Abstract
Background
The centromere kinesin motor protein CENP-E plays a crucial role in mitosis, and is an appealing molecular target in cancer. GSK923295A is an allosteric inhibitor of CENP-E that is undergoing clinical evaluation.
Procedures
GSK923295A was evaluated against the 23 cell lines in the Pediatric Preclinical Testing Program (PPTP) in vitro panel using 96 hour exposures to concentrations ranging from 1.0 nM to 10.0 μM. GSK923295A was also tested in vivo against the PPTP acute lymphoblastic leukemia (ALL) and solid tumor xenograft panels using a Day 1–3 and Day 8–10 schedule that was repeated at Day 21. The agent was administered via the intraperitoneal (IP) route at a daily dose of 125 mg/kg.
Results
The median IC50 for all PPTP cell lines was 27 nM, with the median IC50 for the ALL panel being the lowest (18 nM) and for the neuroblastoma panel the highest (39 nM). Excessive toxicity was observed for each of the 8 xenografts of the ALL panel in NOD/SCID mice. Thirty-five solid tumor xenograft models were considered evaluable. GSK923295A induced significant differences in EFS distribution compared to controls in 32 of 35 evaluable solid tumor xenografts tested. Objective responses were noted in 13 of 35 solid tumor xenografts, including 9 with maintained complete responses (MCR), and 3 with complete response (CR).
Conclusions
GSK923295A demonstrated significant antitumor activity against solid tumor models, inducing complete responses in Ewing sarcoma, rhabdoid and rhabdomyosarcoma xenografts. These results suggest that CENP-E may be a valuable therapeutic target in pediatric cancer.
Keywords: Preclinical Testing, Developmental Therapeutics, GSK923295A
INTRODUCTION
One of the central features of malignant cells is the acquisition of unlimited proliferative capacity [1]. This high rate of cell division and lack of checkpoint controls relative to non-transformed cells have provided targets for pharmacological intervention, for example by drugs that target microtubules and the mitotic machinery. Although drugs that disrupt the cytoskeleton have proven to be very effective in blocking mitosis and inducing apoptosis, those same components are not exclusive to dividing cells and therefore deleterious consequences for normal tissues are inevitable [2]. There is a clear need to improve the arsenal of available drugs, particularly for the treatment of childhood cancer, to maximize the likelihood of cure while minimizing the long-term deleterious effects of treatment. Novel drugs directed against mitotic components such as the Aurora kinases [3,4], the Polo-like kinases and mitotic kinesins [5,6] represent a new generation of anti-mitotic drugs that hold promise for improved cancer treatment with reduced toxicity [7].
CENP-E is a kinesin protein responsible for chromosome congression to, and alignment on, the metaphase plate, and is required for satisfaction of the mitotic checkpoint allowing the metaphase to anaphase transition [8]. A large motor protein with ATPase activity, CENP-E exhibits a capacity to anchor on the kinetochore and tightly bind microtubules [9]. CENP-E expression is clearly associated with cellular proliferation, and it is highly expressed in a broad range of human cancers. CENP-E kinesin motor domain is responsible for CENP-E interaction with microtubules, while its interaction with BubR1 is thought to integrate kinetochore-microtubule interaction and spindle assembly checkpoint (SAC) signaling [10–12]. Cells which are unable to satisfy the SAC are arrested and as a consequence suffer senescence or apoptosis [13], although defects in mitotic checkpoints per se are linked to aneuploidy and carcinogenesis [14–16].
Since drugs (such as microtubule inhibitors or DNA damaging agents) that inhibit or activate mitotic checkpoint components have proven to be effective in the treatment of cancer, CENP-E represents a promising new target. Moreover, reduced levels of CENP-E activity correlate with a reduced incidence of spontaneous or induced tumors in murine models [17]. GSK923295A is a small molecule specific allosteric inhibitor of CENP-E kinesin motor ATPase activity that stabilizes CENP-E motor domain interaction with microtubules [18]. Both in vitro and in vivo testing demonstrated that GSK923295A induces failure of metaphase chromosome alignment as well as mitotic arrest [18]. Potent in vitro cytotoxicity and in vivo tumor regressions were observed for many of the adult cancer preclinical models evaluated [18], and GSK923295A has progressed to clinical testing in adults with refractory cancers [18].
The aims of the Pediatric Preclinical Testing Program (PPTP) are to test novel drugs with demonstrated preclinical or clinical efficacy in adult cancer against preclinical models of pediatric solid tumors and acute lymphoblastic leukemia (ALL), and in doing so assess their priority for pediatric cancer clinical trials [19]. Since anti-mitotic drugs form the cornerstone of many effective treatment regimens for pediatric cancer, and PPTP has previously reported the preclinical efficacy of two novel drugs that target the mitotic kinesin Eg5 (ispinesib) [20] and Aurora kinase A (MLN8237) [21], it was of interest to test the efficacy of GSK923295A against the PPTP in vitro cell line and in vivo xenograft panels.
MATERIALS AND METHODS
In Vitro Testing
In vitro testing was performed using DIMSCAN, a semiautomatic fluorescence-based digital image microscopy system that quantifies viable (using fluorescein diacetate [FDA]) cell numbers in tissue culture multiwell plates [22]. Cells were incubated in the presence of GSK923295A for 96 hours under aerobic conditions at concentrations from 10 nM to 10 μM and analyzed as previously described [23]. The relative IC50 is the concentration of agent that gives a half-maximal response, while the absolute IC50 values represent the concentration at which the agent reduces cell survival to 50% of the control value [24]. To compare activity between cell lines, the ratio of the median relative IC50 to individual cell line’s relative IC50 value is used (larger values connote greater sensitivity). The lowest T/C% value is the Ymin. Relative In/Out (I/O)% values represent the percentage difference between the Ymin value and the estimated starting cell number and either the control cell number (for agents with Ymin > starting cell number) or 0 (for agents with Ymin < estimated starting cell number). Relative I/O% values range between 100% (no treatment effect) to −100% (complete cytotoxic effect), with a Relative I/O% value of 0 being observed for a completely effective cytostatic agent.
In Vivo Tumor Growth Inhibition Studies
CB17SC-F scid−/− female mice (Taconic Farms, Germantown NY) were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [23,25–27]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [28]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Ten mice (solid tumors) or eight mice (leukemias) were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [hCD45] cells [ALL xenografts] were determined as previously described [28].
Determination of Response
Responses were determined using three activity measures as previously described [19]. For individual mice, progressive disease (PD) was defined as < 50% regression from initial volume during the study period and > 25% increase in initial volume at the end of study period. Stable disease (SD) was defined as < 50% regression from initial volume during the study period and ≤ 25% increase in initial volume at the end of the study. Partial response (PR) was defined as a tumor volume regression ≥50% for at least one time point but with measurable tumor (≥ 0.10 cm3). Complete response (CR) was defined as a disappearance of measurable tumor mass (< 0.10 cm3) for at least one time point. A complete response was considered maintained (MCR) if the tumor volume was <0.10 cm3 at the end of the study period. For treatment groups only, if the tumor response was PD, then PD was further classified into PD1 or PD2 based on the tumor growth delay (TGD) value. TGD values were calculated based on the numbers of days to event. For each individual mouse that had PD and had an event in the treatment groups, a TGD value was calculated by dividing the time to event for that mouse by the median time to event in the respective control group. Median times to event were estimated based on the Kaplan-Meier event-free survival distribution. If a mouse had a TGD value ≤ 1.5, that mouse was considered PD1. If the TGD value was > 1.5, the mouse was considered PD2. Mice that had PD but did not have an event at the end of the study were coded as PD2.
Event-Free Survival
An event in the solid tumor xenograft models was defined as a quadrupling of tumor volume from the initial tumor volume. Event-free survival (EFS) was defined as the time interval from initiation of study to the first event or to the end of the study period for tumors that did not quadruple in volume. The time to event was determined using interpolation based on the formula:
where tx is the interpolated day to event, t1 is the lower observation day bracketing the event, t2 is the upper observation day bracketing the event, V1 is the tumor volume on day t1, V2 is the tumor volume on day t2 and Ve is the event threshold (4 times initial tumor volume for solid tumor xenografts).
Response and event definitions for the ALL xenograft models were determined as described previously [19–21].
Summary Statistics and Analysis Methods
Overall Group Response
Each individual mouse was assigned a score from 0 to 10 based on their response: PD1=0, PD2=2, SD=4, PR=6, CR=8, and MCR=10, and the median for the group determined the overall response (median group response). Studies in which toxicity was greater than 25% or in which the control group was not at least SD, were considered inevaluable and were excluded from analysis. Treatment groups with PR, CR, or MCR are considered to have had an objective response. Agents inducing objective responses are considered highly active against the tested line, while agents inducing SD or PD2 are considered to have intermediate activity, and agents producing PD1 are considered to have a low level of activity against the tested line.
Tumor Volume T/C value
Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if less than 21 days). The mean RTV for control and treatment mice for each study were then calculated and the T/C value was the mean RTV for the treatment group divided by the mean RTV for the control group. For the tumor volume T/C response measure, agents producing a T/C of ≤ 15% are considered highly active, those with a mean tumor volume T/C of ≤ 45% but > 15% are considered to have intermediate activity, and those with mean T/C values > 45% are considered to have low levels of activity [29].
EFS T/C value
An EFS T/C value was defined by the ratio of the median time to event of the treatment group and the median time to event of the respective control group. If the treatment group did not have a median time to event, then EFS T/C was defined as greater than the ratio of the last day of the study for the treatment group divided by the median time to event for the control group. For the EFS T/C measure, agents are considered highly active if they meet three criteria: a) an EFS T/C > 2; b) a significant difference in EFS distributions (p≤ 0.050), and c) a net reduction in median tumor volume for animals in the treated group at the end of treatment as compared to at treatment initiation. Agents meeting the first two criteria, but not having a net reduction in median tumor volume for treated animals at the end of the study are considered to have intermediate activity. Agents with an EFS T/C < 2 are considered to have low levels of activity. Xenografts in which the median EFS for the control line was greater than one-half of the study period or in which the median EFS for the control line did not exist are considered not evaluable for the EFS T/C measure of activity.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies. The Mann-Whitney test was used to test the difference in medians of IC50 values between cell lines of a given histotype compared to the remaining cell lines of the panel. Correlations between results for GSK923295A and ispinesib were evaluated by Spearman’s correlation.
Drugs and Formulation
GSK923295A was provided to the PPTP by Cytokinetics Inc. through the Cancer Therapy Evaluation Program (NCI). For in vitro testing, a stock solution of GSK923295A was prepared in DMSO, with dilutions in culture media. For in vivo testing, the compound was first dissolved in a 1:1 mixture of dimethylacetamide and Cremophor EL, 96% acidified water (pH 5.0 using HCl). GSK923295 was administered at 125 mg/kg i.p., days 1–3 and 8–10 repeated at day 21, as recommended by the drug supplier. GSK923295A was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
GSK923295A In Vitro Testing
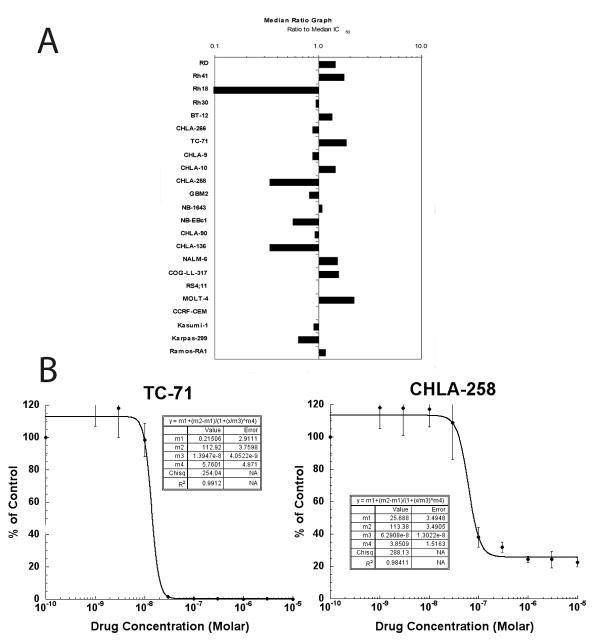
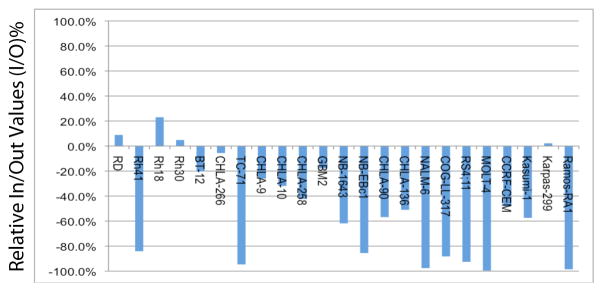
GSK923295A was evaluated against the the 23 cell lines in the PPTP in vitro panel using 96 hour exposures to concentrations ranging from 1.0 nM to 10.0 μM. The median absolute IC50 for all PPTP cell lines was 27 nM, with the median absolute IC50 for the ALL panel being the lowest (18 nM) and with the median absolute IC50 for the neuroblastoma panel being the highest (39 nM) (Table I, Figure 1A). The PPTP cell lines showed two primary patterns of response to GSK923295A in terms of their minimum T/C% (Ymin) values. One group of cell lines showed T/C% values approaching 0%, while another showed T/C% values that reached a plateau far above 0% (Figure 1B). The rhabdomyosarcoma cell lines all had T/C% values far above 0% at the highest GSK923295A concentrations tested (median Ymin = 17.3%). The Relative In/Out values for 3 of the 4 rhabdomyosarcoma cell lines were close to 0%, consistent with a cytostatic effect for these cell lines (Table 1, Figure 2). By contrast, most of the ALL cell lines showed near complete cytotoxicity at higher concentrations, with Relative In/Out values approaching -100% for 4 of 5 cell lines. The Ymin response pattern for GSK925295A was similar to that previously reported by the PPTP for ispinesib [20] (R2=0.83, Supplemental Figure 1). By contrast, there was no correlation between the IC50 values of GSK923295A and ispinesib (R2 = 0.02).
Table I.
Activity of GSK923295A against Cell Lines in the PPTP in Vitro Panel
| Cell Line | Histology | Minimum T/C% | Absolute IC50 (nM) | Relative IC50 (nM) | Relative I/O |
|---|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 13.9 | 18 | 17 | 8.9% |
| Rh41 | Rhabdomyosarcoma | 3.5 | 15 | 14 | −84.1% |
| Rh18 | Rhabdomyosarcoma | 57.4 | >10000 | >10000 | 23.1% |
| Rh30 | Rhabdomyosarcoma | 20.7 | 29 | 26 | 4.9% |
| BT-12 | Rhabdoid | 6.5 | 20 | 19 | −20.3% |
| CHLA-266 | Rhabdoid | 24.6 | 31 | 30 | −5.6% |
| TC-71 | Ewing sarcoma | 0.1 | 15 | 14 | −94.6% |
| CHLA-9 | Ewing sarcoma | 2.7 | 31 | 30 | −26.0% |
| CHLA-10 | Ewing sarcoma | 4.3 | 19 | 18 | −31.9% |
| CHLA-258 | Ewing sarcoma | 22.5 | 81 | 63 | −42.3% |
| GBM2 | Glioblastoma | 8.1 | 33 | 30 | −17.8% |
| NB-1643 | Neuroblastoma | 8.1 | 25 | 23 | −61.8% |
| NB-EBc1 | Neuroblastoma | 3.3 | 48 | 46 | −85.6% |
| CHLA-90 | Neuroblastoma | 12.0 | 30 | 29 | −56.8% |
| CHLA-136 | Neuroblastoma | 14.1 | 81 | 73 | −51.0% |
| NALM-6 | ALL | 0.1 | 18 | 18 | −97.6% |
| COG-LL- 317 | ALL | 0.5 | 17 | 18 | −88.2% |
| RS4;11 | ALL | 1.1 | 27 | 27 | −92.6% |
| MOLT-4 | ALL | 0.0 | 12 | 12 | −99.6% |
| CCRF-CEM | ALL | 3.3 | 27 | 26 | −48.3% |
| Kasumi-1 | AML | 12.2 | 30 | 29 | −57.4% |
| Karpas-299 | ALCL | 9.8 | 43 | 40 | 2.2% |
| Ramos-RA1 | NHL | 0.0 | 23 | 23 | −98.6% |
| Median | 6.5 | 27 | 26 | −51.0% | |
| Minimum | 0.01 | 12 | 12 | −99.6% | |
| Maximum | 57.4 | > 10000 | > 10000 | 23.1% |
Figure 1.
GSK923295A in vitro activity. Relative sensitivity of the cell lines displayed as mean ratios to the median IC50 values, displayed by histiotype (A). The black line indicates the median IC50 (27 nM) for the panel. Bars to the right indicate increased drug sensitivity. Typical growth inhibition curves (B) for two Ewing sarcoma lines TC-71 (left) and CHLA-258 (right) demonstrating different plateau values. Error bars represent standard deviations for each concentration tested.
Figure 2.
Relative In/Out Values for GSK923295. Relative In/Out (I/O)% values represent the percentage difference between the Ymin value and the estimated starting cell number and either the control cell number (for agents with Ymin > starting cell number) or 0 (for agents with Ymin < estimated starting cell number). Relative I/O% values range between 100% (no treatment effect) to −100% (complete cytotoxic effect), with a Relative I/O% value of 0 being observed for a completely effective cytostatic agent.
GSK923295A in Vivo Testing
GSK923295A was tested using a Day 1–3 and Day 8–10 schedule that was repeated at Day 21 (solid tumor panels only). The agent was administered i.p. at a daily dose of 125 mg/kg. Excessive toxicity was observed for each of the 8 xenografts of the ALL panel with mortality of 64.4% (n=59) but GSK923295A was well tolerated for the solid tumor xenografts with mortality of 5.2% (n=367). A complete summary of results is provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
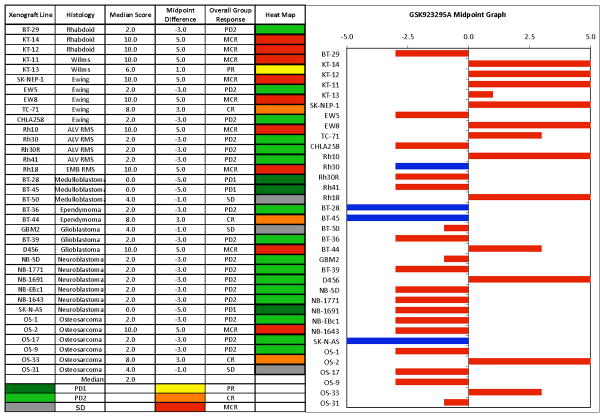
Thirty-five xenograft models were considered evaluable, with two solid tumor xenografts (KT-5 from the Wilms panel and Rh28 from the rhabdomyosarcoma panel) and each of the 8 ALL xenografts excluded from reporting because of excessive toxicity (>25% mortality). GSK923295A induced significant differences in EFS distribution compared to controls in 32 of 35 evaluable solid tumor xenografts tested as shown in Table II. The only xenografts for which there was no significant difference in EFS distribution were Rh30 (rhabdomyosarcoma) and BT-28 and BT-45 (both from the medulloblastoma panel).
Table II.
Activity for GSK923295A against the PPTP in Vivo Panel
| Xenograft Line | Histology | KM Estimate of Median Time to Event | P-value | EFS T/C | Median Final RTV | T/C | P-value | T/C Activity | EFS Activity | Response Activity |
|---|---|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | 38.5 | <0.001 | 2.8 | >4 | 0.34 | <0.001 | Int | Int | Int |
| KT-14 | Rhabdoid | > EP | <0.001 | > 2.0 | 0 | 0.11 | <0.001 | High | High | High |
| KT-12 | Rhabdoid | > EP | <0.001 | > 4.5 | 0 | 0 | <0.001 | High | High | High |
| KT-11 | Wilms | > EP | <0.001 | > 4.0 | 0 | 0 | <0.001 | High | High | High |
| KT-13 | Wilms | > EP | <0.001 | > 3.5 | 0.7 | 0.34 | <0.001 | Int | High | High |
| SK-NEP-1 | Ewing | > EP | <0.001 | > 6.1 | 0 | 0.05 | <0.001 | High | High | High |
| EW5 | Ewing | 13.4 | <0.001 | 1.6 | >4 | 0.52 | <0.001 | Low | Low | Int |
| EW8 | Ewing | > EP | <0.001 | > 4.0 | 0 | 0.11 | <0.001 | High | High | High |
| TC-71 | Ewing | > EP | <0.001 | > 6.4 | 1.7 | 0.15 | <0.001 | High | Int | High |
| CHLA258 | Ewing | 25.7 | <0.001 | 3.8 | >4 | 0.44 | <0.001 | Int | Int | Int |
| Rh10 | ALV RMS | > EP | <0.001 | > 2.8 | 0 | 0.14 | <0.001 | High | High | High |
| Rh30 | ALV RMS | 23.9 | 0.217 | 1.9 | >4 | 0.52 | 0.043 | Low | Low | Int |
| Rh30R | ALV RMS | 34.2 | <0.001 | 3.2 | >4 | 0.61 | 0.003 | Low | Int | Int |
| Rh41 | ALV RMS | 18.6 | <0.001 | 1.5 | >4 | 0.57 | <0.001 | Low | Low | Int |
| Rh18 | EMB RMS | > EP | <0.001 | > 3.6 | 0 | 0.1 | <0.001 | High | High | High |
| BT-28 | Medulloblastoma | 4.1 | 0.974 | 0.8 | >4 | 1.29 | 0.739 | Low | Low | Low |
| BT-45 | Medulloblastoma | 15.3 | 0.068 | 1.2 | >4 | 0.8 | 0.247 | Low | Low | Low |
| BT-50 | Medulloblastoma | > EP | 0.002 | > 1.3 | 0.8 | 0.63 | 0.024 | Low | NE | Int |
| BT-36 | Ependymoma | > EP | 0.011 | > 1.6 | 1.5 | 0.48 | 0.007 | Low | NE | Int |
| BT-44 | Ependymoma | > EP | <0.001 | > 2.8 | 0.2 | 0.05 | <0.001 | High | High | High |
| GBM2 | Glioblastoma | 34.2 | <0.001 | 2.7 | >4 | 0.11 | <0.001 | High | Int | Int |
| BT-39 | Glioblastoma | > EP | <0.001 | > 4.0 | 2.4 | 0.78 | 0.315 | Low | Int | Int |
| D456 | Glioblastoma | > EP | <0.001 | > 6.7 | 0 | 0.02 | <0.001 | High | High | High |
| NB-SD | Neuroblastoma | > EP | <0.001 | > 5.7 | 3.6 | 0.38 | 0.021 | Int | Int | Int |
| NB-1771 | Neuroblastoma | 9.2 | 0.002 | 1.9 | >4 | 0.43 | 0.002 | Int | Low | Int |
| NB-1691 | Neuroblastoma | 11.3 | <0.001 | 2 | >4 | 0.37 | <0.001 | Int | Low | Int |
| NB-EBc1 | Neuroblastoma | 15.9 | <0.001 | 2.3 | >4 | 0.5 | <0.001 | Low | Int | Int |
| NB-1643 | Neuroblastoma | 14.5 | 0.006 | 2.6 | >4 | 0.22 | 0.002 | Int | Int | Int |
| SK-N-AS | Neuroblastoma | 6.1 | 0.056 | 1.2 | >4 | 0.67 | 0.052 | Low | Low | Low |
| OS-1 | Osteosarcoma | > EP | <0.001 | > 1.4 | 1.9 | 0.49 | <0.001 | Low | NE | Int |
| OS-2 | Osteosarcoma | > EP | <0.001 | > 1.2 | 0.8 | 0.61 | 0.001 | Low | NE | High |
| OS-17 | Osteosarcoma | > EP | <0.001 | > 1.4 | 1.7 | 0.7 | 0.024 | Low | NE | Int |
| OS-9 | Osteosarcoma | > EP | <0.001 | > 2.2 | 2.6 | 0.44 | <0.001 | Int | Int | Int |
| OS-33 | Osteosarcoma | > EP | <0.001 | > 2.7 | 1.1 | 0.26 | <0.001 | Int | Int | High |
| OS-31 | Osteosarcoma | > EP | <0.001 | > 3.0 | 1.2 | 0.4 | <0.001 | Int | Int | Int |
The EFS T/C values met criteria for high activity for the time to event measure of activity (EFS T/C > 2 with final tumor volume less than starting tumor volume) in 10 of 35 evaluable solid tumor xenografts. The rhabdomyosarcoma, rhabdoid tumor, Wilms tumor, and Ewing sarcoma panels each had two models with high activity. Intermediate activity for the time to event activity measure (also requiring EFS T/C > 2) was observed in 12 xenograft models, including 3 neuroblastoma and 3 osteosarcoma models. Low activity was observed for 8 models, including 3 from the neuroblastoma panel. Five models were inevaluable for the EFS T/C activity measure due to slow tumor growth in control animals, including 3 xenografts from the osteosarcoma panel, Table II.
Objective responses were noted in 13 of 35 solid tumor xenografts, including 9 with maintained complete responses (MCR), 3 with complete response (CR), and 1 with partial response (PR). Three of 5 Ewing sarcoma xenografts achieved MCR or CR, as did 2 of 3 rhabdoid tumor models, and 2 of 5 rhabdomyosarcoma models. An inevaluable xenograft (Rh28) would have been scored as MCR had there not been excessive toxicity in 4 of 10 treated animals. Two osteosarcoma xenografts (OS-2 and OS-33) met criteria for CR or MCR, related in part to the relatively small tumor volume at which treatment was started for the osteosarcoma xenografts and related also to the PPTP definition of CR. For the neuroblastoma panel, the best response was progressive disease with growth delay (PD2), which was observed for 5 of 6 xenografts. A single ALL xenograft (ALL-3) treated with 50% (62.5 mg/kg) of the dose administered to the solid tumors achieved an objective response (PR, data not shown).
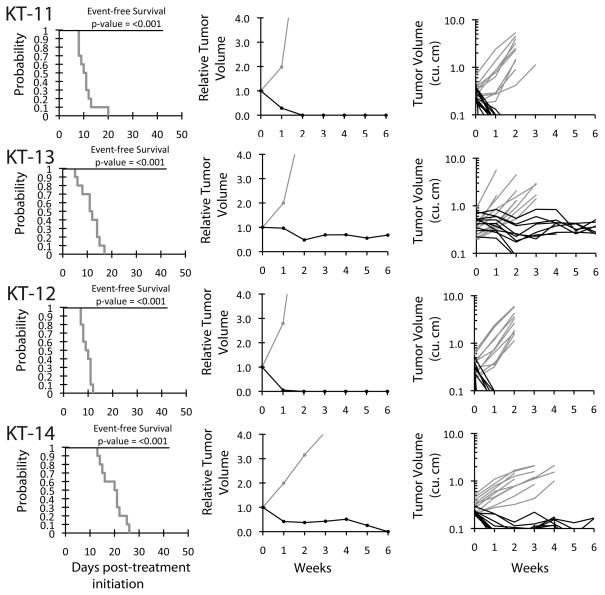
The in vivo testing results for the objective response measure of activity are presented in Figure 3 in a ‘heat-map’ format as well as a ‘COMPARE’-like format, based on the scoring criteria described in the Material and Methods and the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease). Objective responses were seen in 13 of 35 solid tumor models, with examples of kidney tumor response shown in Figure 4 (Wilms tumors KT-11, and KT-13 and rhabdoid tumors KT-12, and KT-14).
Figure 3.
GSK923295A in vivo objective response activity. Left: The colored ‘heat map’ depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥ 2 but < 6, and low activity by a score of < 2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Figure 4.
GSK923295A activity against individual kidney tumor xenografts (KT-11, KT-13 Wilms tumors; KT-12, KT-14 rhabdoid tumors of the kidney). Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Gray lines correspond to control animals and black lines to drug treated animals.
DISCUSSION
The response of the PPTP cell lines to GSK923295A is reminiscent of the response that was previously observed for ispinesib [20]. The PPTP previously demonstrated that the response of the PPTP cell lines for ispinesib and vincristine are highly related in terms of the minimum T/C% (Ymin) values for each agent [30]. To analyze GSK923295A and ispinesib response patterns in vitro, the Ymin values for the two agents were compared. There was a highly significant correlation between the Ymin values for GSK923295A and ispinesib (R2=0.83). Although the contribution to this correlation by the cell line Rh18, which has Ymin above 50% for both GSK923295A and ispinesib, is considerable, when Rh18 is omitted from the analysis there remains a significant correlation between the Ymin values for the two agents (R2=0.53).
The strong correlation between Ymin responses to ispinesib and GSK923295A suggests that the PPTP cell lines have a shared pattern of response to these two agents in terms of whether the cell lines undergo a cytotoxic response versus a cytostatic/partial cytotoxic response. The pattern of response does not show complete histotype dependence, although the ALL cell lines have a high percentage of near complete cytotoxic responders. The similar activity patterns for GSK923295A, ispinesib and vincristine against the PPTP’s in vitro panel is consistent with NCI-60 cell line panel testing results, for which similar activity profiles for antimitotic agents such as vincristine and the kinesin spindle protein inhibitor S-trityl-L-cysteine (STLC) were also observed [31,32]. The two different patterns of response observed for the PPTP cell lines to these diverse anti-mitotic agents suggest that the cellular decision-making process for a cytotoxic response (e.g., apoptosis) versus a cytostatic/partial cytotoxic response lies downstream of the primary mitotic effect of the compounds. However, the biological basis for the similar responses of the PPTP cell lines to these anti-mitotic inhibitors is not clear.
Objective responses were noted in 13 of 35 solid tumor xenografts, including 9 with MCRs, 3 with CRs, and 1 with PR. Three of 5 Ewing sarcoma xenografts achieved MCR or CR, as did 2 of 3 rhabdoid tumor models, and 2 of 5 rhabdomyosarcoma models. For the neuroblastoma panel, the best response was progressive disease with growth delay (PD2), which was observed for 5 of 6 xenografts. The determinants of this differential sensitivity of diverse tumor histotypes to GSK923295A currently remain unresolved. However, similar to the cell line panels, the xenograft responses to GSK923295A were remarkably concordant with those to ispinesib. Out of 14 xenografts common to both studies, 9 achieved identical median group responses, while the median group responses of 3 out of the remaining 5 xenografts differed by only a single category. The median group responses of these 14 xenografts showed a significant correlation between the two drugs (r=0.78; p=0.001). Again, these data indicate a shared in vivo response to two drugs targeting different components of the mitotic machinery.
The ALL xenograft panel was inevaluable because of toxicity. There is no obvious reason that would explain why the NOD/SCID mice did not tolerate GSK923295A as well as the SCID mice used for most of the solid tumor testing. GSK923295A is relatively impotent towards murine CENP-E [18], and so drug-related toxicity is not expected for mice at the dose selected for testing. Treatment for the ALL xenografts was administered only on Days 1–3 and Days 8–10, and most of the toxicity was observed at Day 21 or thereafter. If responses at Day 21 were evaluated, then 6 of 8 ALL xenografts had sufficient numbers of evaluable treated animals to assess response. Among these, 5 of 6 xenografts met criteria for CR at Day 21, whereas 1 model (ALL-4) met criteria for PR as best response. The two xenografts with excessive toxicity by Day 21 were ALL-3 and ALL-19, and a repeat experiment with ALL-3 at 50% dose achieved a PR. Thus, GSK923295A, like other anti-mitotic agents such as vincristine and ispinesib [19,20], demonstrated substantial remission-inducing anti-leukemia activity against the PPTP ALL xenografts, although for unexplained reasons the dose used for testing exceeded that which can be tolerated by leukemia-bearing NOD/SCID mice.
Our studies highlight similarities between GSK923295A and ispinesib. The dose limiting toxicities for ispinesib have been largely hematologic but this agent has not demonstrated clinical efficacy in phase 2 trials [33,34]. As these two drugs demonstrated similar activity in the PPTP preclinical models it may suggest that GSK923295A may also have little human efficacy. However, GSK923295A demonstrates a significantly different pattern of tumor responses compared to vincristine, previously evaluated against the same in vivo panel of solid tumors [19]. Comparison of these data sets shows that the response to GSK923295A and vincristine differed by at least two response categories (e.g. PD2 vs PR) in 16 of 30 solid tumor models. In 10 models GSK923295A was superior to vincristine, whereas it was inferior in 6 models. Superiority was particularly noticeable in 3 of 5 Ewing sarcoma models.
In conclusion, GSK923295A exhibited substantial in vitro and in vivo activity against the PPTP’s preclinical models. These data further indicate the relevance of targeting the process of mitosis for treatment of childhood malignancies. Although the similarity between ispinesib and GSK293295A is of concern, as the former drug has not demonstrated efficacy against adult malignancies, the novel spectrum of activity compared to vincristine in these solid tumor models suggests that GSK293295A may have a role in treatment of childhood cancers. A key issue in relating the observed high level of preclinical activity to the clinical setting will be the relationship between the drug exposures achieved in the SCID mice used for solid tumor testing compared to those achieved in humans at tolerable doses.
Supplementary Material
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute. GSK923295A was provided by Cytokinetics, Inc. In addition to the authors represents work contributed by the following: Sherry Ansher, Catherine A. Billups, Ingrid Boehm, Joshua Courtright, Mila Dolotin, Edward Favours, Henry S. Friedman, Debbie Payne-Turner, Charles Stopford, Chandra Tucker, Jianrong Wu, Joe Zeidner, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospital.
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors consider that there are no actual or perceived conflicts of interest.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JR, Patrick DR, Dar MM, et al. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7(2):107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 3.Garuti L, Roberti M, Bottegoni G. Small molecule aurora kinases inhibitors. Curr Med Chem. 2009;16(16):1949–1963. doi: 10.2174/092986709788682227. [DOI] [PubMed] [Google Scholar]
- 4.Gautschi O, Heighway J, Mack PC, et al. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14(6):1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 5.Vijapurkar U, Wang W, Herbst R. Potentiation of kinesin spindle protein inhibitor-induced cell death by modulation of mitochondrial and death receptor apoptotic pathways. Cancer Res. 2007;67(1):237–245. doi: 10.1158/0008-5472.CAN-06-2406. [DOI] [PubMed] [Google Scholar]
- 6.Haque SA, Hasaka TP, Brooks AD, et al. Monastrol, a prototype anti-cancer drug that inhibits a mitotic kinesin, induces rapid bursts of axonal outgrowth from cultured postmitotic neurons. Cell Motil Cytoskeleton. 2004;58(1):10–16. doi: 10.1002/cm.10176. [DOI] [PubMed] [Google Scholar]
- 7.Warner SL, Gray PJ, Von Hoff DD. Tubulin-associated drug targets: Aurora kinases, Polo-like kinases, and others. Semin Oncol. 2006;33(4):436–448. doi: 10.1053/j.seminoncol.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Yen TJ, Compton DA, Wise D, et al. CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 1991;10(5):1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaar BT, Chan GK, Maddox P, et al. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139(6):1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan GK, Jablonski SA, Sudakin V, et al. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol. 1999;146(5):941–954. doi: 10.1083/jcb.146.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao Y, Desai A, Cleveland DW. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. The Journal of cell biology. 2005;170(6):873–880. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X, Abrieu A, Zheng Y, et al. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol. 2000;2(8):484–491. doi: 10.1038/35019518. [DOI] [PubMed] [Google Scholar]
- 13.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7(5):637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Tao W. The mitotic checkpoint in cancer therapy. Cell Cycle. 2005;4(11):1495–1499. doi: 10.4161/cc.4.11.2130. [DOI] [PubMed] [Google Scholar]
- 15.Suijkerbuijk SJ, van Osch MH, Bos FL, et al. Molecular causes for BUBR1 dysfunction in the human cancer predisposition syndrome mosaic variegated aneuploidy. Cancer research. 2010;70(12):4891–4900. doi: 10.1158/0008-5472.CAN-09-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanks S, Coleman K, Reid S, et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nature genetics. 2004;36(11):1159–1161. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- 17.Weaver BA, Silk AD, Montagna C, et al. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11(1):25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Wood KW, Lad L, Luo L, et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(13):5839–5844. doi: 10.1073/pnas.0915068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 20.Carol H, Lock R, Houghton PJ, et al. Initial testing (stage 1) of the kinesin spindle protein inhibitor ispinesib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53(7):1255–1263. doi: 10.1002/pbc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55(1):26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frgala T, Kalous O, Proffitt RT, et al. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 23.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(1):37–45. doi: 10.1002/pbc.21214. [DOI] [PubMed] [Google Scholar]
- 24.Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharmaceut Statist. 2010 doi: 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- 25.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 26.Graham C, Tucker C, Creech J, et al. Evaluation of the antitumor efficacy, pharmacokinetics, and pharmacodynamics of the histone deacetylase inhibitor depsipeptide in childhood cancer models in vivo. Clin Cancer Res. 2006;12(1):223–234. doi: 10.1158/1078-0432.CCR-05-1225. [DOI] [PubMed] [Google Scholar]
- 27.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 28.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 29.Plowman JCR, Alley M, Sausville E, et al. US-NCI testing procedures. In: Feibig HHBA, editor. Relevance of tumor models for anticancer drug development. Basel; Karger: 1999. pp. 121–135. [Google Scholar]
- 30.Kang M, Smith MA, Morton CL, et al. National Cancer Institute Pediatric Preclinical Testing Program: Model description for in vitro cytotoxicity testing. Pediatr Blood Cancer. 2010 doi: 10.1002/pbc.22801. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paull KD, Lin CM, Malspeis L, et al. Identification of novel antimitotic agents acting at the tubulin level by computer-assisted evaluation of differential cytotoxicity data. Cancer Res. 1992;52(14):3892–3900. [PubMed] [Google Scholar]
- 32.DeBonis S, Skoufias DA, Lebeau L, et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol Cancer Ther. 2004;3(9):1079–1090. [PubMed] [Google Scholar]
- 33.Lee CW, Belanger K, Rao SC, et al. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2008;26(3):249–255. doi: 10.1007/s10637-007-9097-9. [DOI] [PubMed] [Google Scholar]
- 34.Knox JJ, Gill S, Synold TW, et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168) Invest New Drugs. 2008;26(3):265–272. doi: 10.1007/s10637-007-9103-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.