Abstract
Objective
Increasing evidence has accumulated showing the role of APOBEC3G (A3G) and 3F (A3F) in the control of HIV-1 replication and disease progression in humans. However, very few studies have been conducted in HIV-infected children. Here, we analyzed the levels of A3G and A3F expression and induced G-to-A hypermutation in a group of children with distinct profiles of disease progression.
Methodology/Principal Findings
Perinatally HIV-infected children were classified as progressors or long-term non-progressors according to criteria based on HIV viral load and CD4 T-cell counts over time. A group of uninfected control children were also enrolled in the study. PBMC proviral DNA was assessed for G-to-A hypermutation, whereas A3G and A3F mRNA were isolated and quantified through TaqMan® real-time PCR. No correlation was observed between disease progression and A3G/A3F expression or hypermutation levels. Although all children analyzed showed higher expression levels of A3G compared to A3F (an average fold of 5 times), a surprisingly high A3F-related hypermutation rate was evidenced in the cohort, irrespective of the child's disease progression profile.
Conclusion
Our results contribute to the current controversy as to whether HIV disease progression is related to A3G/A3F enzymatic activity. To our knowledge, this is the first study analyzing A3G/F expression in HIV-infected children, and it may pave the way to a better understanding of the host factors governing HIV disease in the pediatric setting.
Introduction
Human immunodeficiency virus type 1 (HIV-1) infection in children often progresses rapidly to acquired immunodeficiency syndrome (AIDS). The majority of infected children under 15 years of age develop AIDS, and most die [1]. The risk of pediatric disease progression in mother-to-child transmission (MTCT) has been associated with different factors, such as mother with advanced HIV-1 disease, as well as immunological, virological and host-specific factors [2], [3].
A new class of host restriction factors has been described to play an important role in restricting intracellular viral replication. Enzymes of the apolipoprotein B mRNA-editing catalytic polypeptide (APOBEC) family convert cytidine to uridine in the transient (−)ssDNA replication intermediate, which is later reflected as G-to-A changes in the (+)strand [4], [5], [6], [7]. As these mutations usually occur at very high frequency in sequences, this phenomenon is often called hypermutation. Dinucleotide motifs within DNA are preferentially targeted by APOBEC3G (A3G) and APOBEC3F (A3F), resulting in proviral DNA GG to AG and GA to AA substitutions, respectively. A3G and A3F exhibit potent anti-HIV-1 activity [8], [9], [10], [11], [12], [13] and are expressed in lymphocytes, the major target cells for HIV-1 infection [9], [11]. HIV-1 Vif counteracts A3G/F by preventing their encapsidation into virions and by inducing their proteossomal degradation in the producer cell [14], [15]. The effects of A3G/F proteins likely modify both the efficiency of HIV-1 transmission and disease progression [16], but their role in these processes is not well understood.
A3G has been referred to as the major contributor to hypermutation in vivo and in vitro [12], [13], [17], [18], [19], [20], [21], [22], [23]. However, depending on the HIV genomic region analyzed, varying effects of A3G can be found. For example, hypermutation analyses in the HIV protease (PR) region of the pol gene have indicated a proportionally large number of G-to-A changes associated to A3F [24], [25].
Evidence suggests that individuals who express high levels of A3G mRNA tend to control the levels of HIV-1 viremia [26], [27]. It has also been suggested that levels of A3G expression decrease with disease progression [28]. A recent study showed that the frequency of hypermutated viral genomes in elite suppressors was not significantly different from that observed in patients on highly active antiretroviral therapy (HAART), and no correlation was found between A3G expression and the rate of hypermutation [20]. Differently from the previous report, the level of hypermutation was correlated with A3G expression in other studies [28], [29].
In the present study, we analyzed A3G and A3F mRNA expression levels, as well as G-to-A hypermutation contexts in HIV-1 proviral protease sequences from infected children with different profiles of disease progression.
Methods
Ethics Statement
All parents/guardians of the children read and signed an informed consent to participate to this study. This study was approved by the Institutional Review Board of the Federal University of Rio de Janeiro.
Patients and Samples
Seventeen HIV-1-infected children with different profiles of disease progression regularly followed-up at the Pediatric Unit of Federal University of Rio de Janeiro since birth were enrolled in this study. All clinical and laboratory data, including CD4+ T-cell counts and HIV-1 viral load at every 3–4 months, were retrieved from a previous prospective study. All patients have been infected perinatally and therefore had known time of infection. Stored peripheral blood mononuclear cell (PBMC) samples were thawed and used for mRNA and proviral DNA extraction (see below). Six children were classified as long-term nonprogressors (LTNP), defined as over 8 years of age, with CD4≥25%, and under no antiretroviral therapy, a well-accepted definition for pediatric patients [30]; the remaining children (11 patients) were classified as typical progressors. Demographical, clinical, laboratory and follow-up data for all pediatric patients are summarized in Table 1. For A3G and A3F expression level determination, nine samples of HIV-negative children (NC) from the same previous study were used as a negative control group to assess A3G/F expression in the absence of HIV infection.
Table 1. Demographic and clinical characteristics of HIV-infected children under study.
| Patient ID | Age* | Treatment experience* | CDC classification* | Follow-up Data | Sample Data | ||||
| Period | Highest logHIV-1 VL | LowestCD4 T-cell% | Date | Log HIV-1 VL | CD4+T-cell% | ||||
| N35/LTNP | 10.2 | No | A1 | 1994–2003 | 4.59 | 28 | 04/2003 | 3.73 | 27 |
| N52/LTNP | 10.6 | Yes | A1 | 1992–2002 | 4.88 | 27 | 11/2002 | 3.88 | 33 |
| N64/LTNP | 11.3 | No | A1 | 1993–2004 | 4.40 | 31 | 05/2003 | 4.08 | 33 |
| N70/LTNP | 13.7 | No | A1 | 1996–2004 | 4.30 | 30 | 11/2003 | 4.34 | 32 |
| N72/LTNP | 7.7# | No | N1 | 2000–2007 | 2.58 | 29 | 12/2003 | 2.23 | 30 |
| N82/LTNP | 6.8# | No | N1 | 1998–2007 | 4.91 | 33 | 07/2004 | 4.85 | 34 |
| N48/P | 6.3 | Yes | A3 | 2001–2004 | 4.28 | 17 | 02/2004 | 3.64 | 19 |
| N58/P | 5.8 | No | A2 | 2000–2004 | 5.49 | 15 | 07/2003 | 5.49 | 25 |
| N59/P | 5.6 | Yes | A2 | 2000–2005 | 5.04 | 5 | 09/2003 | 4.75 | 14 |
| N61/P | 12.2 | Yes | A2 | 1998–2004 | 4.77 | 15 | 02/2003 | 2.86 | 23 |
| N67/P | 8.2 | Yes | B2 | 1999–2005 | 5.28 | 10 | 04/2004 | 2.34 | 10 |
| N75/P | 6.8 | Yes | B2 | 2000–2004 | 5.15 | 19 | 08/2003 | 1.96 | 22 |
| N25/P | 3.4 | Yes | C3 | 2001–2003 | 6.45 | 1 | 06/2003 | 5.26 | 19 |
| N27/P | 3.0 | Yes | C3 | 2001–2003 | 6.41 | 9 | 10/2002 | 5.30 | 15 |
| N28/P | 3.3 | Yes | C3 | 2001–2003 | 6.97 | 6 | 09/2002 | 5.79 | 24 |
| N33/P | 3.0 | Yes | C3 | 2001–2003 | 7.53 | 4 | 12/2003 | 4.83 | 35 |
| N62/P | 1.9 | Yes | B3 | 2002–2003 | 5.49 | 6 | 12/2002 | 5.23 | 8 |
*At sample collection.
#Both patients had CD4%>25% at the age of 8 years old.
HIV proviral DNA hypermutation
Genomic DNA from PBMC was isolated using the Illustra Blood GenomicPrep Mini Spin Kit (GE Healthcare). Nested PCR was then performed to amplify the HIV-1 proviral protease (PR) genomic region corresponding to HXB2 coordinates 2268–2564, as previously described [31]. HIV PR region was chosen because the first half of the pol gene is a highly frequent target of hypermutation-associated changes [21], [23], [32]. PCR-amplified products were cloned into pMOSBlue Blunt-ended PCR cloning kit (GE Healthcare) and a minimum of 10 clones from each patient were isolated by colony PCR and sequenced, therefore providing a sensitivity of 10% for detecting hypermutated sequences. Hypermutation was quantified using Hypermut 2.0 [33]. A sequence was considered hypermutated when the p-value was ≤0.05 in the Fisher's exact test that compared the number of G-to-A mutations by A3G/F versus a control context. In order to avoid overestimation of hypermutation by comparing different viral strains, we have used the plasma-derived genomic viral sequence generated by RT-PCR, representative of each patient's circulating virus, as the control context. We have also used the HIV-1 clone HXB2 as a reference for context in Hypermut for comparison of results. All patients were infected with HIV-1 subtype B viruses, as determined by phylogenetic analysis (not shown). All plasma-derived bulk sequences and proviral clonal sequences analyzed were submitted to the GenBank sequence database and were assigned the accession numbers JF950037 to JF950234.
APOBEC3G and 3F mRNA expression analyses
Patient's PBMC also had their RNA extracted and purified for A3G and A3F mRNA expression analyses through TaqMan®-based real time PCR. Total RNA was extracted with Trizol®, precipitated with lithium chloride and quantified in a Nanodrop® apparatus. Two micrograms of RNA were subject to cDNA synthesis with Superscript RT and oligo-d(T) (Invitrogen, CA). For a subset of the samples, we have also primed the RT reactions with random hexameric primers, using the same amount of RNA. Primers and probes for A3G, A3F and the internal control GAPDH were synthesized through the Assay-on-DemandTM (Applied Biosystems). Real time PCR reactions were conducted in an ABI 7500 apparatus (Applied Biosystems) in triplicate and with three different dilutions for each sample for determination of the reaction efficiency. Only reactions with efficiency superior to 95% were used in the calculations. Results were expressed as the relative number of A3G or A3F mRNA copies per 104 copies of GAPDH mRNA.
Statistical analyses
Demographic and laboratory data (CD4+ T-cell counts and HIV viral load) were compared among the two patient groups (typical progressors versus non-progressors) using Student's t tests, whereas for A3G and A3F expression level comparisons the Mann-Whitney U test was used. The definition of HIV-1 hypermutated sequences conducted in Hypermut was implemented within the software package using Fisher's exact tests. We have also compared A3G and A3F mRNA expression levels between patients for which hypermutation was detected and those with no hypermutation. Finally, we have stratified patients according to HIV viral load levels (below and above 10,000 copies/ml) and according to their treatment status (treatment-naïve or experienced), and compared the groups with respect to A3G/F expression. Calculations were performed in StatsDirect v.2.7.2 [34]. In all cases, p-values were considered significance at the ≤0.05 level.
Results
Demographic and clinical characteristics of the HIV-infected children analyzed in this study are depicted in Table 1. Among the LTNP children, the lowest CD4+ T-cell percentage values during all time of follow-up ranged from 27 to 33%, whereas for progressors they ranged from 1 to 19%. The average age of the groups at the time of sample collection was 10.0 for LTNP and 5.4 for progressors (p = 0.005). As expected, CD4+ counts were higher among LNTP (p<0.001) when compared with the progressor group, but there was no difference in HIV viral load between the 2 groups (p = 0.50). Children of the negative control group (NC) averaged 4.7 years of age, ranging from 0.1 to 11.2 years (not shown).
APOBEC3G and 3F G-to-A hypermutation events in HIV proviral sequences were examined in the two disease progression groups of HIV-infected children. An alignment of all proviral clonal sequences referenced by their respective plasma viral bulk sequence can be seen in Figure S1. Hypermutated proviral sequences were detected more frequently among LTNP patients (50%) when compared with the P group (27%) (Table 2 and Figure S1), yet this difference was not statistically significant. Although less frequently, the percentage of clones with hypermuted sequences was higher in the P group patients. Results obtained using HXB2 instead of the patient's consensus plasma HIV sequence as reference for hypermutation determination were not significantly different (not shown). We could not find a clear correlation between hypermutation and the different patterns of disease progression. In both groups, the most frequent changes were GA to AA, surprisingly indicating a predominance of A3F-associated hypermutation (Table 2 and Figure S1). The hypermutated clonal viral sequence with the lowest p-value (higher significance) obtained in Hypermut from all patients had evidence of simultaneous A3G and A3F activity with the exception of sample N52-5, for which only A3G activity was detected (Figure S1).
Table 2. Frequency of G-to-A hypermutation in HIV-1-infected children with distinct profiles of disease progression.
| PATIENTGROUP | NO. OFPATIENTS | NO. OFCLONES | HYPERMUTATION (%) | AVERAGE G-to-A CONTEXT (%) | ||
| Patients | Clones | GG (A3G) | GA(A3F) | |||
| LTNP | 6 | 60 | 3 | 1 (10) | 50 | 0 |
| 1 (10) | 37 | 83 | ||||
| 1 (10) | 39 | 91 | ||||
| Total | 6 | 60 | 50% | 10% | 42% | 58% |
| P | 11 | 121 | 3 | 2 (20) | 35 | 5 |
| 6 (60) | 26 | 86 | ||||
| 1 (10) | 32 | 13 | ||||
| Total | 11 | 121 | 27% | 30% | 31% | 35% |
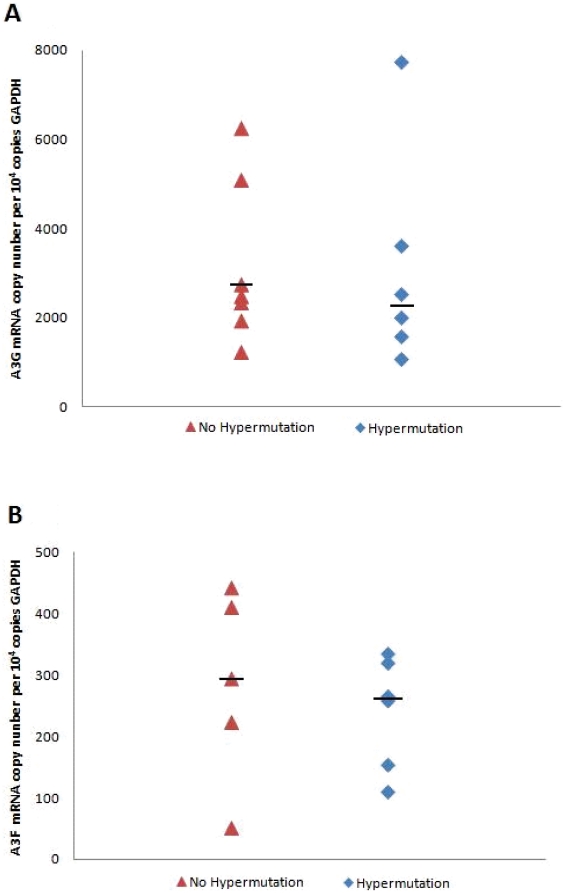
We next investigated whether the G-to-A hypermutation changes were related to the levels of A3G and A3F mRNA expression. All patient samples with detected hypermutation (n = 6), as well as seven additional, randomly chosen samples of children with no signs of hypermutation, were selected for expression analysis. In all samples, A3G expression was higher than that of A3F (p<0.0001; Mann-Whitney U test), with fold variations ranging from 5 to 30 X (Figures 1A and B). Levels of both A3G and A3F expression did not differ significantly when comparing subjects with detected hypermutation with those where no hypermutation was detected (p = 0.84 and 0.66 for A3G and A3F, respectively; Mann-Whitney U test).
Figure 1. APOBEC3G (A) and APOBEC3F (B) mRNA expression levels relative to GAPDH mRNA copy numbers of patients with or without evidenced hypermutation.
Horizontal bars depict the median value for each group.
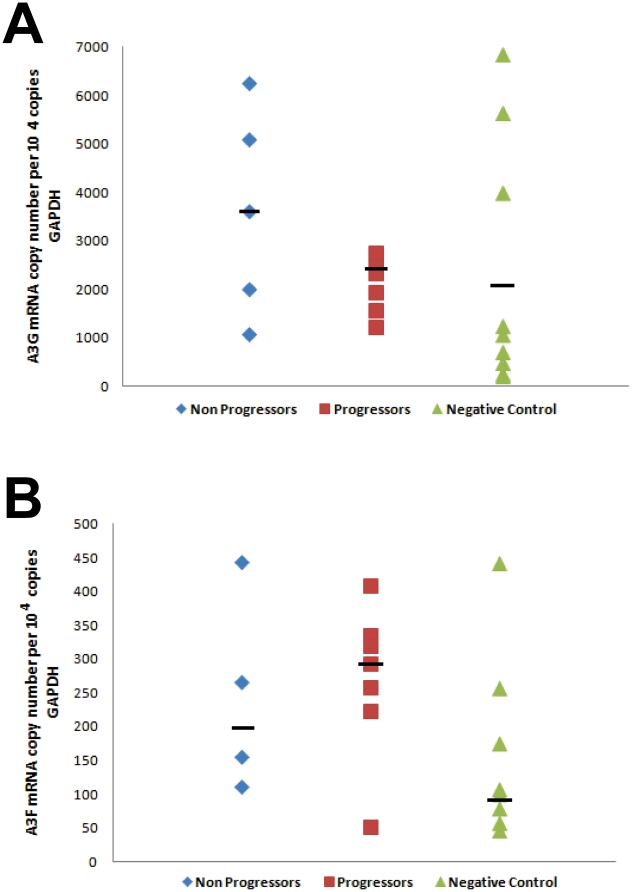
We wanted to further assess whether A3G and A3F expression levels were correlated with disease progression in HIV+ children. Nine samples of uninfected children were used as a negative control (NC) group. Results for A3G and A3F expression in each group are depicted in Figures 2A and B, respectively. There was no clear correlation between both A3G and A3F expression and disease progression in any group comparison done. Of note, when median A3G expression values were compared among groups, we found LTNP to be the highest A3G expressers (3600±2131/104 cp GAPDH), followed by progressors (2626±1809) and NC (1049±2302). In our casuistic, the three highest A3G-expressing uninfected children had median expression values significantly higher than the median of the remaining NC (p = 0.02; Mann-Whitney U test). With respect to A3F expression, the progressors group was the higher expresser (270±113/104 cp GAPDH), followed by LTNP (243±148) and NC (157±134). However, none of the median expression values was significantly different when the disease progression groups were compared. Of interest, we also failed to detect a significant correlation between A3G and A3F expression levels in both HIV+ and NC children groups (data not shown; R2 = 0.16 and 0.03, respectively).
Figure 2. APOBEC3G (A) and APOBEC3G (B) mRNA expression levels relative to GAPDH mRNA copy numbers of patients with different profiles of HIV disease progression.
Horizontal bars depict the median value for each group.
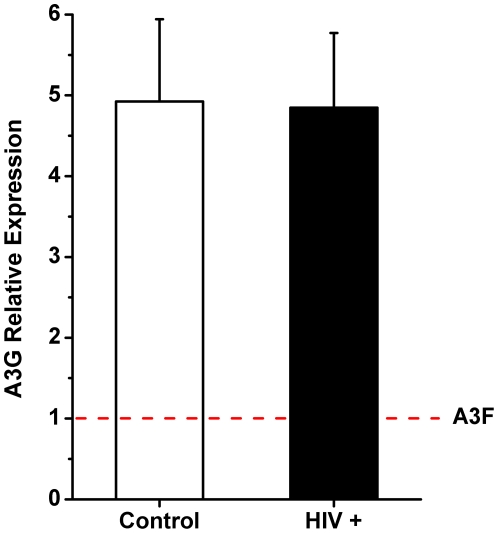
Recent reports have indicated that A3F mRNA expression levels might be largely underestimated when one uses oligo-d(T)-primed cDNA synthesis, due to the presence of repetitive elements in the long 3′ untranslated region of its mRNA [35]. To test for that possibility, we have subjected a subset of our patient's samples to random hexameric primer-based cDNA synthesis, which is reported to be more efficient under these circumstances [35]. Total RNA from plasma of 6/11 progressors, 2/6 nonprogressors and 5/9 HIV-negative controls were re-synthesized with random primers and subject to the same mRNA A3G and A3F quantification protocol. Indeed, the fold difference between A3G and A3F changed significantly, with an average fold difference of 5x more A3G compared to A3F (range 2–9X) (Figure 3), as opposed to the 15x fold difference seen with oligo-d(T)-primed reactions. These results suggest that A3F mRNA levels were underestimated in the previous experiments. However, the new procedure did not change the relative profiles of A3G and A3F expression among different groups, with HIV+ subjects displaying higher expression levels than negative controls (data not shown). The differences observed between groups were not statistically significant, as in the previous experiments.
Figure 3. Average APOBEC3G to APOBEC3F mRNA expression levels quantified from eight HIV+ children (6 progressors and 2 nonprogressors) and five HIV− controls.
A3F expression levels were arbitrarily set at 1.
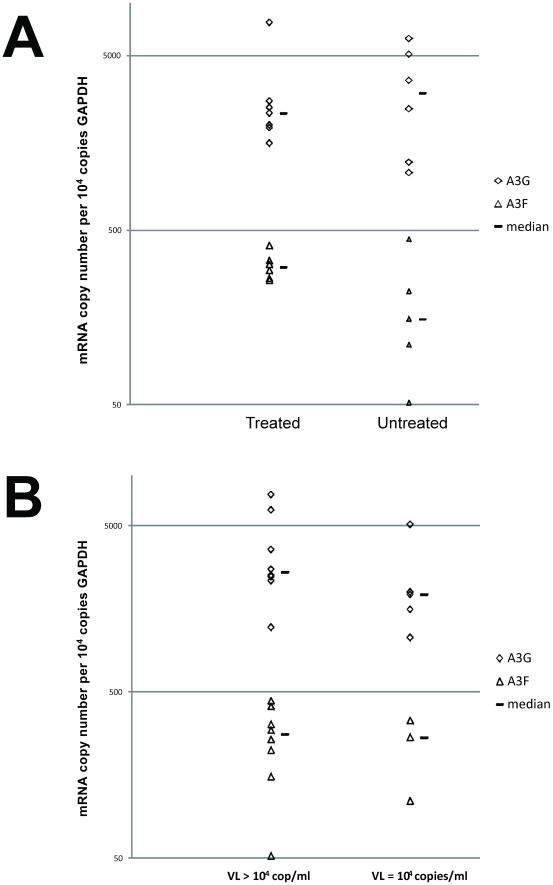
We finally assessed the effect of antiretroviral treatment and of the HIV-1 viral load levels on A3G/F mRNA expression in our casuistic. When children were categorized in treated versus untreated, irrespective of their disease progression profile, there were no significant differences in the levels of either A3G or A3F mRNA expression levels (Figure 4A; p = 0.80 and 0.16, respectively). Children with low HIV VL (below 10,000 copies/ml) also did not show different levels of A3G/F expression when compared to those with VL above that cut-off (Figure 4B; p = 0.25 and 0.71, respectively).
Figure 4. APOBEC3G and APOBEC3F mRNA expression levels relative to GAPDH mRNA copy numbers comparing patients stratified according to antiretroviral treatment exposure (A) and HIV viral load (B).
Horizontal bars depict the median value for each group.
Discussion
Previous studies have addressed G-to-A hypermutation and APOBEC3G sequence variations on disease progression in infected children [36], [37]. Our study, however, describes for the first time the analysis of APOBEC3G and 3F mRNA expression levels in an infected pediatric setting. Here, we investigated whether hypermutation and APOBEC expression were associated with different patterns of disease progression in this small cohort of pediatric patients.
We were unable to correlate HIV hypermutation levels to disease progression in children. Although our study has included a small number of individual proviral sequences from each patient, the lack of correlation between delayed disease progression and hypermutation found here is in agreement with recent findings in adults. Gandhi et al. found no correlation between hypermutation in elite suppressors when compared to that in HIV+ patients undergoing HAART, after analyzing a large number of HIV-1 sequences [20]. This observation suggests that hypermutation levels are not directly related to the intrinsic control of viremia seen in LTNP. Indeed, we found one LNTP child with low HIV viral load (3.73 log10/mL of plasma) in which no hypermutation was detected. Conversely, two samples from progressors, with hypermutated sequences, had HIV VL over 5 log10/mL. Our results further suggest a lack of correlation between HIV VL and hypermutation (data not shown). While some other reports have also failed to correlate HIV VL with either A3G-mediated hypermutation [29], [38] or to A3G expression levels [20], [26], [27], [39] in adults, different studies showed correlation between A3G mRNA expression or G-to-A hypermutation and disease progression [28], [32], and this issue is warranted further investigation.
Of interest, we have found a high contribution of A3F-mediated hypermutation in the HIV-1 PR pol genomic region of viruses from our children, a finding previously reported in infected adults. A recent study by Armitage et al. has shown that, whereas A3G hypermutates HIV proviruses roughly evenly across the genome, A3F promotes higher numbers of changes at definite regions, the largest being the pol PR region [23]. Indeed, A3F-mediated hypermutation in HIV PR has been previously described [9], [24]. These observations might explain, at least in part, the relatively high observation of A3F-mediated hypermutation events in our patients.
A re-analysis of A3G and A3F expression levels with random primed-cDNA synthesis in a subset of HIV+ and negative control children for which cellular RNA was still available showed that the A3F levels were underestimated, with fold differences to A3G levels three times lower than those estimated with oligo-d(T)-primed reactions. This is in agreement with data of Refsland et al. [35], which suggest that the long A3F mRNA 3′UTR presents repetitive elements that can impair oligo-d(T)-primed cDNA synthesis. Moreover, our confirmatory results further corroborate the importance of A3F-mediated hypermutation in the pediatric cohort studied.
Cho et al. have previously reported a positive correlation between the expression of A3G and A3F in adults [39]. In our pediatric casuistic, we failed to detect such correlation, both in HIV+ and in uninfected children. It is plausible that the differences observed between their study and ours are related to the age groups analyzed. Children's immune systems are not completely developed, and HIV infection during development may also impair specific immune components. A3G and A3F expression levels might not correlate well in a partially-developed immune system. In this view, it has been shown that distinct CD4+ T-helper lymphocyte subtypes show differences in the levels of A3G expression [40]. Th1 effector cells express higher levels of A3G and A3F mRNA, and of A3G proteins, than Th2 cells. In addition, HIV-1 virions produced in Th1 cells carry increased amounts of incorporated A3G compared to those produced in Th2 cells [40]. As a result of lower ratios of CD4+ Th1 cells observed in children compared to adults [41], children might have a proportionally higher A3F-mediated hypermutation activity. Finally, differential expression of the A3 proteins has been shown in different human tissues [35]. In particular, tissues such as the thymus and the spleen, which carry a large amount of T cells, and have significantly different activities in children and adults, can explain discrepancies in the levels of A3G and A3F seen in both groups.
We cannot rule out the possibility that differences in A3G and A3F protein activity are due to amino acid polymorphisms, to protein stability or to differential interaction with Vif taking place in the individuals analyzed here. Indeed, a number of Vif mutants have been recently shown to lack A3G-counteracting activity, and even interfere with wild type Vif molecules [42]. Conversely, amino acid changes in several A3 coding regions, including A3G and A3F, also alter susceptibility to HIV-1 Vif [43], [44]. Additional studies in the A3G and A3F coding sequences will improve our understanding of the role of each protein in hypermutation. At the protein level, it is recognized that A3G exists in two forms within cells, aggregated into low (LMM) or high molecular mass (HMM) complexes [45]. While the first form is active in virus deamination, the second is inactive. It has been shown that selected cytokines, like IL-2, IL-7 and IL-15, induce a shift of A3G into HMM complexes, rendering CD4+ T-cells more permissive to HIV infection. Other cytokines, such as TNFα, upregulate A3G in cells but leave them in LMM complexes, active against HIV [45]. Again here, differences in the immune system of children compared to that of adults might account for discrepancies in the amount of enzymatically-active forms of A3 proteins.
Another important issue to be considered here is the fact that many different A3 proteins display anti-HIV activity, as reviewed by Albin and Harris [46]. Although A3G and A3F have been recognized as major players in HIV restriction, other members of the APOBEC family such as A3DE and A3H have been shown to harbor anti-HIV activity, as well as sensitivity to lentiviral Vif proteins [43], [46], [47]. In this scenario, we cannot rule out the possibility that other A3 proteins with similar target contexts to A3F, particularly A3H [48], [49], are contributing to the higher non-A3G-mediated hypermutation seen herein.
Previous studies have reported higher levels of A3G expression in subjects infected by HIV or HCV compared to uninfected individuals [27], [50]. Our results are in agreement with those studies, and extend this observation to A3F. In our control group, however, the three highest A3G-expressing uninfected children had median expression values significantly higher than the median of the remaining NC, suggesting that A3G expression may vary substantially between individuals depending on various unrelated infections and on other immunological factors. It has been recently shown that infection by influenza A virus upregulates A3G, but not A3F expression in infected cells [51]. Therefore, the possibility that varying, unrecognized clinical conditions might alter A3G expression levels in individuals cannot be ruled out, and may prevent the establishment of clear associations between HIV infection and A3G expression.
We failed to observe any association between HIV VL or treatment experience and A3G/F mRNA expression in our patients. This is agreement with the report by Ulenga et al., which were not able to correlate hypermutation with VL in infected adults [29]. A previous report failed to find differences in A3G-mediated hypermutation between patients with undetectable VL by natural means (among elite supressors) or with the use of antiretroviral therapy [20], indicating that treatment per se does not influence A3G expression levels. Further studies are warranted to define specific biological or chemotherapeutical circumstances under which APOBEC expression is modulated.
Overall, we were unable to find any correlations between A3G or A3F expression levels, A3G- or A3F-induced hypermutation and disease progression profiles in HIV-infected children. This is however, to the best of our knowledge, the first study of A3G and A3F mRNA expression conducted in pediatric HIV/AIDS patients. The study of different disease progression profiles in the pediatric setting is also worth mentioning. Interestingly, we found evidence of a more pronounced A3F activity, not previously reported in adults, that merits further investigation. We believe this study helps contributing to a better understanding of the role of restriction factors and host genetics in an infectious disease pediatric setting.
Supporting Information
HIV-1 protease nucleotide alignments of bulk and clonal proviral sequences of each patient analyzed in the study. The bulk sequence of each patient is used as a reference at the top of each alignment. Dots represent nucleotide identities.
(TIF)
Acknowledgments
We are indebted to Prof. Marcos H. Sorgine (Instituto de Bioquímica Médica, UFRJ) for helpful discussions on the implementation and validation of the real-time PCR experiments. We would also like to thank the HIV/AIDS Staff at the Instituto de Pueircultura e Pediatria Martagão Gesteira, UFRJ, for providing care and clinical information on the HIV-infected children studied here. This study is part of the PhD Thesis by AOA at the Graduate Program in Genetics, UFRJ.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the Brazilian AIDS Department (Ministry of Health) and by the Brazilian Research Council (CNPq). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Foster C, Waelbrouck A, Peltier A. Adolescents and HIV infection. Curr Opin HIV AIDS. 2007;2:431–436. doi: 10.1097/COH.0b013e3282ced150. [DOI] [PubMed] [Google Scholar]
- 2.Abrams EJ, Wiener J, Carter R, Kuhn L, Palumbo P, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. Aids. 2003;17:867–877. doi: 10.1097/00002030-200304110-00012. [DOI] [PubMed] [Google Scholar]
- 3.Alexander L, Cuchura L, Simpson BJ, Andiman WA. Virologic and host characteristics of human immunodeficiency virus type 1-infected pediatric long term survivors. Pediatr Infect Dis J. 2006;25:135–141. doi: 10.1097/01.inf.0000199299.00345.83. [DOI] [PubMed] [Google Scholar]
- 4.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 5.Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, et al. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 6.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, et al. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, et al. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop KN, Holmes RK, Malim MH. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J Virol. 2006;80:8450–8458. doi: 10.1128/JVI.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 11.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. Embo J. 2004;23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zennou V, Bieniasz PD. Comparative analysis of the antiretroviral activity of APOBEC3G and APOBEC3F from primates. Virology. 2006;349:31–40. doi: 10.1016/j.virol.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, et al. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Sarkis PT, Luo K, Yu Y, Yu XF. Regulation of Apobec3F and human immunodeficiency virus type 1 Vif by Vif-Cul5-ElonB/C E3 ubiquitin ligase. J Virol. 2005;79:9579–9587. doi: 10.1128/JVI.79.15.9579-9587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu YL, Greene WC. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol. 2008;26:317–353. doi: 10.1146/annurev.immunol.26.021607.090350. [DOI] [PubMed] [Google Scholar]
- 17.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, et al. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 18.Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem. 2007;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 19.Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, et al. G–>A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J Virol. 2005;79:1975–1980. doi: 10.1128/JVI.79.3.1975-1980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi SK, Siliciano JD, Bailey JR, Siliciano RF, Blankson JN. Role of APOBEC3G/F-mediated hypermutation in the control of human immunodeficiency virus type 1 in elite suppressors. J Virol. 2008;82:3125–3130. doi: 10.1128/JVI.01533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kijak GH, Janini LM, Tovanabutra S, Sanders-Buell E, Arroyo MA, et al. Variable contexts and levels of hypermutation in HIV-1 proviral genomes recovered from primary peripheral blood mononuclear cells. Virology. 2008;376:101–111. doi: 10.1016/j.virol.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Land AM, Ball TB, Luo M, Pilon R, Sandstrom P, et al. Human immunodeficiency virus (HIV) type 1 proviral hypermutation correlates with CD4 count in HIV-infected women from Kenya. J Virol. 2008;82:8172–8182. doi: 10.1128/JVI.01115-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armitage AE, Katzourakis A, de Oliveira T, Welch JJ, Belshaw R, et al. Conserved footprints of APOBEC3G on Hypermutated human immunodeficiency virus type 1 and human endogenous retrovirus HERV-K(HML2) sequences. J Virol. 2008;82:8743–8761. doi: 10.1128/JVI.00584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janini M, Rogers M, Birx DR, McCutchan FE. Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4(+) T cells. J Virol. 2001;75:7973–7986. doi: 10.1128/JVI.75.17.7973-7986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris RS, Liddament MT. Retroviral restriction by APOBEC proteins. Nat Rev Immunol. 2004;4:868–877. doi: 10.1038/nri1489. [DOI] [PubMed] [Google Scholar]
- 26.Jin X, Brooks A, Chen H, Bennett R, Reichman R, et al. APOBEC3G/CEM15 (hA3G) mRNA levels associate inversely with human immunodeficiency virus viremia. J Virol. 2005;79:11513–11516. doi: 10.1128/JVI.79.17.11513-11516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulenga NK, Sarr AD, Thakore-Meloni S, Sankale JL, Eisen G, et al. Relationship between human immunodeficiency type 1 infection and expression of human APOBEC3G and APOBEC3F. J Infect Dis. 2008;198:486–492. doi: 10.1086/590212. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez-Perez JA, Ormsby CE, Hernandez-Juan R, Torres KJ, Reyes-Teran G. APOBEC3G mRNA expression in exposed seronegative and early stage HIV infected individuals decreases with removal of exposure and with disease progression. Retrovirology. 2009;6:23. doi: 10.1186/1742-4690-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulenga NK, Sarr AD, Hamel D, Sankale JL, Mboup S, et al. The level of APOBEC3G (hA3G)-related G-to-A mutations does not correlate with viral load in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2008;24:1285–1290. doi: 10.1089/aid.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen K, McSherry G, Petru A, Frederick T, Wara D, et al. A descriptive survey of pediatric human immunodeficiency virus-infected long-term survivors. Pediatrics. 1997;99:E4. doi: 10.1542/peds.99.4.e4. [DOI] [PubMed] [Google Scholar]
- 31.Soares EA, Santos AF, Sousa TM, Sprinz E, Martinez AM, et al. Differential drug resistance acquisition in HIV-1 of subtypes B and C. PLoS ONE. 2007;2:e730. doi: 10.1371/journal.pone.0000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pace C, Keller J, Nolan D, James I, Gaudieri S, et al. Population level analysis of human immunodeficiency virus type 1 hypermutation and its relationship with APOBEC3G and vif genetic variation. J Virol. 2006;80:9259–9269. doi: 10.1128/JVI.00888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose PP, Korber BT. Detecting hypermutations in viral sequences with an emphasis on G –> A hypermutation. Bioinformatics. 2000;16:400–401. doi: 10.1093/bioinformatics/16.4.400. [DOI] [PubMed] [Google Scholar]
- 34.StatsDirectLtd . Liverpool: StatsDirect Ltd; 2008. StatsDirect statistical software. 2.7.2 ed. [Google Scholar]
- 35.Refsland EW, Stenglein MD, Shindo K, Albin JS, Brown WL, et al. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res. 2010;38:4274–4284. doi: 10.1093/nar/gkq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Maio FA, Rocco CA, Aulicino PC, Bologna R, Mangano A, et al. Effect of HIV-1 Vif variability on progression to pediatric AIDS and its association with APOBEC3G and CUL5 polymorphisms. Infect Genet Evol. 2011;11:1256–1262. doi: 10.1016/j.meegid.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Koulinska IN, Chaplin B, Mwakagile D, Essex M, Renjifo B. Hypermutation of HIV type 1 genomes isolated from infants soon after vertical infection. AIDS Res Hum Retroviruses. 2003;19:1115–1123. doi: 10.1089/088922203771881211. [DOI] [PubMed] [Google Scholar]
- 38.Piantadosi A, Humes D, Chohan B, McClelland RS, Overbaugh J. An analysis of the percent of HIV-1 sequences that are hypermutated and markers of disease progression in a longitudinal cohort, including one individual with a partially defective Vif. J Virol. 2009. [DOI] [PMC free article] [PubMed]
- 39.Cho SJ, Drechsler H, Burke RC, Arens MQ, Powderly W, et al. APOBEC3F and APOBEC3G mRNA levels do not correlate with human immunodeficiency virus type 1 plasma viremia or CD4+ T-cell count. J Virol. 2006;80:2069–2072. doi: 10.1128/JVI.80.4.2069-2072.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vetter ML, Johnson ME, Antons AK, Unutmaz D, D'Aquila RT. Differences in APOBEC3G expression in CD4+ T helper lymphocyte subtypes modulate HIV-1 infectivity. PLoS Pathog. 2009;5:e1000292. doi: 10.1371/journal.ppat.1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, et al. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 42.Walker RC, Jr, Khan MA, Kao S, Goila-Gaur R, Miyagi E, et al. Identification of dominant negative human immunodeficiency virus type 1 Vif mutants that interfere with the functional inactivation of APOBEC3G by virus-encoded Vif. J Virol. 2010;84:5201–5211. doi: 10.1128/JVI.02318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JL, Pathak VK. Identification of specific determinants of human APOBEC3F, APOBEC3C, and APOBEC3DE and African green monkey APOBEC3F that interact with HIV-1 Vif. J Virol. 2010;84:12599–12608. doi: 10.1128/JVI.01437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albin JS, LaRue RS, Weaver JA, Brown WL, Shindo K, et al. A single amino acid in human APOBEC3F alters susceptibility to HIV-1 Vif. J Biol Chem. 2010;285:40785–40792. doi: 10.1074/jbc.M110.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stopak KS, Chiu YL, Kropp J, Grant RM, Greene WC. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J Biol Chem. 2007;282:3539–3546. doi: 10.1074/jbc.M610138200. [DOI] [PubMed] [Google Scholar]
- 46.Albin JS, Harris RS. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert Rev Mol Med. 2010;12:e4. doi: 10.1017/S1462399409001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larue RS, Lengyel J, Jonsson SR, Andresdottir V, Harris RS. Lentiviral Vif degrades the APOBEC3Z3/APOBEC3H protein of its mammalian host and is capable of cross-species activity. J Virol. 2010;84:8193–8201. doi: 10.1128/JVI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gourraud PA, Karaouni A, Woo JM, Schmidt T, Oksenberg JR, et al. APOBEC3H haplotypes and HIV-1 pro-viral vif DNA sequence diversity in early untreated human immunodeficiency virus-1 infection. Hum Immunol. 2011;72:207–212. doi: 10.1016/j.humimm.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li MM, Wu LI, Emerman M. The range of human APOBEC3H sensitivity to lentiviral Vif proteins. J Virol. 2010;84:88–95. doi: 10.1128/JVI.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komohara Y, Yano H, Shichijo S, Shimotohno K, Itoh K, et al. High expression of APOBEC3G in patients infected with hepatitis C virus. J Mol Histol. 2006;37:327–332. doi: 10.1007/s10735-006-9059-0. [DOI] [PubMed] [Google Scholar]
- 51.Pauli EK, Schmolke M, Hofmann H, Ehrhardt C, Flory E, et al. High level expression of the anti-retroviral protein APOBEC3G is induced by influenza A virus but does not confer antiviral activity. Retrovirology. 2009;6:38. doi: 10.1186/1742-4690-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HIV-1 protease nucleotide alignments of bulk and clonal proviral sequences of each patient analyzed in the study. The bulk sequence of each patient is used as a reference at the top of each alignment. Dots represent nucleotide identities.
(TIF)