Abstract
Aims
This study assessed overdose and naloxone administration knowledge among current or former opioid abusers trained and untrained in overdose–response in the United States.
Design and participants
Ten individuals, divided equally between those trained or not trained in overdose recognition and response, were recruited from each of six sites (n = 62).
Setting
US-based overdose training and naloxone distribution programs in Baltimore, San Francisco, Chicago, New York and New Mexico.
Measurements
Participants completed a brief questionnaire on overdose knowledge that included the task of rating 16 putative overdose scenarios for: (i) whether an overdose was occurring and (ii) if naloxone was indicated. Bivariate and multivariable analyses compared results for those trained to untrained. Responses were also compared to those of 11 medical experts using weighted and unweighted kappa statistics.
Findings
Respondents were primarily male (72.6%); 45.8% had experienced an overdose and 72% had ever witnessed an overdose. Trained participants recognized more opioid overdose scenarios accurately (t60 = 3.76, P < 0.001) and instances where naloxone was indicated (t59 = 2.2, P < 0.05) than did untrained participants. Receipt of training and higher perceived competency in recognizing signs of an opioid overdose were associated independently with higher overdose recognition scores. Trained respondents were as skilled as medical experts in recognizing opioid overdose situations (weighted kappa = 0.85) and when naloxone was indicated (kappa = 1.0).
Conclusions
Results suggest that naloxone training programs in the United States improve participants’ ability to recognize and respond to opioid overdoses in the community. Drug users with overdose training and confidence in their abilities to respond may effectively prevent overdose mortality.
Keywords: Cocaine, harm reduction, injection drug use, naloxone distribution, opioids, overdose
BACKGROUND
Overdose is a frequent consequence of heroin and pharmaceutical opioid abuse. It is the single greatest cause of mortality among injecting drug users (IDU) in the United States [1], far exceeding deaths attributable to acquired immunodeficiency syndrome (AIDS), hepatitis and other morbidities [2]. Moreover, the incidence of drug overdose has been increasing in the United States since the 1990s [3–5]. History of non-fatal opioid overdose ranges from 41% [6] in Baltimore IDUs to 48% in San Francisco IDUs [7]. Much of the substantial mortality and morbidity is avoidable. Fatal overdose is usually not instantaneous; it occurs over a period of 1–3 hours [8]. Given the relatively long window of opportunity in which to intervene and the fact that the vast majority of overdoses occur in the presence of others [1,9–12], efforts to mobilize immediate medical intervention are optimal, i.e. rescue breathing and/or administration of naloxone hydrochloride (hereafter naloxone), a short-acting opioid antagonist. Appropriate responses by bystanders require training in overdose recognition and rescue breathing, as well opioid overdose victim [13].
If injected soon after an opioid overdose, naloxone prevents or reverses the effects of opioids on the brain and restores breathing. When given intramuscularly (i.m.), the onset of action is approximately 2 minutes. In the absence of the agonist effects of opioids, naloxone exhibits essentially no pharmacological activity. The US Food and Drug Administration approved naloxone in 1971 for complete or partial reversal of narcotic depression. It is an inexpensive, non-scheduled drug available by prescription only through medical professionals (i.e. physicians, physician assistants, dentists, advanced practice registered nurses, podiatrists), and is routinely carried by emergency medical technicians. Many states and localities are trying to expand access to naloxone to other first-line responders (e.g. police, firefighters) and, as a public health intervention for reducing overdose deaths, to IDUs and their peers. To do so, several states (i.e. New Mexico, New York, Illinois, Maryland, Oregon, California, Massachusetts, Connecticut) have legal pilot or established programs for naloxone distribution among drug users and claim impressive declines in their overdose mortality numbers, 17–20% in some cases [14–17]. Additionally, a number of states have initiated ‘good Samaritan’ laws to protect from legal recourse citizens who carry and administer naloxone and medical professionals who prescribe it (e.g. Connecticut, Illinois, New York).
Scientific research into the practice of overdose prevention training and naloxone distribution for opioid abusers is limited, and few well-designed studies have evaluated interventions. In addition, critics often claim that non-medical professionals may use naloxone inappropriately, even when trained, which may result in high costs to society [18]. Lives may be lost if naloxone is not administered appropriately, and misuse of naloxone in non-overdose situations could translate to wasted resources. There are also concerns about consistency and safety of the curriculum, the trainee’s retention of information and liability in the event of an adverse event or misuse of the medication. These issues and concerns often impede adequate funding for implementation, evaluation and expansion of naloxone programs [19].
In an effort to add to the literature on naloxone distribution, this study sought to assess overdose and naloxone administration knowledge among current and former opioid abusers trained and untrained by six naloxone distribution programs across the United States. The premise underlying this study is that appropriate response to an overdose requires sufficient knowledge to recognize when an overdose is occurring, to differentiate between opioid versus non-opioid overdose and non-overdose situations, and to respond appropriately. The ability to distinguish signs of an opioid overdose quickly, to which the bystander can respond actively and directly by performing rescue breathing and/or administering naloxone, from a non-opioid overdose or a non-overdose situation where use of naloxone would be inappropriate, could mean the difference between life and death for the victim. The goals of naloxone training programs, therefore, are to maximize the appropriateness of the reaction and minimize the time to overdose recognition and response.
METHODS
Evaluation sites
Six sites were selected for this evaluation to include both new and established programs, with varied geographic representation. Site liaisons were program directors and project managers, while those recruiting the participants in this study were peer trainers and site staff. The training programs at three siteswere new (Baltimore, Citiwide and Positive Health Project in New York) and three were well established (Chicago, New Mexico, San Francisco). More detail on the projects is included in an appendix (http://www.harmreduction.org/article.php?id=700; http://cira.med.yale.edu/addiction/; http://www.anypositivechange.org/Appendix.pdf).
Summary of training programs
Generally, the six training programs followed similar outlines for content with differences arising in delivery, setting and several topics covered in the curriculum (Table 1). Trainings were brief and took place in diverse settings ranging from syringe exchange programs to street corners to private homes (Fig. 1). Training curricula tended to include both didactic and interactive components (e.g. use of resuscitation dummy, instructor demonstration, practice rousing a victim). All sites covered opioid overdose symptom recognition and response, some focusing more upon immediate administration of naloxone, others concentrating more heavily upon rescue breathing, but all incorporating contacting 911 (the US emergency medical service) at some point in the response sequence.
Table 1.
Program and curriculum components of six study sites: overdose prevention and response programs.
| Site | Training setting | Training type | Participant remuneration |
Average estimated length of training (minutes) |
Aspects of overdose recognition curriculum |
||
|---|---|---|---|---|---|---|---|
| Non-opioid (e.g. stimulant) overdose covered |
‘Heavy nod’ versus overdose differentiation |
Evaluation/ knowledge test |
|||||
| Chicago, Illinois | Needle exchange, mobile van, street | One-on-one, pairs; DVD | None | 15–30 (15–20: training, 10: DVD) | Partial | Yes | After training |
| State of New Mexico | Drug treatment centers, needle exchanges, peer’s homes, street settings | One-on-one, classroom | $5–10 | 20–30 (one-on-one), 120 (classroom) | No | Yes | No |
| San Francisco, California | SRO hotels*, needle exchange programs | One-on-one | None | 15 | Explicitly | Yes | No |
| Baltimore, Maryland | Needle exchange, community centers, mobile unit | One-on-one in mobile unit; Classroom | None | 20–30 (mobile), 90 (classroom) | Explicitly | Yes | After training |
| New York, New York (Positive Health Project) | Multi-service facility | Classroom | $4 metro card | 30–45 | No | Yes | After training |
| New York, New York (Citiwide) | Street, hotel room, drop | One-on-one, classroom | $4 metro card | 15–20 (one-on-one), 60 (classroom) | Partial | Yes | After training |
SRO hotels = single room occupancy hotels.
Figure 1.
One of many community-based naloxone distribution and overdose prevention training programs held across the state of New Mexico. Photo courtesy of Philip Fiuty
Of note, all programs included content that addressed explicitly differentiating an opioid overdose from a ‘heavy nod’, and four programs required some form of evaluation to verify participant comprehension of the training curriculum. Only two programs addressed explicitly the identification and response to non-opioid (e.g. stimulant) overdoses. Site-specific curriculum and program overviews are detailed in an appendix (http://www.harmreduction.org/article.php?id=700; http://cira.med.yale.edu/addiction/; http://www.anypositivechange.org/Appendix.pdf); the site liaisons provided all data.
Study procedure
Staff from sites that conducted overdose prevention training were approached at a national conference on opioid overdose; all agreed to take part in the study. Each sitewas asked to recruit five participants who had been trained in their program and five participants who had not been trained in their program. Recruitment of both groups could be accomplished at the same site, as training sites were co-located within programs where former and active drug users congregate (e.g. needle exchanges, drop-in sites). Study staff explained the procedures and administered informed consent. Participants completed independently a hard copy of the study instrument, although site staff were available for questions or to administer it if literacy posed a problem. Participants were remunerated $10 for study participation.
Evaluation tool
Sixteen unique experiences that IDUs described as an overdose experience were used as the ‘cases’ evaluated by study participants. A complete description of the development and validation of the evaluation tool can be found elsewhere [20]. Briefly, the 16 cases were selected from a pool of 60 unique experiences reported by IDUs as part of a study on public injecting [21]. Eleven medical experts in overdose recognition and treatment were asked to designate each case as either: (i) opioid overdose; (ii) non-opioid overdose; (iii) not an overdose; or (iv) not enough information/unsure, and whether naloxone was indicated. Collapsing the non-opioid overdose and non-overdose categories and using latent class analysis [22,23], agreement among the experts was calculated to arrive at collectively agreed-upon designations. These assignments became the ‘gold standard’ and comprised the evaluation tool: nine cases were deemed opioid overdoses and seven were deemed non-opioid or non-overdose scenarios. All opioid overdose cases required naloxone; non-opioid or non-overdose scenarios did not. Possible scores for overdose recognition and naloxone indication knowledge each ranged from 0 to 16.
In addition to the 16 scenarios, the instrument included items pertaining to participants’ gender, background (e.g. volunteer, current/former drug user, outreach worker), history of overdose recognition and response training and self-rated expertise in overdose recognition and response (1, low to 5, high). To preserve participant anonymity and minimize response burden, no data were gathered on age, race, ethnicity or drugs used.
Data analysis
Descriptive statistics (means, medians, standard deviations) were conducted to characterize the sample’s drug use and overdose experiences. Kappas, weighted kappas and their 95% confidence intervals (CIs) were calculated to compare agreement among overdose and naloxone recognition codes assigned by medical experts, trained and untrained participants. Bivariate analyses (t-tests, χ2 tests and, when appropriate, non-parametric Kruskal–Wallis and Mann–Whitney U-tests) were conducted to compare trained to untrained participants on all demographic and outcome variables. Multivariable regression analyses (linear, logistic) were conducted to explore variables associated with knowledge (i.e. opioid overdose, naloxone indication and overall overdose scores) and having responded to overdose. In all models, non-automated backward and stepwise modeling techniques were employed. Due to small sample size, and drawing upon recommendations from the literature for analyses in such situations [24–26], models considered only variables yielding significant bivariate associations with outcomes, resulting in four or fewer possible independent variables. Sufficient power (> 0.8) to detect at least one significant independent regression variable was achieved, based on expected effect sizes [25,27]. However, sample size precluded a more comprehensive and conclusive test of associations with additional variables or smaller effects; regression findings are necessarily presented as exploratory in nature.
This study was approved by the Human Investigations Committee of Yale University School of Medicine.
RESULTS
Sixty-two respondents took part in the study between October 2005 and July 2006 (Table 2). Respondents were predominantly male (72.6%), and current or former drug users (85.5%). Fourteen (22.6%) were syringe exchange staff. Approximately half (n = 30) had been trained in responding to drug overdose, a median of 8 months (range 1–80 months) prior to evaluation. History of experiencing an overdose was common (45.8%, n = 27), with respondents reporting a median of three previous overdoses [interquartile range (IQR) = 4]. History of witnessing an overdose was also reported by 72% (n = 44) of respondents, witnessing amedian of 7.5 (IQR = 11) overdoses. Having responded to an overdose (e.g. calling 911, administering naloxone, injecting with milk, etc.) was reported by 62.3% of all respondents (n = 38) and by 86.4% of those who reported witnessing an overdose. Of those reporting having ever responded to an overdose, they had done so a median of four times (IQR = 4), with half (n = 19, 50%) occurring within the past year. Trained and untrained respondents did not differ in demographics or overdose experience. They differed, however, in their self-rated level of expertise in identifying symptoms of opioid overdose (t60 = 2.14, P < 0.05) and identifying when to use naloxone (t60 = 5.03, P < 0.0001), with trained respondents having higher perceived competency in recognizing and responding to opioid overdoses (Table 3). The groups did not differ in self-rated level of expertise in identifying non-opioid overdose symptoms (t58 = 0.59, P = 0.56).
Table 2.
Study population socio-demographics and overdose experience, by training history.
| Variable | Trained n (%) |
Untrained n (%) |
Test statistic, P-value |
|---|---|---|---|
| Site | |||
| Chicago | 6 | 5 | 1.43, P = 0.96 |
| Baltimore | 4 | 6 | |
| New York City: Citiwide | 5 | 6 | |
| New York City: Positive Health Project | 5 | 5 | |
| New Mexico | 5 | 5 | |
| San Francisco | 5 | 5 | |
| Total sample | 30 | 32 | |
| Male | 22 (73.3) | 23 (71.9) | 0.02, P = 0.90 |
| Respondent’s background† | |||
| Medical professional | 0 | 1 (3.1) | ‡ P = 1.0 |
| Needle exchange program employee | 9 (30.0) | 5 (15.6) | 1.83, P = 0.18 |
| Outreach worker | 2 (6.7) | 1 (3.1) | ‡ P = 0.61 |
| Current or former drug user | 25 (83.3) | 28 (87.5) | 0.22, P = 0.64 |
| Other (client) | 0 | 2 (6.3) | ‡ P = 0.49 |
| Trained in responding to drug overdose | 30 (100) | 3 (9.4)* | P < 0.0001 |
| History of overdose | 14 (50) | 13 (41.9) | 0.38, P = 0.53 |
| Number of overdoses experienced (median, IQR) | 2 (4) | 3 (3) | 1.14, P = 0.25 |
| Last overdose experienced | |||
| Within past year | 4 (28.6) | 4 (36.4) | 0.65, P = 0.72 |
| 1–5 years ago | 6 (42.9) | 3 (27.3) | |
| 6+ years ago | 4 (28.6) | 4 (36.4) | |
| History of witnessing an overdose | 22 (75.9) | 22 (68.8) | 0.38, P = 0.54 |
| Number of overdoses ever witnessed (median, IQR) | 9 (12) | 7 (8) | 1.09, P = 0.28 |
| History of responding to a drug overdose | 21 (72.4) | 17 (53.1) | 2.41, P = 0.12 |
| Number of overdoses ever responded to (median, IQR) | 4.5 (6) | 2 (5) | 1.21, P = 0.23 |
Three respondents who were not trained by the recruiting program stated that they had received training in overdose symptom recognition and response (one during professional CPR training, one at a long-term drug treatment facility, one in Vancouver, British Columbia). Because these trainings were not aimed specifically at overdose recognition and response or they occurred more than 9 years ago, these respondents were considered as untrained participants in our analyses.
More than one response was possible.
Fisher’s exact test applied. IQR: interquartile range.
Table 3.
Overdose and naloxone knowledge scores by training status.
| Scores | Trained n = 30 Mean (SD) |
Untrained n = 32 Mean (SD) |
Test statistic, P-value |
|---|---|---|---|
| Self-rated level of expertise | |||
| Identifying opioid overdose symptoms | 3.77 (.94) | 3.22 (1.07) | t60 = 2.14, P < 0.05 |
| Identifying when to use naloxone | 3.83 (.91) | 2.63 (.98) | t60 = 5.03, P < 0.0001 |
| Identifying non-opioid overdose symptoms (e.g. cocaine intoxication) | 3.39 (1.13) | 3.22 (1.16) | t58 = 0.59, P = 0.56 |
| Overdose symptom recognition | |||
| Correctly identified as opioid overdose (nine items) | 7.13 (1.38) | 5.18 (2.55) | t60 = −3.76, P < 0.001 |
| Correctly identified as not an overdose/non-opioid overdose (seven items) | 4.63 (1.40) | 4.28 (1.94) | t60 = −0.81, P = 0.42 |
| Total score (16 items) | |||
| Average percentage correct: recognition of overdose | 11.77 (1.92) | 9.47 (3.48) | t48.93 = −3.24, P < 0.005 |
| Naloxone indication | 85.2% | 68.3% | |
| Correctly indicated for opioid overdose (nine items) | 6.93 (1.76) | 5.77 (2.30) | t59 = −2.20, P < 0.05 |
| Correctly not indicated for opioid overdose (seven items) | 6.60 (0.72) | 5.32 (1.94) | t38.32 = −3.43, P < 0.001 |
| Total score (16 items) | 13.53 (1.96) | 11.09 (2.32) | t59 = −4.38, P < 0.0001 |
| Average percentage correct: naloxone indication | 84.6% | 69.3% |
SD: standard deviation.
Overdose and naloxone knowledge
Knowledge of opioid overdose symptoms was significantly higher among trained compared to untrained respondents (Table 3: 7.1 of nine situations recognized versus 5.2, t60 = −3.76, P < 0.001). However, trained and untrained respondents had similar indecision in recognizing non-overdose and non-opioid overdose situations (4.6 versus 4.3 of seven situations, t60 = −0.81, P = 0.42). Overall, overdose knowledge was high for trained respondents who scored 85.2% correct compared to the 68.3% correct among untrained respondents (11.7 versus 9.5 of 16 correct, t48.93 = −3.24, P < 0.005). Trained respondents showed greater aptitude in recognizing when naloxone was indicated (13.5 versus 11.1 of 16, t59 = −4.38, P < 0.0001). There were no significant differences in knowledge scores by site.
Naloxone response: ‘missed opportunities’, ‘wasted resources’
Fewer (2.1 versus 3.2) opioid situations that required naloxone administration were missed by trained respondents compared to those untrained (t59 = −2.20, P < 0.05). That is, of the opioid overdose situations, untrained respondents were more likely than trained respondents to miss the opportunity to respond appropriately with naloxone. In a similar vein, trained participants were less likely to recommend administration of naloxone in situations where it was not indicated (i.e. in non-overdose and non-opioid overdose events): 0.4 versus 1.7 of seven situations (t38.32 = −3.43, P < 0.001). Hence, resources—measured in response time and naloxone—were less likely to be wasted by trained respondents than by untrained respondents.
Multivariable analyses
Table 4 presents results from exploratory multivariable analyses of the knowledge and reported overdose–response outcomes. In a multiple linear regression, the only statistically significant independent predictor of greater opioid overdose knowledgewas receipt of training in overdose response (βst, standardized beta = 0.41, P < 0.001). For the outcome of overall overdose knowledge score (opioid or non-opioid overdose), the independent predictors were receipt of training (βst = 0.47, P < 0.001) and self-rated level of expertise in recognizing signs of opioid overdose (βst = 0.24, P < 0.05). Having ever responded to overdose of any kind was predicted independently by self-rated level of expertise in recognizing signs of opioid overdose [adjusted odds ratio (AOR): 2.06 (95% CI: 1.05, 4.03)] and, more strongly, expertise in recognizing signs of non-opioid overdose [AOR: 2.6 (95% CI: 1.31, 5.21)]. Only receipt of overdose training was associated independently with higher naloxone knowledge scores (βst = 0.51, P < 0.001).
Table 4.
Predictors of knowledge of overdose recognition, naloxone administration and history of response: multiple linear and logistic regressions.
| Outcome | Receipt of training |
Perceived competency in recognizing signs of an opioid overdose |
Perceived competency in recognizing signs of a non-opioid overdose |
|---|---|---|---|
| Knowledge of opioid overdose symptom recognition | βst = 0.41, P < 0.001 | – | – |
| Knowledge of naloxone indication | βst = 0.51, P < 0.001 | – | – |
| Overall overdose knowledge (opioid, non-opioid, non-overdose) recognition | βst = 0.47, P < 0.001 | βst = 0.24, P < 0.05 | – |
| History of ever treating or responding to an overdose | – | AOR 2.06 (95% CI: 1.05, 4.03), P < 0.05 | AOR 2.6 (95% CI: 1.31, 5.21), P < 0.001 |
AOR = adjusted odds ratio; 95% CI = 95% confidence interval; βst = standardized beta.
Responses to overdose situations and time since training
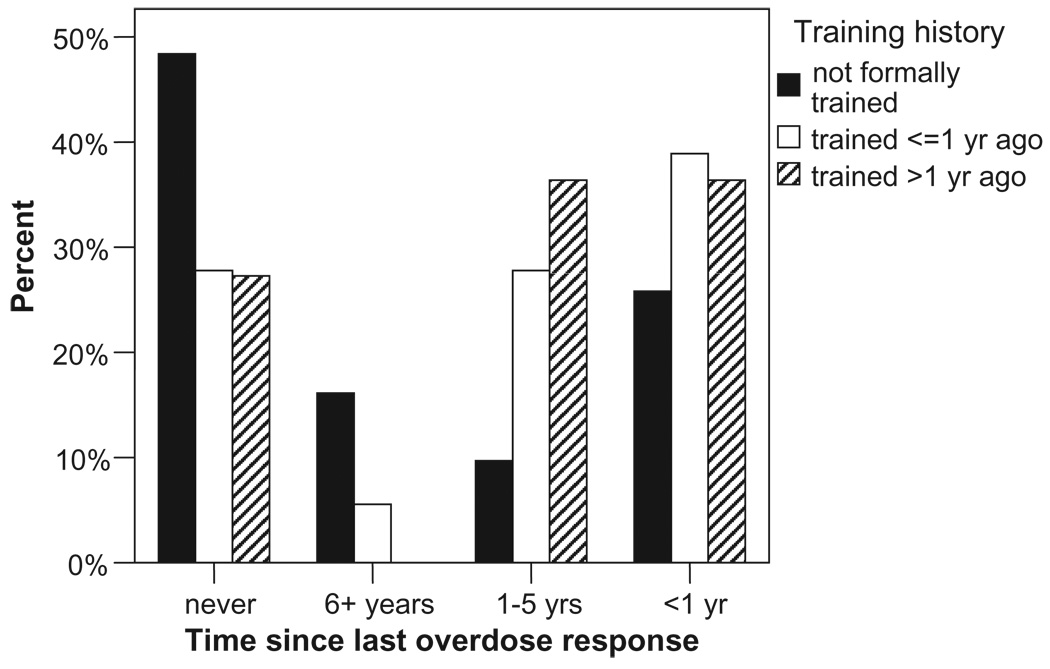
Untrained participants were significantly more likely to report having never responded to an overdose (Fig. 2, 48.4% versus 27.6%, χ23 = 8.62, P < 0.05). A linear trend indicated that trained participants were more likely to have responded recently than those who had not been trained (trained: 37.9% ≤ 1 year ago, 31.0% 1–5 years ago versus untrained: 25.8% and 9.7%, respectively, χ21 linear trend = 3.94, P < 0.05). People who had been trained over 1 year prior to assessment were as likely to report having responded to an overdose that occurred within the past year as to an overdose that occurred 1–5 years ago [53.8% versus 50% past year responded, 38.5% versus 50% 1–5 years ago responded, χ22 = not significant]. These data suggest a continued level of involvement in overdose–response for those who had been trained. Additionally, people trained within the past year appeared to be applying their training: the highest proportion of last overdoses responded to for this group occurred in the past year (38.9%).
Figure 2.
Time since last reported overdose–response by participants’ overdose–response training history
Witnessing and responding to overdose
More recently witnessing an overdose was associated marginally with having been trained in overdose recognition and response [OR: 2.71 (95% CI: 0.95, 7.71)]. Among those who had recently witnessed an overdose, trained participants also reported having responded to a median of four overdoses compared to only 1.5 overdoses among the untrained participants (Mann–Whitney U = 119.5, Wilcoxon’sW = 329.5, Z = −2.00, P < 0.05).
Comparison of participants to medical experts
Examining the 962 raw ratings, 43.9% (n = 422) were assigned to opioid overdose, 39% (n = 375) to not an overdose/non-opioid overdose and 17.2% (n = 165) to unsure/not enough information. Compared to the medical experts, trained respondents assigned a comparable proportion of opioid overdose ratings (47.7 versus 51.7, χ21 = 0.842, P = 0.36) to the same 16 putative overdose scenarios. The medical experts and trained respondents assigned differing proportions of codes to the non-opioid/non-overdose category and to the unsure/not enough information category. Notably, the medical experts assigned approximately 10% more codes to the ‘don’t know/unsure/not enough information’ category, compared to trained respondents (25.6% versus 16.0% of codes). Weighted kappas for the three overdose rating categories comparing medical examiners to trained respondents were high: 0.85 (weighted kappa 95% CI: 0.72, 0.98), whereas those comparing medical experts to untrained respondents [weighted kappa: 0.48 (95% CI: 0.18, 0.77)], and trained to untrained respondents [weighted kappa: 0.56 (95% CI: 0.22, 0.89)] were lower, with larger variances.
There was perfect agreement (kappa = 1.0) between the medical experts and trained respondents in their recommendations for administration of naloxone. In contrast, agreement between the untrained respondents and medical experts or trained respondents, the kappa was 0.75 with a wide confidence interval (0.44, 1.0).
DISCUSSION
This study is the first multi-site evaluation of overdose training and naloxone distribution programs in the United States. It is also the first study to employ a psychometrically valid instrument to measure improvements in overdose recognition and naloxone administration knowledge. We found that people trained in overdose recognition and naloxone administration were comparable to medical experts in identifying situations in which an opioid overdose was occurring and when naloxone should be administered. Training programs improved recognition and response to opioid overdoses significantly, so that fewer opioid overdoses would be missed and fewer overdoses would be responded to inappropriately by trained participants.
Our findings suggest that non-opioid overdose knowledge is low, even among people who have been trained to recognize and respond to overdose situations. A review of training curricula at each site (see appendix mentioned previously) found that most of the training programs (four of six) do not address explicitly identification and responding to non-opioid overdoses, so the low non-opioid overdose knowledge scores in this study may be expected. Several recent studies have highlighted the role that cocaine plays in opioid overdose deaths [28–30]. Overdoses with cocaine and heroin in combination, cocaine-only and cocaine, heroin and alcohol combinations are often experienced by drug users, yet their epidemiology is not well understood [29,31]. Areas that have historically reported high abuse of cocaine and other stimulants such as the US eastern seaboard [32], California [33] and Canada [34–36] may find muted effects of naloxone training programs on the prevalence of fatal drug overdose in their community. Curricula to recognize and respond to non-opioid overdose, especially cocaine intoxication, should be incorporated into naloxone trainings. For example, curricula could include explicitly addressing and practicing recognition of signs of cocaine/stimulant overdoses and a clear directive to call 911 in such circumstances.
The association between more recently witnessing an overdose and having been trained in overdose recognition and response may indicate that the people being trained by the programs are those exposed to settings in which the risk of overdose is high. In other words, the programs appear to be reaching their target population. Given relatively high knowledge scores among untrained participants, the association is less likely due to inability to recognize an overdose when it takes place. Among those who had recently witnessed an overdose, the greater median number of overdoses responded to by trained participants may not be due to success of training but rather to a reluctance of untrained participants to do anything that could potentially be harmful to the victim. Further evaluation studies pairing witnessed overdose and their specific response might elucidate this point.
It is important to note the relatively high opioid overdose symptom knowledge amongst untrained participants. While this may reveal a diffusion of knowledge from trained to untrained members in the community, it may also reflect the drug user community’s awareness of, concern about and organic efforts to educate others about overdose risk. Programs for overdose recognition and response often arise directly from or are built upon such cumulative foundations, influencing program mobilization and thus their efficacy. Further, diffusion of public health messages from those trained to those untrained increases program effectiveness and overdose risk minimization within the community.
Finally, this study found that people who have been trained in overdose–response techniques and who feel confident in their ability to recognize an opioid overdose may effectively prevent overdose mortality. Self-efficacy, or one’s perceived ability to engage in a specific behavior, is a prominent predictor of behavior change and action [37–39]. The finding that perceived competency (i.e. self-efficacy) in recognizing opioid overdose was associated independently with greater knowledge of overdose recognition suggests that mechanisms to improve confidence in one’s abilities such as attending trainings and practicing newly acquired skills exert an influence on knowledge, a key mediator of behavior change. Moreover, self-efficacy to recognize both opioid and non-opioid overdoses were associated independently with ever responding to overdose, suggesting links between self-efficacy and behavior. For drug users, there may be a sense of empowerment and other important psychosocial benefits gained through receipt of overdose recognition and naloxone training. Future studies should further explore these potential benefits.
Cost-effectiveness of health interventions such as diagnostic screening is a function of the prevalence of the problem being alleviated, the frequency and cost of a false positive, and the frequency and cost of a false negative [40–42]. In the case of naloxone training programs, the prevalence of opioid overdose is high among drug users but may differ by location depending on, among other things, accessibility to drugs other than opioids. This study demonstrated that the frequency of a false positive (i.e. ‘wasted resources’) and the frequency of a false negative (i.e. ‘missed opportunity’)were minimized with training. The cost of a false positive could range from the cost of the naloxone dose ($1.75–3.10 per 1 mg dose wholesale; Safetyworks: http://www.1800safety2.com) to death, if the case was a non-opioid overdose. The cost of a false negative could range from nothing to death. Mitigating the influence of these costs is the capacity to train drug users and people who spend time around drug users in overdose recognition and response. This study provides key evidence, without having to conduct a large, costly, multi-site study or a complex cost–effectiveness analysis, that naloxone training programs are effective and probably cost–effective.
This study has several important limitations. Sample size was small, and power was insufficient to test for site-specific differences. For the main knowledge outcomes, however, the large effect sizes (Cohen’s d = 0.97 for overdose knowledge, d = 1.1 for naloxone indication knowledge) were detected with confidence using this sample size. Nevertheless, a larger scale evaluation could examine site and program-specific effects more thoroughly, provide adequate power for multivariable regressions to test hypotheses generated by our exploratory models and return results with greater external validity than our small study provides. This study is observational in nature and thus is open to the limitations of such designs including self-report and reporting bias. Moreover, the non-random sampling strategy employed to recruit subjects at each site may have introduced bias. We made every effort to convey the importance of recruiting a random sample of participants trained and untrained at each site. In all cases, the programs’ time and staff constraints would have made orchestration of a non-random selection-biased recruitment effort unlikely. Another limitation pertains to the lack of specificity in the data obtained, namely that the overdoses witnessed were not necessarily the same overdoses that were responded to by participants (i.e. unpaired overdose situations). Instead, interpretation is restricted to associations between participants’ reports of witnessing and responding to overdoses in general. Finally, due to time and space constraints at the sites, the evaluation instrument collected limited demographic variables. Thus, residual confounding may have influenced the findings.
In conclusion, this study reports initial evidence of the effectiveness of overdose training and naloxone distribution programs in opioid overdose recognition and response. People trained through these programs identify opioid overdoses and indications for naloxone as well as medical experts and consistently scored higher in knowledge of overdose and naloxone indication scenarios than their untrained counterparts. Efforts to develop and incorporate curricula for recognizing and responding to non-opioid overdoses, especially cocaine intoxication, are needed. Expansion of overdose training and naloxone distribution programs for drug-using populations is warranted.
Acknowledgements
The authors are grateful to Jeannette Ickovics for support of this research, to Min Kim for his assistance in organizing and analyzing data, to Mark Kinzly for assisting with pilot testing the evaluation tool and liaising with sites and to Darlene Palmer, Karina Lavoie, Melissa Prevost and Catherine Hankins for assistance in obtaining overdose scenarios. We are especially thankful to study participants and staff of the evaluation sites: Pete Morse, Emalie Huriaux (DOPE Project, San Francisco); Susan Sherman, Monique Rucker (Johns Hopkins School of Public Health and the City of Baltimore); Bernie Lieving, Phillip Fiuty, Diana McCague (New Mexico Department of Health); Sharon Stancliff, Jason Farrell (Harm Reduction Coalition, Positive Health Project, New York); Dan Bigg, Suzanne Carlberg-Racich, Big John (Chicago Recovery Alliance); and Dahlia Heller, Rafi Toruella (Citiwide Harm Reduction Services, New York). We acknowledge with appreciation the 11 medical experts who took part in this study: Phillip Coffin, Brian Edlin, Niels Tangherlini, Sharon Stancliff, Bernie Lieving, R. Douglas Bruce, Sarz Maxwell, Karl Sporer, Josh Bamberger, Dan Ciccarone and one expert who preferred to remain anonymous. This evaluation was conducted with support from an institutional training grant for T. C. G.: NIMH 5T32MH020031.
References
- 1.Latkin CA, Hua W, Tobin K. Social network correlates of self-reported non-fatal overdose. Drug Alcohol Depend. 2004;73:61–67. doi: 10.1016/j.drugalcdep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Sporer KA. Acute heroin overdose. Ann Intern Med. 1999;130:584–590. doi: 10.7326/0003-4819-130-7-199904060-00019. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Unintentional poisoning deaths—United States, 1999–2004. MMWR. 2007;56:93–96. [PubMed] [Google Scholar]
- 4.Garfield J, Drucker E. Fatal overdose trends in major US cities: 1990–1997. Addict Res Theory. 2001;9:425–436. [Google Scholar]
- 5.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 6.Tobin KE, Latkin CA. The relationship between depressive symptoms and nonfatal overdose among a sample of drug users in Baltimore, Maryland. J Urban Health. 2003;80:220–229. doi: 10.1093/jurban/jtg025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seal KH, Kral AH, Gee L, Moore LD, Bluthenthal RN, Lorvick J, et al. Predictors and prevention of nonfatal overdose among street-recruited injection heroin users in the San Francisco Bay Area, 1998–1999. Am J Public Health. 2001;91:1842–1846. doi: 10.2105/ajph.91.11.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sporer KA. Strategies for preventing heroin overdose. BMJ. 2003;326:442–444. doi: 10.1136/bmj.326.7386.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strang J, Powis B, Best D, Vingoe L, Griffiths P, Taylor C, et al. Preventing opiate overdose fatalities with take-home naloxone: pre-launch study of possible impact and acceptability. Addiction. 1999;94:199–204. doi: 10.1046/j.1360-0443.1999.9421993.x. [DOI] [PubMed] [Google Scholar]
- 10.Powis B, Strang J, Griffiths P, Taylor C, Williamson S, Fountain J, et al. Self-reported overdose among injecting drug users in London: extent and nature of the problem. Addiction. 1999;94:471–478. doi: 10.1046/j.1360-0443.1999.9444712.x. [DOI] [PubMed] [Google Scholar]
- 11.Davidson PJ, McLean RL, Kral AH, Gleghorn AA, Edlin BR, Moss AR. Fatal heroin-related overdose in San Francisco, 1997–2000: a case for targeted intervention. J Urban Health. 2003;80:261–273. doi: 10.1093/jurban/jtg029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson PJ, Ochoa KC, Hahn JA, Evans JL, Moss AR. Witnessing heroin-related overdoses: the experiences of young injectors in San Francisco. Addiction. 2002;97:1511–1516. doi: 10.1046/j.1360-0443.2002.00210.x. [DOI] [PubMed] [Google Scholar]
- 13.Sporer KA, Kral AH. Prescription naloxone: a novel approach to heroin overdose prevention. Ann Emerg Med. 2007;49:172–177. doi: 10.1016/j.annemergmed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Seal KH, Thawley R, Gee L, Bamberger J, Kral AH, Ciccarone D, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: a pilot intervention study. J Urban Health. 2005;82:303–311. doi: 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis. 2006;25:89–96. doi: 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- 16.Galea S, Worthington N, Piper TM, Nandi VV, Curtis M, Rosenthal DM. Provision of naloxone to injection drug users as an overdose prevention strategy: early evidence from a pilot study in New York City. Addict Behav. 2006;31:907–912. doi: 10.1016/j.addbeh.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Scott G, Thomas SD, Pollack HA, Ray B. Observed patterns of illicit opiate overdose deaths in Chicago, 1999–2003. J Urban Health. 2007;84:292–306. doi: 10.1007/s11524-007-9157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobin KE, Gaasch WR, Clarke C, MacKenzie E, Latkin CA. Attitudes of emergency medical service providers towards naloxone distribution programs. J Urban Health. 2005;82:296–302. doi: 10.1093/jurban/jti052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigg D. Data on take home naloxone are unclear but not condemnatory. BMJ. 2002;324:678. doi: 10.1136/bmj.324.7338.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green TC, Grau LE, Heimer R. Medical Expert Agreement of Drug User Reported Overdose Symptoms; International Harm Reduction Association; London. 2006. [accessed 27 November 2007]. Available at: http://www.ihra.net/uploads/downloads/Conferences/Vancouver2006/Vancouver2006ConferenceAbstractBook.pdf. [Google Scholar]
- 21.Green TC, Hankins CA, Palmer D, Boivin JF, Platt R. Ascertaining the need for a supervised injecting facility (SIF): the burden of public injecting in Montreal, Canada. J Drug Issues. 2003;33:713–732. [Google Scholar]
- 22.Hui SL, Zhou XH. Evaluation of diagnostic tests without gold standards. Stat Methods Med Res. 1998;7:354–370. doi: 10.1177/096228029800700404. [DOI] [PubMed] [Google Scholar]
- 23.Toft N, Jorgensen E, Hojsgaard S. Diagnosing diagnostic tests: evaluating the assumptions underlying the estimation of sensitivity and specificity in the absence of a gold standard. Prev Vet Med. 2005;68:19–33. doi: 10.1016/j.prevetmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell SE. Sample size and multiple regression analysis. Psychol Methods. 2000;5:434–458. doi: 10.1037/1082-989x.5.4.434. [DOI] [PubMed] [Google Scholar]
- 26.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 27.Soper DS. [accessed 6 June 2007];The Free Statistics Calculators Website. Available at: http://www.danielsoper.com/statcalc/ [Google Scholar]
- 28.Galea S, Nandi A, Coffin PO, Tracy M, Markham PT, Ompad D, et al. Heroin and cocaine dependence and the risk of accidental non-fatal drug overdose. J Addict Dis. 2006;25:79–87. doi: 10.1300/J069v25n03_10. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein KT, Bucciarelli A, Piper TM, Gross C, Tardiff K, Galea S. Cocaine- and opiate-related fatal overdose in NewYork City, 1990–2000. BMC Public Health. 2007;7:31. doi: 10.1186/1471-2458-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaye S, Darke S. Non-fatal cocaine overdose among injecting and non-injecting cocaine users in Sydney, Australia. Addiction. 2004;99:1315–1322. doi: 10.1111/j.1360-0443.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 31.Coffin PO, Galea S, Ahern J, Leon AC, Vlahov D, Tardiff K. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–98. Addiction. 2003;98:739–747. doi: 10.1046/j.1360-0443.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 32.Boylan RT, Ho V. Tracking variations in cocaine deaths across U.S. cities. Addict Res Theory. 2004;12:461–468. [Google Scholar]
- 33.Ochoa KC, Hahn JA, Seal KH, Moss AR. Overdosing among young injection drug users in San Francisco. Addict Behav. 2001;26:453–460. doi: 10.1016/s0306-4603(00)00115-5. [DOI] [PubMed] [Google Scholar]
- 34.Poulin C, Fralick P, Whynot EM, El Guebaly N, Kennedy D, Bernstein J, et al. The epidemiology of cocaine and opiate abuse in urban Canada. Can J Public Health. 1998;89:234–238. doi: 10.1007/BF03403924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monga N, Rehm J, Fischer B, Brissette S, Bruneau J, El Guebaly N, et al. Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug Alcohol Depend. 2007;88:1–8. doi: 10.1016/j.drugalcdep.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Hankins C, Alary M, Parent R, Blanchette C, Claessens C. Continuing HIV transmission among injection drug users in Eastern Central Canada: the SurvUDI Study, 1995 to 2000. J Acquir Immune Defic Syndr. 2002;30:514–521. doi: 10.1097/00126334-200208150-00007. [DOI] [PubMed] [Google Scholar]
- 37.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 38.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Grau LE, Bluthenthal RN, Marshall P, Singer M, Heimer R. Psychosocial and behavioral differences among drug injectors who use and do not use syringe exchange programs. AIDS Behav. 2005;9:495–504. doi: 10.1007/s10461-005-9020-3. [DOI] [PubMed] [Google Scholar]
- 40.Villari P, Fattore G, Siegel JE, Paltiel AD, Weinstein MC. Economic evaluation of HIV testing among intravenous drug users. An analytic framework and its application to Italy. Int J Technol Assess Health Care. 1996;12:336–357. doi: 10.1017/s0266462300009673. [DOI] [PubMed] [Google Scholar]
- 41.Paltiel AD, Walensky RP, Schackman BR, Seage GR, III, Mercincavage LM, Weinstein MC, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 42.Jekel JF, Katz DL, Elmore JG, Wild D. Epidemiology, Biostatistics and Preventive Medicine. 3rd edn. Philadelphia: W.B. Saunders Co; 2001. [Google Scholar]