SUMMARY
White adipose tissue regulates metabolism; the importance of this control is highlighted by the ongoing pandemic of obesity and associated complications such as diabetes, atherosclerosis, and cancer. White adipose tissue maintenance is a dynamic process, yet very little is known about how pharmacologic stimuli affect such plasticity. Combining in vivo lineage marking and BrdU labeling strategies, we found that rosiglitazone, a member of the thiazolidinedione class of glucose-lowering medicines, markedly increases the evolution of adipose progenitors into adipocytes. Notably, chronic rosiglitazone administration disrupts the adipogenic and self-renewal capacities of the stem cell compartment and alters its molecular characteristics. These data unravel unknown aspects of adipose dynamics and provide a basis to manipulate the adipose lineage for therapeutic ends.
INTRODUCTION
Adipose tissue is a central component of metabolic control (Spiegelman and Flier, 2001). A surfeit or a deficiency of adipose tissue compromises its ability to maintain metabolic homeostasis. Indeed, the current epidemic of obesity is accompanied by a proportionate increase in a multitude of untoward sequelae; notable among them are diabetes, hyperlipidemia, hypertension, cardiovascular disease, and cancer (Kopelman, 2000). Thus, appropriate homeostatic balance of the adipocyte pool (e.g., number, mass, formation, replacement) is essential for health.
Evolving evidence, including human retrospective analyses in the wake of atmospheric radioisotope discharges, indicates that adipocytes constantly turn over and are replenished (Spalding et al., 2008). External stimuli, such as calorie excess, appear able to influence this flow, possibly stimulating stem cell behaviors (Faust et al., 1978; Joe et al., 2009). Some evidence indicates that the thiazolidinedione (TZD) family of anti-diabetes drugs remodel adipose tissue (de Souza et al., 2001). TZDs are potent adipogenic factors in vitro (Hiragun et al., 1988; Sandouk et al., 1993). In vivo, these molecules reduce blood sugar but their administration is often accompanied by the unpleasant side effect of weight gain (Yki-Jarvinen, 2004). This may in part be explained by histological studies indicating that TZDs induce recruitment of new adipocytes; it is postulated that this mobilization is a component of the beneficial effects of TZDs (Hallakou et al., 1997; de Souza et al., 2001; Fonseca, 2003). Therefore, TZDs may provide a powerful tool to unravel mechanisms underlying adipose tissue plasticity. Defining whether and how these molecules act on stem cells may provide an impetus to further exploit control of adipose progenitor activity as a therapeutic strategy for obesity and diabetes.
Through inducible genetic marking, lineage tracing and flow methodologies, termed AdipoTrak, we recently identified progenitors that are central to adipose development (Tang et al., 2008). Friedman, Rossi, and their respective colleagues, by means of flow cytometry-based approaches, also identified adipose stem cells that have many similar characteristics to those we identified with AdipoTrak (Rodeheffer et al., 2008; Joe et al., 2009). Here we investigated the possibility that pharmacologic stimuli affect the behavior of adipose lineage and adipose progenitors in adult mice. We found that rosiglitazone (Rosi), a TZD class drug, stimulates both the differentiation and the proliferation of the progenitors. Moreover, chronic TZD administration results in a progenitor pool in which adipogenesis and self-renewal are compromised.
RESULTS
TZDs Stimulate In Vivo Adipogenic Differentiation
An inherent feature of the AdipoTrak system is the ability to trace the life cycle of the adipose lineage. In this inducible system, the tet-transactivator (Dox-off) was recombined into the endogenous PPARγ locus, a master gene in adipocyte biology (Tang et al., 2008; Tontonoz and Spiegelman, 2008). We then combined the PPARγ-tTA allele with two complementary reporter systems, TRE-H2B-GFP as well as TRE-Cre; R26RlacZ (Soriano, 1999; Tumbar et al., 2004). The first arrangement, “PPARγ-GFP”, drives expression of a nuclear-localized GFP within the adipose lineage (Kanda et al., 1998; Hadjantonakis and Papaioannou, 2004). H2B-GFP is thought stable in post-mitotic cells (Schaniel and Moore, 2009); however, if the system is suppressed by Dox, the GFP signal is diminished upon cell death or proliferation and, in fat depots, if progenitors leave the SV compartment and differentiate into mature buoyant adipocytes. The “PPARγ-R26R” is a binary (on or off) indelible marking system that is present in PPARγ-expressing cells and all descendants. The indelible marking is achieved when Cre is driven in PPARγ-expressing cells, and, by recombination, deletes the translational stop signal present upstream of lacZ in the ROSA26 locus, allowing the permanent expression of β-galactosidase, the level of which solely depends on the ROSA26 promoter. When integrated, these systems allow assessment of adipose lineage dynamics.
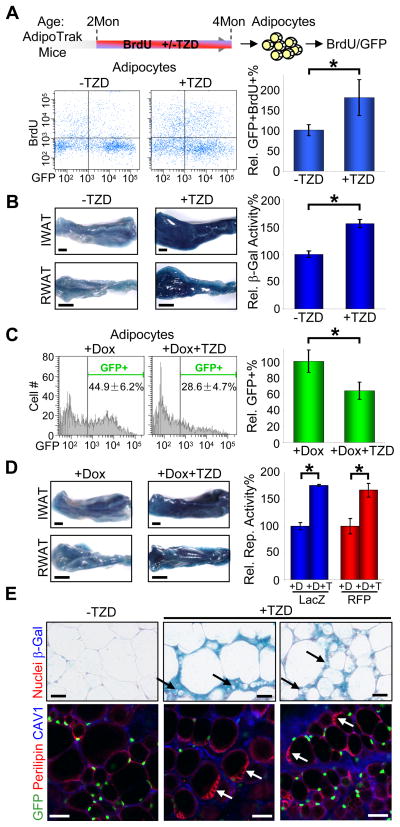
TZDs are widely prescribed for Type II diabetes. In patients as well as animal models, TZDs often increase fat mass and alter fat morphology with the appearance of small adipocytes (Hallakou et al., 1997; Okuno et al., 1998; de Souza et al., 2001). To investigate whether these changes might in part be secondary to the recruitment of new adipocytes from a proliferating source, we administered BrdU in the drinking water of AdipoTrak mice and then randomized the mice to either placebo or Rosi, a widely prescribed TZD (Figure 1A). We then digested adipose depots and separated the resultant cells with centrifugation into the floated fraction containing buoyant adipocytes and the pelleted stomal-vascular fraction (SVF) containing adipose stem cells (Tang et al., 2008). We next evaluated the amount of BrdU incorporated into floated GFP+ adipocytes with flow cytometry. We found that TZDs increased the amount of BrdU present in floated AdipoTrak-marked adipocytes approximately 2-fold (Figure 1A).
Figure 1. TZDs Stimulate New Adipocyte Formation.
(A) Two-month old AdipoTrak mice were treated with or without TZD while receiving BrdU-water for 2 month, and adipocyte nuclei were probed for BrdU uptake with flow cytometry. Top diagram is the experimental design. X-axis of the flow profiles is GFP intensity, and Y-axis is BrdU (bottom left panels). The percent change of +TZD BrdU+GFP+ events compared to −TZD is displayed in the graph on the bottom right. n = 4 per cohort
(B–E) Two-month old AdipoTrak mice were treated as indicated for 2 months and examined for reporter expression. (B) IWAT and RWAT were stained (left panels) or quantified (the graph on the right) for β-galactosidase activity. n = 4 per cohort (C) Adipocyte nuclei were analyzed for GFP with flow cytometry. The relative percentages of GFP+ nuclei are graphed on the right. See also Figure S1. n = 4 to 6 per cohort (D) IWAT and RWAT were stained (left panels) or quantified (blue, the graph on the right) for β-galactosidase activity. AdipoTrak mice carrying a R26RRFP reporter were similarly treated, and total RFP from all adipocytes isolated from each experimental group were quantified and displayed as a relative value in the graph on the right (red). (E) Adipose tissues were stained with X-Gal, sectioned and counterstained with nuclear fast red (top panels). Black arrows point to small adipocytes rarely seen in non-treated controls. Adipose tissues were examined for GFP, perilipin (lipid droplets) and caveolin-1 (CAV1, cytoplasmic membrane) (bottom panels). White arrows point to adipocytes that contain multiple lipid droplets rarely seen in non-treated controls.
*p < 0.05 by two-tailed Student’s t-Test. Error bars indicate SEM. Scale bars: 2mm (B left, D left); 50 μm (E)
We further explored the effects of TZDs on in vivo adipose specification and formation of new adipocytes with the β-galactosidase reporter system. To that end, we treated AdipoTrak mice with a 2-month course of placebo or Rosi. We then determined the levels of adipose depot β-galactosidase and observed a significant TZD-induced increase in X-gal staining and β-galactosidase activity (Figure 1B). Since the R26R system is binary (either on or off), these data indicate that TZDs induce the formation of a significant number of new cells.
We continued to probe the TZD-dependent effects on adipose depots by administering Dox to suppress the AdipoTrak system. We randomized AdipoTrak mice to receive a two-month course of either Dox alone or Dox with Rosi and then interrogated adipocyte GFP levels with flow cytometry. We found that Rosi reduced the percentage and fluorescent intensity of adipocytes that expressed the GFP reporter in the presence of Dox (Figures 1C, S1). These data support the notion of TZD-induced adipocyte turnover and formation, thus paralleling the TZD-induced increase in adipocyte BrdU incorporation and lacZ reporter described above (Figures 1A, 1B).
To evaluate whether the newly formed adipocytes derived from an AdipoTrak-marked source, we examined expression of the permanent PPARγ-R26R marker in the Dox-suppressed state. Nonetheless, we again detected a roughly 1.5-fold increase in β-galactosidase staining and activity even with Dox-induced suppression (Figure 1D). We supplemented these studies by replacing the R26RlacZ with an R26RRFP allele (Madisen et al., 2010), and similar results were observed (Figure 1D). In the TZD-treated R26RlacZ and GFP reporter samples (with or without Dox), histological analyses revealed clusters of small intensely labeled cells, which could be nascent adipocytes, as well as cells that contained multiple lipid droplets, potentially due to adipocyte remodeling (Figure 1E).
TZDs Stimulate Progenitor Proliferation
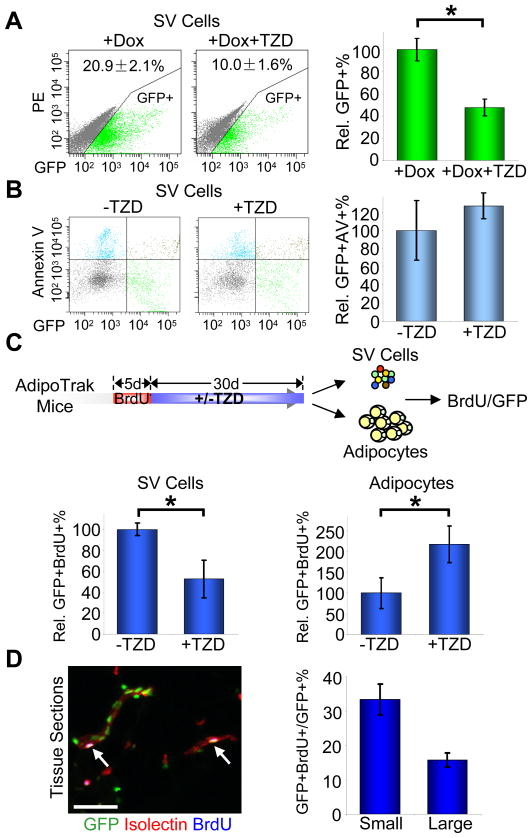
To further investigate the possible source of the Rosi-induced new adipocytes, we tracked the stem cell compartment combining AdipoTrak marking, Rosi treatment, and Dox suppression. In concert with the observed changes in adipocytes, we found that the number, percentage, and fluorescent intensity of the SV progenitors were significantly reduced by Rosi (Figures 2A, S2). We next evaluated whether TZDs might alter the rate of cell death in SV progenitors, which could account for the observed GFP reduction. However TZDs did not significantly change the apoptotic rate of the progenitors (Figure 2B). These data support the notion that TZDs couple SV progenitor proliferation, adipogenic differentiation, and evolution of new adipocytes. To examine such a potential flow from SV progenitors to adipocytes, we pre-labeled mice with BrdU, stopped BrdU, and during a 1-month chase administered placebo or Rosi (Figure 2C). After this chase, we quantified BrdU levels in both the SV and the adipocyte fractions, and found that Rosi, compared to placebo, induced a significant reduction in the progenitor BrdU level accompanied by a commensurate increase in adipocyte BrdU content (Figure 2C). We also found that the progenitors that retained label, a hallmark of stem cells, after TZD treatment were present in the vasculature (Figure 2D). Together these data indicate that TZDs stimulate SV-resident AdipoTrak-marked progenitors to divide and differentiate into new adipocytes.
Figure 2. TZDs stimulate in vivo progenitor proliferation and differentiation.
(A–B) Adult AdipoTrak mice were treated as indicated for 2 months, and adipose SV cells were analyzed for GFP (A) or the apoptotic marker Annexin V (B) with flow cytometry. (A) X-axis is GFP intensity; Y-axis is PE channel to illustrate the distribution of GFP+ cells. The percentage of GFP+ cells are as indicated at top of the flow profiles, and displayed as percent changes in the graph on the right. See also Figure S2. n = 4~6 per cohort (B) X-axis is GFP intensity; Y-axis is Annexin V. The relative percentages of GFP+/Annexin V+ cells compared to −TZD are shown on the right. n = 4 per cohort
(C) AdipoTrak mice were injected with BrdU for 5 consecutive days, and then treated with or without TZD for 1 month. Top diagram shows the experimental design. Isolated SV cells (bottom left) and adipocyte nuclei (bottom right) were analyzed for BrdU incorporation with flow cytometry. The relative percentages of GFP+/BrdU+ cells are displayed at the bottom. n = 3~4 per cohort
(D) Adipose tissues from mice treated as in (C) were sectioned and stained for GFP, isolectin (red) and BrdU (blue). White arrows indicate label-retaining progenitors located in the vascular niche. The percent of GFP+BrdU+ cells in GFP+ cells on small (1–2 cells in diameter) or relatively larger (3–5 cells in diameter) blood vessels (as stained positive for isolectin) were quantified and displayed on the right.
*p < 0.05 by two-tailed Student’s t-Test. Error bars indicate SEM. Scale bars: 50μm (D left)
TZDs Exhaust the Progenitor Pool
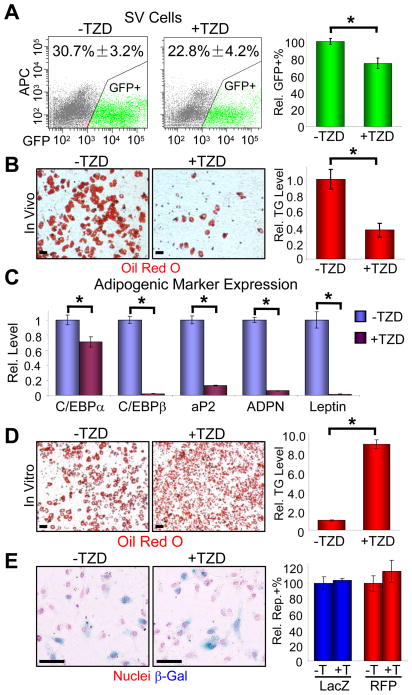
TZDs are a potent proliferative and adipogenic stimulus to adipose progenitors in vivo (Figures 1, 2). To assess the effect of chronic TZD administration on the progenitor pool, we treated AdipoTrak mice with a two-month course of placebo or Rosi and evaluated the SV fraction for GFP expression. These studies were done in the absence of Dox, to eliminate the potential confounds of suppressing the reporter system as described above. Interestingly, we also observed a significant decrease in the number and intensity of GFP positive progenitors present in the SV fraction even in the non-suppressed state (Figures 3A, S3). To determine possible functional consequences of this reduction, we again treated mice with placebo or Rosi for 2 months, isolated total SV as well as GFP positive cells (an equal number of GFP positive cells were plated from placebo and Rosi mice) and evaluated their adipogenic potential in culture. Notably, in either case, chronic in vivo TZD administration reduced in vitro adipogenesis based upon Oil Red O staining and adipogenic marker quantification (Figures 3B, 3C, not shown). This reduction contrasts with the potent pro-adipogenic effect of in vitro TZD administration, for example such as that observed when Rosi was added to primary cultures of SV or GFP positive sorted cells isolated from mice that did not receive Rosi (Figure 3D, not shown). One possible explanation for the reduced SV GFP expression observed after chronic TZD exposure was that TZDs decreased the number of progenitors present in the niche. However, the reduced adipogenic potential of the GFP-sorted cells indicates that this was not the only perturbation. Another possibility was that the reduced GFP levels resulted from altered behavior of the progenitor pool, for example a division in which GFP is no longer expressed in a subset of progeny. To distinguish between changes in progenitor number from changes in progenitor behavior, we turned to lineage tracing methodology. To that end, we repeated the AdipoTrak chronic TZD studies but in this case quantified the number of SV cells that expressed the indelible lacZ or RFP markers. Notably, and in contrast to the reduced stem cell GFP expression (Figure 3A), TZDs had no effect on the number or percentage of lineage traced, β-galactosidase or RFP positive, cells present in the SV fraction (Figure 3E). These data support the notion that chronic TZD administration depletes the adipogenic capacity of the progenitor pool and induces a progenitor division whereby some descendants display altered properties.
Figure 3. TZDs exhaust the progenitor pool.
(A–C) Adult AdipoTrak mice were treated with or without TZD for 2 months, and the adipose SV cells were analyzed for GFP (A) or sorted for GFP+ cells, plated in equal numbers, and cultured in insulin (B–C). (A) The percentages of GFP+ cells are as indicated at top of the flow profiles. X-axis is GFP intensity; Y-axis is APC channel to illustrate cell distribution. The relative percentages of GFP+ cells, compared to −TZD, are shown on the graph on the right. See also Figure S3. n = 4 per cohort (B–C) Adipogenesis was assayed with Oil Red O staining (left two panels (B), fat stains red), triglyceride quantification (graph on the right, (B)), and qPCR analyses of a panel of adipogenic markers (C). ADPN: adiponectin.
(D) SV cells from untreated AdipoTrak mice were isolated, cultured in insulin with or without TZD. Adipogenesis was assayed by Oil Red O staining (left two panels) and triglyceride quantification (right panel).
(E) AdipoTrak R26RlacZ or R26RRFP reporter mice were treated with or without TZD for 2 months, and adipose SV cells were examined for β-galactosidase by X-gal staining (left panels) and cell counting (2,500~5,500 cells, blue in the graph) or RFP with flow cytometry (red in the graph).
*p < 0.05 by two-tailed Student’s t-Test. Error bars indicate SEM. Scale bars: 50 μm (B, D, E)
Chronic TZDs Alter the Molecular Signature of Adipose Progenitors
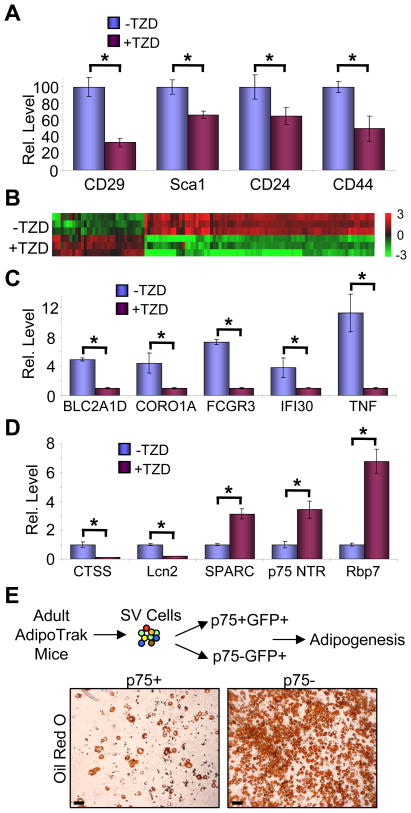
We next began to probe the molecular underpinnings of the changes induced in the progenitor pool by 2 months of TZD administration. We interrogated AdipoTrak-labeled progenitors derived from control or Rosi-treated mice with a panel of relevant cell surface markers using flow cytometry. Although markers of other lineages were unchanged, we found that chronic TZD treatment significantly decreased the expression of several key adipogenic stem cell markers (e.g., Sca1, CD29, CD24) as well as mesenchymal stem cell markers (e.g., CD44) (Figure 4A, not shown). These data further support the notion that long-term TZD treatment alters the stem and progenitor properties of the AdipoTrak-labeled progenitor pool.
Figure 4. In vivo TZD treatment alters the molecular characteristics of adipose progenitors.
(A–D) Adult AdipoTrak mice were treated with or without TZD for 2 months, and adipose GFP+ SV cells were analyzed with a panel of cell surface markers (A) or sorted and interrogated with gene expression profiling (B) or qPCR (C–D). (A) Some markers reduced by TZD are displayed as percentage of GFP+ cells that co-expressed the marker of interest. (B) The heat map illustrates 133 genes in which TZD induced differential expression. The color bar on the right indicates gene expression level in log 2 scale. n = 3–4 mice per cohort, 3 cohorts for each experimental group (C–D) qPCR analyses of selected genes identified from microarray that are involved in immune response (C) or have established roles in fat formation or obesigenic responses (D).
(E) GFP+ SV cells were stained with an anti-p75 NTR antibody, and sorted into p75− and p75+ fractions; equal numbers of cells from each fraction were plated, incubated in insulin, and stained with Oil Red O.
*p < 0.05 by two-tailed Student’s t-Test. Error bars indicate SEM. Scale bars: 50 μm (E)
We also performed expression profiling, sorting and comparing the molecular signature of GFP+ progenitors isolated from mice treated with or without TZD. We identified a battery of genes whose expression was significantly different between the two populations (Figure 4B); qPCR on selective genes validated the microarray data (Figures 4C, 4D). Many of the genes down-regulated by TZDs were secreted cytokines and factors that regulate immune responses (e.g., TNF, CCLs, etc.). TZDs have anti-inflammatory properties, and these data raise the possibility that TZDs might exert such effects through adipose progenitors even though the progenitors do not belong to the hematopoietic lineage (Tang et al., 2008). Notably, many of the genes that were identified have previously established roles in fat formation or obesigenic responses and they were regulated in a manner consistent with a potential role in the altered adipogenic ability (Figure 4D). For example, levels of cathepsin S (CTSS), a gene that promotes adipogenesis and is increased in obesity and associated diseases (Taleb and Clement, 2007), were significantly reduced by TZDs. In contrast, genes such as SPARC (secreted protein, acidic, cysteine-rich), an inhibitor of adipogenesis and obesity (Kos and Wilding, 2010; Nagaraju and Sharma, 2011), were increased by TZDs as were TZD targets such as RBP7 (retinol-binding protein 7) (Zizola et al., 2008). We extended these studies by further examining the potential role of one of the up-regulated genes, p75 NTR (p75 neurotrophin receptor), in progenitor adipogenesis. Because chronic TZDs reduced adipogenesis, we hypothesized that genes that were up-regulated in this state might themselves be adipogenic inhibitors. We selected p75 as it is a known stem cell marker regulating differentiation in other lineages, and because it is a cell surface marker thereby allowing direct investigation of its function within the adipose lineage using cell sorting. To that end, we compared the adipogenic potential of AdipoTrak GFP+ cells further sorted into p75+ and p75− populations (Figure 4E). Consistent with the hypothesis, p75+ progenitors had significantly lower adipogenic potential than progenitors that were p75− (Figure 4E). Taken together, these studies provide initial molecular insights into how in vivo TZD administration alters the adipogenic behavior and stem cell properties of the AdipoTrak-marked progenitor pool.
DISCUSSION
Adipose depots regulate metabolism and much interest stems from the potential of altering adipose biology to therapeutic ends; the vascular location of adipose progenitors indicates that they might be therapeutically accessible (Tang et al., 2008; Zeve et al., 2009). To explore this notion, we examined effects of TZDs on adipose lineage and stem cell dynamics as several lines of evidence indicated a potential role (Hiragun et al., 1988; Lehmann et al., 1995; de Souza et al., 2001; Tang et al., 2008; Tontonoz and Spiegelman, 2008; Choi et al., 2010). Although TZDs are effective at lowering blood glucose levels, side effects and concerns that TZDs increase cardiovascular risk have hastened the need to find alternative therapeutics (Nissen and Wolski, 2007; Home et al., 2009). A better understanding of whether and how TZDs modulate the adipose lineage may shed light on their insulin-sensitizing efficacy, and may also help to develop the next generation insulin-sensitizers.
Our studies indicate that TZDs enhance the homeostatic flow of cells from the progenitor compartment to adipocytes. For example, BrdU and AdipoTracking studies indicate that TZDs roughly double the number of adipocytes formed during treatment compared to controls. A parallel stimulation was also observed in the progenitor fraction with marked changes in the ephemeral AdipoTrak reporter supporting an evolution from the progenitor compartment to differentiated cells. As an independent measure of the notion that TZDs induce new adipocytes to emanate from the progenitor fraction, we pre-labeled mice with BrdU and then quantified possible TZD-dependent changes in BrdU levels in the stem and mature adipocyte compartments. We found a significant reduction in BrdU in the progenitors and a commensurate increase in adipocytes. Together the studies indicate that TZDs activate the progenitors to proliferate, differentiate and form new adipocytes.
Notably, chronic TZD administration significantly altered the progenitor pool, decreasing the number of GFP positive AdipoTrak-marked cells present in the SV fraction and reducing the adipogenic potential of the total SV pool and of sorted GFP positive cells. One possibility for these effects was that TZDs were such a potent adipogenic stimulus that they induced progenitors to become adipocytes without a commensurate replenishment of the stem cell population. That is they uncoupled the normal homeostatic balance of the progenitor pool. Lineage tracing with the indelible reporter system indicated that the number of traced SV-resident cells that derived from the progenitors was unchanged by TZDs. This supports the notion that progenitor division appeared to be coordinated with adipogenic exodus. Apparently the progeny of some of these divisions were different from those produced during homeostasis; GFP expression and adipogenic capacity were reduced and cell surface marker and gene expression were changed in a manner consistent with the observed phenotypic changes. These data support potential TZD-induced divisions in which daughter cells have lost some of the properties of the mother cell. These observations open up potential new avenues to manipulate adipocyte number and fat biology.
White adipose tissue plays a key role in regulating metabolism. A thorough understanding of the physiology and pharmacological responses of adipose tissue may help to develop strategies for obesity and associated metabolic diseases. We found that the TZD class of diabetes drugs alters the dynamics of adipocytes and progenitors, and indicate that adipose progenitors are therapeutically accessible. Further characterization of lineage dynamics may provide additional means to regulate adipocyte specification and turnover.
EXPERIMENTAL PROCEDURES
Animals
Mice were housed in a 12:12 light:dark cycle and chow and water were provided ad libitum. R26RRFP is available in the Jackson Laboratory (Stock #007905). Doxycycline (0.5mg/ml in 1% sucrose) was provided in the drinking water and protected from light. BrdU was I.P. injected (100mg/kg body mass) or provided in the drinking water (0.5mg/ml in 1% sucrose) and protected from light. Mice were fed with rosiglitazone at 0.0075% in normal chow (4% fat mouse diet from Harlan TEKLAD) when desired. Rosi intake was estimated to be 15mg/kg body mass/day. All animals were maintained under the guidelines of the U.T. Southwestern Medical Center Animal Care and Use Committee according to NIH guidelines.
SV and Adipocyte Fractionation
The fractionation of the stromal-vascular cells and adipocytes was as described (Tang et al., 2008) except that the SVF pellet was resuspended in erythrocyte lysis buffer (0.83% NH4Cl in H2O) for 8 minutes to lyse red blood cells before being subjected to flow cytometry or culture. Adipocyte nuclei were extracted as described (Tang et al., 2008).
Flow Cytometry Analysis
Cell surface marker analysis and sorting of GFP+ and GFP− cells were performed on live cells stained with propidium iodide (PI, 1 μg/ml) or 7-AAD (1 μg/ml) to exclude dead cells that incorporated dyes. Isolated and fixed adiocyte nuclei were similarly stained and gated for dye positive nuclei events to exclude non-nucleus debris. BrdU detection of SV cells was performed with APC BrdU Flow Cytometry Kit according to manufacturer’s instructions. Adipocyte nuclei were fixed in nuclei wash buffer (Tang et al., 2008) containing 10% formaldehyde prior to the BrdU detection, and spun at 3000g.
Gene Expression Microarrays
Adipose GFP+ SV cells were sorted from 6 cohorts of adult AdipoTrak mice (3~4 mice per cohort), either treated with or without Rosi for 2 months. These GFP+ SV cells were then subjected to gene expression microarrays performed on the Illumina Mouse-6 V1.1 BeadChip arrays by the UTSWMC Microarray Core as described (Tang et al., 2008). Genes considered to have differential expression values between the −TZD and +TZD groups showed a ≥2-fold change in expression (at p<0.05).
Supplementary Material
HIGHLIGHTS.
Thiazolidinedions (TZDs) stimulate new adipocyte formation in white adipose tissue.
TZDs stimulate the proliferation of adipose progenitors and differentiation into new adipocytes.
Chronic TZDs treatment disrupts the adipogenic ability and self-replenishment of the adipose progenitor pool.
TZDs alter the molecular characteristics of adipose progenitors.
Acknowledgments
We thank Dr. Luis Parada, Dr. Michelle Tallquist, Shawna Kennedy and members of the Graff Lab. This study was supported by NIH and NIDDK (R01 DK066556, R01 DK064261 and R01 DK088220), the AHA postdoctoral (W.T. and J.S.) and predoctoral (D.Z.) fellowship grants. J.M.G. is a founder of Reata Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, Burkey BF. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50:1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279–286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;115(Suppl 8A):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, Dugail I, Morin J, Auwerx J, Ferre P. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393–1399. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- Hiragun A, Sato M, Mitsui H. Preadipocyte differentiation in vitro: identification of a highly active adipogenic agent. J Cell Physiol. 1988;134:124–130. doi: 10.1002/jcp.1041340115. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Kanda T, Sullivan KF, Wahl GM. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kos K, Wilding JP. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol. 2010;6:225–235. doi: 10.1038/nrendo.2010.18. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Nagaraju GP, Sharma D. Anti-cancer role of SPARC, an inhibitor of adipogenesis. Cancer Treat Rev. 2011 doi: 10.1016/j.ctrv.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Sandouk T, Reda D, Hofmann C. Antidiabetic agent pioglitazone enhances adipocyte differentiation of 3T3-F442A cells. Am J Physiol. 1993;264:C1600–1608. doi: 10.1152/ajpcell.1993.264.6.C1600. [DOI] [PubMed] [Google Scholar]
- Schaniel C, Moore KA. Genetic models to study quiescent stem cells and their niches. Ann N Y Acad Sci. 2009;1176:26–35. doi: 10.1111/j.1749-6632.2009.04608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Taleb S, Clement K. Emerging role of cathepsin S in obesity and its associated diseases. Clin Chem Lab Med. 2007;45:328–332. doi: 10.1515/CCLM.2007.083. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell. 2009;5:472–481. doi: 10.1016/j.stem.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizola CF, Schwartz GJ, Vogel S. Cellular retinol-binding protein type III is a PPARgamma target gene and plays a role in lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E1358–1368. doi: 10.1152/ajpendo.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.