Abstract
Background
Termination of persistent atrial fibrillation (AF) is a valuable ablation endpoint, but is difficult to anticipate. We evaluated whether temporal and spatial indices of AF regularization predict intra-procedural AF termination and outcome.
Objective
To test whether temporospatial organization of AF after pulmonary vein isolation (PVI) predicts whether subsequent stepwise ablation will terminate persistent AF or predict outcome.
Methods
In 75 patients with persistent AF, we measured AF cycle length (AFCL), temporal regularity index (TRI, a spectral measure of timing regularity) and spatial regularity index (SRI, cycle-to-cycle variations in spatial vector) between right atrial appendage, proximal and distal coronary sinus before and during stepwise ablation to the endpoint of AF termination.
Results
AF termination was achieved in 59 patients (79%) by ablation. AF terminated during PVI in 11 patients, who were excluded from analysis. In the remaining 48 patients, TRI and SRI increased during stepwise ablation, as compared to 16 patients without termination (p < 0.05). AFCL prolonged in both groups. From ROC analysis of the first 22 patients (training set), a post-PVI TRI increase predicted AF termination in the latter 42 patients (test set) with PPV 96 %, NPV 53 %, sensitivity 71 % and specificity 91 %. Results were similar for SRI. After 36 months, higher arrhythmia-free outcome was observed in patients in whom PVI caused temporospatial regularization in AF.
Conclusions
Temporal and spatial regularization of persistent AF after PVI identifies patients in whom stepwise ablation subsequently terminates AF and prevents recurrence.
Keywords: Atrial Fibrillation, Ablation, Termination, Organization, Spectral Analysis, Signal Processing
INTRODUCTION
Termination1, 2 of AF to sinus rhythm or atrial tachycardia has been shown to predict long-term success of persistent AF ablation in several trials 3–10. Pulmonary vein isolation (PVI) is a cornerstone of ablation for AF, yet the termination of persistent AF often requires additional ablation 1, 2. Although pre-procedural factors, such as AF cycle length (AFCL)3, 11, f-wave amplitude in ECG lead V112, LA diameter9 and duration of continuous AF predict the likelihood of AF termination, they are of limited intraprocedural predictive value.
Intraprocedurally, it would be useful to develop indices that predict whether persistent AF will terminate with additional ablation after PVI. Notably, ECG indices of AF organization rise after ablation,13 anti-arrhythmic medications and atrial defibrillation14, 15. Measured inside the atrium, the temporal organization of AF16 rises when the PV that perpetuates paroxysmal AF is isolated17. However, temporal and spatial organization of AF18, 19 have not been studied in relation to AF termination or post-ablation outcome, or in persistent AF. We hypothesized that temporal and spatial regularization of intra-atrial electrograms would predict procedural termination of AF and freedom from recurrence.
METHODS
Study Population
We performed a retrospective analysis of consecutive patients undergoing first ablation for symptomatic drug-refractory persistent AF between September 2006 and March 2009. Since PVI is a cornerstone of AF ablation1, our goal was to identify parameters to apply post-PVI that predict subsequent termination of AF to sinus rhythm or organized atrial tachycardia by ablation. Thus, we focused on patients whose AF persisted despite PVI. Patients were classified into two groups, AF Termination and AF Non-Termination, depending on the procedural outcome.
Electrophysiologic Study
Antiarrhythmic medications were discontinued for > 5 half-lives prior to ablation, with the exception of amiodarone. Prior to the procedure, patients had taken oral anticoagulants (target INR 2–3) for at least 1 month, and transesophageal echocardiography was performed to exclude thrombus. For ablation, a 3.5-mm externally irrigated-tip catheter (Thermocool, Biosense Webster, Diamond Bar, California) was used. After transeptal access, a single bolus of 50IU/kg body weight of heparin was administered with additional heparin if the procedure lasted for more than 4 hours. Surface electrocardiogram (ECG) and endocardial electrograms were continuously monitored and stored (Labsystem Pro; Bard, Natick MA). Signals were band-pass filtered as follows: ECGs from 0.05 to 100 Hz; PV electrograms from 100–250Hz; other intracardiac electrograms from 30–250Hz.
Ablation Procedure
Radiofrequency energy was delivered with a power of 25–35 watts using irrigation rates of 5–60ml/min to limit the temperature to ≤40°C. Ablation was performed in stepwise fashion as described20. Wide circumferential PVI was performed to abolish or dissociate electrical activity in all PVs. Electrogram-guided ablation in the left atrium was performed at sites of complex fractionated atrial electrograms (CFAE)21,22, to transform CFAE into discrete electrograms, abolish electrograms or slow local CL. Linear ablation was performed if AF persisted, targeting the LA roof then mitral isthmus to reduce or abolish local electrograms with subsequent confirmation of bidirectional block. Ablation was directed to the right atrium if we observed RA CL < LA CL after LA ablation.10. Following restoration of sinus rhythm, isolation of PVs was confirmed and conduction block was verified across each line23.
Procedural end point
The procedural endpoint was AF termination to an intermediate atrial tachycardia or sinus rhythm as described20. If termination was not achieved by the stepwise ablation protocol, DC cardioversion was performed to restore sinus rhythm and the patient was assigned to the AF Non-Termination group. Additional ablation was performed to ensure PV isolation and linear conduction block.
Electrogram Processing
All analyses were blinded to clinical and procedural information and performed using software written in Labview (National Instruments, Austin, TX). Intra-atrial electrograms were analyzed in 8.2 second windows at 5 different stages of the procedure (details below): (I) baseline: prior to ablation, (II) after PVI: 5 minutes after all 4 PVs were electrically isolated, (III) after LA ablation: one minute prior to termination (LA termination patients) or before commencing RA ablation, (IV) after RA ablation: one minute prior to conversion to atrial tachycardia or sinus rhythm (RA termination patients) or DC cardioversion (non-termination patients), and (V) during atrial tachycardia (termination patients only). At each stage the following three indexes were measured: AFCL, Temporal Regularity Index (TRI) and Spatial Regularity Index (SRI). After PVI TRI/SRI were calculated from single records, 5 minutes after PVI.
AF Cycle Length
We measured AFCL from the proximal coronary sinus, using our sliding correlation approach to eliminate ventricular artifact, followed by autocorrelation analyses of AF CL as we 24, 25 and others 26 have described.
Temporal Regularity Index
Cycle-to-cycle regularity in AF was measured by the width of the spectral dominant frequency (DF) measured in the proximal coronary sinus, similar to the regularity or organizational index 16, 27. First, we applied a Hanning window 28. Each signal was rectified, bandpass filtered from 1–10 Hz then analyzed using a 8192-point fast Fourier transform to compute its power spectrum. DF was assigned between 3–10 Hz (CL range 333–100ms). TRI was calculated as the ratio of the area under the 5 largest peaks, each for a 1-Hz window, to the total area from 2.5 Hz to 10.5 Hz. ‘Organized’ atrial activity with regularly timed and shaped electrograms produce a narrow dominant frequency peak 15, resulting in a high TRI15, 29 (figure 1: “Atrial Tachycardia”). Conversely, ‘disorganized’ AF produces less discrete peaks in the power spectrum and a lower TRI (figure 1 “Baseline”).
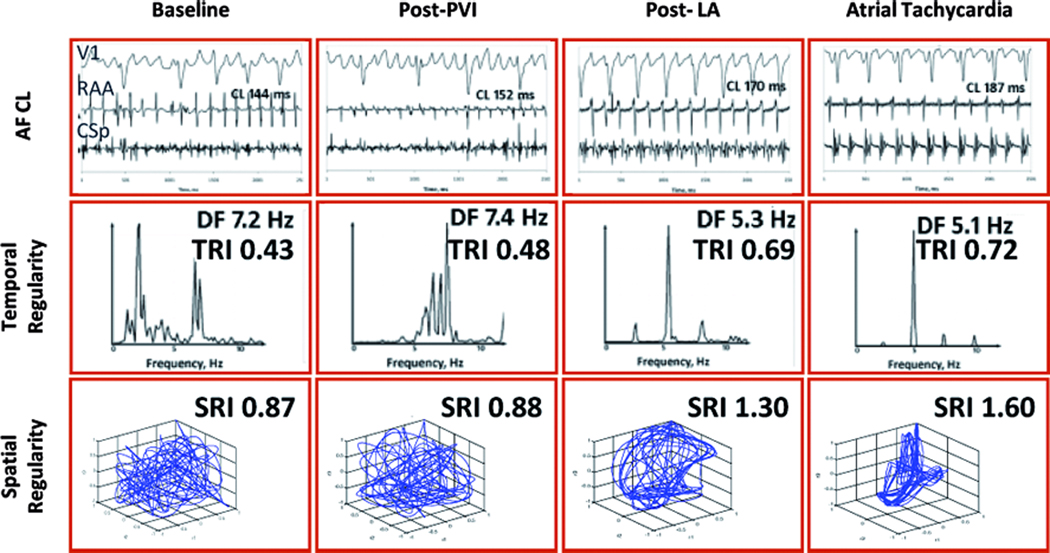
Figure 1.
AF Regularization precedes AF Termination in a 34 year old man with continuous AF for 36 months, LA diameter 43 mm and LVEF 55 %. RAA CL prolongs after PVI and continues to do so to the point of AF termination. Intracardiac electrograms, CSp frequencies spectra and spatial activation vector appear to organize during ablation, which is quantitatively confirmed by the increase in the TRI and SRI. RAA=right atrial appendage; CSp=proximal coronary sinus; DF=dominant frequency; TRI=temporal regularity index; SRI=spatial regularity index.
Spatial Regularity Index
We quantified cycle-to-cycle reproducibility in the spatial vector between right atrial appendage, proximal and distal coronary sinus during AF as described 25, 29, 30. Spatial regularity was computed from simultaneous plots of correlation time series for RAA against proximal and distal CS. In each, we identified the greatest excursion for each cycle and computed the Pythagorean resultant, i.e. perfect spatial regularity =√(12+12+12)=1.73 (figure 1: atrial tachycardia exhibits loops that reach the (1,1) point), while spatial irregularity gives lower values (figure 2: baseline and post-PVI).
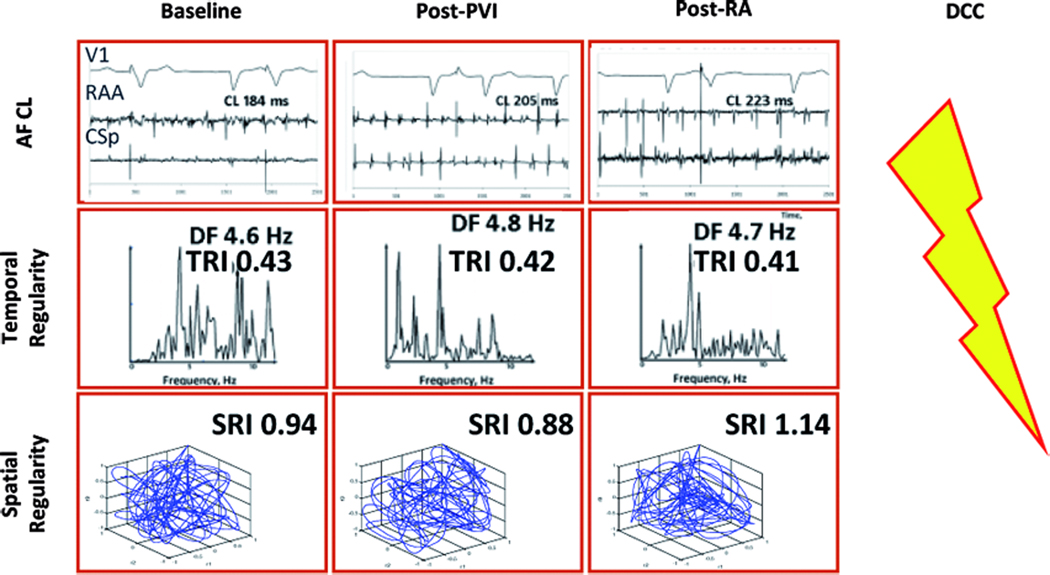
Figure 2.
Lack of AF regularization and absence of AF termination in a 75 year old man with longstanding persistent AF, left atrial diameter 45mm and LVEF 45 %. Notably, there is no increase in TRI or SRI after PVI and subsequent ablation, even though baseline AFCL is relatively long and prolongs with ablation. Same abbreviations as in Figure 1.
Follow-up
All patients were monitored in hospital for 3–5 days post procedure. Anti-arrhythmic drugs were discontinued 3 months after the index procedure, in the absence of a continued indication. Patients were re-evaluated at 1, 3, 6, 9 and 12 months, and in the absence of AF or symptoms, followed up with their referring physician. At each visit, ambulatory 24–48 hour monitoring was performed to detect asymptomatic arrhythmias. In the event of arrhythmia recurrence, patients were offered a trial of drug therapy then additional ablation. Additional ablation procedures commenced with verification of PV isolation, identification of gaps in each line, then repeated prior steps.
Clinical outcome
Success after first procedure was defined as maintenance of sinus rhythm during follow-up after the index ablation, with a post procedural blanking period of 3 months.
Success after the last procedure was defined as maintenance of sinus rhythm (absence of symptomatic and asymptomatic atrial fibrillation or atrial tachycardia) without and with antiarrhythmic drugs during follow-up after last ablation, with a post procedural blanking period of 3 months.
Statistical Analysis
Data are expressed as mean ± SD for continuous variables and absolute numbers and percentages for categorical variables. Categorical variables were compared with chisquared or Fisher exact test. For AFCL, TRI and SRI one-way ANOVA was performed, followed by paired or unpaired t-tests using GraphPad Prism, version 3.00, for Windows. The initial third (n=22) of patients were used as a ‘training set’ to calculate receiver operating characteristics (ROC) to define an optimal cutpoints for AF termination. Cutpoints were applied prospectively to subsequent patients (n=42) (‘test set’). Logistic regression analysis was performed using SYSTAT 12 (SYSTAT Inc). For continuous variables, the ROC curve was derived and the optimal cutoff chosen. Raw odds ratios were calculated in univariate analysis. Variables with p < 0.25 were included in the multivariate logistic regression model. All tests were 2-tailed, and statistical significance was established at p < 0.05. Cumulative event rates (i.e. arrhythmia recurrence) were calculated using the Kaplan-Meier method.
RESULTS
A total of 75 consecutive patients (age 58±11 years, 11 women) who underwent a first ablation for symptomatic drug-refractory persistent AF were studied (Table 1). The overall rate of AF termination was 79% (n=59). AF terminated during PV isolation in n=11 who were excluded from the analysis. Among the 64 patients whose AF continued after PVI, AF subsequently terminated during stepwise ablation in n=48 and did not terminate in n=16. There were no major procedural complications.
Table 1.
Clinical Characteristics of Study Population (n=64)
| Age (years) | 58 ± 11 |
| Sex (male/female) | 55/9 |
| History of AF (months) | 81 ± 65 (7 – 360) |
| Duration of Continuous AF (months) | 25 ± 27 (1 – 120) |
| Long-lasting Persistent AF (>12 month) (%) | 65 |
| LVEF (%) | 56 ± 13 |
| Left Ventricular dysfunction (LVEF<50%) (n) | 17 |
| LVEDD (mm) | 54 ± 7 |
| LVESD (mm) | 36 ± 9 |
| LAD (mm) | 46 ± 7 |
| Structural Heart Disease | 17 |
| Coronary Artery Disease | 5 |
| Hypertension | 18 |
| Number of failed AAD | 2.1 ± 1.0 |
| Administration of amiodarone | 16 |
| Number of electrical cardioversions | 1.8 ± 2.3 |
| Surface ECG AFCL (ms) | 151 ± 18 |
AF = atrial fibrillation; LVEF = left ventricular ejection fraction; LVEDD = left ventricular end diastolic diameter; LVESD = left ventricular end systolic diameter; LAD = Left Atrial Diameter (antero-posterior); AAD = antiarrhythmic drugs; AFCL = AF cycle length
Figure 1 shows data from a 34 year old man with continuous AF for 36 months in whom ablation terminated AF. Baseline AFCL of 144ms increased progressively at each ablation step to AF termination. In parallel, a progressive narrowing in spectra and regularization of the cycle-to-cycle spatial vectors were observed, and TRI and SRI increased accordingly. Figure 2 shows an example of a 75 year old man with longstanding persistent AF in whom ablation failed to terminate AF. Despite a high baseline AFCL (184ms) that increased during rigorously performed ablation, AF termination was not achieved and the patient required electrical cardioversion. Notably, while AFCL prolonged during the procedure, AF temporal and spatial regularity indices did not increase.
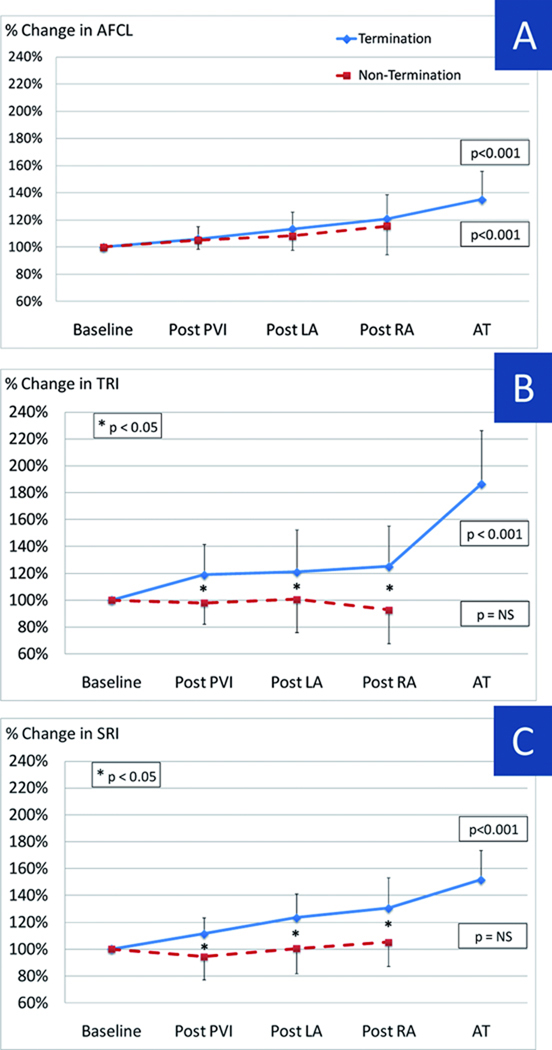
Similar findings were seen in the entire population. Figure 3 shows relative changes in AFCL, TRI and SRI at different stages of ablation, expressed as percentage of baseline.
Figure 3.
AFCL, TRI and SRI relative changes throughout ablation process expressed as percentage variation from baseline. AFCL significantly prolongs after PVI and continues to do so throughout the ablation process in AF termination and non-termination groups (A). Both TRI (B) and SRI (C) progressively increase after PVI in patients with termination. Importantly, regularity indexes do not display significant changes during the procedure in patients without termination. In addition, statistical significance between termination and non termination groups is reached already after PVI. Same abbreviations as in Figure 1.
Atrial Fibrillation Cycle Length
Figure 3A illustrates that AFCL prolonged progressively during stepwise ablation but, in these patients with continued AF post-PVI, that this occurred in patients with and without subsequent AF termination (p<0.001 ANOVA). Baseline AFCL (169±23ms vs 167±25ms, p=0.76) and AFCL prolongation (compared to baseline) did not differ between Termination and Non-Termination groups (p=0.75). Although not a primary study analysis, baseline AFCL for all patients (n=75) trended longer in patients with any AF termination (i.e. even during PVI) than those without termination (161±23ms vs 150±22ms, p=0.08), as we have shown 3. Comparable evolution was obtained when AFCL was assessed from RAA.
Temporal Regularization Index
TRI increased progressively during stepwise ablation in patients with (p < 0.001) but not those without (p=0.67; Figure 3B) AF termination. Notably, patients with AF termination showed an abrupt post-PVI increase in TRI that was absent in patients without AF termination (119±23% vs 98±15%, p < 0.001). Subsequent ablation steps caused modest further elevations in TRI in patients with but not in patients without AF termination.
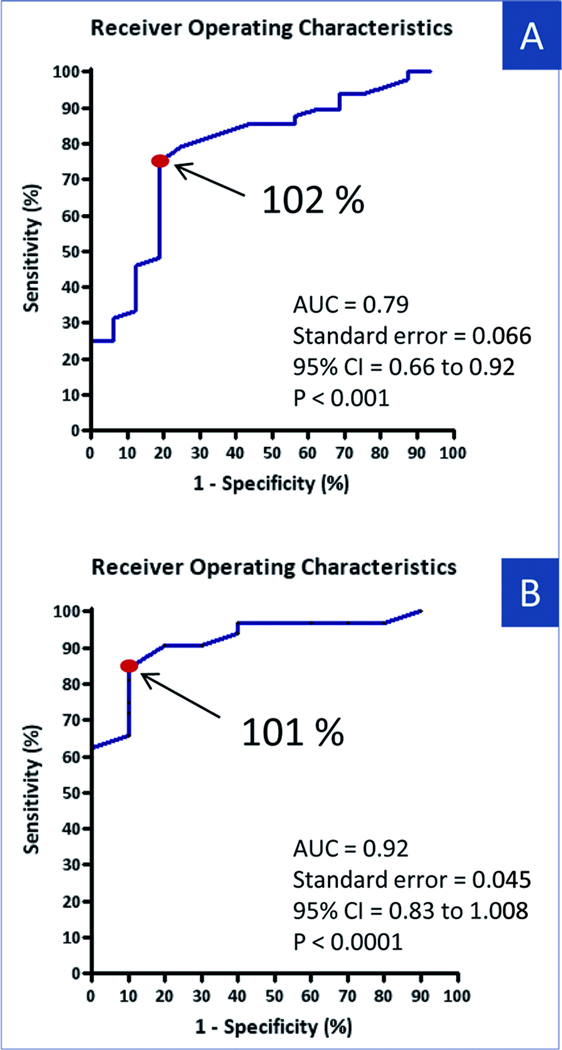
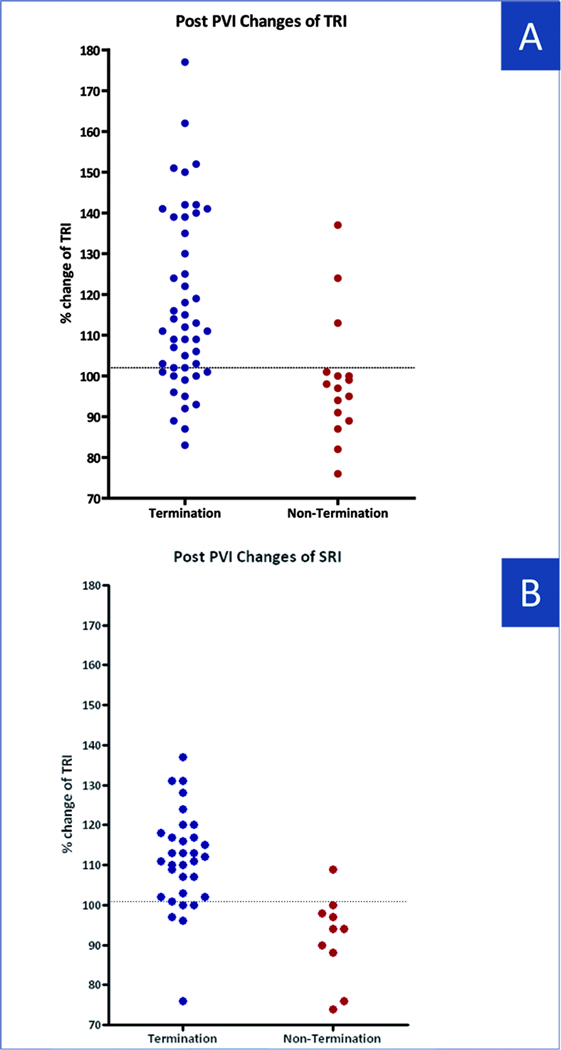
ROC analysis of the TRI increase (baseline to post-PVI) in the training set gave an optimal cutoff of ≥ 102% of baseline. Applied prospectively to the test set (n=42), this cutpoint provided 96% positive predictive value (PPV), 53% negative predictive value (NPV), 71% sensitivity and 91% specificity % for termination. The scatterplot in figure 4A illustrates TRI changes (normalized to pre-ablation values) after PVI in patients with and without AF termination (ROC-derived cutoff indicated). For the entire population, the ROC-derived cutoff provided 92% PPV, 52% NPV, 75% sensitivity and 81% specificity for AF termination (Figure 5A).
Figure 5.
Termination of Persistent AF according to Regularity Indexes Receiver Operating Characteristic Curve of after PVI change in TRI (A) and SRI (B) expressed as changes from baseline for AF termination of persistent AF by catheter ablation in the entire population. Arrows shows optimal cutoff point for sensitivity and specificity. AUC=area under the ROC; CI=confidence interval; other abbreviations as in Figure 1.
Spatial Regularity Index
Figure 3C shows spatial regularity during step-wise ablation for all patients. Cycle-to-cycle spatial AF regularity increased during ablation in patients with (p < 0.001) but not without (p=0.48) AF termination. SRI increased abruptly after PVI in AF Termination (111 ± 12%) but not Non-Termination (94±17%; p < 0.01) groups, and remained separated throughout the procedure (Figure 4B).
Figure 4.
Changes in Regularity Indexes after PVI as compared to Baseline Scatter plot of changes in TRI (A) and SRI (B) in patients with and without AF termination. Horizontal line indicates optimal diagnostic cutoff to predict AF termination. Same abbreviations as in Figure 1.
In the training set, an SRI cutoff of ≥101 % of baseline provided AUC=0.89. Applying this prospectively to the test set provided 94% PPV, 67% NPV, 85% sensitivity and 90% specificity for termination. In 10% of patients in the termination group and 89% of patients in the non termination group, SRI was lower after PVI than at baseline. For the entire population, the ROC derived cutoff was 101% (AUC=0.92), with 96% PPV, 64% NPV, 84% sensitivity and 90% specificity for termination (Figure 5B).
Predictors of AF termination
In univariate analysis, AF termination was associated with post-PVI increase in TRI (119±23% vs 98±15%: patients with vs without AF termination; p < 0.001), post-PVI increase in SRI (111±12% vs 94±17%; p < 0.01), and LVEDD (52±6% vs 59±7%; p=0.005). In multivariate analyses, post-PVI TRI≥101% of baseline (OR 14.1; 95%CI 2.9–68.5; p=0.001) and absence of hypertension (OR 4.8; 95%CI 1.0–22.7; p=0.05) independently predicted AF termination. This model provided a sensitivity and specificity of 84% and 44% respectively with AUC =0.86 (p=0.001).
Follow-up and Clinical Outcome
With 22±7 month follow-up after the first procedure, freedom from AF was achieved in 47% of patients (n=29). A redo procedure was performed in 52% (n=17) of the patients. Only 2 patients underwent more than 2 procedures (3 and 4 respectively). Two of the 64 patients in this study lived in another country and were lost to follow-up.
After 1.3±0.6 procedures and 19±8 month follow-up, overall clinical success rate was 73% (n=45; 50%, n=31 without AAD and 23%, n=14 with AAD). Success was more frequent in the Termination than the Non-Termination group, 83% (31% taking AAD) vs. 40% (33% taking AAD) respectively (p=0.02) (Table 2).
Table 2.
Follow-up and success rate
| All patients (n=62) |
Term (n=47) |
Non-Term (n=15) |
P Value | TRI ≥ 101% (n=45) |
TRI < 101% (n=17) |
P Value | SRI ≥ 101% (n=28) |
SRI < 101% (n=12) |
P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean FU (months) | 22 ± 7 | 23 ± 7 | 21 ± 7 | 24 ± 6 | 20 ± 6 | 22 ± 5 | 18 ± 5 | |||
| Patients free of AF/AT during FU after the index procedure |
29 (47%) | 24 (51%) | 5 (33%) | 0.25 | 23 (51%) | 6 (35%) | 0.26 | 15 (54%) | 3 (25%) | 0.17 |
| Taking AAD | 7 | 6 | 1 | 1.00 | 5 | 2 | 1.00 | 5 | 1 | 0.65 |
| Patients free of AF/AT after all procedures |
45 (73%) | 39 (83%) | 6 (40%) | 0.02 | 36 (80%) | 9 (53%) | 0.03 | 25 (89%) | 5 (42%) | 0.002 |
| Taking AAD | 14 | 12 | 2 | 0.48 | 10 | 4 | 1 | 9 | 3 | 0.72 |
2 lost FU
Regularity Indices and Clinical Outcome
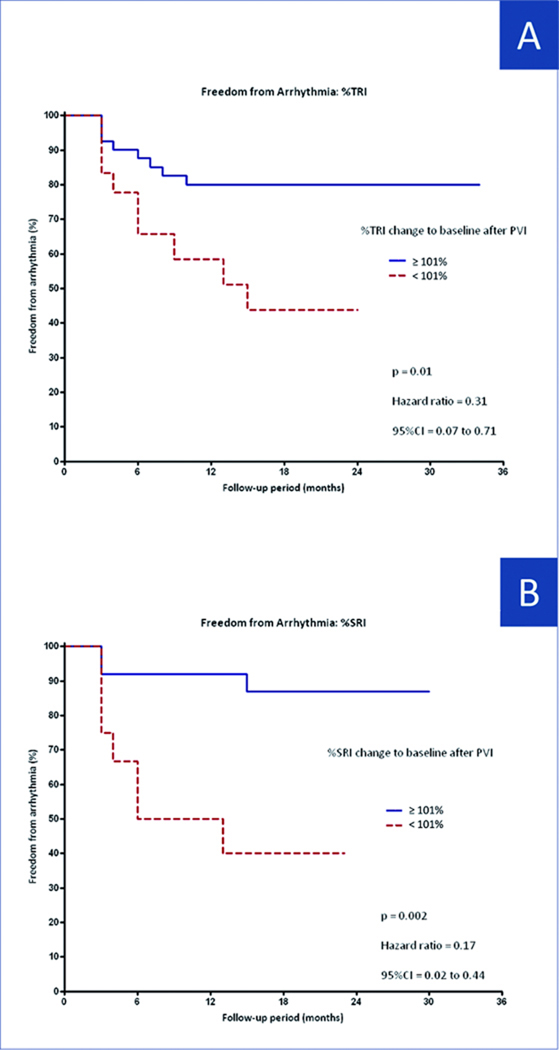
Success after last procedure was more common in patients with post-PVI TRI≥101% (of baseline) compared with patients with TRI < 101% (80% vs 47%, p=0.03; Figure 6A). This cutoff provided 82% positive predictive value, 50% negative predictive value, 80% sensitivity and 53% specificity for clinical success (p=0.03, odds ratio: 4.5, 95% CI: 1.36 to 14.95).
Figure 6.
Freedom From Arrhythmia Kaplan-Meier curve analysis of freedom form arrhythmia after last ablation procedure according to after PVI change in TRI ≥ or < 102% (A) and in SRI ≥ or < 101% (B). Abbreviations as in Figure 5.
Similarly, patients with post-PVI SRI ≥ 101% of baseline had a significantly higher success after the final ablation (83% vs 30%; p=0.003) (Figure 6B). This cutoff provided 83% PPV, 70% NPV, 89% sensitivity and 58% specificity for clinical success (p=0.003, odds ratio: 11.7, 95% CI: 2.22 to 61.30).
Predictors of success after the last ablation procedure
In univariate analysis, freedom from AF recurrence was associated with post-PVI increase in TRI (117±22% in patients with vs 103±14% in patients without AF termination; p=0.03), a post-PVI increase in SRI (111±12% vs 100±14%; p=0.02), LA diameter (45±6mm vs 49±9mm; p=0.05), procedure time (274±66min vs 316±78min; p < 0.05), and AF Termination during index procedure (85% vs 50%; p=0.005). On multivariate analysis, procedural duration≤312min (OR 8.2; 95%CI 1.9–36.0; p=0.005) and post-PVI TRI≥101% of baseline (OR 4.8; 95%CI 1.5–28.4; p=0.012) independently predicted freedom from arrhythmia after the last ablation. This model provided a sensitivity and specificity of 81% and 44% for procedural success, respectively, with AUC=0.80 (p=0.001).
DISCUSSION
In patients undergoing persistent AF ablation, intra-procedural regularization in electrogram timing and spatial activation after PVI identifies patients in whom AF is likely to terminate with further ablation and who are subsequently likely to remain free of recurrent AF. A lack of regularization after PVI is less specific. Mechanistically, these data emphasize a primary role of the PVs in sustaining persistent AF such that PVI regularizes AF even if it does not terminate AF. These results provide a quantitative intra-procedural tool to identify patients in whom AF may and may not terminate by stepwise ablation after the component of PV isolation.
Temporospatial Regularization Provides Mechanistic Insights into AF Termination
Patients whose AF terminated by ablation showed temporospatial AF regularization at the first stage (PVI), while patients whose AF did not terminate showed minimal regularization at any stage of ablation despite progressive AFCL prolongation. These results suggest that patients in whom PV isolation causes temporospatial AF regularization may have AF mechanisms predominantly near the PVs. These findings complement our prior work that isolating successive PVs organizes paroxysmal AF to the point of termination11. These results must be interpreted in the context of our stereotypical stepwise ablation sequence, in which PVI is always performed first, although considerable data emphasize the importance of PVI1.
Patients without procedural AF termination showed no increase in temporospatial regularization after PVI or additional extensive ablation. It is possible that ablation was performed less effectively in these patients, although the same operators performed all cases and PVI and lines of block were confirmed in all patients. An alternative explanation is that the mechanisms perpetuating AF in these patients lie further from the PVs. In this interpretation, AFCL prolongation may reflect increased pathlength due to ablation rather than slowing of perpetuating mechanisms, although further mapping is required to address this issue. Of interest, AFCL predicted AF termination in the entire cohort (i.e. including those terminated by PVI), but not in patients whose AF persisted after PVI.
Technical considerations
Indices of AF temporal and spatial regularity have been used in the non-ablation setting by many laboratories14, 15, 31 including our own 32. We selected 8.192s (=213ms) recordings because such long durations are stable over time 24. We have validated vector regularity analyses in several studies 25, 33. We selected right atrial appendage and coronary sinus electrodes for analysis since these sites span large portions of the left and right atrium and interatrial septum and are stable.
Clinical Application
We acknowledge that the desirability of AF termination by ablation remains controversial. Five groups have reported improved outcome when AF is terminated by ablation3, 5–9 while others did not4, 21, 34. In patients whose AF shows temporal or spatial regularization after PVI, AF termination is likely to occur by stepwise ablation with 92% and 96% PPV, respectively, and high freedom from AF on 2 year follow-up. If post-PVI organization is not observed, procedural AF termination is less likely, albeit with modest NPV(52% and 64% for TRI and SRI, respectively). These results provide further motivation to re-confirm the completeness of PVI in all such patients.
Limitations
All patients received stepwise ablation and we cannot determine if the ablation sequence should be varied or if steps can be eliminated if AF regularizes after PVI. Second, we did not assess the predictive value of SRI or TRI after each ablation step to avoid diluting statistical power, and because PVI is performed by most laboratories and thus a useful stage to assess efficacy. Technically, increased TRI is not a ‘pure’ index of temporal regularity, because it also indicates regularization in cycle-to-cycle electrogram shape. The spatial vector spans both atria, and may thus dilute organization within the left atrium alone. Future work should compare spatial vectors during AF ablation. Another limitation is that it is difficult to ensure completeness of some ablation steps until pacing can be performed. Finally, the rate of AF termination in this study is slightly lower than in prior publications since we did not include patients in whom PVI terminated AF35.
CONCLUSIONS
In patients with persistent AF in whom AF continues despite PVI, regularized temporospatial activation predicts AF termination on stepwise ablation. Increased temporal or spatial regularity after PVI provide ≥90 % positive predictive accuracy for AF termination by ablation, independent of AFCL prolongation. SRI and TRI may therefore be useful intraprocedural indices to identify patients in whom stepwise ablation terminates AF with high long-term clinical success.
Supplementary Material
Acknowledgements
We are grateful to Valérie Aurillac for statistical assistance, and to the nursing staff at l’Hôpital Haut-Lévêque for their help during the clinical research protocol.
This work was supported by grants to AF from Fondation Vaudoise de Cardiologie (Switzerland) and the European Heart Rhythm Association, and to SMN from Doris Duke Charitable Foundation, National Institutes of Health (R01 HL83359), and Biosense-Webster. Dr. Narayan holds equity in Topera Inc; Topera does not fund any research including that presented here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Références
- 1.Calkins H, Brugada J, Packer D, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation.European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) Heart Rhythm. 2007 Jun;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. Epub 2007 Apr 30. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill MD, Wright M, Knecht S, et al. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J. 2009 May;30:1105–1112. doi: 10.1093/eurheartj/ehp063. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo S, Lellouche N, Wright M, et al. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J Am Coll Cardiol. 2009 Aug 25;54:788–795. doi: 10.1016/j.jacc.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 4.Elayi CS, Verma A, Di Biase L, et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008 Dec;5:1658–1664. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Fiala M, Chovancik J, Nevralova R, et al. Termination of long-lasting persistent versus short-lasting persistent and paroxysmal atrial fibrillation by ablation. Pacing Clin Electrophysiol. 2008 Aug;31:985–997. doi: 10.1111/j.1540-8159.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 6.Lo LW, Tai CT, Lin YJ, et al. Predicting factors for atrial fibrillation acute termination during catheter ablation procedures: implications for catheter ablation strategy and long-term outcome. Heart Rhythm. 2009 Mar;6:311–318. doi: 10.1016/j.hrthm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Rostock T, Steven D, Lutomsky B, et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol. 2008 Jun 3;51:2153–2160. doi: 10.1016/j.jacc.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Chugh A, Good E, et al. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010 Mar;7:295–302. doi: 10.1016/j.hrthm.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Haissaguerre M, Hocini M, Sanders P, et al. Catheter Ablation of Long-Lasting Persistent Atrial Fibrillation: Clinical Outcome and Mechanisms of Subsequent Arrhythmias. Journal of Cardiovascular Electrophysiology. 2005b;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 10.Hocini M, Nault I, Wright M, et al. Disparate Evolution of Right and Left Atrial Rate During Ablation of Long-Lasting Persistent Atrial Fibrillation. J Am Coll Cardiol. 2010;55:1007–1016. doi: 10.1016/j.jacc.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haissaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004 Jun 22;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 12.Nault I, Lellouche N, Matsuo S, et al. Clinical value of fibrillatory wave amplitude on surface ECG in patients with persistent atrial fibrillation. J Interv Card Electrophysiol. 2009 Oct;26:11–19. doi: 10.1007/s10840-009-9398-3. [DOI] [PubMed] [Google Scholar]
- 13.Raine D, Langley P, Murray A, Furniss SS, Bourke JP. Surface Atrial Frequency Analysis in Patients with Atrial Fibrillation: Assessing the Effects of Linear Left Atrial Ablation. Journal of Cardiovascular Electrophysiology. 2005;16:838–844. doi: 10.1111/j.1540-8167.2005.40456.x. [DOI] [PubMed] [Google Scholar]
- 14.Bollmann A, Kanuru NK, McTeague KK, Walter PF, DeLurgio DB, Langberg JJ. Frequency Analysis of Human Atrial Fibrillation Using the Surface Electrocardiogram and Its Response to Ibutilide. Am J Cardiol. 1998;81:1439–1445. doi: 10.1016/s0002-9149(98)00210-0. 1998/6/15. [DOI] [PubMed] [Google Scholar]
- 15.Everett TH, IV, Moorman JR, Kok L-C, Akar JG, Haines DE. Assessment of Global Atrial Fibrillation Organization to Optimize Timing of Atrial Defibrillation. Circulation. 2001b;103:2857–2861. doi: 10.1161/01.cir.103.23.2857. 2001/6/12. [DOI] [PubMed] [Google Scholar]
- 16.Everett I, Thomas H, Akar JG, Kok L-C, Moorman JR, Haines DE. Use of global atrial fibrillation organization to optimize the success of burst pace termination. J Am Coll Cardiol. 2002;40:1831–1840. doi: 10.1016/s0735-1097(02)02476-2. 2002/11/20. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Sanders P, Jais P, et al. Organization of frequency spectra of atrial fibrillation: relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17:382–388. doi: 10.1111/j.1540-8167.2005.00414.x. 2006 Apr. [DOI] [PubMed] [Google Scholar]
- 18.Konings K, Kirchhof C, Smeets J, Wellens H, Penn O, Allessie M. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. 1994/4/1. [DOI] [PubMed] [Google Scholar]
- 19.Botteron GW, Smith JM. Quantitative assessment of the spatial organization of atrial fibrillation in the intact human heart. Circulation. 1996;93:513–518. doi: 10.1161/01.cir.93.3.513. [DOI] [PubMed] [Google Scholar]
- 20.Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005 Nov;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004a;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. 2004/6/2. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, O'Neill MD, Hocini M, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008 Mar 11;51:1003–1010. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 23.Hocini M, Jais P, Sanders P, et al. Techniques, Evaluation, and Consequences of Linear Block at the Left Atrial Roof in Paroxysmal Atrial Fibrillation: A Prospective Randomized Study. Circulation. 2005;112:3688–3696. doi: 10.1161/CIRCULATIONAHA.105.541052. 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 24.Narayan SM, Krummen DE, Kahn AM, Karasik PL, Franz MR. Evaluating Fluctuations in Human Atrial Fibrillatory Cycle Length Using Monophasic Action Potentials. Pacing Clin Electrophysiol. 2006d;29:1209–1218. doi: 10.1111/j.1540-8159.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown JP, Krummen DE, Feld GK, Narayan SM. Using Electrocardiographic Activation Time and Diastolic Intervals to Separate Focal from Macroreentrant Atrial Tachycardias. J Am Coll Cardiol. 2007;49:1965–1973. doi: 10.1016/j.jacc.2006.10.080. [DOI] [PubMed] [Google Scholar]
- 26.Dibs SR, Ng J, Arora R, Passman RS, Kadish AH, Goldberger JJ. Spatiotemporal characterization of atrial activation in persistent human atrial fibrillation: multisite electrogram analysis and surface electrocardiographic correlations--a pilot study. Heart Rhythm. 2008 May;5:686–693. doi: 10.1016/j.hrthm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal Periodicity During Atrial Fibrillation in the Isolated Sheep Heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. 1998/9/22. [DOI] [PubMed] [Google Scholar]
- 28.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of Left-to-Right Atrial Frequency Gradient in Paroxysmal but Not Persistent Atrial Fibrillation in Humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. 2004/11/16. [DOI] [PubMed] [Google Scholar]
- 29.Hoppe BL, Kahn AM, Feld GK, Hassankhani A, Narayan SM. Separating Atrial Flutter from Atrial Fibrillation with Apparent ECG Organization Using Dominant and Narrow F-wave Spectra. J Am Coll Cardiol. 2005;46:2079–2087. doi: 10.1016/j.jacc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 30.Narayan SM, Hassankhani A, Feld GK, Bhargava V. Separating Non-Isthmus From Isthmus Dependent Atrial Flutter Using Wavefront Variability. J Am Coll Cardiol. 2005b 45;:1269–1279. doi: 10.1016/j.jacc.2004.12.070. April 19, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008 Apr 9;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummen DE, Peng K, Bullinga JR, Narayan SM. Centrifugal Gradients of Rate and Organization in Human Atrial Fibrillation. Pacing & Clinical Electrophysiology. 2009;32:1366–1378. doi: 10.1111/j.1540-8159.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn A, Krummen DE, Feld GK, Narayan SM. Localizing Circuits of Atypical Atrial Flutter Using Preferred ECG Planes of Atrial Activation. Heart Rhythm. 2007;4:445–451. doi: 10.1016/j.hrthm.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009 Mar 3;53:782–789. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 35.Haissaguerre M, Sanders P, Hocini M, et al. Catheter Ablation of Long-Lasting Persistent Atrial Fibrillation: Critical Structures for Termination. Journal of Cardiovascular Electrophysiology. 2005a;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.