 25 and 1 cases, respectively; del(17)(p13) was found in 35/704 (5%) patients and associated in 5 and in 1 cases with del(13)(q14) and trisomy 12, respectively.
Table 1. Biological, molecular and cytogenetic features of CLL patients included in the study.
| Variable | N° of positive/investigated patients (%) | Correlation with TTFT |
| ZAP-70 | 367/1011 (36.3%) | P<0.0001 |
| CD38 | 306/1051 (29.1%) | P<0.0001 |
| IGHV * | 405/1126 (36%) | P<0.0001 |
| No FISH alteration ** | 238/704 (33.8%) | P<0.0001 |
| Del(13)(q14) | 287/704 (40.7%) | |
| Trisomy 12 | 98/704 (13.9%) | |
| Del(11)(q23) | 46/704 (6.5%) | |
| Del(17)(p13) | 35/704 (5%) |
Identification of stereotyped sequences
To identify stereotyped HCDR3 sequences occurring in our dataset, we performed a global alignment analysis which allowed (a) to compare each of our cases with publicly available data [10], [22], [23] and (b) to investigate the occurrence of new putative stereotypes within the proprietary database. Using this approach, stereotyped sequences were found in 357/1126 (31.7%) of the patients, 64.7% (231/357) of which were UM (P<0.0001), further supporting previous evidence [10], [22], [23].
Among patients with stereotyped HCDR3, 294 (82.3%) belonged to previously described subsets. In particular, the most recurrent subsets identified in our study were #1 (32 pts), #4 (29 pts), #7 (22 pts), #2 (20 pts), #3 (16 pts) and #9 (15 pts) (Table S1). Of note, the global alignment procedures performed on pair-wise sequences of our dataset allowed the identification of 31 new putative subsets in 63 patients (63/357;17.7%) recorded with a progressive code from G1 to G31 (see Table S2).
Correlation between cellular and molecular features with stereotyped BCRs in CLL
We then evaluated the prevalence of known molecular, biological and cytogenetic markers in the most represented and characterized stereotyped subsets of our dataset (Table 2). Subset #1 (IGHV1-5-7/IGHD6-19/IGHJ4) was the most frequent in our cohort. Despite the relatively heterogeneous gene usage, it was associated with UM IGHV genes in all of the cases (32pts). Subset #1 patients were more frequently ZAP-70 and CD38 positive compared to all the other patients (P<0.0001) or those utilizing the same IGHV genes without stereotyped HCDR3 (P = 0.0002 and P<0.0001 for ZAP-70 and CD38, respectively). However, no significant association between subset #1 and ZAP-70/CD38 positivity was identified when compared to patients with the UM IGHV configuration (data not shown). Considering only patients (18/32;56.3%) evaluated by FISH, we observed a higher prevalence of unfavorable deletions (7/18; 38.9%), particularly del(17)(p13) (5/18; 27.8%) (Table 2). Notably, the prevalence of del(17p)(p13) in subset #1 patients was significantly higher than that found in all the remaining patients (30/686;4.3%) (P = 0.0012). To avoid that the prevalent UM status in subset #1 may represent a bias factor, we compared the frequency of del(17)(p13) between subset #1 and all the remaining UM patients (22/244; 9%) confirming the previous association (P = 0.0266). Furthermore, comparing subset #1 patients with those showing the same IGHV gene usage without homologous HCDR3, we observed that del(17)(p13) retained its significant correlation (P = 0.0064). The percentage (median 78%: range 33.5–99) of malignant cells carrying the del(17)(p13) in subset #1 patients did not differ significantly in the remaining ones (30pts) having the del(17)(p13) (data not shown).
Table 2. The molecular, cytogenetic and clinical characterization of the most representative and described subsets.
| Subset | N° of pts | Binet A* | UM | CD38 | ZAP-70 | FISH-neg | del(13) | 12 | del(11) | del(11) and del(13) | del(17) | del(17) and del(13) |
| #1 | 32 | 13 | 32/32 | 21/32 | 22/30 | 4/18 | 2/18 | 5/18 | 2/18 | 0/18 | 3/18 | 2/18 |
| #2 | 20 | 12 | 10/20 | 3/17 | 5/15 | 1/13 | 10/13 | 0/13 | 0/13 | 2/13 | 0/13 | 0/13 |
| #3 | 16 | 8 | 16/16 | 6/14 | 10/13 | 6/11 | 0/11 | 3/11 | 0/11 | 0/11 | 2/11 | 0/11 |
| #4 | 29 | 19 | 1/29 | 0/25 | 3/24 | 13/23 | 9/23 | 1/23 | 0/23 | 0/23 | 0/23 | 0/23 |
| #5 | 5 | 2 | 5/5 | 3/4 | 2/4 | 0/2 | 0/2 | 1/2 | 0/2 | 1/2 | 0/2 | 0/2 |
| #6 | 5 | 2 | 5/5 | 3/3 | 1/3 | 2/4 | 1/4 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 |
| #7 | 22 | 10 | 22/22 | 12/21 | 16/21 | 4/13 | 4/13 | 1/13 | 2/13 | 2/13 | 0/13 | 0/13 |
| #8 | 8 | 5 | 8/8 | 6/7 | 7/7 | 1/7 | 0/7 | 5/7 | 0/7 | 0/7 | 1/7 | 0/7 |
| #9 | 15 | 7 | 15/15 | 6/14 | 6/10 | 1/9 | 2/9 | 2/9 | 2/9 | 1/9 | 1/9 | 0/9 |
| #10 | 11 | 5 | 10/10 | 8/10 | 10/10 | 1/7 | 0/7 | 6/7 | 0/7 | 0/7 | 0/7 | 0/7 |
*Patients in Binet A with complete follow-up.
A number of additional correlations between distinct stereotyped HCDR3 and cytogenetic/molecular features were evidenced in our panel. The cytogenetic profile of subset #2 (IGHV3-21) was characterized by a very strong prevalence of del(13)(q14) (12/13; 92.3%), found as the sole abnormality in 10 patients and associated with the del(11)(q23) in the remaining two (Table 2). When compared to the whole panel (313/684; 45.7%), this finding was statistically significant (P = 0.0009). Of note, differently from another group [13] we did not observe any significant difference between patients admitted to Institutions from North or South Italy in terms of IGHV3-21 usage (20/548 and 14/578 respectively) or subset #2 (13 and 6, respectively) prevalence (Table S3).
Subset #4 (IGHV4-34) was characterized by an almost complete M IGHV configuration and negative ZAP-70/CD38 expression (Table 2). In addition, we found that subset #4 patients were characterized by a low incidence of genomic aberrations. In fact, among the 23 cases investigated by FISH, 13 (56.5%) were negative for the most common cytogenetic lesions; this finding was statistically significant when compared to all patients (225/681; P = 0.02) or those with M IGHV configuration (142/420; P = 0.0408). Trisomy 12 was strongly associated with both subset #8 (IGHV4-39/IGHD6-13/IGHJ5) (5/7; 71.4%) and subset #10 (IGHV4-39 and IGHV2-5/IGHD2-2/IGHJ6) (6/7; 85.7%), confirming its higher prevalence in IGHV4-39 stereotyped patients [17], [20]. Specifically, trisomy 12 was significantly associated with subset #8 and #10 either when all patients or only those with UM IGHV configuration were considered (data not showed). Finally, subsets #8 and #10 were strongly associated with ZAP-70 positivity when all patients were considered (P = 0.0008 and P<0.0001 respectively). Instead, only subset #10 retained its significant association with ZAP-70 considering only UM patients (P = 0.017). Moreover, a significant association between subset #8 and #10 and CD38 positivity was found when compared to all patients (P = 0.003 and P = 0.0013 respectively), but not to UM patients.
Clinical relevance of stereotyped subsets
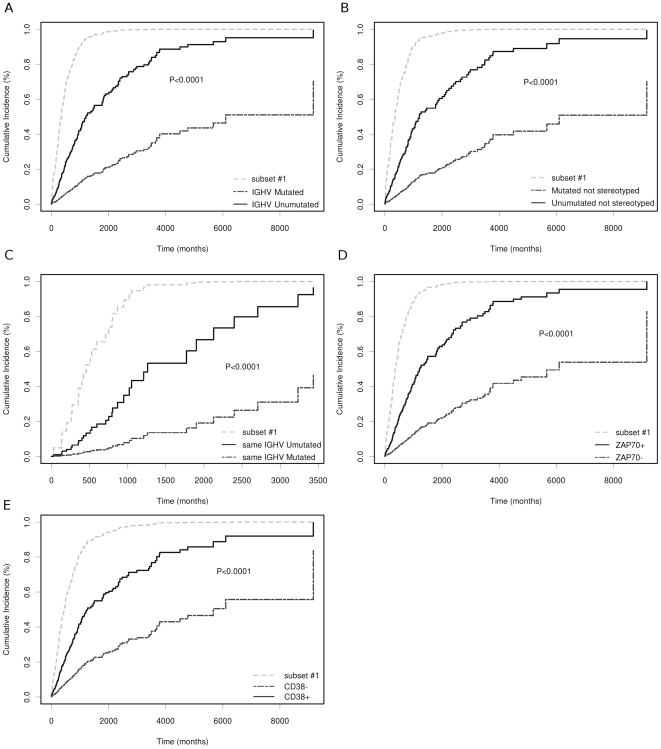
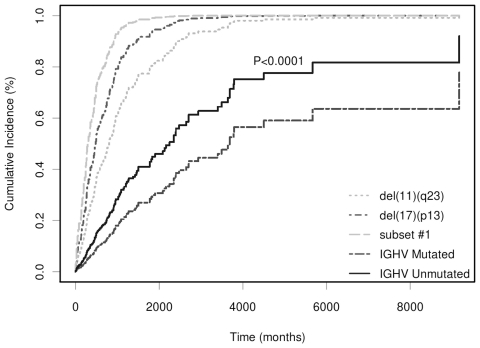
We investigated whether the stereotype configuration was correlated with disease progression. For this purpose only Binet A patients were considered. Subset #1 (13 patients, see Table 2) exhibited a significantly reduced TTFT when compared to all UM patients (P<0.0001), UM non-stereotyped patients (P<0.0001), or UM patients with the same IGHV gene usage (P<0.0001) (Figure 1A–C). Additionally, subset #1 was associated with an increased risk of earlier treatment compared to the presence of ZAP-70 and CD38 positivity (P<0.0001) (Figure 1D–E). The clinical course of subset #1 patients appeared to be similar to that of patients with del(17p) or del(11q) (Figure 2). A multivariate analysis using a proportional hazard model showed that subset #1 retained independence (P = 0.0132) from del(17p), del(11q), IGHV configuration and ZAP-70 or CD38 positivity. Moreover, considering only UM patients, the prognostic power of subset #1 maintained its significant independence from ZAP-70, del(17p), del(11q) and CD38 (P = 0.0102).
Figure 1. TTFT analysis of subset #1.
A: Subset #1 patients compared with all UM and M patients. B: Subset #1 compared with all non-stereotyped UM and non-stereotyped M patients. C: Subset #1 compared with all UM and M patients showing the same IGHV usage of subset #1. D and E: Subset #1 patients compared with the remaining cases grouped according to ZAP-70 and CD38 expression positivity, respectively.
Figure 2. TTFT analysis of subset #1.
Subset #1 patients were compared to those positive for del(17)(13p) and del(11)(q23), and those with UM or M IGHV status.
In agreement with previous reports [13], [19], [22], subset #2 (IGHV3-21) stereotyped patients had an unfavorable clinical course compared to all other patients (P = 0.00033) or to those utilizing non-stereotyped IGHV3-21 sequences (P = 0.052). In addition, subset #2 did not show a more unfavorable clinical course compared to UM patients (P>0.05).
Patients in subset #4 were characterized by a long TTT. In fact, only 2/19 patients in Binet A had been treated at the time of the study: of these, one was highly positive for ZAP-70 and the other was the only one with UM IGHV configuration.
Finally, likely due to the low number of cases in our series, we were unable to confirm the previously described [10] favorable or unfavorable clinical outcome of subset #5 (IGHV1-69/IGHD3-10/IGHJ6) or subset #3 (IGHV1-69/IGHD2-2/IGHJ6), respectively.
Discussion
In order to contribute to the elucidation of HCDR3 stereotyping in CLL, we characterized the BCR repertoire in a comprehensive panel of 1126 patients with the following aims: (a) to investigate whether HCDR3 stereotyped sequences might be correlated with molecular and cytogenetic profiles; and (b) to evaluate the putative clinical relevance in terms of TTFT for the most represented stereotypes.
In our study, we identified a total of 31.7% stereotyped HCDR3 sequences using an amino acid sequence alignment approach according to previously reported criteria [10]. This percentage was slightly higher than those reported to date [10], [22], [23], a finding in all likelihood related to the assessment of proprietary database stereotypes against previously reported ones. In fact, limiting the analysis only to patients included in our cohort, the percentage decreased to 28.3%, as 31 novel putative stereotyped sequences were identified after comparison with the published registries. Therefore, such a procedure (auto-matching and matching with published data) may represent an optimal and unbiased approach to perform stereotyped BCR characterization in CLL.
Our study revealed that subset #1, known to be the most frequent (9% of all stereotyped cases and 7.9% of all UM patients in our series) and characterized by UM IGHV configurations, was significantly associated with del(17)(p13). Notably, subset #1 exhibited a more unfavorable clinical course than other patients with an UM IGHV configuration, independently of the presence of other adverse prognostic factors, such as del(17)(p13), del(11)(q23), ZAP-70 and CD38 positivity or the usage of IGHV genes. The finding that subset #1 shows the worst clinical outcome as found in patients exhibiting 11q23 or 17p13 deletion suggests that it might represent a reliable marker for high risk CLLs in the early stage of the disease.
As regards subset #2 (IGHV3-21), we confirmed its more unfavorable clinical outcome. However, differently from previously reported data [13], we did not observe a significant difference in the geographical distribution of IGHV3-21 across Italy in our cohort of patients. Moreover, we found the presence of del(13)(q14) in virtually all patients tested by FISH (12/13); this finding is in accordance with data recently published by Marincevic et al [18], suggesting that this association could be considered subset-specific. In addition, we did not observe a strong association between del(11)(q23) and subset #2 as described by the same authors [18]. This discrepancy could be partially explained by the lower number of subset #2 patients analyzed by FISH in our study. However, it should be noted that all but one of the subset #2 cases in our panel were Binet A, whereas 70% of those from Marincevic et al. [18] were either in advanced clinical stages or no information was provided, thus preventing any definitive comparison.
In our study, we described a recurrent favorable cytogenetic profile and the indolent course in subset #4 patients. This finding is in agreement with data reported by some authors [10], [18], but not by others [17], leaving this aspect still controversial. Finally, we confirmed that trisomy 12 was correlated with IGHV4-39 stereotyped HCDR3 subsets #8 and #10 [17], [20] showing that these two subsets were particularly associated with higher CD38 expression.
In conclusion, our study indicates that distinct stereotyped HCDR3 regions of BCR in CLL are characterized by specific cytogenetic and/or molecular profiles and clinical course. Further validation in larger and prospective series of patients may help to better clarify distinct biological and clinical features of specific stereotyped subsets.
Supporting Information
Predictive value of Binet A, IGHV gene status, CD38, ZAP-70 and the most common genomic aberrations evaluated by FISH. Cases were subdivided according to Binet classification (A), CD38 expression (B), FISH (C), IGHV gene status (D) and ZAP-70 expression (E) before determining TTFT.
(TIF)
(A) IGHV distribution and association with mutated (M) or unmutated (UM) IGHV configuration. IGHV bars were ordered according to the total number of patients belonging to each subset. Among the most represented IGHV genes, there was a higher prevalence of M configuration in IGHV3-23 (84/95; 88.4%), of IGHV4-34 (97/108; 89.8%), (73/83; 87.9%) of IGHV3-7 (73/83; 87.9%) and of IGHV3-30 (49/66; 74.2%) cases (representing 42% of all M patients); conversely, 91.4% (106/116) of IGHV1-69 patients were UM (representing 26.1% of all UM CLL gene usage). The IGHV3-21 gene was present in only a small fraction of cases of our panel (34/1126, 3%; 14 UM and 20 M), confirming its low prevalence in a Mediterranean cohort of CLL patients. (B) IGHD distribution and association with M and UM IGHV configuration. IGHD bars were ordered by the total number of patients belonging to each subset. IGHD gene distribution was similar to that described for other cohorts. IGHD3-3 was the most used IGHD gene and it was significantly associated with the UM configuration (108/133; 81.2%). On the contrary, IGHD3-10 (67/95; 70.5%), IGHD2-15 (43/49; 87.8%), IGHD1-26 (50/59; 54.7%), and IGHD3-22 (64/97; 66%) were significantly associated with the M configuration. (C) IGHJ gene distribution and association with IGHV mutational status. IGHJ4 and 6 were the most represented IGHJ gene and they were associated with M (361/498; 72.5%) and UM (189/353; 53.5%) mutational status, respectively.
(TIF)
BCR molecular features of previously described subets.
(XLS)
Clinical and molecular features of 31 new putative subsets
(XLS)
Distribution of IGHV3-21 in patients from North and South Italy.
(XLS)
Acknowledgments
In addition to the listed Authors, the following Investigators participated in this study as part of the GISL - Gruppo Italiano Studio Linfomi: Dr Di Tonno, Struttura di Ematologia Azienda Ospedaliera Di Venere, Bari, Italy; Dr Guarini, Unità di Oncoematologia IRCCS Istituto Tumori Giovanni Paolo II, Bari, Italy; Dr Quarta, Divisione di Ematologia, Presidio Ospedaliero Di Summa Perrino, Brindisi, Italy; Prof Abbadessa, Unità di Oncoematologia Ospedale S. Anna e S. Sebastiano, Caserta, Italy; Dr Gugliemo, Unità di Oncoematologia Presidio Ospedaliero Garibaldi Nesima, Catania, Italy; Dr Iuliano, Unità di Oncologia Ospedale Civile Giannettasio, Rossano Calabro (CS), Italy; Prof Gobbi, Unità Operativa Clinica Ematologica Dipartimento di Medicina Interna E Specialià Mediche (DIMI), Università degli Studi, Genova, Italy; Prof Carella, Divisione di Ematologia I - Monoblocco 11 Piano-Ponente, Azienda Ospedaliera Universitaria S. Martino, Genova, Italy; Dr Brugiatelli, Divisione di Ematologia, Azienda Ospedaliera Papardo, Messina. Italy; Dr Bertoldero, Dipartimento di Oncologia ed Ematologia Oncologica, Ospedali Civile di Mirano, Dolo Mirano (VE), Italy; Dr Ferrara, Unità di Ematologia con Trapianto di Midollo Osseo, A.O.R.N. Antonio Cardarelli; Dr Iannitto, Divisione di Ematologia con Trapianto di Midollo Osseo Policlinico Universitario Giaccone, Palermo, Italy; Dr Angrilli, Dipartimento di Ematologia, Ospedale Spirito Santo, Pescara, Italy; Dr Vallisa, Unità di Oncologia Medica ed Ematologia, Ospedale Civile Guglielmo da Saliceto, Piacenza, Italy; Prof Nobile, Azienda Ospedaliera Bianchi Melacrino Morelli, Reggio Calabria, Italy; Dr Musto, IRCCS Centro di Riferimento Oncologico della Basilicata, Rionero in Vulture (PZ), Italy; Dr Mauro and Prof Foà, Università La Sapienza, Roma, Italy; Dr Cox, Reparto di Ematologia ed Immunoematologia Azienda Ospedaliera S. Andrea, Roma, Italy; Dr Andriani, Unità di Ematologia, Ospedale S. Giacomo, Roma, Italy; Dr Cascavilla, Struttura Complessa di Ematologia IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG), Italy; Dr Russo, Divisione di Ematologia con Trapianto di Midollo Presidio Ospedaliero San Vincenzo, Taormina (ME), Italy; Dr Riezzo, Divisione di Ematologia Presidio Ospedaliero di Trani, Trani, Italy; Dr Pinotti, Unità di Oncologia Medica Ospedale di Circolo, Varese, Italy.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from Associazione Italiana Ricerca sul Cancro (AIRC) (to AN - IG4569, M. Ferrarini - IG10492 and F. Morabito - RG6432) and AIRC-Special Program Molecular Clinical Oncology “5 per mille”, grant 9980, 2010-15 (to AN, M. Ferrarini and F. Morabito); Ricerca Finalizzata from Italian Ministry of Health 2006 (to GC, F. Morabito and M. Ferrarini) and 2007 (to GC); FIRB Grant no. RBIP06LCA9 (to M. Ferrarini); Fondazione Matarelli, Milano (to AN); Progetto Compagnia San Paolo (to GC); Associazione Italiana Leucemie (AIL), Milano; Assistenza e Studio Malati Ematologici (ASME), Milano Italy. ML was supported by a fellowship from Fondazione Italiana Ricerca sul Cancro (FIRC). The authors thank Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) for financial and administrative assistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Dighiero G. CLL biology and prognosis. Hematology Am Soc Hematol Educ Program. 2005:278–284. doi: 10.1182/asheducation-2005.1.278. [DOI] [PubMed] [Google Scholar]
- 3.Kay NE, O'Brien SM, Pettitt AR, Stilgenbauer S. The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia. 2007;21:1885–1891. doi: 10.1038/sj.leu.2404802. [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Ferracin M, Cimmino A, Di LG, Shimizu M, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 6.Zenz T, Mertens D, Kuppers R, Dohner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10:37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 7.Damle RN, Temburni S, Calissano C, Yancopoulos S, Banapour T, et al. CD38 expression labels an activated subset within chronic lymphocytic leukemia clones enriched in proliferating B cells. Blood. 2007;110:3352–3359. doi: 10.1182/blood-2007-04-083832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 9.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood. 2007;109:259–270. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 11.Tobin G, Thunberg U, Johnson A, Eriksson I, Soderberg O, et al. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted Vlambda2-14 gene use and homologous CDR3s: implicating recognition of a common antigen epitope. Blood. 2003;101:4952–4957. doi: 10.1182/blood-2002-11-3485. [DOI] [PubMed] [Google Scholar]
- 12.Widhopf GF, Rassenti LZ, Toy TL, Gribben JG, Wierda WG, et al. Chronic lymphocytic leukemia B cells of more than 1% of patients express virtually identical immunoglobulins. Blood. 2004;104:2499–2504. doi: 10.1182/blood-2004-03-0818. [DOI] [PubMed] [Google Scholar]
- 13.Bomben R, Dal BM, Capello D, Benedetti D, Marconi D, et al. Comprehensive characterization of IGHV3-21-expressing B-cell chronic lymphocytic leukemia: an Italian multicenter study. Blood. 2007;109:2989–2998. doi: 10.1182/blood-2006-10-051110. [DOI] [PubMed] [Google Scholar]
- 14.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117:1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packham G, Stevenson F. The role of the B-cell receptor in the pathogenesis of chronic lymphocytic leukaemia. Semin Cancer Biol. 2010;20:391–399. doi: 10.1016/j.semcancer.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Tobin G, Thunberg U, Karlsson K, Murray F, Laurell A, et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004;104:2879–2885. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 17.Athanasiadou A, Stamatopoulos K, Gaitatzi M, Stavroyianni N, Fassas A, et al. Recurrent cytogenetic findings in subsets of patients with chronic lymphocytic leukemia expressing IgG-switched stereotyped immunoglobulins 21. Haematologica. 2008;93:473–474. doi: 10.3324/haematol.11872. [DOI] [PubMed] [Google Scholar]
- 18.Marincevic M, Cahill N, Gunnarsson R, Isaksson A, Mansouri M, et al. High-density screening reveals a different spectrum of genomic aberrations in chronic lymphocytic leukemia patients with ‘stereotyped’ IGHV3-21 and IGHV4-34 B-cell receptors. Haematologica. 2010;95:1519–1525. doi: 10.3324/haematol.2009.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stella S, et al. Geographic patterns and pathogenetic implications of IGHV gene usage in chronic lymphocytic leukemia: the lesson of the IGHV3-21 gene. Blood. 2005;105:1678–1685. doi: 10.1182/blood-2004-07-2606. [DOI] [PubMed] [Google Scholar]
- 20.Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res. 2009;15:4415–4422. doi: 10.1158/1078-0432.CCR-08-3266. [DOI] [PubMed] [Google Scholar]
- 21.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200:519–525. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bomben R, Dal BM, Capello D, Forconi F, Maffei R, et al. Molecular and clinical features of chronic lymphocytic leukaemia with stereotyped B cell receptors: results from an Italian multicentre study. Br J Haematol. 2009;144:492–506. doi: 10.1111/j.1365-2141.2008.07469.x. [DOI] [PubMed] [Google Scholar]
- 23.Murray F, Darzentas N, Hadzidimitriou A, Tobin G, Boudjogra M, et al. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: implications for the role of antigen selection in leukemogenesis. Blood. 2008;111:1524–1533. doi: 10.1182/blood-2007-07-099564. [DOI] [PubMed] [Google Scholar]
- 24.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 26.Cutrona G, Colombo M, Matis S, Fabbi M, Spriano M, et al. Clonal heterogeneity in chronic lymphocytic leukemia cells: superior response to surface IgM cross-linking in CD38, ZAP-70-positive cells. Haematologica. 2008;93:413–422. doi: 10.3324/haematol.11646. [DOI] [PubMed] [Google Scholar]
- 27.Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 28.Fabris S, Mosca L, Todoerti K, Cutrona G, Lionetti M, et al. Molecular and transcriptional characterization of 17p loss in B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2008;47:781–793. doi: 10.1002/gcc.20579. [DOI] [PubMed] [Google Scholar]
- 29.Forconi F, Potter KN, Wheatley I, Darzentas N, Sozzi E, et al. The normal IGHV1-69-derived B-cell repertoire contains stereotypic patterns characteristic of unmutated CLL. Blood. 2010;115:71–77. doi: 10.1182/blood-2009-06-225813. [DOI] [PubMed] [Google Scholar]
- 30.Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 31.Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predictive value of Binet A, IGHV gene status, CD38, ZAP-70 and the most common genomic aberrations evaluated by FISH. Cases were subdivided according to Binet classification (A), CD38 expression (B), FISH (C), IGHV gene status (D) and ZAP-70 expression (E) before determining TTFT.
(TIF)
(A) IGHV distribution and association with mutated (M) or unmutated (UM) IGHV configuration. IGHV bars were ordered according to the total number of patients belonging to each subset. Among the most represented IGHV genes, there was a higher prevalence of M configuration in IGHV3-23 (84/95; 88.4%), of IGHV4-34 (97/108; 89.8%), (73/83; 87.9%) of IGHV3-7 (73/83; 87.9%) and of IGHV3-30 (49/66; 74.2%) cases (representing 42% of all M patients); conversely, 91.4% (106/116) of IGHV1-69 patients were UM (representing 26.1% of all UM CLL gene usage). The IGHV3-21 gene was present in only a small fraction of cases of our panel (34/1126, 3%; 14 UM and 20 M), confirming its low prevalence in a Mediterranean cohort of CLL patients. (B) IGHD distribution and association with M and UM IGHV configuration. IGHD bars were ordered by the total number of patients belonging to each subset. IGHD gene distribution was similar to that described for other cohorts. IGHD3-3 was the most used IGHD gene and it was significantly associated with the UM configuration (108/133; 81.2%). On the contrary, IGHD3-10 (67/95; 70.5%), IGHD2-15 (43/49; 87.8%), IGHD1-26 (50/59; 54.7%), and IGHD3-22 (64/97; 66%) were significantly associated with the M configuration. (C) IGHJ gene distribution and association with IGHV mutational status. IGHJ4 and 6 were the most represented IGHJ gene and they were associated with M (361/498; 72.5%) and UM (189/353; 53.5%) mutational status, respectively.
(TIF)
BCR molecular features of previously described subets.
(XLS)
Clinical and molecular features of 31 new putative subsets
(XLS)
Distribution of IGHV3-21 in patients from North and South Italy.
(XLS)