Abstract
Research on the pathogenesis of asthma has concentrated on initial stimuli, genetic susceptibilities, adaptive immune responses, and end-organ alterations (particularly in airway mucous cells and smooth muscle) as critical steps leading to disease. Recent evidence indicates that the innate immune cell response to respiratory viruses also contributes to the development of inflammatory airway disease. We further develop this concept by raising the issue that the interaction between host airway epithelial cells and respiratory viruses is another aspect of innate immunity that is also a critical determinant of asthma. We also introduce a rationale for how antiviral performance at the epithelial cell level might be improved to prevent acute infectious illness and chronic inflammatory disease caused by respiratory viruses.
Introduction
One of the major tasks facing medical research is to define the pathogenesis of chronic inflammatory diseases. In the case of asthma, the approach to understanding chronic inflammation has implicated a broad array of cell types, cell-cell interactions, and cellular products. One leading scheme for integrating this information is based on the classification of the adaptive immune system, and especially the responses of T helper (Th) cells into T helper type 1 (Th1) cells that mediate delayed-type hypersensitivity reactions and selectively produce interleukin (IL)-2 and interferon (IFN)-γ, and Th2 cells that promote B-cell dependent humoral immunity and selectively produce IL-4, IL-5, and IL-13. Under this scheme, an up-regulated Th2 and perhaps a down-regulated Th1 response is thought to drive the development of asthma. The newer contributions of Th17 (IL-17-producing) and Treg (IL-10- and TGF-β-producing) subsets of T cells are also proposed to contribute to inflammatory airway disease by skewing the system towards a Th2 response [1, 2].
In general, the Th2 hypothesis is based on observations of the response to allergen challenge in mouse models of asthma and in humans with allergic asthma [3, 4]. However, it has been pointed out that a Th2-biased response does not account for the epidemiological link between respiratory viral infection and the subsequent development of asthma [5]. Indeed, the broader issue of the relationship between acute viral infection and chronic inflammatory disease remains uncertain. In an effort to understand this issue, we identified the likely steps leading from viral infection to inflammatory disease using respiratory viral infection and asthma as a template for this process (as outlined in Fig. 1). Here, we review three major advances that lead to a substantial revision of this virus-disease connection. First, we develop the experimental and clinical evidence that the link between acute infection and chronic disease of the airway unexpectedly depends on immune cells of the innate rather than the adaptive immune system; second, we extend this concept to the airway epithelial cell and the proposal that high-level viral replication at this cellular site is required to trigger the innate immune cell activation that in turn drives asthma; and third, we introduce strategies that could improve antiviral defense at the airway epithelial cell level and thereby help to prevent acute infectious illness and chronic asthmatic disease. We conclude by showing how these advances provide for a new virus-disease paradigm.
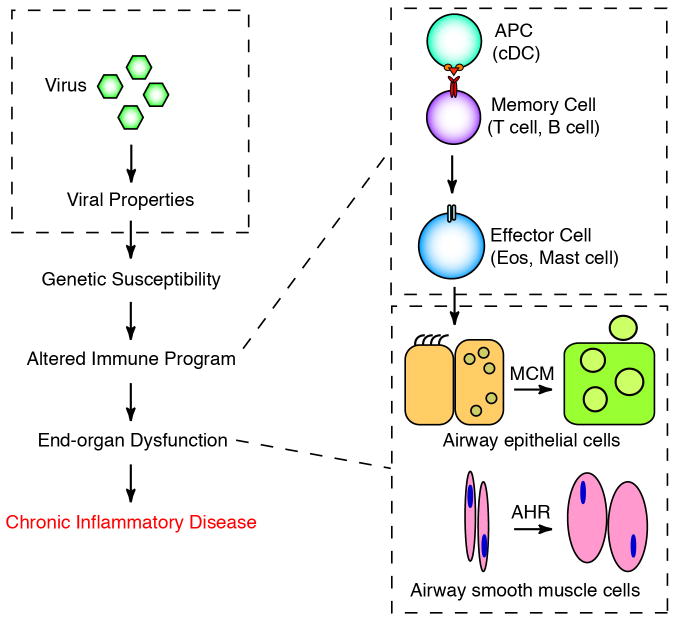
Fig. 1. Conventional scheme for how respiratory viruses trigger chronic asthma.
Critical steps leading from initial respiratory virus infection to chronic inflammatory disease include viral properties, genetic susceptibility, altered immune program, and end-organ dysfunction. The immune program was felt to depend on the development of an adaptive immune response involving antigen-presenting cells (especially conventional dendritic cells (cDCs), memory cells (especially T cells and B cells), and effector cells (especially eosinophils and mast cells). End-organ dysfunction involves a transition from epithelial precursor cells such ciliated cells and Clara cells to mucous cells (mucous cell metaplasia, MCM) and increased mass and contractility of airway smooth muscle cells (airway hyperreactivity, AHR). Modified from ref. [5].
Introducing the innate immune cells for chronic postviral disease
One of the initial objectives for understanding the role of respiratory viruses in the pathogenesis of asthma was to define the immune program for postviral disease. This goal required a high-fidelity experimental model of postviral asthma in humans, where respiratory syncytial virus (RSV) is implicated. However, we recognized (and confirmed) the shortcomings of using RSV for an experimental model in mice [6], and therefore substituted the corresponding mouse paramyxovirus, Sendai virus (SeV). The change in approach provided for cardinal features of human disease, including acute bronchiolitis followed by chronic (perhaps lifelong) airway inflammation, mucus overproduction, and hyperreactivity that depend on genetic susceptibility [7-9]. We used this model to identify a new immune axis that translates viral infection into chronic airway disease. When the acute lung disease appears in this model (at 3 weeks after viral inoculation), it depends on an immune response that features expression and activation of the high-affinity IgE receptor (FcεRI) on conventional lung dendritic cells (cDCs) and consequent CCL28 production to recruit IL-13-producing CD4+ T cells to the airways [10, 11]. In addition, when the chronic lung disease develops fully (at 7 weeks after inoculation), it is driven instead by an innate immune response that relies on invariant natural killer T (iNKT) cells that are programmed to activate macrophages to produce IL-13 [12, 13]. The interaction between iNKT cells and macrophages depends on contact between the semi-invariant Vα14Jα18-TCR on lung iNKT cells and the oligomorphic MHC-like protein CD1d on macrophages as well as NKT cell production of IL-13 that binds to the IL-13 receptor (IL-13R) on the macrophage. This innate immune axis is also activated in the lungs of humans with severe asthma or COPD based on detection of increased numbers of iNKT cells and alternatively-activated (M2) IL-13-producing macrophages in the lung [12, 14-16]. Together, the findings identify an adaptive immune response that mediates acute disease and an innate immune response that drives chronic obstructive lung disease in experimental and clinical settings (as summarized in Fig. 2).
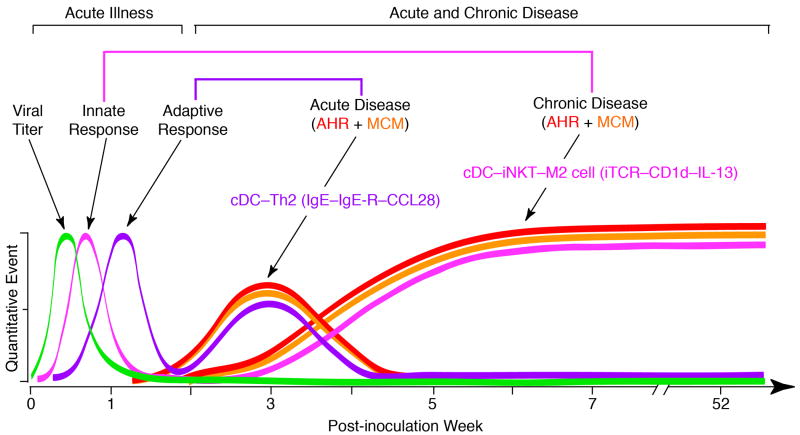
Fig. 2. Time course for immune events after respiratory viral infection.
Viral replication leads to increased viral levels followed by innate and adaptive immune responses that eventually clear virus to noninfectious levels. The acute illness is followed by the development of acute and chronic disease that are both characterized by airway hyperreactivity (AHR) and mucous cell metaplasia (MCM). Acute disease is manifest at 3 weeks after viral inoculation and is driven by an adaptive immune response that includes cDCs and Th2 cells with IgE–high-affinity IgE receptor interaction and CCL28 production. Chronic disease is fully manifest at 7 weeks after inoculation and is driven by an innate immune response that includes cDCs, iNKT cells, and M2 cells with semi-invariant TCR–CD1d interaction and IL-13 production. Modified from ref. [5].
The viral and immune mechanisms for postviral asthma (for SeV and related viruses) are still under study, but even at this stage, we recognize that a critical element of the experimental model is the severity of acute infection. As discussed further in the next section, this requirement for severity may be the reason why others missed the effect of SeV and other viruses in experimental models and in humans with milder degrees of acute illness. In contrast, we tailored our experimental approach to match clinical observations from our group and others that the children with the most severe manifestations of viral bronchiolitis are the ones that are marked for the subsequent development of chronic asthma [17-19]. A natural corollary of this issue is that the severity of illness often depends on the level of virus and in turn on the flux between viral replication and clearance in the primary host cell. Since this cell is most often the airway epithelial cell (and within this cell population, it is often the ciliated mucosal epithelial cell) [20], this additional component of innate mucosal immunity was a prime candidate for further study.
Moving upstream to the airway epithelial cell
Despite being the primary home to respiratory viruses (or perhaps because of it), airway epithelial cells contain a potent antiviral system based mainly on IFN production and subsequent expression of IFN-stimulated genes (ISGs). An abbreviated scheme for this complex IFN-based network features a master regulator known as STAT1 (as diagrammed in Fig. 3). If this network is genetically defective (e.g., due to STAT1 deficiency) in mice or man, the host often succumbs to lethal viral infection [21]. Moreover, the magnitude of this antiviral IFN response, as judged by the level of IFN production and signaling, appears to correlate with the level of protection against infection. For example, in a study of peripheral blood mononuclear cells (PBMCs) obtained at the time of birth, it was found that the level of IFN-γ production in response to RSV infection can predict the likelihood of respiratory tract infection in the first year of life [22]. In this case, the higher the IFN production, then the less likely the child is to develop an infection, and in turn, the less likely to develop serious respiratory or wheezing illnesses. Similarly, decreased induction of IFN-α production was found in RSV-inoculated PBMCs that were isolated from children and adults with asthma versus normal control subjects [23]. A comparable deficiency in IFN-γ production was detected in human rhinovirus (HRV)-inoculated PBMCs isolated from asthmatics compared to normal controls [24]. Others have also reported that circulating plasmacytoid dendritic cells (pDCs) from asthmatics produce less IFN-α than cells from normals after inoculation with influenza A virus (IAV) [25].
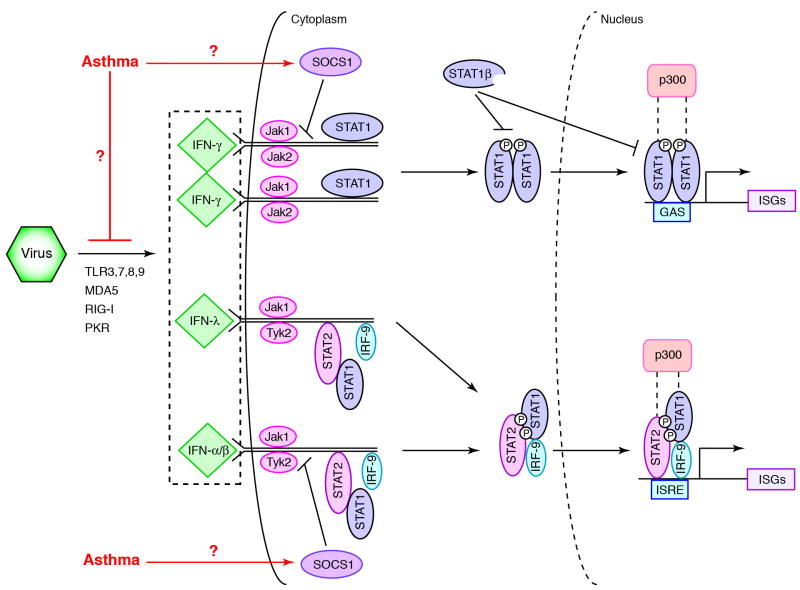
Fig. 3. Scheme for virus-induced IFN production and signal transduction.
Viral replication causes Toll-like receptor (TLR) 3, 7, 8, and 9, melanoma differentiation-associated gene 5 (MDA5), retinoic acid-inducible gene I (RIG-I), and protein kinase R (PKR)-dependent production of three types of IFNs that trigger IFN signaling. IFN-γ signaling begins when IFN-γ dimer binds to its heterodimeric receptor (IFNGR) and triggers activation of Jak1 and Jak2 tyrosine kinases and consequent receptor phosphorylation. This step enables recruitment of STAT1 and subsequent release of the phosphorylated STAT1-homodimer. Activated Stat1 homodimer translocates to the nucleus where it binds to the gamma-activation site (GAS) and activates (in concert with p300) transcription of interferon-stimulated genes (ISGs). IFN-λ- and IFN-α/β-driven gene expression is initiated by activation of the IFN-λ receptor (IL-10R2/IL-28AR)) or IFN-α/β receptor (IFNAR) and subsequent activation of IL10R2- or IFNAR1-associated Tyk2 and IFNLR1- or IFNAR2-associated Jak1 with consequent IL-10R2 or IFNAR1 phosphorylation and recruitment of STAT2. Phosphorylation of Stat2 enables recruitment of Stat1 and release of the phosphorylated STAT1-STAT2-heterodimer. This heterodimer in concert with IRF-9 forms a complex that binds to the interferon stimulated response element (ISRE) and increases ISG transcription. SOCS1 and STAT1β (a truncated form of STAT1) decrease signaling as indicated. Asthma may down-regulate these pathways by direct or indirect actions on IFN production or signaling as indicated. Modified from ref. [33].
This concept that there is a deficiency in IFN-dependent control of respiratory viruses in asthma has been extended to the study of airway epithelial cells in experimental models and in humans. In particular, a deficiency of IFN signaling in airway epithelial cells will likely compromise host defense against respiratory viruses and promote the subsequent development of experimental asthma in mouse models [26-33]. These findings imply that a deficiency in IFN-dependent antiviral defense at the level of airway epithelial cells might also be found in humans with more severe infections and subsequent asthma. In support of this possibility, studies from the labs of Donna Davies and Sebastian Johnston show a deficiency in IFN-β and IFN-λ, production in response to HRV inoculation in airway epithelial cells cultured from asthmatic versus normal subjects [34, 35]. A recent follow-up study indicated that the bronchial epithelial cell response to IFN-β in asthma was no different from normal [36], suggesting that the defect lies in IFN production rather than signaling. However, others reported that asthma is also characterized by a genetic increase in the levels of an endogenous inhibitor of IFN signaling known as SOCS1 [37], suggesting that a signaling defect may also be found in some patients with asthma. Meanwhile, there is evidence that control of viral replication and production of IFN may also be defective in airway epithelial cells from patients with other types of inflammatory airway disease, e.g., COPD and cystic fibrosis, but any possible abnormalities or underlying mechanisms still need to be better defined [38-40].
Even in the case of asthma, not all reports agree on a defect in IFN-dependent control of viral replication. In a study from James Gern's lab, airway epithelial cells from asthmatic and control subjects exhibited no difference in viral levels after inoculation with HRV [41]. Similarly, a study from Homer Boushey's and Pedro Avila's labs found no difference in HRV levels in asthma versus normal subjects, and in this case, cells were cultured under air-liquid interface conditions to better achieve ciliated cell differentiation [42]. Moreover, reports from two different labs (including Johnston's lab) show no statistical difference in HRV levels in asthma versus control subjects after controlled inoculation in vivo [43, 44]. Other reports indicate that the levels of IFN-γ in airway epithelium or IFN-λ in sputum are similar in asthmatic versus normal subjects [45, 46]. However, we do not yet have comprehensive reports of IFN (type I, II, and/or III) level or signal during viral infection. In fact, precise quantification of IFN and corresponding viral level in vivo are clinical biomarkers with significant methodological challenges.
In addition to technical concerns, the divergent results in studies of cultured airway epithelial cells could also be explained by the proposal for a close relationship between level of viral replication, subsequent acute illness, and finally chronic disease. In particular, it appears that HRV replicates relatively inefficiently in well-differentiated airway epithelial cells and in mouse in vivo models as well [47, 48]. A lower level of viral replication could explain why the anti-HRV response has not appeared to be reproducible in vitro and why HRV infection has not appeared to be sufficient for postviral asthma in vivo in experimental models in mice. By contrast, SeV is particularly adapted for vigorous viral replication in the experimental mouse model, and this element of the model is essential for the subsequent illness and development of chronic obstructive lung disease. Similarly in humans, despite the difficult in comparing viral levels in asthmatics versus normals, if one concentrates on just the asthmatic population that is susceptible to virus-induced disease, there remains a tight relationship between viral load and severity of illness [44].
Improving innate immunity
Taken together, there appears to be a direct relationship between viral level and both the severity of acute illness and the likelihood of chronic disease in experimental models and in humans with asthma. Moreover, the capacity of the host to control viral level appears to be deficient in asthma, perhaps at the level of IFN production and/or signaling in airway epithelial cells and likely in immune cells in the airway as well. Even if some of these tenets turn out to be wrong, there still stands to be significant benefit for the normal and the asthmatic host to improve control over viral infection. So the question still remains as to how best to achieve that goal given the absence of vaccines for asthmagenic viruses. In that regard, nearly as soon as it was recognized that physiologic levels of IFNs were required for normal host defense, it was proposed that excessive levels of IFNs might provide therapeutic benefit. Indeed, transgenic overexpression of IFN-encoding genes in mice may protect against experimental infection and inflammatory disease, and administration of recombinant IFN is commonly used for infectious, autoimmune, and cancerous conditions in humans [49]. Unfortunately, this approach is limited by toxicity, so that excessive IFN might harm the normal host [50]. In fact, this strategy suggests that there is little safe reserve in the IFN system that can be utilized for benefit in vivo. For these reasons and others, it might be more prudent to aim at increasing the efficiency of endogenous IFN and thereby potentiate downstream signal transduction [33].
Although an improvement in IFN efficacy might be desirable, it is made difficult by the complexity of the IFN signaling pathway. Nonetheless, support for an approach to increase IFN signaling can be found in ongoing experimental work directed at STAT1. In particular, a double-cysteine substitution of native STAT1 (designated STAT1-CC) leads to a markedly increased responsiveness to IFN stimulation [51]. The consequence is a marked improvement in ISG expression and control of viral replication, at least in vitro. Initial experiments suggest that a similar benefit can be achieved in a transgenic mouse model in vivo. Thus, current efforts aim to develop therapeutics that mimic this benefit and thereby correct a possible defect in host defense that might contribute to chronic inflammatory diseases such as asthma. In particular, the assay methods developed for studies of IFN signaling can also be used to identify small molecular weight compounds that mimic Stat1-CC and enhance IFN efficacy in a manner that has distinct advantages over current therapies, including IFN itself [52].
Conclusion
Here we summarize new work on the pathogenesis of asthma to support three breakthrough issues: (1) respiratory viral infections drive long-term activation of an innate immune cell response and in turn chronic obstructive lung disease in an experimental model that resembles virus-induced asthma in humans; (2) this type of innate immune response depends on the severity of acute infection and in turn the tissue levels of virus, so that proper control of virus by airway epithelial cells via IFN-dependent signaling would prevent asthma under normal conditions but promote it under deficient conditions; and (3) smart mechanisms exist for improving IFN signaling as a means for improving antiviral defense and preventing severe infection and consequent asthma. These concepts lead to a revised scheme for the steps leading from respiratory viral infection to asthma (as summarized in Fig. 4). The model also provides a framework for therapeutic strategies that improve antiviral defense at the level of airway epithelial cells and thereby overcomes common respiratory viruses that pose a serious public health problem in terms of acute respiratory illness and chronic airway disease
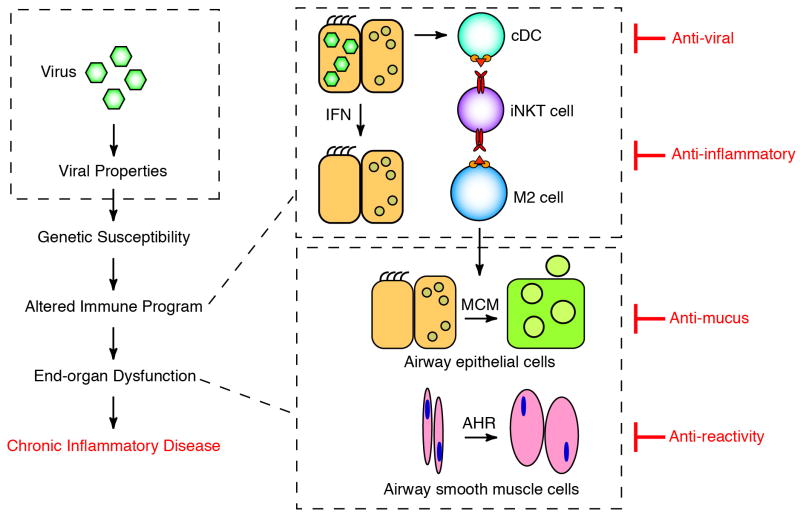
Fig. 4. Revised scheme for how viruses might trigger chronic asthma.
This scheme is modified from the conventional scheme depicted in Fig. 1 to include the control of viral level in airway epithelial cells based on IFN production and signaling. The diagram also indicates proposed strategies for therapeutic intervention, including an antiviral approach that aims to improve epithelial control of viral levels.
Research highlights.
Placing antiviral defense in the context of innate mucosal immunity
Understanding how a defect in antiviral defense causes asthma
Identifying the precise defect in antiviral defense in asthma
Devising a strategy to improve antiviral defense
Acknowledgments
Our research on this topic is supported by grants from the National Institutes of Health (National Heart, Lung, and Blood Institute and National Institute of Allergy and Infectious Diseases).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol. 2008;20:697–702. doi: 10.1016/j.coi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson MS, Pesce JT, Ramalingam TR, et al. Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol. 2008;181:6942–6954. doi: 10.4049/jimmunol.181.10.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poston RN, Chanez P, Lacoste JY, et al. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. Am Rev Respir Dis. 1992;145:918–921. doi: 10.1164/ajrccm/145.4_Pt_1.918. [DOI] [PubMed] [Google Scholar]
- 4.Ying S, Durham SR, Corrigan CJ, et al. Phenotype of cells expressing mRNA for TH2-type (interleukin 4 and interleukin 5) and TH1-type (interleukin 2 and interferon γ) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol. 1995;12:477–487. doi: 10.1165/ajrcmb.12.5.7742012. [DOI] [PubMed] [Google Scholar]
- 5.Holtzman MJ, Byers DE, Benoit LA, et al. Immune pathways for translating viral infection into chronic airway disease. Adv Immunol. 2009;102:245–276. doi: 10.1016/S0065-2776(09)01205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 7**.Walter MJ, Morton JD, Kajiwara N, et al. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–175. doi: 10.1172/JCI14345. This article established that an acute respiratory viral infection can lead to chronic inflammatory lung disease in an experimental model system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Patel AC, Morton JD, Kim EY, et al. Genetic segregation of airway disease traits despite redundancy of chloride channel calcium-activated (CLCA) family members. Physiol Genomics. 2006;25:502–513. doi: 10.1152/physiolgenomics.00321.2005. This article established the genetic susceptibility of the process leading from an acute respiratory viral infection to chronic inflammatory lung disease in an experimental model system and genetically segregated two distinct disease traits for chronic obstructive lung disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyner JW, Kim EY, Ide K, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest. 2006;116:309–321. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Grayson MH, Cheung D, Rohlfing MM, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. This article established the up-regulation of high-affinity IgE receptors on lung dendritic cells using a mouse model of respiratory viral infection and showed how this immune event leads to chronic inflammatory lung disease in an experimental model system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung DS, Ehlenbach SJ, Kitchens RT, et al. Cutting Edge: CD49d+ neutrophils induce FcεRI expression on lung dendritic cells in a mouse model of postviral asthma. J Immunol. 2010;185:4983–4987. doi: 10.4049/jimmunol.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic inflammatory lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. This article established the innate immune basis for how an acute respiratory viral infection can lead to chronic inflammatory lung disease in an experimental model system and in humans with asthma and chronic obstructive pulmonary disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoit LA, Holtzman MJ. New immune pathways from chronic post-viral lung disease. Ann NY Acad Sci. 2010;1183:195–210. doi: 10.1111/j.1749-6632.2009.05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agapov E, Battaile JT, Tidwell R, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009;41:379–384. doi: 10.1165/rcmb.2009-0122RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byers DE, Holtzman MJ. Alternatively activated macrophages as cause or effect in asthma. Am J Respir Cell Mol Biol. 2010;43:1–4. doi: 10.1165/rcmb.2009-0407ED. [DOI] [PubMed] [Google Scholar]
- 16.Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest. 2010 doi: 10.1378/chest.10-2132. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 18**.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. This article and related ones from this research group provided the epidemiologic evidence for a connection between respiratory viral infection and asthma in childhood. [DOI] [PubMed] [Google Scholar]
- 19.Castro M, Schweiger T, Yin-Declue H, et al. Cytokine response after severe respiratory syncytial virus bronchiolitis in early life. J Allergy Clin Immunol. 2008;122:725–733. doi: 10.1016/j.jaci.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibricevic A, Pekosz AS, Walter MJ, et al. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Dupuis S, Jouanguy E, Al-Hajjar S, et al. Impaired response to interferon-a/b and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. This article and related ones from this research group established the requirement for IFN signaling in antiviral host defense in humans by identifying the consequences for genetic deficiency of this signaling pathway. [DOI] [PubMed] [Google Scholar]
- 22.Sumino KC, Visness CM, Schwarz J, et al. Antiviral interferon responses at birth can predict respiratory illness in the first year of life. Am J Respir Crit Care Med. 2010;181:A2503. [Google Scholar]
- 23.Gelhar K, Bilitewski C, Reinitz-Rademacher K, et al. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos NG, Stanciu LA, Papi A, et al. A defective type I response to rhinovirus in atopic asthma. Thorax. 2002;57:328–332. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill MA, Bajwa G, George TA, et al. Counterregulation between the FceRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Look DC, Keller BT, Rapp SR, Holtzman MJ. Selective induction of intercellular adhesion molecule-1 by interferon-γ in human airway epithelial cells. Am J Physiol. 1992;263:L79–L87. doi: 10.1152/ajplung.1992.263.1.L79. This article was the first in a series to establish the functional and molecular biology of the IFN signal transduction system in airway epithelial cells. [DOI] [PubMed] [Google Scholar]
- 27.Look DC, Pelletier MR, Holtzman MJ. Selective interaction of a subset of interferon-g response element binding proteins with the intercellular adhesion molecule-1 (ICAM-1) gene promoter controls the pattern of expression on epithelial cells. J Biol Chem. 1994;269:8952–8958. [PubMed] [Google Scholar]
- 28.Look DC, Pelletier MR, Tidwell RM, et al. Stat1 depends on transcriptional synergy with Sp1. J Biol Chem. 1995;270:30264–30267. doi: 10.1074/jbc.270.51.30264. [DOI] [PubMed] [Google Scholar]
- 29.Walter MJ, Look DC, Tidwell RM, et al. Targeted inhibition of interferon-g-dependent ICAM-1 expression using dominant-negative Stat1. J Biol Chem. 1997;272:28582–28589. doi: 10.1074/jbc.272.45.28582. [DOI] [PubMed] [Google Scholar]
- 30.Look DC, Roswit WT, Frick AG, et al. Direct suppression of Stat1 function during adenoviral infection. Immunity. 1998;9:871–880. doi: 10.1016/s1074-7613(00)80652-4. [DOI] [PubMed] [Google Scholar]
- 31.Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and type I interferon responsiveness. J Virol. 2005;79:9315–9319. doi: 10.1128/JVI.79.14.9315-9319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Shornick LP, Wells AG, Zhang Y, et al. Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J Immunol. 2008;180:3319–3328. doi: 10.4049/jimmunol.180.5.3319. This article provides the best evidence to date for the proposed role of the airway epithelial cell system in mediating mucosal immunity and antiviral host defense in vivo. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Hinojosa ME, Holtzman MJ. Viral and host strategies to take advantage of the innate immune response. Am J Respir Cell Mol Biol. 2010;43:507–510. doi: 10.1165/rcmb.2009-0213ED. [DOI] [PubMed] [Google Scholar]
- 34*.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. This article provided evidence that a defect in airway epithelial cell production of IFN after viral infection might be found in human asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-l production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 36.Cakebread JA, Xu Y, Grainge C, et al. Exogenous IFN-b has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.01.023. in press. [DOI] [PubMed] [Google Scholar]
- 37.Harada M, Nakashima K, Hirota T, et al. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- 38.Zheng S, De BP, Choudhary S, et al. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003;18:619–30. doi: 10.1016/s1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 39.Schneider D, Ganesan S, Comstock AT, et al. Increased cytokine resonse of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:332–340. doi: 10.1164/rccm.200911-1673OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bochkov YA, Hanson KM, Keles S, et al. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Souza N, Favoreto S, Wong H, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMore JP, Weisshaar EH, Vrtis RF, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–252. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampath D, Castro M, Look DC, Holtzman MJ. Constitutive activation of an epithelial signal transducer and activator of transcription (Stat1) pathway in asthma. J Clin Invest. 1999;103:1353–1361. doi: 10.1172/JCI6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bullens DM, Decraene A, Dilissen E, et al. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy. 2008;38:1459–1467. doi: 10.1111/j.1365-2222.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- 47.Bartlett NW, Walton RP, Edwards MR, et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newcomb DC, Sajjan US, Nagarkar DR, et al. Human rhinovirus 1B exposure induces phophatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borden EC, Sen GC, Uze G, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biggioggero M, Gabbriellini L, Meroni PL. Type I interferon therapy and its role in autoimmunity. Autoimmunity. 2010;43:248–254. doi: 10.3109/08916930903510971. [DOI] [PubMed] [Google Scholar]
- 51**.Zhang Y, Takami K, Lo MS, et al. Modification of the Stat1 SH2 domain broadly improves interferon efficacy in proportion to p300/CREB-binding protein coactivator recruitment. J Biol Chem. 2005;280:34306–34315. doi: 10.1074/jbc.M503263200. This article provided the initial molecular strategy for enhancement of IFN signal transduction and consequent improvement in antiviral host defense. [DOI] [PubMed] [Google Scholar]
- 52.Heilman C. NIAID influenza antiviral development workshop: new generation. 2009:1–31. www3.niaid.nih.gov/topics/Flu.