Abstract
Reactive oxygen species (ROS) play an important role in physiological and pathological processes. In recent years, a feed-forward regulation of the ROS sources has been reported. The interaction between main cellular sources of ROS, such as mitochondria and NADPH oxidases, however, remain obscure. This work summarizes the latest findings on the role of crosstalk between mitochondria and NADPH oxidases in pathophysiological processes. Mitochondria have the highest levels of antioxidants in the cell and play an important role in the maintenance of cellular redox status, thereby acting as an ROS and redox sink and limiting NADPH oxidase activity. Mitochondria, however, are not only a target for ROS produced by NADPH oxidase but also a significant source of ROS, which under certain condition may stimulate NADPH oxidases. This crosstalk between mitochondria and NADPH oxidases, therefore, may represent a feed-forward vicious cycle of ROS production which can be pharmacologically targeted under conditions of oxidative stress. It has been demonstrated that mitochondria-targeted antioxidants break this vicious cycle, inhibiting ROS production by mitochondria and reducing NADPH oxidase activity. This may provide a novel strategy for treatment of many pathological conditions including aging, atherosclerosis, diabetes, hypertension and degenerative neurological disorders in which mitochondrial oxidative stress seems to play a role. It is conceivable that the use of mitochondria-targeted treatments would be effective in these conditions.
Introduction
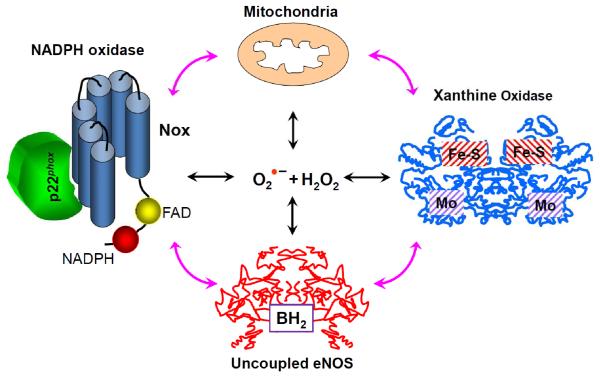
Over the past several years, it has become clear that reactive oxygen species (ROS) play an important role in both physiological and pathological processes.1, 2 Superoxide () and hydrogen peroxide (H2O2) have been implicated in redox regulation of cell differentiation, proliferation, migration and vasodilatation.3-6 Under normal physiological conditions, production of ROS is highly restricted to specific subcellular sites and is down regulated by a number of negative feed-back mechanisms.7-10 Production of ROS “in the wrong place at the wrong time” or generation of ROS in excessive amounts results in oxidative stress leading to cellular dysfunction and apoptosis which contributes to atherosclerosis,11 heart failure,12 hypertension,13 ischemia/reperfusion injury,14 cancer,15 aging16 and neurodegeneration.17 While there are numerous enzyme systems that produce ROS in mammalian cells, four enzymatic systems seem to predominate. These include the NADPH oxidases,18 xanthine oxidase,19 uncoupled NO synthase 20 and the mitochondrial electron transport chain.16 There is a substantial interplay between these sources, such that activation of one can lead to activation of the others (Figure 1). This can lead to feed forward processes, which further augment ROS production and oxidative stress.21 The phenomenon of ROS-induced ROS production is very well documented: H2O2 activates production by phagocytic and non-phagocytic NADPH oxidases;22 peroxynitrite uncouples eNOS switching from NO to production and increases production of mitochondrial ROS;23, 24 H2O2 induces transformation of XDH into XO, a source of H2O2 and .25 The interplay between specific ROS sources, however, is not clear. Crosstalk between two major ROS sources, mitochondria and NADPH oxidases, is of particular interest.
Figure 1.
Interaction of various sources of reactive oxygen species ROS. The production of ROS from any one source can lead to activation of the NADPH oxidases, conversion of xanthine dehydrogenase to xanthine oxidase, can stimulate the production of mitochondrial ROS or result in uncoupling of the endothelial nitric oxide synthase (eNOS).
Mitochondrial function and production of mitochondrial ROS
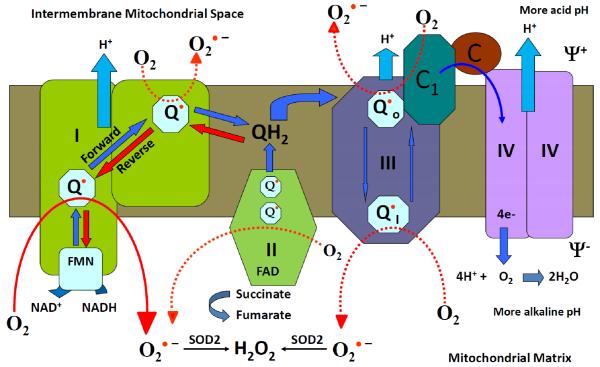
It is generally assumed that the major biological function of mitochondria is ATP synthesis by oxidative phosphorylation.26 This process is based on aerobic oxidation of hydrogen and is much more efficient than anaerobic metabolism of glucose. It is based on transfer of electrons through the mitochondrial respiratory chain (Figure 2). Electrons can be supplied by either NADH at complex I or by succinate at complex II. Ubiquinone mediates electron transfer to complex III, which in turn reduces complex IV. Complex IV couples oxygen reduction to water and the proton pump, transporting protons (H+) from the matrix to the intermembrane space. Respiring mitochondria generate the proton motive force across the inner membrane which results in a negative charge inside and produces a pH gradient.27
Figure 2.
Schematic presentation of the mitochondrial electron transport chain and production of mitochondrial .
At several sites of the respiratory chain, electrons “leak” to O2 creating 28, 29 (Figure 1). The main sources of mitochondrial ROS under physiological conditions are complexes I and II, which produce mainly on the matrix side, where it is rapidly dismutated to H2O2 by mitochondrial Mn-SOD (SOD2).30, 31 Other sources of mitochondrial may include alpha-ketoglutarate dehydrogenase, pyruvate dehydrogenase,32 glycerol 3-phosphate dehydrogenase, fatty acid beta-oxidation,33 and complex III.34, 35 H2O2 is a neutral molecule and will easily leave mitochondria regardless of mitochondrial energization. The amount of mitochondrial H2O2 is in the range of 0.1% to 2% of the electron flow.16
Until recently, the functional significance of mitochondria-derived ROS, particularly in vascular cells, has received little attention. This is partly due to low metabolic activity and the lack of information regarding regulation of mitochondrial ROS compared with other enzymes like NADPH oxidase.18 However, a paradigm shift has occurred in recent years, focusing greater attention on a potential key role of mitochondrial ROS in cell signaling.36
A new concept is emerging that mitochondria are more than just “ATP cows”37, 38 and ROS production by mitochondria is a part of their physiological function.1 This process is likely to be highly regulated and we are just beginning to uncover the specific molecular mechanisms. Reverse electron transport from complex II to complex I is likely to be a major pathway for mitochondrial ROS production. It is stimulated by complex II substrate succinate and can be inhibited by proton ionophore CCCP, rotenone or the complex II inhibitors malonate or oxaloacetate (Figure 2).39, 40 It has been recently shown that this pathway strongly depends on the pH gradient across the inner membrane (ΔpH).41 Activation of mitochondrial ATP-sensitive potassium channels (mitoKATP) increases production of mitochondrial ROS 42, 43 and is likely to be associated with an increase of ΔpH. In this review, we are particularly interested in reverse electron transport because it can be regulated by redox-sensitive mitoKATP and mitochondrial ATP level.44, 45
Ischemia and apoptosis trigger production by complex III.34 This may occur due to inhibition of complex IV and overreduction of the electron transport chain in cases of hypoxia or NO-mediated inhibition of complex IV which can be simulated by treatment with the complex III inhibitor antimycin A.46 The contribution of complex III in production of mitochondrial under normal physiological conditions is, however, not clear. It is possible that production by complex III does not depent on mitochondrial transmembrane potential as much as reverse electron transport.41 For example, uncoupling of mitochondria with antimycin A may inhibit production of mitochondrial ROS by reverse electron transport but stimulate production by complex III.47, 48
Mitochondrial manganese superoxide dismutase (SOD2) is a key scavenger of in the mitochondrial matrix. It is a nuclear-encoded protein that forms a homotetramer with each subunit binding one manganese atom. SOD2 plays critical roles in regulating redox-sensitive signaling pathways and controlling mitochondrial .49 By inhibiting the reaction of with 4Fe-4S clusters, this enzyme prevents inactivation of aconitase, complex I and complex II.50 SOD2 is inactivated by ONOO− 51 and its activity is decreased with age 52. Expression of SOD2 is upregulated by various cytokines and agonists in a redox-dependent manner 53. SOD2 overexpression attenuates H2O2-induced apoptosis,54 decreases lipid peroxidation and reduces the age-related decline in mitochondrial ATP.55
Mitochondria are not only one of the major sources of and H2O2 in vascular cells 56, 57 but are also the targets of cellular ROS 56. Mitochondrial membranes, proteins, and mtDNA are particularly sensitive to oxidative damage.58, 59 ROS modify mitochondrial proteins, leading to their inactivation, as in the case of SOD2 and aconitase, or alter their function, as occurs with cytochrome c 60-62. Superoxide reacts with 4Fe-4S clusters of complex I, complex II and aconitase, resulting in the release of Fe3+ and altered protein function 16. It has been shown that oxidative damage to complex I and complex II, presumably at the level of 4Fe-4S clusters, increases mitochondrial production. Interestingly, a decrease in complex II activity due to oxidative modification increases its production by 3-4 fold.63
Enhanced production of mitochondrial ROS is linked to mitochondrial dysfunction.64 Mitochondrial oxidative stress not only alters the ability of the cell to generate energy but also affects cellular redox signaling 56. ROS generated in the mitochondrial respiratory chain have been proposed as secondary messengers for activation of NFκB by TNF-α and IL-1.65 Mitochondrial ROS, therefore, not only enhance cellular oxidative stress, but can represent an important modulator of cellular function. Growth factor receptor transactivation and its downstream signaling in response to H2O2 are abrogated by mitochondrial targeted antioxidants, but not by nontargeted counterparts, suggesting the involvement of mitochondrial oxidants in these events.66
Mitochondrial protein kinase C epsilon (PKCε) has been shown to play an important role in modulation of mitochondrial function. This enzyme is known to phosphorylate and activate the mitoKATP. Moreover PKCε is exquisitely redox sensitive and therefore is a very good candidate to transduce signals from extramitochondrial ROS leading to mitochondrial ROS production.45 Indeed, our data implicate activation of mitoKATP in stimulation of mitochondrial ROS.42 We, therefore, hypothesize that ROS produced by extramitochondrial NADPH oxidases stimulate PKCε, which then phosphorylates and activates mitoKATP.
Mitochondria-targeted antioxidants
Recent studies have demonstrated that decrease of mitochondrial ROS by overexpression of SOD2 protects against mitochondrial oxidative damage and myocardial dysfunction 67-69. Low-molecular weight antioxidants, such as α-tocopherol and N-acetylcysteine, also decrease mitochondrial oxidative damage in vitro 70. In vivo, however, these traditional antioxidants have limited mitochondrial accumulation.71 A major continued challenge, therefore, is to develop mitochondria-targeted antioxidant agents that can prevent mitochondrial oxidative damage and mitochondrial dysfunction 71. Antioxidants can be targeted to mitochondria by several methods: (i) preferential accumulation in mitochondria because of their hydrophobicity and positive charge (hydrophobic cations), (ii) binding with high affinity to an intra-mitochondrial constituent, and (iii) metabolic conversions by specific mitochondrial enzymes to reveal an active entity.72
The membrane potential of mitochondria within living cells is negative inside (−140 mV). As this membrane potential is far larger than in other organelles within cells, lipophilic cations such as triphenylmethylphosphonium (TPMP) selectively accumulate within mitochondria 71. Antioxidants conjugated to TPMP, therefore, can be targeted to mitochondria. Due to their positive charge, agents such as MitoTEMPOL 73 are concentrated in the mitochondrial matrix by 1000-fold.74 It has been reported that MitoTEMPOL is readily reduced to its hydroxylamine (MitoTEMPOL-H) by a direct reaction with ubiquinol.73 MitoTEMPOL-H may react with producing MitoTEMPOL similar to the reaction of TEMPONE-H.75 A previous report showing lack of scavenging by MitoTEMPOL-H was likely due to the artifact associated with direct reduction of cytochrome C by MitoTEMPOL-H.76 Our spin trapping experiments unambiguously demonstrate scavenging of by hydroxylamine mitoTEMPO-H.21
While mitoTEMPOL acts as an SOD-mimetic converting molecules into H2O2, 21 the benefit of such agents probably exceeds that of simply scavenging superoxide, because it seems by preventing mitochondrial damage, we reduce mitochondrial electron leak and thus inhibit production of all ROS, including superoxide, hydrogen peroxide and peroxynitrite. Recently, it has been shown that pretreatment of endothelial cells with the mitochondria-targeted SOD mimetic mito-CP significantly reduced H2O2- and lipid peroxide-induced cellular oxidative stress 77. Mito-CP inhibited peroxide-induced inactivation of complex I and aconitase, while restoring the mitochondrial membrane potential. In contrast, the “untargeted” carboxy proxyl (CP) did not protect the cells from peroxide-induced oxidative stress and apoptosis.
The pharmacology of mitochondria-targeted antioxidants is not well understood. For example, previously described mitoquinone (MitoQ10) 78 may have prooxidant and proapoptotic properties due to redox cycling and generation of by quinone. 79, 80 Nitroxides such as TEMPO have very low toxicity making them perfect candidates for conjugation with triphenylmethylphosphonium for in vivo use, 81 but antioxidants such as Mito-CP are esters and potentially can be hydrolyzed into inactive 3-carboxyproxyl (CP) 77 and triphenylmethylphosphonium. Furthermore, micromolar concentrations of tetraphenylphosphonium inhibit oxidation of pyruvate, malate, 2-oxoglutarate and glutamate in heart mitochondria, 82 suggesting that triphenylmethylphosphonium conjugates should be used at submicromolar concentrations and tested for side effects on respiration. We have recently described in vivo applications of the mitochondria-targeted SOD mimetic mitoTEMPO, which is resistant to hydrolysis, and low doses of mitoTEMPO (25 nM in vitro and 0.7 mg/kg/day in vivo) did not reveal side effects on respiration.
The cationic, arginine-rich SS (Szeto-Schiller) tetrapeptides Dmt-D-Arg-Phe-Lys-NH2 and D-Arg-Dmt-Lys-Phe-NH employ the targeted delivery of antioxidants to the inner mitochondrial membrane 83 and have been shown to be very effective in diminishing mitochondrial ROS, inhibiting mitochondrial permeability transition, reducing cytochrome c release,84 attenuating mitochondrial swelling, inhibiting oxidative cell death, and reperfusion injury.72 Notably, these small peptides were concentrated 1000-fold across the inner mitochondrial membrane and also readily crossed the cell membrane. Preclinical studies support the use of these peptides for ischemia-reperfusion injury and neurodegenerative disorders. Although peptides have often been considered to be poor drug candidates, the few that have been studied are promising agents for the treatment of diseases.84
Gramicidin S-TEMPO conjugates (GS-TEMPO) preferentially accumulates in the mitochondria due to high-affinity GS binding with the inner mitochondrial membranes. The gramicidin segment was used to target the nitroxide to mitochondria because antibiotics of this type have a high affinity for bacterial membranes and because of the close relationship between bacteria and mitochondria. In a rat model of hemorrhagic shock, delayed treatment with XJB-5-131 has been shown to prolong survival time.85
NADPH oxidases
NADPH oxidases are a family of enzyme complexes whose primary function is to catalyze the transfer of electrons from NADPH to molecular oxygen via their “Nox” catalytic subunit, generating and H2O2. The Nox enzymes contribute to numerous biological and pathological processes including hearing and balance (Nox3), blood pressure regulation, inflammation, cell growth (Nox1/Nox2), and differentiation (Nox4).86 The Nox proteins vary in terms of their mode of activation and localization.87 Nox1 is expressed in smooth muscle cells, but is also present in other vascular cells. Nox2, previously known as gp91phox, is present in endothelial and phagocytic cells.88-91 Nox3 is expressed in the brain and inner ear.86 Nox4 is constitutively expressed and active in vascular smooth muscle and endothelial cells. 92, 93 Nox5 has been identified in human immature lymphatic tissues, in human endothelial cells, and it is activated by Ca2+ binding to EF-hand motifs. The Duox1/Duox2 proteins are described as having a dual nature due to an extracellular peroxidase domain in addition to the EF-hand Ca2+ binding and gp91phox homology domains. Originally isolated from the thyroid, they produce the H2O2 that is used to oxidize iodide during thyroid hormone synthesis.94
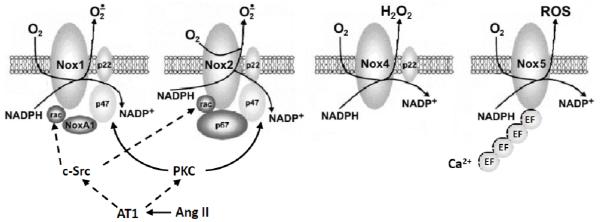
It is important that Nox isoforms have not only different regulation and specific subcellular localization but also generate distinct ROS. For example, Nox4 is responsible for the basal production of H2O2, 95, 96 Nox1 and Nox2 generates ,95 and Nox5 produces H2O2 in a Ca2+ dependent fashion 97 (Figure 3).
Figure 3.
Structural homology of the vascular NADPH oxidases.
It has been recently reported that Nox4 may be expressed in the mitochondria of rat kidney cortex 98 and in the mitochondria of cardiac myocytes.99 Ago et al. reported higher expression of Nox4 in mitochondrial fraction of cardiac myocytes compared to microsomal fraction.100 Confocal microscopy showed significant co-localization of Nox4 with mitochondrial F1F0-ATP synthase as well as p22phox subunit of NADPH oxidases. Cysteine residues of mitochondrial proteins were more oxidized in Nox4-transgenic mice. Nox4 expression did correlate with dihydroethidium staining for superoxide and cardiac damage.100 These studies, however, remain highly controversial since they were not able to directly demonstrate the Nox4 activity in mitochondrial preparations but showed crude NADH dependent ROS production which is known to be mediated by mitochondrial complex I. The assessment of Nox4 activity by superoxide measurements with dihydroethidium is also questionable since several groups have reported that Nox4 primarily generates H2O2 but not superoxide.95, 96 Our studies did not show the presence of Nox1, Nox2, Nox4 and p22phox subunits in the mitochondria of endothelial cells.42 It has been previously shown that Nox4 is specifically localized in focal adhesions, along stress fibers, and in the nucleus. Nox4 is co-localized with the p22phox subunit which is required for Nox4 activity.93, 101 Unfortunately, Ago et al. did not present co-localization of p22phox and cardiac mitochondria. It is possible that mitochondrial localization of Nox4 reported by Block et al.98 and Ago et al. 100 differ from previous publications 93, 101 due to distinct Nox4 antibodies used for immunostaining while many authors raised concerns regarding the specificity of some Nox4 antibodies. Although it may be intriguing to suggest the role for Nox4 in mitochondrial oxidative stress, the lack of data on mitochondrial p22phox and the absence of specific measurements of mitochondrial Nox4 activity do not support this hypothesis. It is also important that mitochondria do not require any Nox isoform to produce ROS as described above and ROS production by mitochondria can significantly surpass the amount of ROS produced by Nox4, particularly in the heart. The proposed role of Nox4 in cardiac pathology 100 may also conflict with Nox4-mediated cardiac protection against chronic load-induced stress by enhancing angiogenesis.102 Therefore, the data on mitochondrial expression of Nox4 and its functional significance should be taken with caution and further studies of mitochondrial Nox4 are required. Meanwhile, it is conceivable that cytoplasmic Nox4 may contribute to redox sensitive upregulation of mitochondrial ROS produced in the electron transport chain via activation of PKCε, mitoKATP or modulation of thioredoxin 2 activity as described below.
Angiotensin II is the major effector hormone of the renin–angiotensin system which plays an important role in the activation of vascular NADPH oxidases by PKC and c-Src dependent pathways.103 Initial activation of the angiotensin AT1 receptor leads to PKC-mediated phosphorylation of p47phox. This leads to c-Src activation and stimulation of the epidermal growth factor receptor (EGFR), which evokes phosphatidylinositol 3-kinase-dependent production of phosphatidylinositol (3,4,5)-trisphosphate and, in turn, activates Rac1 subunit of NADPH oxidase.104 Nox4 and Nox5 do not require p47phox or Rac1 subunits.105 Thus, in vascular cells, AngII primarily increases activity of Nox1 or Nox2 (Figure 3).106 Activation of c-Src is redox sensitive and stimulated by H2O2,4 which appears to represent a feed-forward mechanism whereby H2O2-mediated activation of c-Src amplifies NADPH oxidase activity of Nox1 and Nox2.
A correlation between endothelial Nox2 expression and hypertension has been reported.107 Aortic Nox2 is elevated in stroke-prone SHR, in rats exposed to aldosterone plus salt and in AngII-infused mice. 108 The SOD mimetic TEMPOL inhibits redox-dependent Nox2 expression and improves pulmonary hypertension in renin transgenic rats.109 A chimeric peptide that inhibits the association of p47phox with Nox2 in NADPH oxidase (gp91ds-tat) attenuates AngII-induced hypertension and decreases aortic production in AngII-treated rats.110 It was found that this peptide inhibits the NADPH oxidase in vivo and was very effective in inhibiting Nox2 function in vitro.
Angiotensin-converting enzyme inhibitors and type I angiotensin-receptor blockers also reduce age-related mitochondrial dysfunction, attenuate hypertension-induced renal mitochondrial dysfunction, and protect against cardiac mitochondrial dysfunction in the setting of acute ischemia. 111-113 We have found that depletion of the p22phox subunit prevents mitochondrial dysfunction and the increase of mitochondrial ROS caused by AngII.42 These findings suggest that AngII can alter mitochondrial function via activation of NADPH oxidases.12, 42 The molecular mechanisms underlying the interplay between the NADPH oxidases and the mitochondria remain undefined.
Stimulation of mitochondrial ROS by NADPH oxidases
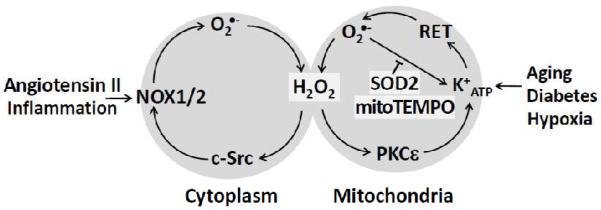
We have previously reported that AngII increases production of mitochondrial ROS and decreases mitochondrial membrane potential, respiratory control ratio, and low molecular weight thiols content. Activation of NADPH oxidases is an early response of endothelial cells to AngII.114 Angiotensin II binds to the AngII type 1 receptor, leading to rapid-generation of ROS through PKC-dependent activation of NADPH oxidases. The deleterious effects of AngII on mitochondrial function are associated with increased cellular production and decreased endothelial NO• bioavailability. Interestingly, our results indicate that AngII-mediated mitochondrial dysfunction is dependent on activation of vascular NADPH oxidases and opening of the mitoKATP channels. Indeed, Paul Brookes’ group showed that mitoKATP channels are activated by and H2O2 but not other peroxides.44 Our data support activation of mitoKATP by ROS coming from NADPH oxidases because inhibition of NADPH oxidases and PKC by apocynin and chelerythrine completely prevented mitochondrial dysfunction induced by AngII. Apocynin is known to block the activation of NADPH oxidases, and chelerythrine selectively inhibits PKC. Both inhibitors dramatically attenuated mitochondrial ROS generation in response to AngII. Most importantly, depletion of p22phox, an essential component for NADPH oxidase function, with siRNA led to a significant decrease in ROS production in mitochondria isolated from AngII treated cells. Treatment with the mitoKATP channels blocker 5-HD and glibenclamide prevented the increase in mitochondrial H2O2, attenuated the decrease in mitochondrial membrane potential, and preserved respiratory control ratio and low molecular weight thiols content induced by AngII. 42 Taken together, these results showed that stimulation of mitochondrial ROS by AngII requires the full enzymatic activity of NADPH oxidases and depends on activation of redox sensitive mitoKATP channels (Figure 4).42
Figure 4.
Proposed crosstalk between mitochondria and NADPH oxidases.
Our data also clearly implicate mitoKATP channels in AngII–mediated mitochondrial dysfunction. As we observed, 5-HD, a specific inhibitor of mitoKATP channels, and glibenclamide, a non-selective inhibitor of ATP-sensitive potassium channels, suppressed AngII-induced mitochondrial H2O2 production and membrane potential depolarization, prevented the decrease in mitochondrial RCR and reduced thiols content. The mechanism by which AngII regulates mitoKATP channels activity is unclear. An involvement of and PKC in activation of mitoKATP channels has been suggested in vascular smooth muscle cells and cardiac cells.115, 116 AngII activated NADPH oxidase-derived is capable of stimulating the opening of the mitoKATP channels via a direct action on the sulfhydryl groups of this channel.117 Opening of these channels has been proposed to increase potassium influx causing matrix alkalinization, swelling, mild mitochondrial uncoupling and ROS production.118 It is conceivable that mitoKATP channels are both downstream and upstream of mitochondrial ROS providing feed-forward regulation.
Interestingly, acute treatment with the mitoKATP channels inhibitor 5-HD after four hours incubation with AngII brought back endothelial production to baseline levels and restored NO• bioavailability in endothelial cells. The decline in generation and recovery of NO• production by 5-HD implies that mitochondrial ROS indeed enhances endothelial oxidative stress by a feed-forward mechanism (Figure 4).42
Activation of NADPH oxidases by mitochondrial ROS
It has been shown that opening of mitoKATP channels with diazoxide in rat vascular smooth muscle cells depolarized the mitochondrial membrane potential and increased cellular detected by dihydroethidium.115 Activation of mitoKATP channels with diazoxide stimulates production on mitochondrial complex I43; however, dihydroethidium does not detect mitochondrial .21 The increase in dihydroethidium fluorescence indicates production in the cytoplasm by NADPH oxidases.21 These data therefore suggest that stimulation of mitochondrial may increase the activity of NADPH oxidases leading to enhanced production in the cytoplasm. However, the exact mechanism of this process is not clear.
Studies with human embryonic kidney 293T cells have shown that serum withdrawal promotes the production of mitochondrial ROS and the activation of Nox1.119 Mitochondria respond to serum withdrawal within a few minutes, and the ROS produced by the mitochondria trigger Nox1 action by stimulating phosphoinositide 3-kinase (PI3K) and Rac1. Activation of the PI3K/Rac1/Nox1 pathway was evident 4-8 hours after serum withdrawal initiation. Functional analysis suggested that, although the mitochondria contribute to the early accumulation of ROS, the maintenance of the induced ROS levels in the later (4-8 h) phase required the action of the PI3K/Rac1/Nox1 pathway. Serum withdrawal-treated cells eventually lost their viability, which was reversed by blocking either the mitochondria-dependent induction of ROS using rotenone or the PI3K/Rac1/Nox1 pathway using the dominant negative mutants or small interfering RNAs. This suggests that mitochondrial ROS are essential but not sufficient to promote cell death, which requires the sustained accumulation of ROS by the subsequent action of Nox1.119
Recently, effects of mild mitochondrial dysfunction on AngII-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells have been described.120 Mild mitochondrial dysfunction due to mitochondrial DNA damage after 24 h incubation of rabbit aortic smooth muscle with ethidium bromide (EtBr) resulted in 29% less oxygen consumption and 16% greater baseline hydrogen peroxide. The normally observed increase in NADPH oxidase activity after AngII was completely abrogated after EtBr, together with failure to upregulate Nox1 mRNA expression. Similar loss in AngII redox response occurred after 24 h of antimycin A treatment. These results implicate mitochondria in regulation of expression and activity of NADPH oxidase.
The role of mitochondrial ROS was further investigated in endothelial cells with overexpression or depletion of mitochondrial superoxide dismutase (SOD2). Transfection of HAEC with an SOD2 plasmid increased mitochondrial SOD2 activity by 2.4-fold, while depletion of SOD2 with siRNA decreased SOD2 activity by 2.7-fold. In cells transfected with a GFP control plasmid, AngII stimulation doubled NADPH oxidase activity. In contrast, AngII had no effect on NADPH oxidase activity in HAEC transfected with the SOD2 plasmid. Interestingly, depletion of SOD2 increased NADPH oxidase activity in unstimulated cells. Furthermore, AngII stimulation of SOD2 depleted cells resulted in higher activity of NADPH oxidase.21
Analysis of intact cells showed that AngII increased the to a similar extent in non-transfected cells or cells treated with a GFP control plasmid. SOD2 overexpression completely prevented AngII-stimulated production while not affecting production in unstimulated cells. SOD2 depletion enhanced both basal and AngII-stimulated in intact cells. These data confirmed that modulation of mitochondrial by mitoTEMPO or changing SOD2 levels affects the production of cellular by NADPH oxidases. Fluorescence microscopy with mitoSOX showed that SOD2 depletion increased both basal and AngII–stimulated mitochondrial superoxide production, and that this could be inhibited by mitoTEMPO. It is important to note that mitoTEMPO treatment inhibited cellular and mimicked SOD2 overexpression in SOD2 depleted cells, rescuing SOD2 depleted cells.21
Crosstalk between mitochondria and NADPH oxidases
The data described above suggest that activation of NADPH oxidases may increase production of mitochondrial ROS and vice versa: increase of mitochondrial ROS may activate NADPH oxidases. We have suggested that this represents an ongoing feed-forward cycle. Indeed, acute treatment of AngII-stimulated cells with mitochondria-targeted SOD mimetic mitoTEMPO reduced mitochondrial superoxide measured by mitoSOX, completely blocked the increase in NADPH oxidase activity measured in the membrane fraction and abrogated production of cytoplasmic measured in intact cells using dihydroethidium and HPLC.21 It is important that mitoTEMPO or SOD2 overexpression did not affect basal NADPH oxidase activity in unstimulated cells but only inhibited Nox activity in stimulated cells. Since AngII stimulates Nox1 and Nox2 activity, we think that these NADPH oxidase isoforms are particularly important in the crosstalk between mitochondria and NADPH oxidases (Figure 4).
An interesting example of crosstalk between mitochondrial and Nox-derived ROS has been reported in nitrate tolerance.110 Nitrate tolerance was induced by nitroglycerin infusion in male Wistar rats. Isometric tension studies revealed that genetic deletion or inhibition of NADPH oxidases improved endothelial function, whereas nitrate tolerance was unaltered. Vice versa, nitrate tolerance was attenuated by co-treatment with the respiratory chain complex I inhibitor rotenone or the mitochondrial permeability transition pore blocker cyclosporine A. Both compounds improved endothelial function, suggesting a link between mitochondrial and Nox-derived ROS. Mitochondrial respiratory chain-derived ROS are critical for the development of nitrate tolerance, whereas Nox-derived ROS mediate nitrate tolerance-associated endothelial dysfunction.110
Comparison of AngII and diazoxide showed that both mitochondrial ROS and NADPH oxidase contribute to redox-sensitive mitogen-activated protein kinase activation in rat vascular smooth muscle cells in vitro and in rat aorta in vivo.115 Similarly, AngII treatment with diazoxide depolarized the mitochondrial membrane potential and increased cytoplasmic superoxide production measured with DHE, resulting in phosphorylated MAP kinases (ERK1/2, p38, and JNK), which were suppressed by the specific inhibitor of mitoKATP channels 5-hydroxydecanoic acid. These results reveal that stimulation of mitochondrial ROS by NADPH oxidase dependent pathway or directly by diazoxide is required for maintenance of cytoplasmic superoxide production and redox-sensitive activation of MAP kinase.115
Overall, at least six different examples of cross-talk between mitochondrial and Nox-derived reactive oxygen species (ROS) have been reported.121 In the first model, AngII is discussed as a trigger for NADPH oxidase activation with subsequent ROS-dependent opening of mitoKATP channels in endothelial cells, leading to depolarization of mitochondrial membrane potential followed by mitochondrial ROS formation and respiratory dysfunction.42 In the second model, direct stimulation of mitoKATP channels with diazoxide in smooth muscle cells mimicked the effect of AngII on cytoplasmic production of superoxide by NADPH oxidases and redox-sensitive activation of MAP kinase.115 This concept was supported by observations that ethidium bromide-induced mitochondrial damage suppressed AngII-dependent increase in Nox1 and oxidative stress.120 In the third example, hypoxia was used as a stimulator of mitochondrial ROS formation and by using pharmacological and genetic inhibitors, a role of mitochondrial ROS for the induction of NADPH oxidases via PKCε was demonstrated.122 The fourth model was based on cell death by serum withdrawal that promotes the production of ROS in human 293T cells by stimulating both the mitochondria and Nox1.119 These studies showed that mitochondria were responsible for the fast onset of ROS formation followed by a slower but long-lasting oxidative stress condition based on the activation of Nox1 in response to the fast mitochondrial ROS formation. Fifth, cross-talk between mitochondria and Nox2 was shown in nitroglycerin-induced nitrate tolerance involving the mitochondrial permeability transition pore and ATP-sensitive potassium channels.123 Finally, it has been shown that interplay between NADPH oxidase and mitochondria is mediated by the level of mitochondrial superoxide both in vitro and in vivo.21 In this work it was shown that depletion of mitochondrial SOD2 increases the NADPH oxidase activity while SOD2 overexpression attenuates activation of NADPH oxidases.21
Taken together, these studies indicate that the interplay between mitochondrial and NADPH oxidase-derived constitutes a feed-forward cycle in which the NADPH oxidases increase mitochondrial ROS, which further activates the cytoplasmic NADPH oxidases and increases cellular production, diminishing NO• bioavailability and uncoupling eNOS.124 The effect of mitochondrial ROS on NADPH oxidase activity is quite likely mediated by c-Src 5 which can be stimulated by H2O2.4 Indeed, activation of NADPH oxidases has been reported to be a biphasic process in which the first phase requires direct activation by AngII followed by a second phase of sustained activation that is H2O2 dependent.104 This could explain why inhibition of mitochondrial H2O2 by mitoTEMPO results in decrease of NADPH oxidase activity.21 Our current findings indicate that scavenging of mitochondrial using mitochondria-targeted antioxidants can interrupt this vicious cycle and down-regulate NADPH oxidase activity.21 In the present study, we summarized the latest findings on the potential role of the interplay between mitochondria and NADPH oxidases in pathophysiological processes.
The interplay between mitochondrial ROS and NADPH oxidases may be rather specific and does not necessarily represent a general feed-forward mechanism due to an additive effect of ROS from Noxes and ROS from the mitochondria. Both mitochondria and NADPH oxidases are physically associated with the endoplasmic reticulum, and their redox signals can be very site specific without exposing the whole cell to elevated ROS. This crosstalk can be mediated by endoplasmic reticulum stress 125 which normally does not involve xanthine oxidase or uncoupled eNOS localized in the caveolae of the extracellular membrane. The crosstalk between mitochondrial ROS and NADPH oxidases likely plays an important role in normal physiological redox cell signaling. Under normal physiological conditions, production of ROS is highly restricted to specific subcellular sites and is down regulated by a number of negative feed-back mechanisms.7-10 Production of ROS in excessive amounts due to overstimulation by AngII, high glucose, fat or hypoxia results in oxidative stress and transforms this feed-forward redox signaling into a vicious cycle (Figure 4) which contributes to the development of many pathological conditions.21
Hypertension
While ROS do not regulate blood pressure under normal conditions, they clearly contribute to the elevation in blood pressure in the setting of hypertension. ROS mediate the potent vasoconstrictor and hypertrophic effects of AngII and treatment with antioxidants decreases AngII-induced hypertension 126, 127. PEG-SOD very effectively lowers blood pressure in AngII-treated rats, but not in normal rats 128. Blood pressure is normal in mice lacking subunits of the NADPH oxidase, but these animals have a blunted hypertensive response to both AngII and DOCA-salt treatment 129. Genetic overexpression of NADPH oxidase stimulates the hypertensive response to AngII 127, while overexpression of SOD attenuates the rise in blood pressure.126Using mice deficient in the NADPH oxidase subunit p47phox and mice lacking the endothelial NO synthase, it has been found that hypertension is a result of the cascade involving production of ROS from the NADPH oxidases leading to oxidation of tetrahydrobiopterin and uncoupling of endothelial NO synthase (eNOS).20
The role of mitochondrial oxidative stress in hypertension has been suggested in the work of Julian Wider and colleagues using mice overexpressing human thioredoxin 2 (Tg(hTrx2).130 Systolic arterial blood pressure was not different between Tg(hTrx2) and wild-type animals under baseline conditions but the AngII-induced hypertension in Tg(hTrx2) mice was significantly attenuated. Aortic endothelium-dependent relaxation was reduced in wild-type mice after AngII infusion but was nearly unchanged in transgenic mice. Elevated vascular ROS and expression of NADPH oxidase subunits in response to AngII infusion were significantly attenuated in Tg(hTrx2) mice. Mitochondrial superoxide was increased after AngII infusion in wild-type mice but not in Tg(hTrx2) mice. The precise molecular mechanisms of regulation of mitochondrial ROS and NADPH oxidase by thioredoxin 2, however, remain unclear.
Co-infusion of mitochondria-targeted antioxidant mitoTEMPO and AngII attenuated hypertension decreased mitochondrial , reduced cellular NADPH oxidase activity, inhibited vascular production and prevented the loss of endothelial NO.21 Treatment of mice in vivo with mitoTEMPO decreased blood pressure by 30 mm Hg following establishment of both AngII-induced and DOCA-salt hypertension, while a similar dose of non-targeted TEMPOL was not effective. In vivo, mitoTEMPO decreased vascular produced by NADPH oxidases, increased vascular NO• production and improved endothelial-dependent vasorelaxation. Interestingly, transgenic mice overexpressing mitochondrial SOD2 demonstrated attenuated AngII-induced hypertension and reduced vascular oxidative stress similar to mice treated with mitoTEMPO 21 while SOD2+/− mice were predisposed to both age-related and salt-induced hypertension.131 These studies show that mitochondrial is important for the development of hypertension and that antioxidant strategies specifically targeting this organelle could have therapeutic benefit in this and possibly other diseases.21
Atherosclerosis
Oxidative modification of LDL and its transport into the subendothelial space of the arterial wall at the sites of endothelial damage are considered initiating events for atherosclerosis.132 Oxidative modification of LDL results from the interaction of reactive oxygen species and reactive nitrogen species, produced from vascular wall cells and macrophages, with LDL. The resulting increased oxidative and nitrosooxidative stress induces endothelial dysfunction by impairing the bioactivity of endothelial nitric oxide and promotes leukocyte adhesion, inflammation, thrombosis, and smooth muscle cell proliferation - all processes that exacerbate atherosclerosis.133 Studies in mice that are deficient in p47phox and gp91phox NADPH oxidase subunits show that ROS produced by these oxidases contribute to atherosclerosis.134
It has been recently shown that SOD2 deficiency increases endothelial dysfunction in ApoE-deficient mice.11 Mice heterozygous for mitochondrial SOD2 (SOD2(+/−)) with apoE deficiency (apoE(−/−)) had increased formation of atherosclerotic lesions. Mitochondrial dysfunction, resulting from SOD2 deficiency, increased mtDNA damage and accelerated atherosclerosis in apoE knockout mice, consistent with the notion that increased ROS production and DNA damage in mitochondria are early events in the initiation of atherosclerosis. Mitochondrial dysfunction can result in apoptosis, favoring plaque rupture. 132
Taken together, these data may suggest a crosstalk between NADPH oxidases and mitochondrial ROS in the development of atherosclerosis. These data also suggest that NADPH oxidases can be a pharmacological target for treatment of atherosclerosis. Indeed, recent developments in mitochondrial-targeted antioxidants that concentrate on the matrix-facing surface of the inner mitochondrial membrane in order to protect against mitochondrial oxidative damage may have therapeutic potential as a treatment for atherosclerosis.135
Cancer
Oxidative stress plays an important role in malignant transformation and cancer progression.15 The incidence of melanoma is increasing worldwide, and the prognosis for patients with high-risk or advanced metastatic melanoma remains poor despite the advances in the field.136 As prostate cancer and aberrant changes in ROS become more common with aging, ROS signaling may play an important role in the development and progression of this malignancy. Oxidative stress is associated with several pathological conditions including inflammation and infection. Chronic increases in ROS over time are known to induce somatic mutations and neoplastic transformation.137
Melanoma proliferation was reduced by inhibition of NADPH oxidases.138 Accumulating evidence suggests that ROS function as signaling molecules to mediate various growth-related responses including angiogenesis. Vascular endothelial growth factor (VEGF), one of the major angiogenesis factors, is induced in growing tumors and stimulates EC proliferation and migration. NADPH oxidases are activated by various growth factors including VEGF and angiopoietin-1 as well as hypoxia and ischemia, and ROS derived from this oxidase are involved in VEGFR2 autophosphorylation, and diverse redox signaling pathways leading to induction of transcription factors and genes involved in angiogenesis. Dietary antioxidants appear to be effective for treatment of tumor angiogenesis. Recent progress on the role of ROS derived from NADPH oxidases and redox signaling events involved in angiogenesis implicates NADPH oxidases as potential therapeutic targets for tumor angiogenesis.139
Recent studies suggest that mitochondria control Nox1 redox signaling and the loss of control of this signaling contributes to breast and ovarian tumorigenesis.140 Inactivation of mitochondrial genes in rho(0) cells led to down-regulation of Nox1 while the transfer of wild type mitochondrial genes restored Nox1 expression to a level comparable to that in the parental cell line. Superoxide levels were reversed to parental levels in hybrid cells when Nox1 expression was restored by transfer of wild type mitochondria. Increasing mitochondrial superoxide levels also increased the expression of Nox1 in parental cells. Nox1 was highly expressed in breast and ovarian tumors and its expression positively correlated with expression of cytochrome C oxidase encoded by mtDNA. This study demonstrates the existence of cross talk between the mitochondria and NADPH oxidase in ovarian cancer.140
Among the available antioxidants, vitamin E was of the greatest interest to researchers. But collective data from all the different clinical trials, including the α-tocopherol, β-carotene prevention trial (ATBC), the heart outcome prevention evaluation-the ongoing outcomes trial (HOPE-TOO), the prostate, lung, colorectal and ovarian trial (PLCO), and the selenium and vitamin E cancer prevention trial (SELECT), were a complete disappointment due to the conclusion that the overall risks for cancer were unaffected by supplemental dietary antioxidants. Thus, treatment strategies aimed to reduce ROS production, rather than ROS neutralization, might offer an effective means against prostate cancer in particular and other malignancies in general.137
Cancer cells are known to have reduced levels of SOD2 expression and higher mitochondrial potential than non-malignant cells.141 Increased expression of mitochondrial SOD2 appears to have adaptive and radioprotective effects.142 Similarly, mitochondria-targeted antioxidants show promising therapeutic strategies to reduce detrimental effects of radiation exposure.143 However, both SOD2 overexpression and mitochondria-targeted antioxidants may diminish efficacy of chemotherapy because they will counteract the increase of pro-apoptotic oxidative stress in cancer cells.
We have recently shown cross talk between mitochondrial ROS and NADPH oxidases,21 where SOD2 depletion increased NADPH oxidase activity and SOD2 overexpression attenuated activation of NADPH oxidases. Furthermore, treatment with mitochondria-targeted SOD mimetic mitoTEMPO mimics the effect of SOD2 overexpression.21 Because cancer may be associated with both reduced SOD2 141 and increased NADPH oxidase activity, we suggest that crosstalk between NADPH oxidases and mitochondrial ROS may play an important role in malignant transformation and cancer progression. Further studies are required to elucidate the specific role of these interactions in cancer pathophysiology.
Diabetes
Oxidative stress mediated by hyperglycemia-induced generation of ROS contributes significantly to the development and progression of diabetes and related vascular complications.144 Michael Brownlee suggested that the mitochondrial electron transport chain plays a key role in a hyperglycemia-induced overproduction of superoxide and the development of secondary complications such as endothelial dysfunction.145 In ρ°endothelial cells, removal of the mitochondrial electron transport chain completely inhibited hyperglycemia-induced ROS production. Interestingly, NADPH oxidases have been also implicated as a major source of ROS generation in the vasculature in response to high glucose and advanced glycation end-products.146 On the other hand, many studies emphasize the role of mitochondrial dysfunction and mitochondrial ROS in diabetes.147 One theory suggests that overproduction of ROS by NADPH oxidases leads to mitochondrial dysfunction.144 Other theories emphasize that diabetes-induced defects in the electron transport chain promote ROS overproduction.147 Interestingly, diabetes may be associated with increased opening of mitoKATP channels 148 which may be important in reduced insulin secretion and ischemic preconditioning.149 These data potentially suggest the presence of feed-forward interaction between NADPH oxidases and mitochondria in the settings of hyperglycemia and diabetes which may be potentially mediated by activation of mitoKATP channels similar to results recently described in endothelial cells.21 The pathophysiological role of this cross-talk in diabetes has not been fully investigated.
Neurodegeneration
Mitochondrial oxidative stress has been implicated in cognitive longevity.150 Human cognition depends on the ability of the central nervous system to sustain high rates of energy production continuously throughout life while maintaining a healthy internal electrochemical environment. However, the central nervous system is especially susceptible to oxidative stress. The brain contains large amounts of iron, ascorbate, glutamate (a free radical-generating excitatory neurotransmitter), and highly peroxidizable unsaturated fatty acids. The brain consumes large volumes of oxygen (about 20% of whole-body O2 consumption) and has a relatively poor antioxidant defense system. Because the rate of ROS production in human tissues is proportional to the rate of local oxygen consumption,151 and the rate of oxygen consumption in the brain elevates with increasing demand for cognitive functions requiring planning, inductive thinking, and flexible thought,152 the brain is a continuously operating, high-intensity oxidation/antioxidation battleground prone to oxidative imbalance during times of high demand for complex cognitive activity.150
Parkinson’s disease, Alzheimer’s disease and Huntington’s disease are neurodegenerative diseases with distinct clinical and morphological manifestations. However, a common feature of different neurodegenerative diseases is an impairment of mitochondrial energy metabolism in brain cells due to the critical role of mitochondria in glutamate excitotoxicity and other forms of cell death via apoptotic or necrotic pathways. The proposed mechanisms by which mitochondria induce cell death are the Ca2+-dependent disruption of mitochondrial electrical membrane potential (ΔΨ) and opening of the pore with nonspecific permeability, known as the mitochondrial permeability transition pore (mPTP).153 Biochemical studies have suggested the impairment of mitochondrial complex I in Parkinson’s disease. Recent experimental work has modeled this abnormality using complex I inhibitor rotenone. Chronic rotenone exposure increased mitochondrial ROS, impaired mitochondrial respiration, accurately recapitulated the pathological, biochemical, and behavioral features of Parkinson’s disease.154
Recently, NADPH oxidases have been associated with neurodegenerative disorders and related complications.155 For example, NADPH oxidases are activated in brains from Alzheimer’s disease patients and are upregulated in Parkinson’s disease.156 The NADPH oxidases may participate in ROS production in neurons and microglial cells,157 potentially diluting their toxicity.155 In these cell types, increased intracellular oxidative stress and AngII stimulate the expression and the activity of NADPH oxidases.150
It is conceivable that production of mitochondrial ROS can stimulate the expression of NADPH oxidases in the brain resulting in pro-inflammatory and pro-apoptotic vicious cycles. Interestingly, epidemiological studies in humans show both positive and negative effects of the use of antioxidant supplements on healthy cognitive aging and on the risk of developing Alzheimer disease.158 Furthermore, it has been reported that mitochondria-targeted antioxidant SkQ1 reduced learning in the MWM task in Wistar rats but resulted in higher locomotor and exploratory activity and less anxiety.159 Antioxidant enriched diet leads to rapid learning improvements, memory improvements after prolonged treatment and cognitive maintenance. In the brains of aged treated dogs, oxidative damage is reduced and there is some evidence of reduced Alzheimer disease-like neuropathology.158 These data suggest that antioxidant treatments targeting ROS production by mitochondria or NADPH oxidases in the brain may be beneficial; however, further studies are required to minimize the risk of impairment by physiological redox dependent processes in the brain.
Aging
In 1972 Denham Harman suggested that free radical damage of mitochondria can be a key determinant of the aging process.160 It has been shown that mitochondrial dysfunction and damage of mtDNA as a result of endogenous and mitochondrial ROS play an important role in the degenerative processes.161 Although the limitations of this hypothesis has been recently criticized by David Gems and Linda Partridge,162 there are many compelling studies demonstrating that mitochondrial production results in damage to macromolecules in spite of such defensive enzymes as superoxide dismutases and glutathione peroxidase, leading to the progressive dysfunction that we see as senescence.16 Increased mitochondrial uncoupling and cell ATP depletion are evident in human muscle nearly a decade before accumulation of irreversible DNA damage that causes defects in mitochondrial electron transport chain. New evidence points to the reduction in activators of biogenesis (e.g. PGC-1alpha) and to degradation of mitochondria, allowing accumulation of molecular and membrane damage in aged mitochondria. The early dysfunction appears to be reversible based on improved mitochondrial function in vivo and elevated gene expression levels after exercise training. New molecular and in vivo findings regarding the onset and reversibility of mitochondrial dysfunction with age indicate the potential: 1) for diagnostic tools to identify patients at risk for severe irreversible defects later in life; and 2) for development of an intervention to delay the tempo of aging and improve the quality of life of the elderly.163
Recently, the role of NADPH oxidases in the aging process has been emphasized.164 It is well known that the AngII mediated upregulation of NADPH oxidases contributes to age-related cardiovascular phathological conditions such as hypertension, heart failure and diabetes. Interestingly, blocking AngII signalling protects against degenerative processes and promotes longevity in rodents. Altogether these findings open the perspective for exploring AngII signaling in therapeutic interventions in inflammatory diseases and aging-related tissue injury.165 Although it is widely assumed that mitochondria are the predominant source of ROS relevant for the aging process, the role of ROS generated by NADPH oxidases has been largely overlooked in aging theories. From an experimental point of view, there is now abundant evidence for the involvement of NOX enzymes in age-associated diseases.164
We suggest that the role of these distinct sources of ROS can be reconciled on the basis of crosstalk of NADPH oxidases and mitochondrial ROS. The role of Noxes in the aging process itself and their relative contribution as compared to mitochondria need further investigations.
Cardiac dysfunction
Emerging evidence suggests the involvement of NADPH oxidases in cardiac physiological and pathophysiological processes.166 Definitive evidence for the involvement of NADPH oxidases in pathological hypertrophy came from experiments in Nox2−/− mice.167 ROS affect cellular Ca2+ regulation at several levels, notably via redox modifications of key amino acid residues involved in the function and gating properties of intracellular and plasma membrane ion channels and transporters — e.g., L-type channels, the Na+/Ca2+ exchanger, the sarcoplasmic reticulum ATPase and the ryanodine receptor.168 The regulation of Ca2+ in cardiac myocytes is centrally important not only in excitation-contraction coupling but also in many other processes such as the regulation of gene expression and cellular energetics.
Recently, it has been shown that mitochondrial ROS regulate the cardiac sodium channel.169 Mitochondria-targeted antioxidant mitoTEMPO and malonate reduced mitochondrial ROS and normalized the activity of cardiac sodium channels. Furthermore, mice with mitochondria-targeted expression of catalase are resistant to cardiac hypertrophy.170 These data indicate the critical role of mitochondrial ROS in cardiac hypertrophy and failure.
Interestingly, the pathophysiological role of NADPH oxidases and mitochondrial ROS is substantially overlapped. This indicates that not only different sources of ROS contributes to cardiac dysfunctions but also suggest crosstalk between NADPH oxidases and mitochondrial ROS. Indeed, inhibition of type I angiotensin-receptor blockers reduces age-related mitochondrial dysfunction, attenuates hypertension induced renal mitochondrial dysfunction, and protects against cardiac mitochondrial dysfunction in the setting of acute ischemia.111-113 On the other hand, the role of mitochondrial ROS in NADPH oxidase-mediated processes such as cardiomyocyte differentiation and endothelin signaling has been reported.171, 172 These data support the crosstalk of NADPH oxidases and mitochondrial ROS in cardiac pathophysiological processes; however, further studies are necessary.
Future directions
The role of mitochondrial oxidative stress in pathological conditions is very well documented; however, the role of mitochondrial ROS in physiological processes and adaptive responses is less clear. It is conceivable that mitochondrial ROS affect cell proliferation, cell transformation, survival and differentiation via interaction with NADPH oxidases.1 The specific molecular mechanisms of crosstalk between NADPH oxidases and mitochondria have to be further investigated.173 Mitochondria may represent an important node in the regulation of NADPH oxidase expression 120 and activity.21 It is therefore interesting to speculate that mitochondria may provide both feed-forward and feed-back regulations of NADPH oxidases. The mechanisms preventing the development of oxidative stress, however, may decline with age due to mitochondrial impairment associated with reduced mitochondrial membrane potential, diminished redox status and decreased ATP level. This will drive a feed-forward vicious cycle of ROS production by mitochondria and NADPH oxidases. Interestingly, current findings indicate that scavenging of mitochondrial using mitochondria-targeted antioxidants can interrupt this vicious cycle using very low therapeutical doses.21 There are many common conditions including aging, atherosclerosis, diabetes and degenerative neurological disorders in which mitochondrial oxidative stress seems to play a role.16, 174 It is conceivable that mitochondria-targeted interventions would be effective in these conditions.
Acknowledgements
We thank Drs. Lula Hilenski and Anna Dikalova for assistance with manuscript preparation. This work was supported by funding from National Institute of Health grant HL094469.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 35(9):505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2009;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27(1):42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21(4):489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 5.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20(10):2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol. 2003;63(2):325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 7.Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296(1):C116–123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal. 2007;9(3):301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Miura K, Liu X, Zweier JL. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275(50):38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 10.Carey RM. Update on the role of the AT2 receptor. Curr Opin Nephrol Hypertens. 2005;14(1):67–71. doi: 10.1097/00041552-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi M, Runge MS, Faraci FM, Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26(10):2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4(2):51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens. 2007;1(1):30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Sadek HA, Nulton-Persson AC, Szweda PA, Szweda LI. Cardiac ischemia/reperfusion, aging, and redox-dependent alterations in mitochondrial function. Arch Biochem Biophys. 2003;420(2):201–208. doi: 10.1016/j.abb.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266(1):6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3(1):13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 17.Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6(5):661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 18.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 19.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107(10):1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 20.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107(1):106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li WG, Miller FJ, Jr., Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276(31):29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278(25):22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 24.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33(11):1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 25.McNally SJ, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Free Radic Biol Med. 2005 doi: 10.1161/01.ATV.0000170827.16296.6e. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3-4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 27.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955;217(1):409–427. [PubMed] [Google Scholar]
- 28.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 29.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278(8):5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 30.Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353(Pt 2):411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24(36):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 45(7-8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 35.Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med. 2009;46(9):1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Gutterman DD. Mitochondria and reactive oxygen species: an evolution in function. Circ Res. 2005;97(4):302–304. doi: 10.1161/01.RES.0000179773.18195.12. [DOI] [PubMed] [Google Scholar]
- 37.Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33(6):755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 38.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Ackrell BA, Kearney EB, Mayr M. Role 3f oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem. 1974;249(7):2021–2027. [PubMed] [Google Scholar]
- 40.Lambert AJ, Buckingham JA, Boysen HM, Brand MD. Diphenyleneiodonium acutely inhibits reactive oxygen species production by mitochondrial complex I during reverse, but not forward electron transport. Biochim Biophys Acta. 2008;1777(5):397–403. doi: 10.1016/j.bbabio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doughan AK, Harrison DG, Dikalov SI. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial Dysfunction. Linking Mitochondrial Oxidative Damage and Vascular Endothelial Dysfunction. Circ Res. 2008;102(4):488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 43.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291(5):H2067–2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 44.Queliconi BB, Wojtovich AP, Nadtochiy SM, Kowaltowski AJ, Brookes PS. Redox regulation of the mitochondrial K(ATP) channel in cardioprotection. Biochim Biophys Acta. doi: 10.1016/j.bbamcr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295(2):H874–882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piskernik C, Haindl S, Behling T, Gerald Z, Kehrer I, Redl H, Kozlov AV. Antimycin A and lipopolysaccharide cause the leakage of superoxide radicals from rat liver mitochondria. Biochim Biophys Acta. 2008;1782(4):280–285. doi: 10.1016/j.bbadis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Panov A, Schonfeld P, Dikalov S, Hemendinger R, Bonkovsky HL, Brooks BR. The neuromediator glutamate, through specific substrate interactions, enhances mitochondrial ATP production and reactive oxygen species generation in nonsynaptic brain mitochondria. J Biol Chem. 2009;284(21):14448–14456. doi: 10.1074/jbc.M900985200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudin AP, Malinska D, Kunz WS. Sites of generation of reactive oxygen species in homogenates of brain tissue determined with the use of respiratory substrates and inhibitors. Biochim Biophys Acta. 2008;1777(7-8):689–695. doi: 10.1016/j.bbabio.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9(3):343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 50.Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L189–198. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- 51.Quijano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J Biol Chem. 2001;276(15):11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- 52.Wu JL, Wu QP, Yang XF, Wei MK, Zhang JM, Huang Q, Zhou XY. L-malate reverses oxidative stress and antioxidative defenses in liver and heart of aged rats. Physiol Res. 2008;57(2):261–268. doi: 10.33549/physiolres.931161. [DOI] [PubMed] [Google Scholar]
- 53.Shatrov VA, Brune B. Induced expression of manganese superoxide dismutase by non-toxic concentrations of oxidized low-density lipoprotein (oxLDL) protects against oxLDL-mediated cytotoxicity. Biochem J. 2003;374(Pt 2):505–511. doi: 10.1042/BJ20030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79(6):859–868. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64(11):1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38(10):1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93(6):573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 58.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94(2):514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86(9):960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 60.Chen YR, Deterding LJ, Sturgeon BE, Tomer KB, Mason RP. Protein oxidation of cytochrome C by reactive halogen species enhances its peroxidase activity. J Biol Chem. 2002;277(33):29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 61.Brookes PS, Zhang J, Dai L, Zhou F, Parks DA, Darley-Usmar VM, Anderson PG. Increased sensitivity of mitochondrial respiration to inhibition by nitric oxide in cardiac hypertrophy. J Mol Cell Cardiol. 2001;33(1):69–82. doi: 10.1006/jmcc.2000.1276. [DOI] [PubMed] [Google Scholar]
- 62.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31(12):1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 63.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280(51):42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 64.Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a “complex” problem. Antioxid Redox Signal. 2007;9(10):1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- 65.Schulze-Osthoff K, Los M, Baeuerle PA. Redox signalling by transcription factors NF-kappa B and AP-1 in lymphocytes. Biochem Pharmacol. 1995;50(6):735–741. doi: 10.1016/0006-2952(95)02011-z. [DOI] [PubMed] [Google Scholar]
- 66.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem. 2004;279(33):35079–35086. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 67.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55(3):798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 68.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara T, Oikawa S, Kinugawa S, Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113(14):1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 69.Banmeyer I, Marchand C, Clippe A, Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 2005;579(11):2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 70.Ernster L, Forsmark P, Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992;3(4):241–248. [PubMed] [Google Scholar]
- 71.Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41(2):235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 72.Kagan VE, Wipf P, Stoyanovsky D, Greenberger JS, Borisenko G, Belikova NA, Yanamala N, Samhan Arias AK, Tungekar MA, Jiang J, Tyurina YY, Ji J, Klein-Seetharaman J, Pitt BR, Shvedova AA, Bayir H. Mitochondrial targeting of electron scavenging antioxidants: Regulation of selective oxidation vs random chain reactions. Adv Drug Deliv Rev. 2009;61(14):1375–1385. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trnka J, Blaikie FH, Smith RA, Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med. 2008;44(7):1406–1419. doi: 10.1016/j.freeradbiomed.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 74.Skulachev VP. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry (Mosc) 2007;72(12):1385–1396. doi: 10.1134/s0006297907120139. [DOI] [PubMed] [Google Scholar]
- 75.Dikalov S, Skatchkov M, Bassenge E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6, 6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem Biophys Res Commun. 1997;231(3):701–704. doi: 10.1006/bbrc.1997.6174. [DOI] [PubMed] [Google Scholar]
- 76.Trnka J, Blaikie FH, Logan A, Smith RA, Murphy MP. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic Res. 2009;43(1):4–12. doi: 10.1080/10715760802582183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39(5):567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 78.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 79.O’Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem. 2006;281(52):39766–39775. doi: 10.1074/jbc.M608268200. [DOI] [PubMed] [Google Scholar]
- 80.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007;9(11):1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 81.Namiecinski M, Pulaski L, Kochman A, Skolimowski J, Bartosz G, Metodiewa D. Cytotoxicity, cytoprotection and neurotoxicity of novel deprenyl-related propargylamines, stable nitroxide free radicals, in vitro and in vivo. In Vivo. 2004;18(2):171–180. [PubMed] [Google Scholar]
- 82.Mildaziene V, Baniene R, Marcinkeviciute A, Nauciene Z, Kalvenas A, Zimkus A. Tetraphenylphosphonium inhibits oxidation of physiological substrates in heart mitochondria. Mol Cell Biochem. 1997;174(1-2):67–70. [PubMed] [Google Scholar]
- 83.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8(2):E277–283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rocha M, Hernandez-Mijares A, Garcia-Malpartida K, Banuls C, Bellod L, Victor VM. Mitochondria-targeted antioxidant peptides. Curr Pharm Des. 16(28):3124–3131. doi: 10.2174/138161210793292519. [DOI] [PubMed] [Google Scholar]
- 85.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35(9 Suppl):S461–467. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- 86.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 87.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 88.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87(1):26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 89.Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 90.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90(11):1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 91.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82(7):810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 92.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109(2):227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 93.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24(4):677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 94.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47(9):1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 286(15):13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-Dependent NOX5 NADPH oxidase Contributes to Vascular Oxidative Stress in Human Coronar Artery Disease. J Am Coll Cardiol. 2008;52(22) doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106(34):14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 107(35):15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 106(7):1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105(3):249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA. 107(42):18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144(6):2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 104.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91(5):406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 105.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18(1):69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 106.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88(9):888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 107.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100(7):1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 108.Park YM, Lim BH, Touyz RM, Park JB. Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. J Korean Med Sci. 2008;23(6):1039–1045. doi: 10.3346/jkms.2008.23.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2008;294(6):H2659–2668. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- 110.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res. 2001;89(5):408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 111.de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CG. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. Faseb J. 2003;17(9):1096–1098. doi: 10.1096/fj.02-0063fje. [DOI] [PubMed] [Google Scholar]
- 112.de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1616–1625. doi: 10.1152/ajpregu.00615.2005. [DOI] [PubMed] [Google Scholar]
- 113.Monteiro P, Duarte AI, Goncalves LM, Providencia LA. Valsartan improves mitochondrial function in hearts submitted to acute ischemia. Eur J Pharmacol. 2005;518(2-3):158–164. doi: 10.1016/j.ejphar.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 114.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 115.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45(3):438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 116.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45(5):860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 117.Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001;89(12):1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- 118.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290(1):H406–415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 119.Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem. 2006;281(47):36228–36235. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- 120.Wosniak J, Jr., Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11(6):1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 121.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797(6-7):897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 122.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45(9):1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008;10(8):1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 124.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC., Jr Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med. 2006;41(5):810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11(10):2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 126.Wang HD, Johns DG, Xu S, Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282(5):H1697–1702. doi: 10.1152/ajpheart.00914.2001. [DOI] [PubMed] [Google Scholar]
- 127.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112(17):2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 128.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95(3):588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 129.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40(4):511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of Angiotensin II-Induced Vascular Dysfunction and Hypertension by Overexpression of Thioredoxin 2. Hypertension. 2009;54(2):338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol. 2007;102(1):255–260. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 132.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100(4):460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 133.Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-Usmar VM. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic Biol Med. 2000;28(12):1780–1794. doi: 10.1016/s0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 134.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24(9):471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 135.Victor VM, Apostolova N, Herance R, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: mitochondria-targeted antioxidants as potential therapy. Curr Med Chem. 2009;16(35):4654–4667. doi: 10.2174/092986709789878265. [DOI] [PubMed] [Google Scholar]
- 136.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic Review of Medical Treatment in Melanoma: Current Status and Future Prospects. Oncologist. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282(6):C1212–1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 139.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266(1):37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005;4(12):1367–1373. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- 141.Huang Y, Peng J, Oberley LW, Domann FE. Transcriptional inhibition of manganese superoxide dismutase (SOD2) gene expression by DNA methylation of the 5′ CpG island. Free Radic Biol Med. 1997;23(2):314–320. doi: 10.1016/s0891-5849(97)00095-6. [DOI] [PubMed] [Google Scholar]
- 142.Murley JS, Kataoka Y, Miller RC, Li JJ, Woloschak G, Grdina DJ. SOD2-mediated effects induced by WR1065 and low-dose ionizing radiation on micronucleus formation in RKO human colon carcinoma cells. Radiat Res. 175(1):57–65. doi: 10.1667/RR2349.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, Kagan VE. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172(6):706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82(1):9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 145.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 146.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57(2):460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- 147.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 88(2):229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Quast U, Stephan D, Bieger S, Russ U. The impact of ATP-sensitive K+ channel subtype selectivity of insulin secretagogues for the coronary vasculature and the myocardium. Diabetes. 2004;53(Suppl 3):S156–164. doi: 10.2337/diabetes.53.suppl_3.s156. [DOI] [PubMed] [Google Scholar]
- 149.Pomerantz BJ, Robinson TN, Heimbach JK, Calkins CM, Miller SA, Banerjee A, Harken AH. Selective mitochondrial KATP channel opening controls human myocardial preconditioning: too much of a good thing? Surgery. 2000;128(2):368–373. doi: 10.1067/msy.2000.107423. [DOI] [PubMed] [Google Scholar]
- 150.Glade MJ. Oxidative stress and cognitive longevity. Nutrition. 26(6):595–603. doi: 10.1016/j.nut.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 151.Loft S, Astrup A, Buemann B, Poulsen HE. Oxidative DNA damage correlates with oxygen consumption in humans. Faseb J. 1994;8(8):534–537. doi: 10.1096/fasebj.8.8.8181672. [DOI] [PubMed] [Google Scholar]
- 152.Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A. 2002;99(16):10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Panov A, Obertone T, Bennett-Desmelik J, Greenamyre JT. Ca(2+)-dependent permeability transition and complex I activity in lymphoblast mitochondria from normal individuals and patients with Huntington’s or Alzheimer’s disease. Ann N Y Acad Sci. 1999;893:365–368. doi: 10.1111/j.1749-6632.1999.tb07856.x. [DOI] [PubMed] [Google Scholar]
- 154.Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson’s disease. IUBMB Life. 2001;52(3-5):135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 155.Block ML. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25(7):1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Head E. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem Res. 2009;34(4):670–678. doi: 10.1007/s11064-008-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stefanova NA, Fursova A, Kolosova NG. Behavioral effects induced by mitochondria-targeted antioxidant SkQ1 in Wistar and senescence-accelerated OXYS rats. J Alzheimers Dis. 21(2):479–491. doi: 10.3233/JAD-2010-091675. [DOI] [PubMed] [Google Scholar]
- 160.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 161.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 162.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7(3):200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 163.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr Opin Clin Nutr Metab Care. 2007;10(6):688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 164.Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol. 2007;42(4):256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 165.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47(1):15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 167.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105(3):293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 168.Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9(4):409–435. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 169.Liu M, Liu H, Dudley SC., Jr Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107(8):967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial Oxidative Stress Mediates Angiotensin II-Induced Cardiac Hypertrophy and G{alpha}q Overexpression-Induced Heart Failure. Circ Res. 2011;108(7):837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Crespo FL, Sobrado VR, Gomez L, Cervera AM, McCreath KJ. Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells. 2010;28(7):1132–1142. doi: 10.1002/stem.441. [DOI] [PubMed] [Google Scholar]
- 172.Deng W, Baki L, Baumgarten CM. Endothelin signalling regulates volume-sensitive Cl- current via NADPH oxidase and mitochondrial reactive oxygen species. Cardiovasc Res. 88(1):93–100. doi: 10.1093/cvr/cvq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.O’Connor PM, Gutterman DD. Resurrecting hope for antioxidant treatment of cardiovascular disease: focus on mitochondria. Circ Res. 2010;107(1):9–11. doi: 10.1161/CIRCRESAHA.110.223321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27(6):545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]