Abstract
In retinitis pigmentosa (RP), various mutations cause rod photoreceptor cell death leading to increased oxygen levels in the outer retina, progressive oxidative damage to cones, and gradual loss of cone cell function. We have been exploring the potential of overexpressing components of the endogenous antioxidant defense system to preserve cone cell function in rd10+/+ mice, a model of RP. Rd10+/+ mice deficient in superoxide dismutase 1 (SOD1) showed increased levels of superoxide radicals and carbonyl adducts (a marker of oxidative damage) in the retina, and more rapid loss of cone function than rd10+/+ mice with normal levels of SOD1. This suggests that SOD1 is an important component of the antioxidant defense system of cones, but increased expression of SOD1 in rd10+/+ mice increased oxidative damage and accelerated the loss of cone function. Co-expression of SOD1 with glutathione peroxidase 4 (Gpx4), which like SOD1 is localized in the cytoplasm, but not with catalase targeted to the mitochondria, reduced oxidative damage in the retina and significantly slowed the loss of cone cell function in rd10+/+ mice. Gene transfer resulting in increased expression of SOD2, but not co-expression of SOD2 and mitochondrial Gpx4, resulted in high levels of H2O2 in the retina. These data suggest that in order to provide benefit in RP, over-expression of a SOD must be combined with expression of a peroxide detoxifying enzyme in the same cellular compartment.
Keywords: catalase, glutathione peroxidase, photoreceptors, reactive oxygen species, retina, retinitis pigmentosa
Introduction
The retina consists of three layers of nuclei, the ganglion cell layer (GCL), near the surface of the retina adjacent to the vitreous cavity, the inner nuclear layer (INL), which is centrally located, and the outer nuclear layer (ONL). The GCL and INL receive their oxygen supply from the retinal circulation, while the ONL receives its oxygen from the choroidal circulation. The ONL consists solely of photoreceptor nuclei and those of rods vastly outnumber those of cones; rods make up 98% of photoreceptors in mice and 92% in humans, although the percentage varies based upon the position within the retina with a higher percentage of cones in the posterior versus the anterior retina [1].
Retinitis pigmentosa (RP) is a group of diseases in which one of several different mutations results in death of rod photoreceptors. As rods die from the pathogenic mutation, oxygen utilization in the outer retina is reduced, and since choroidal vessels, unlike retinal vessels, are incapable of autoregulation to decrease blood flow when tissue oxygen levels are increased, the oxygen level in the outer retina becomes markedly elevated [2, 3]. The high tissue oxygen levels cause increased accumulation of superoxide radicals from a combination of run-off from the electron transport chain and activation of NADP(H) oxidase [4]. The excess superoxide radicals generate other reactive oxygen species (ROS) and react with NO to generate peroxynitrite [5] leading to progressive oxidative and nitrosative damage to cones resulting in loss of cone function and eventual cone cell death [6, 7]. In several models of RP in which rods die from different mutations, exogenous antioxidants slow cone cell death indicating a potential therapeutic approach in all RP patients despite tremendous heterogeneity in pathogenic mutations [8]. The loss of rods results in night blindness, but patients are still able to function well if illumination is adequate. However, once rods die there is gradual loss of cones accompanied by constriction of visual fields and eventual blindness. If cone survival could be prevented in patients with RP, blindness could be averted.
A complementary approach to antioxidant medication is to bolster the endogenous antioxidant defense system. In most tissues, the first line of defense against oxidative stress is the superoxide dismutases (SODs), SOD1 in the cytoplasm, SOD2 in mitochondria, and SOD3 in the extracellular space. SOD1 is an important component of the antioxidant defense system in the retina because compared to wild type mice, mice deficient in SOD1 are more sensitive to the damaging effects of an intraocular injection of paraquat or exposure to hyperoxia [9]. Additional protection is provided by the glutathione peroxidases (Gpxs), catalase, and a variety of other enzymes. The SODs convert superoxide radicals to H2O2 which is then metabolized by glutathione peroxidases (Gpx) and catalase. Gpx-1 or “classical” Gpx was the first family member to be described and utilizes glutathione (GSH) to reduce H2O2 in the cytoplasm [10], but also provides some protection of mitochondrial membranes from lipid peroxidation [11]; however, the greatest protection from lipid peroxidation comes from Gpx4, which has cytoplasmic and mitochondrial isoforms [12]. Induced expression of murine cytoplasmic Gpx4 by treatment of IRBP/rtTA-TRE/Gpx4 mice with doxycycline reduces paraquat-induced oxidative damage in the retina [13]. Catalase is a cytosolic enzyme that has greater capacity but lower affinity for peroxides than Gpx and thereby handles severe oxidative stress. In this study we sought to determine if SOD1 plays an important role in the antioxidant defense system of retinal cones and whether overexpression of SOD1 could reduce loss of cone cell function in mice with RP.
Materials and Methods
Generation of transgenic mice
Mice were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Research and the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice with targeted disruption of the sod1 gene (sod1−/−), 129S7-Sod1tmlLeb/J and carrying a β-actin promoter/human Sod1 transgene (C57BL/6-TgN(SOD1)3Cje/J mice, Sod1(+/−) mice) were purchased from Jackson Laboratories (Bar Harbor, ME) and crossed with rd10+/+ mice in a C57BL/6 background to obtain Sod1−/−-rd10+/+ mice and Sod1(+/−)-rd10+/+ mice.
TRE/Gpx4(+/−) mice in a C57BL/6 background [13] were crossed with rd10+/+ mice to generate TRE/Gpx4(+/−)-rd10+/+ mice. The MCAT plasmid, also known as poCAT, which contains human Catalase gene with the ornithine transcarbamylase leader sequence (OTC) at its 5′ end and without the peroxisomal localization signal (PLS) at its 3′ end to provide mitochondrial targeting, was generously provide by Dr. Peter S. Rabinovitch (University of Washington, Seattle, WA). The MCAT plasmid was ligated into pTRE2. After sequencing, a fragment containing TRE, MCAT and a 1.2kb β-globin poly A signal was released from pTRE2 to provide the TRE/Catalase construct that was used to generate transgenic mice in the Johns Hopkins University Transgenic Mouse Core Facility. Founder mice were mated with C57BL/6 mice to generate founder lines. Mice from each line were crossed with mice from the interphotoreceptor binding protein promoter/reverse tetracycycline transactivator (IRBP/rtTA) driver line to generate IRBP/rtTA-TRE/Catalase double transgenic mice. Mice from double transgenic lines were given 2 mg/ml in their drinking water and real time PCR was done to identify IRBP/rtTA-TRE/Catalase lines with strong, inducible transgene expression. TRE/Catalase(+/−) mice in a C57BL/6 background were crossed with rd10+/+ mice to generate TRE/Catalase-rd10+/+ mice. Homozygous interphotoreceptor binding protein promoter/reverse tetracycycline transactivator (IRBP/rtTA) transgenic mice were crossed with rd10+/+ mice to generate IRBP/rtTA(+/+)-rd10+/+ mice.
Genotyping of mice
Genotyping was done by PCR of tail DNA using the following primers: human Sod1 (forward:5′-CATCAGCCCTAATCCATCTGA-3′, reverse:5′-CGCGACTAACAATCAAAGTGA-3′); TRE/Gpx4 (forward:5′-CACGCTGTTTTGACCTCC-3′, reverse:5′-GTCTGGCAACTCCTAA-3′); TRE/Catalase (forward:5′-TCTGGAGAAGTGCGGAGATT-3′, reverse: 5′-AGTCAGGGTGGACCTCAGTG-3′), IRBP/rtTA (forward:5′-GTTTACCGATGCCCTTGGAATTGACGAGT-3′, reverse:5′-GATGTGGCGAGATGCTCTTGAAGTCTGGTA-3′). To distinguish sod1−/−, sod1+/− and sod1+/+ mice, a 240-bp fragment was amplified from the wild-type allele with forward, 5′-TGTTCTCCTCTTCCTCATCTCC-3′ and reverse, 5′-ACCCTTTCCAAATCCTCAGC-3′ and a 123-bp fragment was amplified from the wild type allele with forward, 5′-TGAACCAGTTGTGTTGTCAAG-3′ and reverse, 5′-TCCATCACTGGTCACTAGCC-3′. To distinguish homozygous rd10, heterozygous rd10, and wild type mice, the PCR fragment generated with forward, 5′-CTTTCTATTCTCTGTCAGCAAAGC-3′ and reverse, 5′-CATGAGTAGGGTAAACATGGTCTG-3 was digested with Cfol [14].
Mutant rd10 mice with expressions of each transgene
Rd10+/+ mice (Jackson Labs, Bar Harbor, ME) were used in an elaborate mating scheme to generate Sod1(+/−)-TRE/Gpx4(+/−)-rd10+/+ mice, Sod1(+/−)-TRE/Catalase(+/−)-rd10+/+ mice and IRBP/rtTA+/+-rd10+/+ mice. Sod1(+/−)-TRE/Gpx4(+/−)-rd10+/+ mice were crossed with IRBP/rtTA+/+-rd10+/+ mice to generate IRBP/rtTA+/−-rd10+/+ mice that did not carry either the Sod1 or TRE/Gpx4 transgenes, IRBP/rtTA+/−-rd10+/+ mice that carried only the Sod1 transgene, IRBP/rtTA+/−-rd10+/+ mice that carried only the TRE/Gpx4 transgene, or IRBP/rtTA+/−-rd10+/+ mice that carried both the Sod1 and TRE/Gpx4 transgenes. Sod1(+/−)-TRE/Catalase(+/−)-rd10+/+ mice were crossed with IRBP/rtTA+/+-rd10+/+ mice to generate IRBP/rtTA+/−-rd10+/+ mice that did not carry either the Sod1 or TRE/Catalase transgenes, IRBP/rtTA+/−-rd10+/+ mice that carried only the Sod1 transgene, IRBP/rtTA+/−-rd10+/+ mice that carried only the TRE/Catalase transgene, or IRBP/rtTA+/−-rd10+/+ mice that carried both the Sod1 and TRE/Catalase transgenes. Starting at postnatal day (P) 10, mothers of these mice were given 2 mg/ml of doxycycline in their drinking water. At P21, the mice were separated from their mother and given drinking water containing 2 mg/ml of doxycycline. Transgene product was measured by immunoblots of retinal homogenates at P25.
Immunoblots
Whole retinas were dissected and placed in 50 μl of lysis buffer (10mM Tris, pH 7.2, 0.5% Triton X-100, 50 mM NaCl, and 1 mM EDTA) containing a proteinase inhibitor mixture tablet (Roche, Indianapolis, IN). After 3 freeze/thaw cycles and homogenization, samples were microfuged at 14,000xg for 5 minutes at 4°C and the protein concentration of the supernatant was measured using a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). For each sample, 50 μg of protein for the whole retina was resolved by SDS-PAGE and transferred to a nitrocellulose membrane (Hybond-ECL, Amersham Biosciences, Piscataway, NJ). Rabbit polyclonal anti-SOD1 (FL-154, 1:1000,Santa Cruz Biotechnology, Inc.,Santa Cruz, CA) for Sod1−/−-rd10+/+ mice, rabbit polyclonal human specific anti-SOD1 antibody (1:1000, Chemicon International, Temecula, CA), rabbit polyclonal anti-Gpx4 antibody (1:1000, Cayman, Ann Arbor, MI), or rabbit polyclonal anti-human Catalase antibody (1:2000, Athens Research Technology, Athens, GA) were used as primary antibody. The secondary antibody was a horseradish peroxidase (HRP)-coupled goat anti-rabbit IgG (1:2000, Cell Signaling, Danvers, MA). Blots were incubated in SuperSignal Western Pico Lumino/Enhancer solution (Pierce, Rockford, IL) and exposed to X-ray film (Eastman-Kodak, Rochester, NY). To assess loading levels of protein, membranes were stripped and incubated with polyclonal rabbit anti-β-actin antibody (1:5000, Cell Signaling, Danvers, MA) followed by HRP-coupled goat anti-rabbit IgG. A Mitochondrial Isolation Kit for Tissue (Pierce, Rockford, IL) was used according to the manufacturer’s instructions to isolate retinal cytosol and mitochondria for Sod1/Catalase-rd10+/+ mice. For each fraction, 20 μg of protein was run in immunoblots using antibodies for specific human SOD1 or specific human Catalase. A blot was stripped and incubated with mouse monoclonal anti-COX4 (1:5000, Abcam, Cambridge, MA), which is known to localize to mitochondria followed by HRP-coupled anti-mouse IgG (1:2000, Cell Signaling, Danvers, MA).
Assessment of superoxide radicals with hydroethidine
As previously described [5,15], in situ production of superoxide radicals was evaluated using hydroethidine, which in the presence of superoxide radicals is converted to ethidium, which binds DNA and emits red fluorescence at approximately 600nm. Briefly, mice were given two 20 mg/kg intraperitoneal injections 30 minutes apart of freshly prepared hydroethidine (Invitrogen, Carlsbad, CA) and euthanized 18 hours after injection. Eyes were rapidly removed and 10 μm frozen sections were fixed in 4% paraformaldehyde for 20 minutes at RT, rinsed with PBS and counterstained for 5 minutes at RT with the nuclear dye Hoechst 33258 (1:10000; Sigma, St. Louis, MO). After rinsing in PBS, slides were mounted with Aquamount solution and evaluated for fluorescence (excitation: 543nm, emission>590nm) with a LSM 510 Meta confocal microscope. Images were captured using the same exposure time for each section.
ELISA for protein carbonyl content
Retinas were homogenized in lysis buffer and centrifuged at 16,000 x g for 5 minutes at 4°C and the protein concentration of the supernatant was measured using a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Samples were adjusted to 4 mg/ml by dilution with Tris-buffered saline, pH 7.4 (TBS), and protein carbonyl content was determined by ELISA as previously described [7, 14].
Viral vectors
Self complementary hybrid AAV vectors in which a truncated chicken beta actin promoter was used to drive expression of SOD2 (scBNP2.5CBhSod2) or mitochondrial Gpx4 (scBNP2.5CBhmitoGpx4) were prepared. C57BL/6 mice were given a subretinal injection of 2.43 × 109 vp of scBNP2.5CBhSod2, 2.43 × 109 vp of scBNP2.5CBhSod2 + 2.43 × 109 vp of scBNP2.5CBhmitoGpx4 or vehicle (PBS). After 4 weeks the mice were euthanized, retinas were dissected, and H2O2 levels were measured in retinal homogenates.
Measurement of H2O2 levels
Hydrogen peroxide was measured by Amplex Red Hydrogen Peroxide Assay kit (Invitrogen, Carlsbad, CA) using the instructions of the manufacturer. Briefly, retinas were dissected, placed in lysis buffer (10 mM Tris-HCl, pH 7.2, 0.5% Triton X-100, 50 mM NaCl, 1 mM EDTA, with proteinase inhibitor cocktail, Roche, Indianapolis, IN), and freeze-thawed and vortexed three times. After centrifugation, supernatants were diluted with reaction buffer. Each sample was loaded to a 96-well plate well containing 50 μl of reaction buffer with 0.2 units horseradish peroxidase and 0.1 mM Amplex Red reagent. The reaction was incubated for 30 minutes in dark and the absorption was measured at 560 nm. The concentration of hydrogen peroxide in each sample was calculated from a standard curve generated using a stock solution of hydrogen peroxide.
Recording of electroretinograms (ERGs)
An Espion ERG Diagnosys machine (Diagnosys LLC, Littleton, MA) was used to record ERGs as previously described [7, 8, 16, 17]. The mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (100mg/kg body weight) and xylazine (5mg/kg body weight). Pupils were dilated with Midrin P containing 0.5% tropicamide and 0.5% phenylephrine, hydrochloride (Santen Pharmaceutical Co., Osaka, Japan). The mice were placed on a pad heated to 39°C and platinum loop electrodes were placed on each cornea after application of Gonioscopic prism solution (Alcon Labs, Fort Worth, TX). A reference electrode was placed subcutaneously in the anterior scalp between the eyes and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a ganzfeld bowl illuminator that ensured equal illumination of the eyes. Recordings for both eyes were made simultaneously with electrical impedance balanced. Low background photopic ERGs were recorded at 1.48 log cd-s/m2 under a 10 cd/m2 background. Sixty photopic measurements were taken and the average value was recorded.
Statistical analysis
Statistical comparisons were done using Tukey-Kramer’s test for multiple comparisons and unpaired Student’s t-test for two comparisons. Differences test for multiple comparisons were judged statistically significant at P<0.05 or P<0.01.
Results
Deficiency of superoxide dismutase 1 (SOD1) increases superoxide radicals and oxidative damage in the retinas of rd10+/+ mice and accelerates loss of cone function
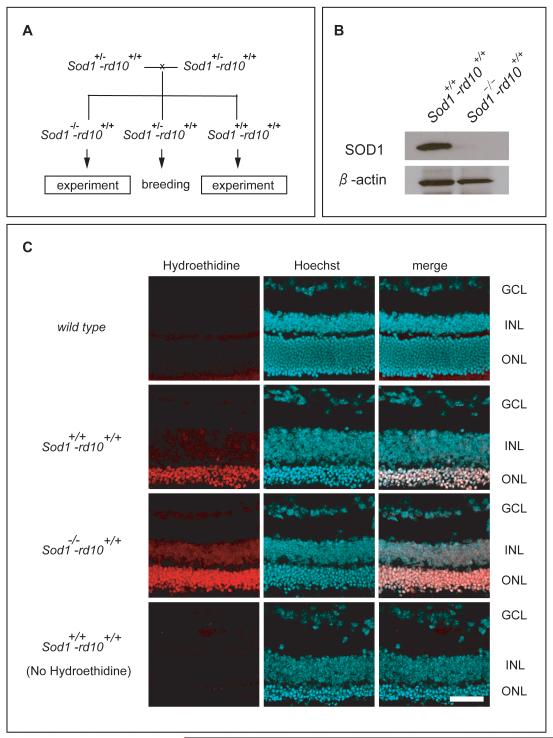
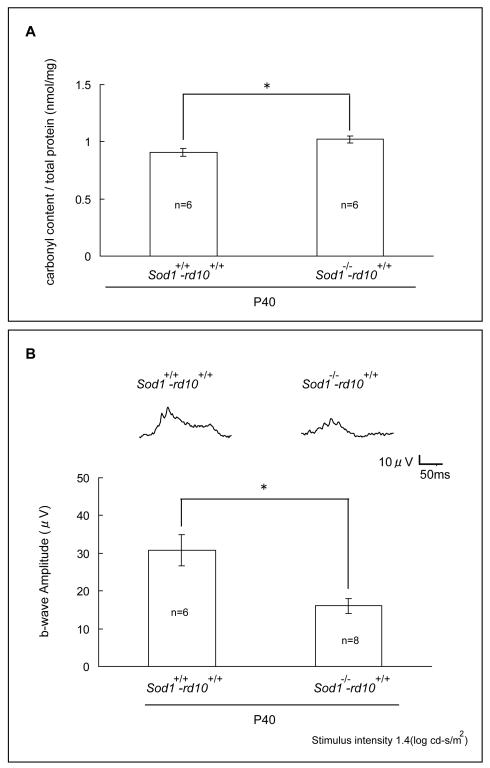
Rd10+/+ mice are homozygous for a mutation in rod phosphodiesterase that causes death of rod photoreceptors between P18 and P35 followed by gradual death of cones from oxidative damage [8, 18]. To determine the effect of deficiency of SOD1 in rd10+/+ mice, a mating scheme (Figure 1A) was devised to generate rd10+/+ mice wild type at the Sod1 allele (Sod1+/+-rd10+/+ mice), Sod1+/−-rd10+/+ mice, and rd10+/+ mice deficient in SOD1 (Sod1−/−-rd10+/+ mice). Immunoblots confirmed Sod1−/−-rd10+/+ mice lacked SOD1 (Figure 1B). Hydroethidine allows visualization of superoxide radicals because in their presence it is converted to ethidium which binds DNA and fluoresces [19]. Eighteen hours after intravenous injection of hydroethidine, there was minimal fluorescence in the retinas of wild type mice (Figure 1C, top row), moderate fluorescence primarily in the remaining outer nuclear layer of the retinas of Sod1+/+-rd10+/+ mice (second row), and strong fluorescence in the retinas of Sod1−/−-rd10+/+ mice (third row). Without injection of hydroethidine, Sod1+/+-rd10+/+ mice showed no fluorescence (Figure 1C, bottom row). At P40, levels of carbonyl adducts on proteins were significantly higher in the retinas of Sod1−/−-rd10+/+ mice compared to Sod1+/+-rd10+/+ mice (Figure 2A). Low background photopic ERGs at P40 showed substantially better waveforms and significantly higher mean photopic b-wave amplitude for Sod1+/+-rd10+/+ mice compared to Sod1−/−-rd10+/+ mice (Figure 2B).
Figure 1. Deficiency of superoxide dismutase 1 (SOD1) increases superoxide radicals in the retinas of rd10+/+ mice.
(A) Heterozygous Sod1 knockout mice that carried two mutant rd10 alleles (Sod1+/−-rd10+/+ mice) were crossed to generate rd10+/+ mice wild type at the Sod1 allele (Sod1+/+-rd10+/+ mice), Sod1+/−-rd10+/+ mice, and rd10+/+ mice deficient in SOD1 (Sod1−/−-rd10+/+ mice).
(B) Immunoblots of retinal homogenates from postnatal day (P) 25 Sod1+/+-rd10+/+ and Sod1−/−-rd10+/+ mice showed a strong band for SOD1 in the former and no detectable band for SOD1 in the latter. Stripping and reprobing the blots with an antibody directed against β-actin showed that loading was equivalent.
(C) At P25, wild type mice (n=4), Sod1+/+-rd10+/+ mice (n=4), and Sod1−/−-rd10+/+ mice (n=4) were given two intraperitoneal injections of 20 mg/kg of hydroethidine and after 18 hours they were euthanized and ocular sections were examined by confocal microscopy. There was minimal fluorescence in the retinas of wild type mice (top row), moderate fluorescence primarily in the remaining outer nuclear layer of the retinas of Sod1+/+-rd10+/+ mice (second row), and strong fluorescence in the retinas of Sod1−/−-rd10+/+ mice (third row). Without injection of hydroethidine, Sod1+/+-rd10+/+ mice showed no fluorescence (bottom row). Scale bar=50 μm
Figure 2. Deficiency of superoxide dismutase 1 (SOD1) significantly increases protein carbonyl content and accelerates loss of retinal function of postnatal day (P) 40 rd10+/+mice.
At postnatal day (P) 40, Sod1+/+-rd10+/+ mice and Sod1−/−-rd10+/+ mice had low background photopic ERGs as described in Methods and were then euthanized and protein carbonyl content in retinal homogenates was measured by ELISA.
(A) The mean (±SEM) carbonyl content per mg retinal protein was significantly greater in Sod1−/−-rd10+/+ mice compared to Sod1+/+-rd10+/+ mice (*p<0.05 by unpaired Student’s t-test).
(B) Representative waveforms are shown for each group and illustrate a substantially better waveform for Sod1+/+-rd10+/+ mice compared to Sod1−/−-rd10+/+ mice. The bars show mean (± SEM) photopic b-wave amplitude, which was significantly higher for Sod1+/+-rd10+/+ mice compared to Sod1−/−-rd10+/+ mice (*p<0.005 by unpaired Student’s t-test).
Co-expression of SOD1 and cytoplasmic Gpx4 in photoreceptors significantly reduces retinal carbonyl content and improves cone function in rd10+/+ mice
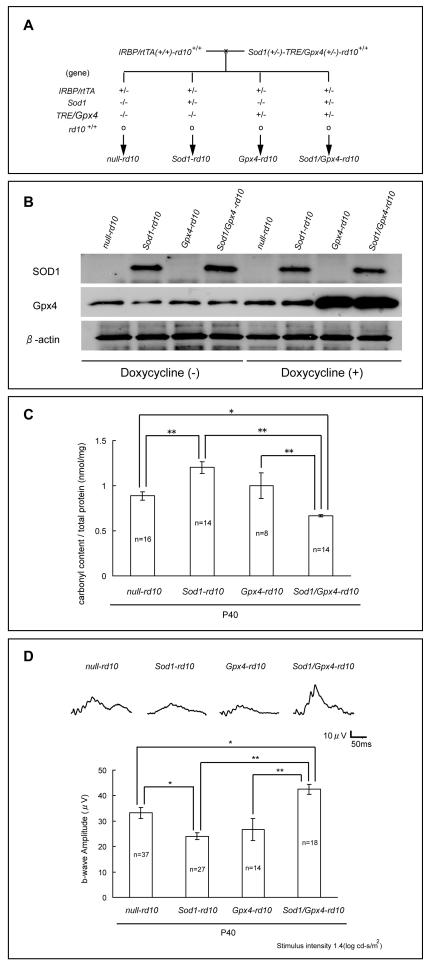
To test the effects of overexpression of SOD1 and Gpx4 on the oxidative damage that occurs in cones of rd10+/+ mice, a crossing scheme was used to generate 4 groups of offspring, null-rd10, Sod1-rd10, Gpx4-rd10, and Sod1/Gpx4-rd10 mice (Figure 3A). Immunoblots of retinal homogenates showed strong expression of human SOD1 in Sod1-rd10 and Sod1/Gpx4-rd10 mice (Figure 3B). Background levels of murine Gpx4 were seen in all mice, but when Gpx4-rd10+/+ or Sod1/Gpx4-rd10+/+ mice were treated with doxycycline, they showed a substantial increase in Gpx4. In doxycycline-treated P40 mice, protein carbonyl content was significantly greater in Sod1-rd10 mice compared to null-rd10 or Sod1/Gpx4-rd10 mice and was significantly less in Sod1/Gpx4-rd10 mice compared to null-rd10, Sod1-rd10 or Gpx4-rd10 mice (Figure 3C). Low background photopic ERGs showed mean photopic b-wave amplitudes that were significantly higher in Sod1/Gpx4-rd10 mice compared to null-rd10, Sod1-rd10, or Gpx4-rd10 mice, and significantly lower in Sod1-rd10 mice than in null-rd10 mice (Figure 3D).
Figure 3. Co-expression of SOD1 and cytoplasmic Gpx4 in photoreceptors significantly reduces carbonyl content and improves cone function at postnatal day (P) 40 in rd10+/+ mice.
(A) Transgenic mice carrying a β-actin promoter/human Sod1 transgene or murine cytoplasmic Gpx4 coupled to the tetracycline response element (TRE) were crossed with rd10+/+ mice. Multiple crosses were done to generate Sod1(+/−)-TRE/Gpx4(+/−)-rd10+/+ mice and homozygous interphotoreceptor retinol binding protein promoter/reverse tetracycline transactivator-rd10+/+ mice (IRBP/rtTA(+/+)-rd10+/+ mice). These two types of mice were crossed to yield 4 groups of offspring, null-rd10, Sod1-rd10, Gpx4-rd10, and Sod1/Gpx4-rd10 mice for which the genotypes are shown.
(B) Null-rd10, Sod1-rd10, Gpx4-rd10, Sod1/Gpx4-rd10 mice were given normal drinking water or water supplemented with 2 mg/ml of doxycycline between postnatal day (P) 10 and P25. Immunoblots of retinal homogenates showed strong expression of human SOD1 in Sod1-rd10 and Sod1/Gpx4-rd10 mice treated with and without doxycycline. Background levels of murine Gpx4 were seen in all mice, but when treated with doxycycline, only Gpx4-rd10+/+ and Sod1/Gpx4-rd10+/+ mice showed a substantial increase in Gpx4. Stripping and reprobing of blots with an antibody directed against β-actin showed that loading was equivalent.
(C) Starting at P10, the mothers of null-rd10, Sod1-rd10, Gpx4-rd10+/+ and Sod1/Gpx4-rd10 mice and after weaning, the mice themselves were given 2 mg/kg of doxycycline in their drinking water. At P40, protein carbonyl content was measured in retinal homogenates by ELISA and the mean (±SEM) carbonyl content per mg retinal protein was significantly greater in Sod1-rd10 mice compared to null-rd10 or Sod1/Gpx4-rd10 mice. Protein carbonyl content was significantly less in Sod1/Gpx4-rd10 mice compared to null-rd10, Sod1-rd10 or Gpx4-rd10 mice.
(D) Low background photopic ERGs were done at P40 in doxycycline-treated null-rd10, Sod1-rd10, Gpx4-rd10 and Sod1/Gpx4-rd10 mice and representative waveforms were substantially better in Sod1/Gpx4-rd10 mice compared to null-rd10, Sod1-rd10, or Gpx4-rd10 mice. The bars show mean (± SEM) photopic b-wave amplitude, which was significantly higher for Sod1/Gpx4-rd10 mice compared to the other 3 types of mice, and was significantly lower for Sod1-rd10 mice compared to null-rd10 mice.
*p<0.05, **p<0.01 by Tukey-Kramer test
Co-expression of SOD1 and mitochondrial-targeted Catalase in photoreceptors does not preserve cone cell function in rd10+/+ mice
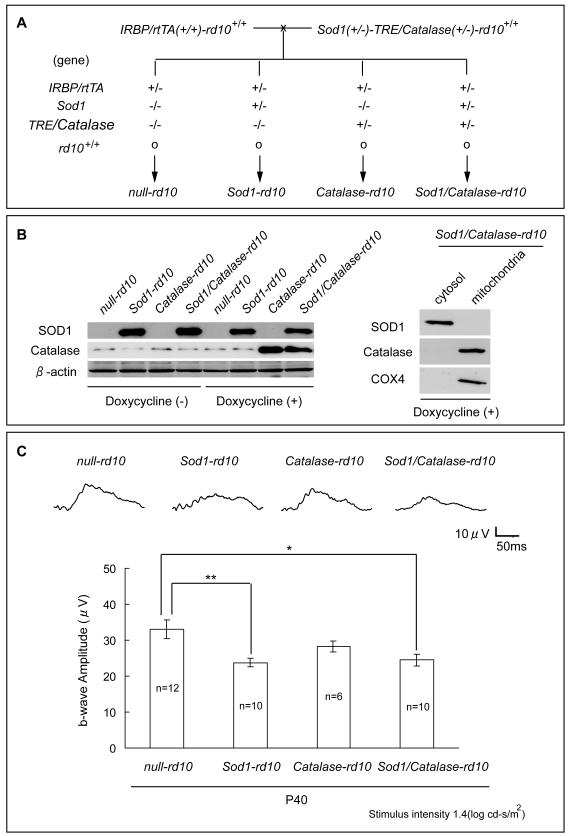
Increased expression of SOD2 increases oxidative stress and promotes cone cell death in rd10+/+ mice, but when SOD2 is co-expressed with Catalase that is targeted to mitochondria, cone function is improved compared to rd10+/+ mice with wild type levels of SOD2 and Catalase [20]. We sought to determine if Catalase targeted to mitochondria reversed the damaging effects of over-expression of SOD1. A mating scheme was designed to generate 4 groups of offspring, null-rd10, Sod1-rd10, Catalase-rd10, and Sod1/Catalase-rd10 mice (Figure 4A). Immunoblots of retinal homogenates showed strong expression of human SOD1 in Sod1-rd10 and Sod1/Catalase-rd10 and strong expression of Catalase in doxycycline-treated Catalase-rd10 and Sod1/Catalase-rd10 mice (Figure 4B). Immunoblots of cytosolic and mitochondrial fractions of retinal homogenates showed that only the cytosolic fraction showed a substantial increase in SOD1 and only the mitochondrial fraction showed a substantial increase in Catalase and COX4, which is known to localize to mitochondria. Low background photopic ERGs at P40 showed a significant reduction in mean photopic b-wave amplitude in Sod1-rd10 mice and Sod1/Catalase-rd10 mice compared to null-rd10 mice (Figure 4C).
Figure 4. Co-expression of SOD1 and mitochondrial Catalase in photoreceptors does not preserve cone cell function at postnatal day (P) 40 in rd10+/+ mice.
(A) Transgenic mice carrying a β-actin promoter/human Sod1 transgene or human Catalase targeted to mitochondria coupled to the tetracycline response element (TRE) were crossed with rd10+/+ mice. Multiple crosses were done to generate Sod1(+/−)-TRE/Catalase(+/−)-rd10+/+ mice and homozygous interphotoreceptor retinol binding protein promoter/reverse tetracycline transactivator-rd10+/+ mice (IRBP/rtTA(+/+)-rd10+/+ mice). These two types of mice were crossed to yield 4 groups of offspring, null-rd10, Sod1-rd10, Catalase-rd10, and Sod1/Catalase-rd10 mice for which the genotypes are shown.
(B) Null-rd10, Sod1-rd10, Catalase-rd10, Sod1/Catalase-rd10 mice were given normal drinking water or water supplemented with 2 mg/ml of doxycycline between postnatal day (P) 10 and P25. Immunoblots of retinal homogenates showed strong expression of human SOD1 in Sod1-rd10 and Sod1/Catalase-rd10 mice treated with and without doxycycline. Catalase-rd10 and Sod1/Catalase-rd10 showed strong bands for Catalase when treated with doxycycline. Stripping and reprobing of blots with an antibody directed against β-actin showed that loading was equivalent. In immunoblots of cytosolic and mitochondrial fractions of retinal homogenates, only the cytosolic fraction showed a substantial increase in SOD1 and only mitochondrial fraction showed a substantial increase in Catalase and COX4, which is known to localize to mitochondria.
(C) Low background photopic ERGs were done at P40 and representative waveforms were substantially better in null-rd10 mice compared to Sod1-rd10 or Sod1/Catalase-rd10 mice. The mean (± SEM) photopic b-wave amplitude was significantly lower for Sod1-rd10 mice and Sod1/Catalase-rd10 mice compared to null-rd10 mice (*p<0.05, **p<0.01 by Tukey-Kramer test).
Increased expression of SOD2, but not SOD2 + mitochondrial Gpx4 increases H2O2 in the retina
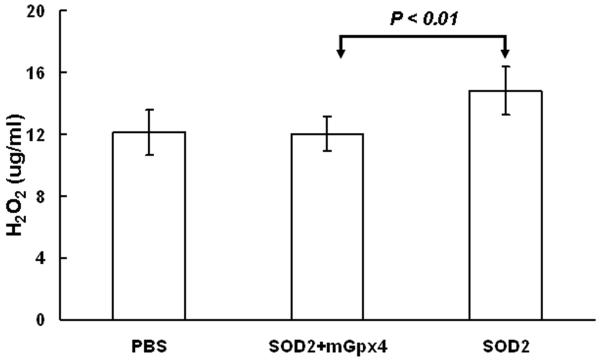
C57BL/6 mice were given a subretinal injection of 2.43 × 109 viral particles (vp) of a self complementary hybrid adeno-associated viral vector that expresses SOD2 in one eye or a subretinal injection of 2.43 × 109 vp of each of two vectors, one that expresses SOD2 and one that expresses mitochondrial Gpx4. Separate mice were given a subretinal injection of vehicle (PBS). After 4 weeks, H2O2 level was measured in retinal homogenates and was significantly higher in retinas from eyes overexpressing SOD2 compared to those overexpression both SOD2 and mitochondrial Gpx4 or controls with normal expression levels (Figure 5).
Figure 5. Retinas with high expression of SOD2 have signficiantly higher Levels of H2O2 than retinas with high expression of SOD2 + mitochondrial Gpx4 or those with normal expression levels.
C57BL/6 mice were given a subretinal injection of 2.43 × 109 viral particles of scBNP2.5CBhSod2 in one eye and 2.43 × 109 vp of scBNP2.5CBhSod2 + 2.43 × 109 vp of scBNP2.5CBhmitoGpx4 in the other eye and separate mice were given a subretinal injection of vehicle (PBS). After 4 weeks the mice were euthanized, the retinas were dissected, and H2O2 was measured in retinal homogenates as described in Methods. The mean level of H2O2 (n=5 in each group) was significantly higher by unpaired t-test in retinas from eyes given a subretinal injection of scBNP2.5CBhSod2 compared to those given a subretinal injection of scBNP2.5CBhSod2 + scBNP2.5CBhmitoGpx4.
Discussion
In this study, we have shown that SOD1 is an important component of the antioxidant defense system in cone photoreceptors, because compared to mice with RP that have normal levels of SOD1, those with RP that are deficient in SOD1 show increased oxidative damage and accelerated loss of cone cell function. It follows that increased expression of this component of the antioxidant defense system might provide benefit in RP; however, this was not the case. Instead mice with RP and overexpression of SOD1 also showed increased oxidative damage and more rapid loss of cone function. This situation was changed when SOD1 was co-expressed with Gpx4; oxidative damage was reduced and cone function was preserved compared to RP mice with unaltered expression of SOD1 and Gpx4. In contrast, co-expression of SOD1 with catalase targeted to mitochondria accelerated loss of cone function in mice with RP. These data indicate that despite its role as a critical member of the antioxidant defense system, overexpression of SOD1 is deleterious unless it is accompanied by increased expression of a peroxide detoxifying enzyme that is expressed in the same cellular compartment.
These data complement and extend a previous study in which we found that overexpression of SOD2 accelerated cone cell damage in mice with RP, but when co-expressed with catalase targeted to the mitochondria, there was strong protection of cones [20]. Increased expression of Gpx4 or mitochondrial catalase alone, was not helpful, but did not accelerate loss of cone function. Thus, it is important to target the SOD system, but it must be combined with increased expression of an enzyme that works in tandem and is in close proximity.
The consequences of overexpression of SOD1 or 2 are not the same in all settings. In Drosophila, if genetic background is identical, flies with increased expression of SOD1 or SOD2 have greater lifespan than those with normal levels [21, 22]. In diabetic mice, overexpression of SOD2 protects from hyperglycemia-induced retinal damage [23] and overexpression of SOD1 reduces kidney damage [24, 25]. Increased levels of SOD2 reduce memory loss in a mouse model of Alzheimer’s disease [26]. Conversely, in a mouse model of ataxia-telangiectasia, overexpression of SOD1 aggrevates the phenotype [27] and in fat-fed mice, increased levels of SOD1 exacerbate atherosclerosis [28]. The Sod1 gene is located on chromosome 21 resulting in overexpression of SOD1 in Down’s syndrome and there is substantial evidence that this increases oxidative stress and contributes to the pathogenesis of the disease (for review, see [29]). Thus, the consequences of increased SOD1 or SOD2 activity seem to vary depending upon the tissue and the disease process.
One possible explanation as to why different tissues react differently to overexpression of SOD1 or 2 may be that they differ in their ability to increase activity of endogenous peroxide detoxifying activity. Increased levels of SOD2 in the retina resulted in high levels of hydrogen peroxide, but levels were normal when SOD2 and mitochondrial Gpx4 were co-expressed. These data suggest that modulation of endogenous peroxide detoxifying enzymes in the retina is either limited or slow, and if levels of an SOD are altered it is prudent to also alter levels of a peroxide detoxifying enzyme in the same cellular compartment. In other tissues, there may be more modulation or greater tolerance for temporary imbalance that is ultimately compensated. It is possible that effects in the retina are influenced by the low percentage of cones and their rapid degeneration in mice with primary rod cell degenerations. The severity and rapidity of the oxidative damage may preclude any modulation of peroxide detoxifying enzymes to handle the increased peroxide load caused by an increase in SOD. Since pigs and humans have a substantially higher percentage of cones and much slower degeneration of cones in the setting of RP compared to rodents, it is possible that there would be sufficient time for modulation of peroxide detoxifying enzymes to re-establish balance leading to an overall enhancement of the antioxidant defense system. This hypothesis should be tested in pigs before gene transfer of an SOD alone is considered for patients with RP.
Another important question raised by our studies is what is the relative value in RP of enhancing the antioxidant capacity in the mitochondrial compartment compared to the cytoplasmic compartment? Our data (present study and [20]) suggest that protection in either compartment provides benefit, but would there be further benefit to simultaneously enhancing protection in both compartments? Thus, our studies have shown that gene transfer to enhance the endogenous antioxidant defense system is a potentially useful strategy, but additional studies are needed to determine the importance of targeting multiple cellular compartments and to determine the capacity of the endogenous system to compensate for imbalances before this strategy can be translated into a treatment for patients with RP.
Acknowledgements
Supported by R01 EY05951 (PAC) and P01 AG01751 (PSR) from NIH and a gift from Dr. and Mrs. William Lake. Shinichi Usui is a Bausch and Lomb Japan Vitreoretinal Research Fellow and was supported by The Osaka Medical Research Foundation for Incurable Diseases. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest. Ophthalmol. Vis. Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- 2.Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- 3.Yu DY, Cringle SJ, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 4.Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A, Miki K, Iwase T, Lu L, Campochiaro PA. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 2009;110:1028–1037. doi: 10.1111/j.1471-4159.2009.06195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komeima K, Usui S, Shen J, Rogers BS, Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008;45:905–912. doi: 10.1016/j.freeradbiomed.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J, Yan X, Dong A, Petters RM, Peng Y-W, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 7.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 9.Dong A, Shen J, Krause M, Akiyama H, Hackett SF, Lai H, Campochiaro PA. Superoxide dismutase 1 protects retinal cells from oxidative damage. J. Cell. Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- 10.Mills GC. Hemoglobin catabolism I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J. Biol. Chem. 1957;229:189–197. [PubMed] [Google Scholar]
- 11.Flohe L, Zimmermann R. The role of GSH peroxidase in protecting the membrane of rat liver mitochondria. Biochem. Biophys. Acta. 1970;223:210–213. doi: 10.1016/0005-2728(70)90149-0. [DOI] [PubMed] [Google Scholar]
- 12.Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Oveson BC, Jo YJ, Lauer T, Usui S, Komeima K, Xie B, Campochiaro PA. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid. Redox. Signal. 2008;11:715–724. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47:624–633. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2008;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 16.Okoye G, Zimmer J, Sung J, P. G, Deering T, Nambu N, Hackett SF, Melia M, Esumi N, Zack DJ, Campochiaro PA. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J. Neuosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno S, Pease ME, Wersinger DMB, Masuda T, Vinores SA, Licht T, Zack DJ, Quigley H, Keshet E, Campochiaro PA. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J. Cell. Physiol. 2008;217:13–22. doi: 10.1002/jcp.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neuosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usui S, Komeima K, Lee SY, Jo Y-J, Ueno S, Rogers BS, Wu Z, Shen J, Lu L, Oveson BC, Rabinovitch PS, Campochiaro PA. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Molec. Ther. 2009;17:778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Molitor J, Tower J. Effects of simultaneous over-expression of Cu/ZnSOD and MnSOD on Drosophila melanogaster life span. Mech. Ageing Devel. 2004;125:341–349. doi: 10.1016/j.mad.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic. Biol. Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Craven PA, Melhem MF, Phillips SL, DeRubertis FR. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes. 2001;50:2114–2125. doi: 10.2337/diabetes.50.9.2114. [DOI] [PubMed] [Google Scholar]
- 25.DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase. Evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- 26.Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hyppocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Nat. Acad. Sci. USA. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakov P, Rotman G, Lotem J, Elson A, Shiloh Y, Groner Y. Elevated Cu/Zn-SOD exacerbates radiation sensitivity and hematopoietic abnormalities of Atm-deficient mice. EMBO J. 2001;20:1538–1546. doi: 10.1093/emboj/20.7.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tribble DL, Gong EL, Leeuwenburgh C, Heinecke JW, Carlson EL, Verstuyft JG, Epstein CJ. Fatty streak formation in fat-fed mice expressing human copper-zinc superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1997;17:1734–1740. doi: 10.1161/01.atv.17.9.1734. [DOI] [PubMed] [Google Scholar]
- 29.Antila E, Westermarck T. On the etiopathogenesis and terapy of Down syndrome. Int. J. Dev. Biol. 1989;33:183–189. [PubMed] [Google Scholar]