Abstract
Sex difference in cardiac contractile function exists which may contribute to the different prevalence in cardiovascular diseases between genders. However, the precise mechanisms of action behind sex difference in cardiac function are still elusive. Given that sex difference exists in insulin-like growth factor I (IGF-1) cascade, this study is designed to evaluate the impact of severe liver IGF-1 deficiency (LID) on sex difference in cardiac function. Echocardiographic, cardiomyocyte contractile and intracellular Ca2+ properties were evaluated including ventricular geometry, fractional shortening, peak shortening, maximal velocity of shortening/relengthening (± dL/dt), time-to-peak shortening (TPS), time-to-90% relengthening (TR90), fura-fluorescence intensity (FFI) and intracellular Ca2+ clearance. Female C57 mice exhibited significantly higher plasma IGF-1 levels than their male counterpart. LID mice possessed comparably low IGF-1 levels in both sexes. Female C57 and LID mice displayed lower body, heart and liver weights compared to male counterparts. Echocardiographic analysis revealed larger LV mass in female C57 but not LID mice without sex difference in other cardiac geometric indices. Myocytes from female C57 mice exhibited reduced peak shortening, ± dL/dt, longer TPS, TR90 and intracellular Ca2+ clearance compared with males. Interestingly, this sex difference was greatly attenuated or abolished by IGF-1 deficiency. Female C57 mice displayed significantly decreased mRNA and protein levels of Na+-Ca2+ exchanger, SERCA2a and phosphorylated phospholamban as well as SERCA activity compared with male C57 mice. These sex differences in Ca2+ regulatory proteins were abolished or overtly attenuated by IGF-1 deficiency. In summary, our data suggested that IGF-1 deficiency may significantly attenuated or mitigate the sex difference in cardiomyocyte contractile function associated with intracellular Ca2+ regulation.
Keywords: IGF-1, cardiomyocytes, sex, intracellular Ca2+, SERCA
INTRODUCTION
Although cardiovascular diseases affect both genders, there are distinct gender-related differences in a broad spectrum of cardiovascular incidence and mortality (Ren, 2006; Shirato and Swan, 2010). Several factors have been speculated to contribute to the gender disparity such as sex hormones, the gender-specific intrinsic organ function, difference in body size and other cardiovascular risk profiles (Ceylan-Isik et al., 2006b; Ren, 2006; Perez-Lopez et al., 2010; Shirato and Swan, 2010). It is well known that premenopausal women possess advantage in the onset and progression of cardiovascular diseases compared with the postmenopausal women and the age-matched men (Ren and Kelley, 2009; Shirato and Swan, 2010). Given that estrogen is a key regulator of maturation, growth, differentiation and function of tissues, including those in the cardiovascular system (Younes and Honma, 2011), the “female advantage” in cardiovascular disease risk may be attributable to the female sex hormone estrogen (Ren and Kelley, 2009; Lombardi et al., 2010; Perez-Lopez et al., 2010). Estrogen exerts its biological actions via estrogen receptor α found in abundance in endometrium, breast cancer cells, ovarian stroma cells and hypothalamus; and estrogen receptor β found in kidney, brain, bone, heart, lungs, intestinal mucosa, prostate and endothelial cells (Billon-Gales et al., 2009; Arnal et al., 2010). Estrogen has been shown to mediate a wide array of beneficial cardiovascular effects including cardiac excitability, regulation of ion channel function and Ca2+ regulatory proteins (Ling et al., 2006; Perez-Lopez et al., 2010). Ovariectomy has been linked to both enhanced and depressed cardiac contractility (Ren et al., 2003; Kravtsov et al., 2007) while selective estrogen receptor modulators are capable of inhibiting L-type Ca2+ current and thus decrease myocyte contraction (Liew et al., 2004). Nonetheless, many of the sex difference in cardiovascular function including cardiac contractile function may be intrinsic to the target organ/tissue and thus be secondary to cardiovascular regulation elicited by sex hormones such as estrogen (Ren and Ceylan-Isik, 2004; Ceylan-Isik et al., 2006a).
Insulin-like growth factor-1 (IGF-1), an important cardiac survival, maintain cardiac function, cardiac growth and energy metabolism in both healthy and failing hearts (Ren et al., 1999). Severe IGF-1 deficiency is associated with the altered body composition, neuroendocrine activation, and cardiac atrophy and compromised cardiac function (Gola et al., 2005; Yakar et al., 2005). Nonetheless, liver IGF-1 deficiency retards cardiac dysfunction in aging (Li et al., 2008) and antagonizes pro-oxidants such as paraquat-induced cardiac contractile dysfunction (Li et al., 2007), indicating a rather unique role of IGF-1 deficiency or depletion in the regulation of cardiovascular function. Given the apparent sex difference in IGF-1 signaling cascade including lower IGF-1 levels in males (Chaler et al., 2009; Gupta et al., 2011), the aim of our present study was to examine the influence of liver IGF-1 deficiency on cardiac contractile function and intracellular Ca2+ handling between males and females. In an effort to explore the potential mechanism(s) of action behind sex difference in cardiac mechanical function, levels of the key intracellular Ca2+ regulatory proteins including sarco(endo)plasmic reticulum Ca2+-ATPase activity (SERCA), Na+-Ca2+ exchanger and phospholamban as well as estrogen receptor β (ERβ) were examined in myocardium from male and female mice.
MATERIALS and METHODS
Experimental animals and genotyping
The experimental procedure was approved by the Institutional Animal Use and Care Committee at the University of Wyoming (Laramie, WY). All animal procedures were in accordance with the National Institutes of Health standard. LID mice on a mixed C57BL/6, FVB/N and 129sv background were generated using Cre/loxP system (Yakar et al., 1999). To determine the presence of the IGF/loxP and Cre transgenes, genomic DNA was isolated from tail clips using a Quick extraction and amplification kit (BioPioneer Inc. San Diego, CA). Homozygous or heterozygous mice for IGF-1/loxP carrying the albumin-Cre transgene were crossed. The homozygous offspring, along with negative controls, were used for our experiment. The mouse genotyping was executed using a double PCR strategy. To identify the genotype IGF-1/loxP, primers of IA6, IA8 and ID3 were used in PCR reaction. Mice that yielded one 0.4 kb band were considered to be negative for IGF-1/loxP, whereas those with one 0.2 kb band were positive. Heterozygous IGF/loxP was identified with the presence of both 0.4 and 0.2 kb bands. To determine the presence of the Cre transgene, primers Cre-5 and Cre-3 were used, which yielded a 0.6 kb band. Mice positive for both IGF-1/loxP and Cre transgenes were deemed LID mice, whereas IGF-1/loxP-negative mice with or without the Cre transgene were used as the LID-negative mice (Li et al., 2007; Li et al., 2008). Male or female LID mice and their negative littermates at 9–10 weeks of age were used. Plasma IGF-1 levels were measured using an ELISA commercial kit from Diagnostic System Laboratory (Webster, TX) as described previously (Li and Ren, 2007).
Isolation of murine cardiomyocytes
Murine cardiomyocytes were isolated as described (Ceylan-Isik et al., 2011). After ketamine/xylazine sedation, hearts were removed and perfused with Ca2+-free Tyrode's solution containing (in mM): NaCl 135, KCl 4.0, MgCl2 1.0, HEPES 10, NaH2PO4 0.33, glucose 10, butanedione monoxime 10, and the solution was gassed with 5% CO2/95% O2. Hearts were digested with Liberase Blendzyme 4 (Hoffmann-La Roche Inc., Indianapolis, IN) for 20 min. Left ventricles were removed and minced before being filtered. Tissue pieces were gently agitated and pellet of cells was resuspended. Extracellular Ca2+ was added incrementally back to 1.20 mM over a period of 30 min. Isolated myocytes were used within 8 hrs of isolation. Normally, a yield of 50–60% viable rod-shaped cardiomyocytes with clear sarcomere striations was achieved. Only rod-shaped myocytes with clear edges were selected for mechanical study.
Cell shortening/relengthening
Mechanical properties of cardiomyocytes were assessed using a SoftEdge MyoCam system (IonOptix Corporation, Milton, MA). In brief, cardiomyocytes were placed in a chamber mounted on the stage of an inverted microscope (Olympus, Model IX-70, Olympus Ltd., Tokyo, Japan) and superfused at 25°C with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 Glucose and 10 HEPES, at pH 7.4. Cells were field stimulated with suprathreshold voltage at frequencies of 0.5 Hz using an FHC stimulator (Brunswick, NE). The myocyte being studied was displayed on a computer monitor using an IonOptix MyoCam camera. IonOptix SoftEdge software was used to capture changes in cell shortening and relengthening. Cell shortening and relengthening were assessed using the following indices: peak shortening, indicative of cell contractile capacity; maximal velocities of cell shortening and relengthening (± dL/dt), indicative of maximal rate of ventricular tension development and decline; time-to-PS (TPS) - indicative of systolic duration; time-to-90% relengthening (TR90) - indicative of diastolic duration (90% rather 100% relengthening was used to avoid noisy signal near baseline). In the case of altering stimulus frequency, the steady-state contraction of myocyte was achieved (usually after the first six beats) before PS amplitude was recorded at 0.1, 0.5, 1.0, 3.0 and 5.0 Hz (Li et al., 2005; Ceylan-Isik et al., 2011).
Intracellular Ca2+ transients
Separate cohorts of myocytes were loaded with fura-2/AM (0.5 μM) for 15 min, and fluorescence intensity was measured with a dual-excitation fluorescence photomultiplier system (IonOptix). Myocytes were placed on an inverted Olympus microscope and imaged through a Fluor 40x-oil objective. Cells were exposed to light emitted by a 75 W mercury lamp and passed through either a 360 nm or a 380 nm filter. The myocytes were stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 nm and 520 nm by a photomultiplier tube after cells were first illuminated at 360 nm for 0.5 sec and then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 nm excitation scan was repeated at the end of the protocol, and qualitative changes in intracellular Ca2+ concentration were inferred from the ratio of the fluorescence intensity at two wavelengths. Intracellular Ca2+ decay was calculated using curve fitting (Ceylan-Isik et al., 2011).
Western blot analysis
Protein expression of estrogen receptor β (ERβ), Na+-Ca2+ exchanger, SERCA2a and phospholamban were examined by Western blot analysis. Left ventricular tissues were homogenized and centrifuged at 70,000 g for 20 min at 4°C. The supernatants were used for immunoblotting. The extracted proteins were separated on 10–15% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After being blocked, the membrane was incubated with rabbit monoclonal anti-ERβ, rabbit polyclonal anti-Na+-Ca2+ exchanger, mouse polyclonal anti-SERCA2a, mouse monoclonal anti-phospho-phospholamban (Ser16) and β-actin (loading control) antibodies at 4°C overnight. Anti-ERβ antibody was purchased from Cell Signaling Technology (Beverly, MA). Anti-NCX antibody was purchased from Swant (Bellinzona, Switzerland). Anti-SERCA2a antibody was from Affinity BioReagents (Golden, CO) and anti-phospholamban antibody was obtained from Abcam (Cambridge, MA). After incubation with the primary antibodies, blots were incubated with horseradish peroxidase-linked secondary antibodies (1:5,000) for 60 min at room temperature. Immunoreactive bands were detected using the Super Signal West Dura Extended Duration Substrate (Pierce, Milwaukee, WI). The intensity of bands was measured with a scanning densitometer (Model GS-800; Bio-Rad) coupled with a Bio-Rad personal computer analysis software (Ceylan-Isik et al., 2011).
SERCA activity measured by 45Ca2+-uptake
Cardiomyocytes were sonicated and solubilized in a tris-sucrose homogenization buffer containing 30 mM Tris·HCl, 8% sucrose, 1 mM PMSF, and 2 mM dithiothreitol (pH 7.1). To determine SERCA-dependent Ca2+ uptake, samples were treated with and without the SERCA inhibitor thapsigargin (10 μM) for 30 min. The difference between the two readings was deemed the thapsigargin-sensitive uptake through SERCA. Uptake was initiated by the addition of an aliquot of supernatant to a solution consisting of (in mM) 100 KCl, 5 NaN3, 6 MgCl2, 0.15 EGTA, 0.12 CaCl2, 30 Tris-HCl (pH 7.0), 10 oxalate, 2 ATP, and 1 μCi 45CaCl2 at 37°C. Aliquots of samples were injected onto glass filters on a suction manifold and washed three times. Filters were removed from the manifold and placed in scintillation fluid. SERCA activity was expressed as counts per million per mg protein (Ceylan-Isik et al., 2011).
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) analysis
Analysis of ERβ, Na+-Ca2+ exchanger, SERCA2a and phospholamban was performed using real time RT-PCR as described previously (Elsherif et al., 2004; Mathiyalagan et al., 2010). In brief, myocardium was homogenized to obtain a clear solution in TriZol and cells were handled in the presence of RNaseIn (Invitrogen, Carlsbad, CA). Total RNA was prepared using RNeasy minikit preparation columns (Qiagen, Valencia, CA). RNA was reverse transcribed with a reverse transcriptase kit (Invitrogen, Carlsbad, CA). SYBR Green (Bio-Rad, USA) real-time quantitative PCR was performed after first strand cDNA synthesis. Designed forward and reverse primers for SERCA, Na+-Ca2+ exchanger and phospholamban were purchased from Integrated DNA Technologies (Coralville, IA) and the ERβ primer was obtained from Qiagen (Qiagen, Valencia, CA) (Table 1). GAPDH was used as the internal control for normalization. Relative differences were evaluated using the cycle time valued and expresses as the fold increase of control. Assuming that the cycle time value is reflective of the initial starting copy and that there is 100% efficacy, a difference of one cycle is calculated from each gene’s standard curve.
Table 1.
General features of male or female C57BL/6 and LID mice
| Genes | Primer |
|---|---|
| Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) | F: GAGGGTCTGCCTGCTGTCA |
| R: CAGAAGGCAGACTTCGAACGA | |
| Na+-Ca2+ exchanger (NCX) | F: GATTCCGTGACTGCCGTTGT |
| R: CCTGGGTAGCTGCTACTTTGCT | |
| Phospholamban (PLB) | F: CAGACCTGCAACATGCCAACT |
| R: GCAGCGGTGCGTTGCT | |
| Estrogen receptor beta (ERβ) | Catalog # QT10761879 (lot #97833315) Sequence information not disclosed per policy of Qiagen |
| GAPDH | F: TGAAGCAGGCATCTGAGGG |
| R: CGAAGGTGGAAGAGTGGGAG | |
Statistical analysis
Data were Mean ± SEM. Statistical significance (p < 0.05) for each variable was determined by a one-way ANOVA followed by the Tukey’s post hoc analysis.
RESULTS
General features and echocardiographic properties of male and female mice
As shown in Table 2, female C57BL/6 mice exhibited significantly lower body, heart and liver weights compared with the age-matched male C57BL/6 mice. This sex difference prevailed in LID mice with the exception of liver weight. Liver IGF-1 deficiency led to overtly increased liver weight and size (normalized to body weight) in both sexes and nullified the sex difference in liver weight in C57BL/6 mice. Neither female sex nor LID affected the cardiac size although the size of heart was significantly enhanced by LID in female mice. Neither female sex nor LID significantly altered the weight and size (normalized to body weight) of kidney. Plasma IGF-1 levels were significantly higher in female C57BL/6 mice while liver IGF-1 gene deletion resulted in a comparable and dramatic drop in plasma IGF-1 levels in both males and females. Echocardiographic examination revealed comparable heart rate, left ventricular end systolic diameter (LVESD), LV end diastolic diameter (LVEDD), LV wall thickness and fractional shortening in male or female C57BL/6 and LID groups. However, the calculated LV mass was significantly lower in female C57BL/6 mice compared with male C57BL/6 mice, the effect of which was not present in LID mice. LID itself did not significantly affect the LV mass. Most likely due to the lower body weight in female mice, the normalized LV mass was comparable among male or female C57BL/6 and LID mice.
Table 2.
General features of male or female C57BL/6 and LID mice
| C57-Male | C57-Female | LID-Male | LID-Female | |
|---|---|---|---|---|
| Body Weight (g) | 27.5 ± 0.6 | 22.7 ± 0.4* | 27.0 ± 0.3 | 23.2 ± 0.5* |
| Heart Weight (Ogah and Bamgboye) | 156 ± 8 | 123 ± 3* | 169 ± 8 | 141 ± 6* |
| HW/BW (mg/g) | 5.69 ± 0.31 | 5.43 ± 0.13 | 6.24 ± 0.27 | 6.11 ± 0.31# |
| Liver Weight (g) | 1.35 ± 0.09 | 0.93 ± 0.15* | 1.56 ± 0.10* | 1.45 ± 0.09# |
| LW/BW (mg/g) | 49.2 ± 3.9 | 40.6 ± 6.6 | 57.8 ± 3.5* | 62.5 ± 3.7* |
| Kidney Weight (g) | 0.35 ± 0.02 | 0.28 ± 0.01 | 0.32 ± 0.02 | 0.26 ± 0.01 |
| KW/BW (mg/g) | 12.6 ± 0.7 | 12.5 ± 0.3 | 11.7 ± 0.7 | 11.4 ± 0.5 |
| Plasma IGF-1 (ng/ml) | 125.7 ± 8.7 | 160.5 ± 8.2* | 46.4 ± 7.1* | 48.2 ± 11.4*,# |
| End Diastolic Diameter (mm) | 2.83 ± 0.12 | 2.53 ± 0.17 | 2.76 ± 0.26 | 2.70 ± 0.10 |
| End Systolic Diameter (mm) | 1.59 ± 0.09 | 1.30 ± 0.09 | 1.39 ± 0.19 | 1.55 ± 0.11 |
| Diastolic Wall Thickness (mm) | 0.88 ± 0.10 | 0.89 ± 0.08 | 0.87 ± 0.05 | 0.78 ± 0.70 |
| Heart Rate (beat per minute) | 459 ± 33 | 465 ± 31 | 451 ± 20 | 462 ±15 |
| LV Mass (Ogah and Bamgboye) | 75.6 ± 7.4 | 60.1 ± 3.5* | 77.4 ± 10.7 | 66.0 ±2.8 |
| Normalized LV Mass (mg/g) | 2.83 ± 0.25 | 2.58 ± 0.15 | 2.89 ± 0.35 | 2.86 ± 0.16 |
| Fractional Shortening (%) | 44.0 ± 1.4 | 48.5 ± 1.3 | 49.2 ± 3.1 | 42.9 ± 2.0 |
BW= Body weight; HW = Heart weight; LW = Liver weight; KW = Kidney weight; Mean ± SEM,
p < 0.05 vs. corresponding male group,
p < 0.05 vs. C57BL/6-Female group, n = 8–10 mice per group.
Mechanical and intracellular Ca2+ properties of cardiomyocytes
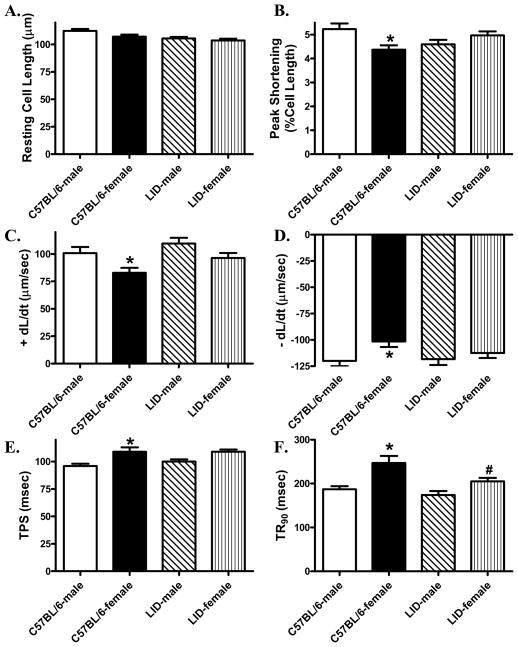
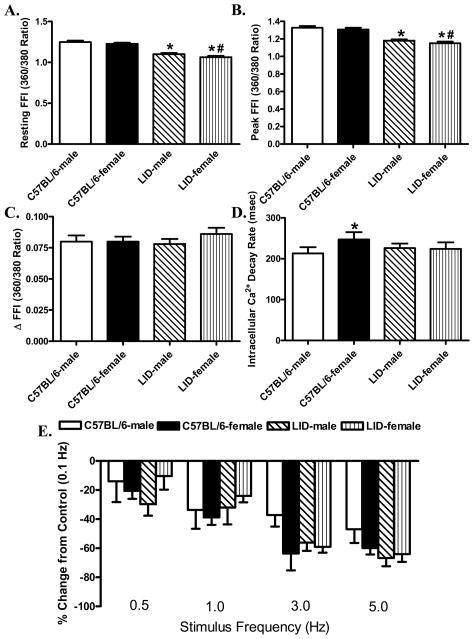
Evaluation of cardiomyocyte mechanical properties revealed similar resting cell length in male or female C57BL/6 and LID mice. Female C57BL/6 mice displayed significantly reduced peak shortening and maximal velocity of shortening/relengthening (± dL/dt) as well as prolonged time-to-PS (TPS) and time-to-90% relengthening (TR90) compared with C57BL/6 male mice. Interestingly, while liver IGF-1 deficiency itself did not affect the cardiomyocyte contractile function in male mice, it nullified the sex difference in cardiomyocyte mechanical function (Fig. 1). To explore to intracellular Ca2+ handling contributes to IGF-1 deficiency-induced effect on sex difference in cardiomyocyte contractile properties, Fura-2 fluorescence microscopy was used to examine the intracellular Ca2+ handling in cardiomyocytes. Our data shown in Fig. 2A-D revealed that resting, peak and the electrically-stimulated rise in intracellular Ca2+ (ΔFFI) were comparable in cardiomyocytes from male or female C57BL/6 and LID mice. Intracellular Ca2+ decay rate was significantly prolonged in female C57BL/6 mice compared with male C57BL/6 mice. Although liver IGF-1 deficiency itself did not affect any of the intracellular Ca2+ handling parameters tested, it significantly attenuated female sex-induced prolongation in intracellular Ca2+ transient clearance.
Fig. 1.
Contractile properties of cardiomyocytes isolated from male or female C57BL/6 and LID mouse hearts. A: Resting cell length; B: Peak shortening (PS, normalized to cell length); C: Maximal velocity of shortening (+ dL/dt); D: Maximal velocity of relengthening (- dL/dt); E: Time-to-PS (TPS); F: Time-to-90% relengthening (TR90); Mean ± SEM, n = 150–166 cells/group, *p < 0.05 vs. corresponding male group, #p < 0.05 vs. C57BL/6-female group.
Fig. 2.
Intracellular Ca2+ transient properties and stimulus frequency-peak shortening response in cardiomyocytes isolated from male or female C57BL6 and LID mouse hearts. A: Resting fura-2 fluorescence intensity (FFI); B: Peak FFI; C: Change in FFI (ΔFFI) in response to electrical stimuli; D: Intracellular Ca2+ decay rate; and E: Influence of stimulus frequency (0.1 – 5.0 Hz) on PS amplitude. PS at each stimulus frequency was normalized to that of 0.1 Hz from the same cell. Mean ± SEM, n = 91-92 (panel A-D) and 21-25 (panel E) cells per group, *p < 0.05 vs. corresponding male group, #p < 0.05 vs. C57BL/6-female group.
Effect of increasing stimulation frequency on cardiomyocyte shortening
Murine hearts beats at high frequencies (~450 beats/min), whereas our baseline stimulus in this study was only set at 0.5 Hz (30 beats/min). To probe possible derangement of cardiac contractile function at higher frequencies, stimulating frequency was increased stepwise from 0.1 Hz to 5.0 Hz pe before steady-state peak shortening was recorded. All values were normalized to peak shortening (Duan et al.) obtained at 0.1 Hz of the same cardiomyocyte. Fig. 2E displays a comparable negative staircase in PS (change from PS obtained at 0.1 Hz) among all four myocyte groups with increasing stimulus frequencies, suggesting minimal effect of female sex and/or IGF-1 deficiency on intracellular Ca2+ cycling.
Influence of IGF-1 deficiency on expression of ERβ and intracellular Ca2+ regulatory proteins
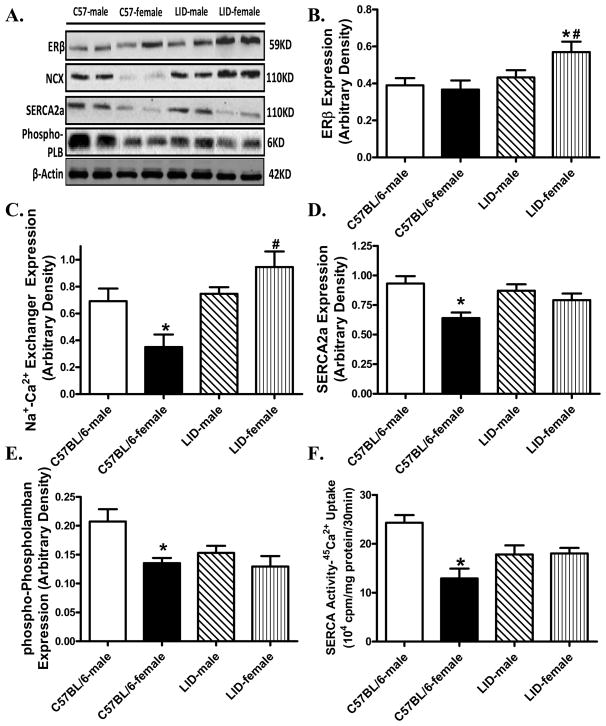
To further determine the potential mechanisms involved in LID-elicited antagonism against sex difference in cardiomyocyte mechanical function, we evaluated the expression and/or activity of the estrogen receptor ERβ, the key intracellular Ca2+ regulatory proteins Na+-Ca2+ exchanger, SERCA2a and the SERCA inhibitor phospholamban in myocardium. As depicted in Fig. 3, Western blot analysis demonstrated significantly downregulated levels of expression of Na+-Ca2+ exchanger, SERCA2a and phosphorylated phospholamban with unchanged ERβ in female C57BL/6 mice compared with male C57BL/6 mice. Although IGF-1 deficiency itself did not affect these intracellular Ca2+ regulatory proteins, it significantly attenuated or mitigated female sex-induced change in Na+-Ca2+ exchanger, SERCA2a and phosphorylated phospholamban. Consistent with the protein expression, SERCA activity measured by 45Ca2+ uptake was significantly decreased in female C57BL/6 mice, the effect of which was lessened by IGF-1 deficiency (with little effect on SERCA activity itself). Last but not least, although LID failed to elicit any effect on ERβ expression in male mice, it significantly enhanced the expression of ERβ in female mice.
Fig. 3.
Protein expression of ERβ, Na+-Ca2+ exchanger (NCX), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a) and phosphorylated phospholamban (PLB) as well as SERCA activity in myocardial tissue from male or female C57BL/6 and LID mice. Panel A: Representative gel blots of ERβ, NCX, SERCA2a, phosphor-PLB and β-actin (loading control) using specific antibodies; Panel B: ERβ expression; Panel C: NCX expression; Panel D: SERCA2a expression; Panel E: phosphorylated (Ser16) PLB expression; and Panel F: SERCA activity measured using 45Ca2+ uptake technique. Mean ± SEM, n = 4–6 hearts per group, *p < 0.05 vs. corresponding male group, #p < 0.05 vs. C57BL/6-female group.
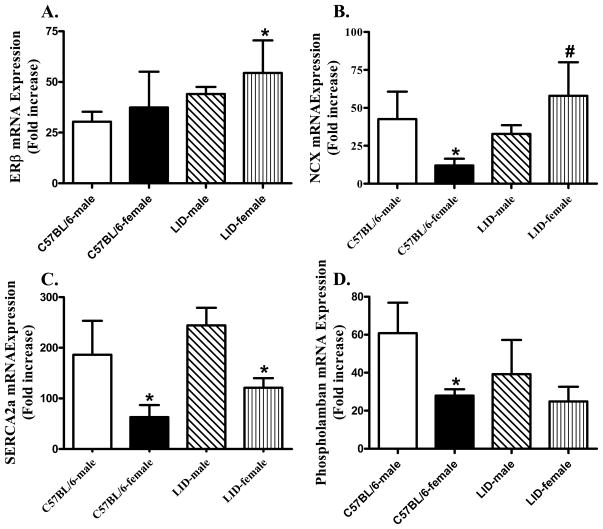
Influence of IGF-1 deficiency on mRNA of ERβ and intracellular Ca2+ regulatory proteins
Fig. 4 illustrates the fold increase in mRNA expression of ERβ, Na+-Ca2+ exchanger, SERCA2a and phospholamban using qRT-PCR. Our result indicated that mRNA expression of Na+-Ca2+ exchanger, SERCA2a and phospholamban was significantly lower in female C57BL/6 mice compared with those in male C57BL/6 mice. Although LID itself did not elicit any notable effect on mRNA levels of these intracellular Ca2+ regulatory proteins, it ablated or partially alleviated the sex difference in mRNA levels of Na+-Ca2+ exchanger and phospholamban but not SERCA2a. Neither sex nor LID significantly affected the mRNA expression of ERβ although the combination of both significantly elevated ERβ mRNA expression.
Fig. 4.
mRNA expression of ERβ, Na+-Ca2+ exchanger (NCX), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), and phospholamban in myocardial tissue from male or female C57BL/6 and LID mice. Panel A: ERβ mRNA expression; Panel B: NCX mRNA expression; Panel C: SERCA2a mRNA expression; and Panel D: Phospholamban mRNA expression. Mean ± SEM, n = 4-6 hearts per group, *p < 0.05 vs. corresponding male group, #p < 0.05 vs. C57BL/6-female group.
DISCUSSION
The salient finding from our study suggested that liver IGF-1 gene deletion attenuated or mitigated the sex difference in cardiomyocyte contractile function possibly due to an intracellular Ca2+ regulation-dependent mechanism. Our data revealed higher plasma IGF-1 levels in female C57BL/6 mice compared with those in male C57BL/6 mice, consistent with the previous notion of a sex difference in circulating IGF-1 levels (Chaler et al., 2009; Gupta et al., 2011). Liver IGF-1 gene deletion significantly lowered the plasma IGF-1 levels to comparable levels in both male and female LID mice. More importantly, we found that female C57BL/6 mice exhibited significantly lower levels (both mRNA and protein) of the intracellular Ca2+ regulator proteins Na+-Ca2+ exchanger, SERCA2a, and phospholamban (phosphorylated) compared to those from male C57 mice. Similarly, SERCA activity measured by 45Ca2+ uptake assay was reduced in female C57BL/6 mice, the effect of which was reversed by IGF-1 deficiency. Measurement of ERβ levels in both mRNA and protein expression did not reveal any significant sex difference in C57BL/6 mice nor did IGF-1 deficiency overtly alter ERβ expression. Interestingly, liver IGF-1 gene deletion significantly enhanced ERβ expression (both mRNA and protein) in female mice.
Epidemiological evidence has demonstrated that premenopausal women possess a lower incidence for cardiovascular diseases compared to the age-match men and postmenopausal women (Ren, 2006; Shirato and Swan, 2010). This female bias is disappeared once menopause starts (Ren, 2006). It has been shown that animal models of ovariectomy exhibited increased vascular reactivity (Ceylan-Isik et al., 2009), heart rate (Marni et al., 2009) and Ca2+ fluxes (Kravtsov et al., 2007). On the other hand, estrogen or estrogen replacement may protect cardiomyocytes from hypoxia-induced Ca2+ overload (Liao et al., 1995), decrease the development of atherosclerosis (Mendelsohn and Karas, 1999; Kim et al., 2006) and increase endothelium-dependent relaxation (Ceylan-Isik et al., 2009). Gender difference in intrinsic cardiomyocytes contractile and intracellular Ca2+ properties have been well documented (Ren and Ceylan-Isik, 2004). Data from our current study revealed that reduced peak shortening, lessened maximal velocity of shortening and relengthening and, prolonged duration of shortening and relengthening in female C57BL/6 mouse cardiomyocytes compared with male C57BL/6 mouse cardiac myocytes. This gender-related myocardial contractile difference is consistent with our previous findings (Zhang et al., 2003; Ceylan-Isik et al., 2006a; Ceylan-Isik et al., 2006b). The mechanisms behind the sex difference in cardiomyocyte contractile function remain unclear but may be associated with in altered intracellular Ca2+ handling as slowed intracellular Ca2+ clearing was seen in female C57BL/6 mice, which was cancelled off by IGF-1 deficiency. In our hand, we failed to observe any difference in the baseline, peak and electrically-stimulated intracellular Ca2+ levels between male and female C57BL/6 mice. The apparent sex difference in cardiomyocyte mechanical and intracellular Ca2+ properties is still elusive but may be associated with sex hormone-induced changes in myofilament Ca2+ sensitivity. Ovarian hormones (e.g., estrogen) are well known to alter myocardial contractile function such as myofilament Ca2+ sensitivity (Bupha-Intr et al., 2007). This is also reflected in the peak shortening-stimulus frequency response where little sex difference was observed. The similar pattern in the negative staircase in peak shortening-stimulus frequency response among male or female C57BL/6 and LID mice may indicate a comparable efficiency in intracellular Ca2+ replenishing from sarcoplasmic reticulum. The fact that cardiomyocyte contractile indices displayed a sex difference in the absence of changes in baseline, peak intracellular Ca2+ and ΔFFI as well as intracellular Ca2+ cycling denotes a possible role of myofilament Ca2+ responsiveness in the sex difference of cardiac contractile function. It is also noteworthy of the subtle but significant difference between whole hearts and cardiomyocytes. This discrepancy between hearts and heart cells may indicate possible contributions from the non-myocyte components such as fibroblasts and cell matrix.
Our echocardiographic result indicated significantly lower calculated LV mass in female C57BL/6 mice compared with that from male C57BL/6 mice. This is consistent with observation from human in that Japanese women display a smaller LV mass compared with age-matched Japanese men (Katayama et al., 2010). In a smaller-scale study performed in Nigerians, women display a trend but insignificant decrease in LV mass compared with age-matched men (Ogah and Bamgboye, 2010). This subtle difference in the LV mass between men and women may be related to the apparent differences in ethnicity and blood pressure (Nigerian participants were hypertensive). Our study did not reveal any sex difference in fractional shortening, again similar that reported in the human setting (Katayama et al., 2010).. Interestingly, the sex difference in LV mass was absent in LID mice. In addition, liver IGF-1 deficiency itself did not significantly affect LV mass. These findings seem to contradict the commonly accepted concept that IGF-1 promotes cardiac growth (Ren et al., 1999) and data from our recent report that IGF-1 overexpression in the heart promotes cardiac hypertrophy (Zhang et al., 2010). Several scenarios may be considered for the paradoxical effect of IGF-1. First, cardiac IGF-1 mRNA expression was found to be normal in these LID mice compared with wild-type controls despite of the severely reduced circulating IGF-1 levels (Yakar et al., 1999). The cardiac growth in LID mice appears to be normal (Yakar et al., 1999). It is possible that locally released IGF-1 may override the reduced circulating IGF-1 levels in LID mice and thus maintain the normal cardiac growth and geometry in LID mice. However, excessive IGF-1 will trigger cardiac hypertrophy (Ren et al., 1999). Secondly, IGF-1 is known to regulate cardiac function and architecture through an endocrine/paracrine fashion (Ren et al., 1999). Severe loss of circulating IGF-1 may turn on other compensatory pro-growth mechanisms to cope with the reduced circulating levels of IGF-1 although further study is warranted.
Perhaps the most intriguing finding from our study is that liver IGF-1 gene deficiency attenuated or abolished the sex difference in cardiomyocyte contractile function, indicating a role of IGF-1 in sex difference in cardiovascular function and thus risk of cardiovascular diseases. IGF-1 is essential in the regulation of growth and cell proliferation (Juul, 2003). Decreased levels of IGF-1 have been reported in chronic heart failure (Broglio et al., 1999; Jankowska et al., 2006) and IGF-1 treatment increased cardiac contractility and reduced ischemia-induced apoptosis in myocytes (Lee et al., 1999). The beneficial effects of cardiac specific overexpression of IGF-1 has been shown to protect cardiac anomalies in sepsis (Zhao et al., 2009), alcoholism (Zhang et al., 2010) and aging (Li and Ren, 2007). Data from our present study depicted that liver IGF-1 deficiency did not lead to any deterioration in cardiac contractile and intracellular Ca2+ property in male mice, suggesting that deficiency of IGF-1 may not be innately harmful to myocardial contractile function at least at relatively young age used in our study (9-10 weeks). Several scenarios may be considered behind the IGF-1 deficiency-induced effect in neutralizing sex difference in cardiac mechanical mechanics. Considering IGF-1 deficiency nullified sex difference in intracellular Ca2+ clearance observed in our current study, regulation through intracellular Ca2+ regulatory proteins including SERCA2a, Na+-Ca2+ exchanger and phospholamban seems to play a major role in LID-mitigated sex difference in cardiomyocyte contractile function. Ca2+ regulatory proteins display different activities in the heart in a sex-dependent manner. Phospholamban is linked to cardiac function and phosphorylation state of phospholamban is reported to be different between sexes, leading to differences in the affinity of the SERCA for Ca2+ reuptake (Dash et al., 2001). In our hand, the phosphorylation of phospholamban is significantly lessened in female C57BL/6 hearts, favoring a lower SERCA function (lessened removal of the phospholamban inhibition on SERCA pump). This was in line with both expression and activity of SERCA observed in our study. Along the same line, SERCA2a mRNA levels were reported to be more abundant in males than those in females (Tappia et al., 2007). It is quite possible that IGF-1 deficiency nullified the sex difference in phospholamban phosphorylation en route to a better SERCA function and intracellular Ca2+ extrusion. In addition, our Western blotting and mRNA data also favored a role of the Na+-Ca2+ exchanger in LID-mediated neutralization of the sex difference in intracellular Ca2+ clearance and cardiomyocyte contractile function. The disparate findings between mRNA and protein in SERCA2a levels (female LID displayed a reduced mRNA level associated with normal protein expression) suggest possible involvement of translational modification for the combined IGF-1 deficiency and sex hormone.
Sex differences in the cardiovascular function have been attributed to the effects of sex steroids. Both estrogen and androgen receptors are found in human and rodent hearts as well as in vascular endothelial and smooth muscle cells, as well as cardiac fibroblasts (Grohe et al., 1997). Estrogens exert their effects via both ERα and ERβ. It has been implicated that induction of ERβ is stronger in female than in male hearts while the increase of ERα is similar in both sexes(Fliegner et al., 2010). Cardiac protective role has been associated with ERβ (Skavdahl et al., 2005; Pedram et al., 2008). It has been shown that, deletion of ERβ in mice led to cardiac hypertrophy in pressure overloaded female mice not male mice (Skavdahl et al., 2005). Although our finding revealed higher ERβmRNA and protein levels in female LID mice, data from our present study are inclusive at this point to suggest a key role of ERβ in the regulation of sex difference in cardiac contractile function. Additional work should focus on the precise role of estrogen receptor signaling in sex difference of cardiac contractile function.
Experimental limitation: Our study suffers from a number of experimental limitations. First, the current study is mostly phenomenological and lacks mechanistic depth with regards to how IGF-1 deficiency mitigates or attenuates sex difference in cardiac function and intracellular Ca2+ handling. Although our data suggest existence of possible interactions among gender, IGF-1 and intracellular Ca2+ regulatory proteins (SERCA, Na+-Ca2+ exchanger and phospholamban), such interaction, if any, seems to occur at the transcriptional level as suggested by our qRT-PCR data. Further study is needed to better understand the transcriptional or epigenetic regulation of sex hormones and IGF-1 on intracellular Ca2+ regulatory or other cardiac proteins governing cardiac contractile function. Furthermore, although LID cancelled off the sex difference in plasma IGF-1 levels and cardiac contractile function, it is premature to draw any conclusion that IGF-1 is one of the major contributing factors for sex difference in cardiac physiology. Although a cardiac regulatory role has been well defined for IGF-1 (Ren et al., 1999; Zhang et al., 2010), little information is available on the interaction between IGF-1 and sex hormones. Estrogen and IGF-1 are known to interact in the brain to regulate a variety of developmental and neuroplastic events involving control of hormonal homeostasis and reproduction (Garcia-Segura et al., 2010). Several molecular mechanisms are speculated for the interaction of estrogen and IGF-I including the cross-regulation of the expression of estrogen and IGF-I receptors, the regulation of estrogen receptor-mediated transcription by IGF-I and the regulation of IGF-I receptor signaling by estrogen (Garcia-Segura et al., 2010). Nonetheless, little is known for the interaction between estrogen and IGF-1 in the heart. Lastly, as evidenced by findings from our laboratory (Li et al., 2007; Li et al., 2008; Ge et al., 2010), IGF-1 deficiency or depletion may have a greater impact on cardiac structure and performance under pathological stress conditions such as aging, alcoholism and oxidative stress.
In conclusion, data from our present study revealed for the first time that IGF-1 deficiency attenuates or abolishes the sex difference in cardiac function possibly through the intracellular Ca2+ regulatory proteins SERCA2a, Na+-Ca2+ exchanger and phosphorylation state of phospholamban. These findings seem to suggest a role of IGF-1 in the interplay between sex hormones and endocrine factors in the maintenance of heart function. In light of gender bias in cardiac function and risk for cardiac event, it is attempting to speculate that a better understanding of IGF-1 and its interaction with sex hormones is of clinical value in the sex-associated prevalence in cardiovascular diseases.
Highlights.
Liver IGF-1 deficiency attenuates or abolishes the sex difference in cardiac contractile function and intracellular Ca2+ handling.
Sex difference in cardiac function is associated with sex difference in intracellular Ca2+ regulatory proteins SERCA2a, Na+-Ca2+ exchanger and phospholamban, which may be alleviated by liver IGF-1 deficiency.
IGF-1 may interact with sex hormones to regulate cardiac contractile function and intracellular Ca2+ handling.
Acknowledgments
This work was supported by NIH INBRE P20 RR16474.
Footnotes
DISCLOSURES - None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnal JF, Fontaine C, Billon-Gales A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- Broglio F, Fubini A, Morello M, Arvat E, Aimaretti G, Gianotti L, Boghen MF, Deghenghi R, Mangiardi L, Ghigo E. Activity of GH/IGF-I axis in patients with dilated cardiomyopathy. Clin Endocrinol (Oxf) 1999;50:417–430. doi: 10.1046/j.1365-2265.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Bupha-Intr T, Wattanapermpool J, Pena JR, Wolska BM, Solaro RJ. Myofilament response to Ca2+ and Na+/H+ exchanger activity in sex hormone-related protection of cardiac myocytes from deactivation in hypercapnic acidosis. Am J Physiol Regul Integr Comp Physiol. 2007;292:R837–843. doi: 10.1152/ajpregu.00376.2006. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Erdogan-Tulmac OB, Ari N, Ozansoy G, Ren J. Effect of 17beta-oestradiol replacement on vascular responsiveness in ovariectomized diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:e65–71. doi: 10.1111/j.1440-1681.2009.05255.x. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, LaCour KH, Ren J. Gender disparity of streptozotocin-induced intrinsic contractile dysfunction in murine ventricular myocytes: role of chronic activation of Akt. Clin Exp Pharmacol Physiol. 2006a;33:102–108. doi: 10.1111/j.1440-1681.2006.04331.x. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, LaCour KH, Ren J. Sex difference in cardiomyocyte function in normal and metallothionein transgenic mice: the effect of diabetes mellitus. J Appl Physiol. 2006b;100:1638–1646. doi: 10.1152/japplphysiol.01273.2005. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Sreejayan N, Ren J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol. 2011;50:107–116. doi: 10.1016/j.yjmcc.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chaler EA, Meazza C, Guercio G, Maceiras M, Rivarola MA, Laarej K, Pagani S, Areny G, Albertini R, Llinares V, Belgorosky A, Bozzola M. Serum IGF-I and IGFBP-3 reference values from a chemiluminescent assay in normal children and adolescents of hispanic and italian origin: presence of sexual dimorphism in IGF-I values. J Pediatr Endocrinol Metab. 2009;22:1127–1135. doi: 10.1515/jpem.2009.22.12.1127. [DOI] [PubMed] [Google Scholar]
- Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33:1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- Duan J, Esberg LB, Ye G, Borgerding AJ, Ren BH, Aberle NS, Epstein PN, Ren J. Influence of gender on ethanol-induced ventricular myocyte contractile depression in transgenic mice with cardiac overexpression of alcohol dehydrogenase. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:607–614. doi: 10.1016/s1095-6433(02)00347-1. [DOI] [PubMed] [Google Scholar]
- Elsherif L, Jiang Y, Saari JT, Kang YJ. Dietary copper restriction-induced changes in myocardial gene expression and the effect of copper repletion. Exp Biol Med (Maywood) 2004;229:616–622. [PubMed] [Google Scholar]
- Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1597–1606. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- Ge W, Li Q, Turdi S, Wang XM, Ren J. Deficiency of insulin-like growth factor 1 reduces vulnerability to chronic alcohol intake-induced cardiomyocyte mechanical dysfunction: Role of AMPK. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M, Bonadonna S, Doga M, Mazziotti G, Giustina A. Cardiovascular risk in aging and obesity: is there a role for GH. J Endocrinol Invest. 2005;28:759–767. doi: 10.1007/BF03347561. [DOI] [PubMed] [Google Scholar]
- Grohe C, Kahlert S, Lobbert K, Stimpel M, Karas RH, Vetter H, Neyses L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;416:107–112. doi: 10.1016/s0014-5793(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Gupta N, Lustig RH, Kohn MA, McCracken M, Vittinghoff E. Sex differences in statural growth impairment in Crohn's disease: Role of IGF-1. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Katayama T, Fujiwara N, Tsuruya Y. Factors contributing to left atrial enlargement in adults with normal left ventricular systolic function. J Cardiol. 2010;55:196–204. doi: 10.1016/j.jjcc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- Kravtsov GM, Kam KW, Liu J, Wu S, Wong TM. Altered Ca(2+) handling by ryanodine receptor and Na(+)-Ca(2+) exchange in the heart from ovariectomized rats: role of protein kinase A. Am J Physiol Cell Physiol. 2007;292:C1625–1635. doi: 10.1152/ajpcell.00368.2006. [DOI] [PubMed] [Google Scholar]
- Lee WL, Chen JW, Ting CT, Ishiwata T, Lin SJ, Korc M, Wang PH. Insulin-like growth factor I improves cardiovascular function and suppresses apoptosis of cardiomyocytes in dilated cardiomyopathy. Endocrinology. 1999;140:4831–4840. doi: 10.1210/endo.140.10.7082. [DOI] [PubMed] [Google Scholar]
- Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–733. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799–806. doi: 10.1111/j.1474-9726.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Yang X, Sreejayan N, Ren J. Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: role of oxidative stress. Rejuvenation Res. 2007;10:501–512. doi: 10.1089/rej.2007.0552. [DOI] [PubMed] [Google Scholar]
- Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang X, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Liao Y, Cooper RS, Mensah GA, McGee DL. Left ventricular hypertrophy has a greater impact on survival in women than in men. Circulation. 1995;92:805–810. doi: 10.1161/01.cir.92.4.805. [DOI] [PubMed] [Google Scholar]
- Liew R, Stagg MA, MacLeod KT, Collins P. Raloxifene acutely suppresses ventricular myocyte contractility through inhibition of the L-type calcium current. Br J Pharmacol. 2004;142:89–96. doi: 10.1038/sj.bjp.0705736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Komesaroff P, Sudhir K. Cellular mechanisms underlying the cardiovascular actions of oestrogens. Clin Sci (Lond) 2006;111:107–118. doi: 10.1042/CS20050084. [DOI] [PubMed] [Google Scholar]
- Lombardi M, Mercuro G, Fini M, Rosano GM. Gender-specific aspects of treatment of cardiovascular risk factors in primary and secondary prevention. Fundam Clin Pharmacol. 2010;24:699–705. doi: 10.1111/j.1472-8206.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- Marni F, Wang Y, Morishima M, Shimaoka T, Uchino T, Zheng M, Kaku T, Ono K. 17 beta-estradiol modulates expression of low-voltage-activated Ca(V)3.2 T-type calcium channel via extracellularly regulated kinase pathway in cardiomyocytes. Endocrinology. 2009;150:879–888. doi: 10.1210/en.2008-0645. [DOI] [PubMed] [Google Scholar]
- Mathiyalagan P, Chang L, Du XJ, El-Osta A. Cardiac ventricular chambers are epigenetically distinguishable. Cell Cycle. 2010;9:612–617. doi: 10.4161/cc.9.3.10612. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Ogah OS, Bamgboye AE. Correlates of left ventricular mass in hypertensive Nigerians: an echocardiographic study. Cardiovasc J Afr. 2010;21:79–85. [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology. 2008;149:3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lopez FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010;17 :511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. Cardiac health and diabetes mellitus in women: problems and prospects. Minerva Cardioangiol. 2006;54:289–309. [PubMed] [Google Scholar]
- Ren J, Ceylan-Isik AF. Diabetic cardiomyopathy: do women differ from men? Endocrine. 2004;25:73–83. doi: 10.1385/ENDO:25:2:073. [DOI] [PubMed] [Google Scholar]
- Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH, Lee KJ, Zeng H. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol. 2003;284:H1800–1807. doi: 10.1152/ajpheart.00866.2002. [DOI] [PubMed] [Google Scholar]
- Ren J, Kelley RO. Cardiac health in women with metabolic syndrome: clinical aspects and pathophysiology. Obesity (Silver Spring) 2009;17:1114–1123. doi: 10.1038/oby.2009.8. [DOI] [PubMed] [Google Scholar]
- Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- Shirato S, Swan BA. Women and cardiovascular disease: an evidentiary review. Medsurg Nurs. 2010;19:282–286. 306. [PubMed] [Google Scholar]
- Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288:H469–476. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- Tappia PS, Dent MR, Aroutiounova N, Babick AP, Weiler H. Gender differences in the modulation of cardiac gene expression by dietary conjugated linoleic acid isomers. Can J Physiol Pharmacol. 2007;85:465–475. doi: 10.1139/y06-104. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Sun H, Zhao H, Pennisi P, Toyoshima Y, Setser J, Stannard B, Scavo L, Leroith D. Metabolic effects of IGF-I deficiency: lessons from mouse models. Pediatr Endocrinol Rev. 2005;3:11–19. [PubMed] [Google Scholar]
- Younes M, Honma N. Estrogen receptor beta. Arch Pathol Lab Med. 2011;135:63–66. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- Zhang B, Turdi S, Li Q, Lopez FL, Eason AR, Anversa P, Ren J. Cardiac overexpression of insulin-like growth factor 1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: Roles of Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med. 2010;49:1238–1253. doi: 10.1016/j.freeradbiomed.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ye G, Duan J, Chen AF, Ren J. Influence of gender on intrinsic contractile properties of isolated ventricular myocytes from calmodulin-induced diabetic transgenic mice. Endocr Res. 2003;29:227–236. doi: 10.1081/erc-120022318. [DOI] [PubMed] [Google Scholar]
- Zhao P, Turdi S, Dong F, Xiao X, Su G, Zhu X, Scott GI, Ren J. Cardiac-specific overexpression of insulin-like growth factor I (IGF-1) rescues lipopolysaccharide-induced cardiac dysfunction and activation of stress signaling in murine cardiomyocytes. Shock. 2009;32:100–107. doi: 10.1097/SHK.0b013e31818ec609. [DOI] [PMC free article] [PubMed] [Google Scholar]