Abstract
Objective
The study addresses risk factors and etiology of pediatric sensory over-responsivity (SOR) in a large sample of twins. We examine, at age two years, (a) the association of temperamental traits with concurrent SOR; (b) the association of prenatal complications with SOR; (c) the association of having a male co-twin with female SOR; and (d) the common and unique genetic etiology of temperament and SOR symptoms.
Method
The sample included 1,026 twin pairs (mean age = 2 years 2 months) from a population-based longitudinal study. Auditory and tactile SOR symptom domains were partially independent and thus were examined separately.
Results
Temperamental negative affect and fear were moderately correlated with auditory and tactile SOR symptoms. Prenatal complications significantly predicted tactile symptoms after controlling for child characteristics. Additionally, females with a male co-twin showed greater SOR at age two than same-sex female dizygotic twins, suggesting a possible risk associated with in utero testosterone exposure. Both auditory and tactile SOR domains were heritable. Bivariate genetic analyses showed that each SOR domain had a similar genetic relationship with fear and negative affect.
Conclusion
The findings suggest partially non-overlapping etiologies and risk factors for tactile versus auditory SOR and indicate that prenatal factors warrant further investigation.
Keywords: sensory over-responsivity, temperament, prenatal complications, testosterone
A small subset of both children and adults report unusually intense, even painful, responses to everyday sensory stimuli that most individuals experience as innocuous. The reactivity of senses to external stimuli appears to vary widely. For instance, most individuals experience no auditory sensation from long florescent light bulbs, but a few find the hum of these lights so uncomfortable that they cannot work in a room with florescent lighting. Clinicians observe this sensory over-responsivity, sometimes called sensory defensiveness, in children with a range of diagnoses, including attention-deficit hyperactivity disorder (ADHD), autism, and Fragile X Syndrome1–3, but sensory over-responsivity, importantly, also occurs in children without any apparent medical condition4. Over-responsivity can occur in response to tactile, visual, auditory, or other modalities of stimuli. Some individuals experience a broad range of sensitivities that are chronic and severe; these sensitivities often begin very early in life and may impact mastering key developmental milestones5. Although these sensitivities may not always elicit strong concern from clinicians, parents and teachers have viewed multiple, severe symptoms of sensory over-responsivity as adversely affecting social interactions, peer and family relationships, and school performance of the child6. In fact, the new diagnostic entity called Sensory Processing Disorder (SPD) has already been acknowledged in some diagnostic classification schemes7–9 and proposed for inclusion in the next installment of the Diagnostic and Statistical Manual of Mental Disorders4,10.
While past research has been predominantly focused on occupational therapy and treatment of SPD, in order to successfully classify SPD, its etiology and relation to other behavioral patterns needs to be thoroughly examined. Although SPD encompasses a wide range of atypical sensory responses including under- and over-responsive sub-types, this study only investigated sensory over-responsivity (SOR). Children who experience SOR “respond to sensory messages more intensely, more quickly, and/or for a longer duration than children with typical sensory responsivity”5. The current study aimed to examine (1) the relationship between SOR and temperament, (2) the effects of individual risk factors such as socio-economic status (SES) and birth events on SOR, (3) the possible role of in utero testosterone exposure in potentiating risk of SOR symptoms in girls with male co-twins, and (4) the distinctiveness of SOR by exploring the common and unique sources of genetic and environmental covariance with temperament traits.
Temperament and Sensory Over-Responsivity
The relationships between different domains of SPD and temperamental qualities have been studied extensively in the literature. Most commonly, sensory symptoms have been found to be positively associated with introversion, difficult temperament, anxiety, and low self-regulation11–13. One study found that sensory modulation problems are associated with facets of difficult temperament such as irritability, hyperactivity and distractibility14, while another study specifically related difficult temperament to auditory and tactile sensory responsiveness12. We previously reported low to moderate correlations between overall SOR and various temperamental and clinical scales13. Attention, activity level, inhibitory control, sadness, anger and soothability all showed low correlations with SOR symptoms, with fear domains showing relatively high correlations (r = 0.50, p < .001). Emotionality, harm avoidance and agoraphobic avoidance are also positively associated with sensory symptoms15. Although these studies examined the relationship between temperament and broader sensory domains such as sensory modulation or sensory responsiveness, these studies did not directly assess SOR. We investigated broad temperamental domains such as negative emotionality, effortful control, and fear rather than clinical conditions because many clinical diagnoses are premature at age two years. Moreover, toddler temperament predicts later childhood internalizing, externalizing, and ADHD diagnoses to a significant degree.
Prenatal and Perinatal Risk Factors
Prenatal and perinatal complications have been implicated as risk factors for SOR; however, the mechanisms underlying these associations are not clearly understood. Prenatal exposure to alcohol, stress, or chronically high cortisol has been shown to increase the prevalence of sensory processing symptoms in rhesus monkeys18. Preliminary studies have shown that children with sensory symptoms were more likely to have had birth and neonatal complications such as prolonged labor, fetal distress and jaundice6,19. Sensory deprivation may also occur during prolonged stays in neonatal intensive care units, making premature or critically ill infants at increased risk of sensory modulation problems12.
Prenatal exposure to testosterone is another potential risk factor for developing SOR. Opposite-sex twin pairs provide a unique model for studying this effect, since hormones such as testosterone can transfer between two fetuses during development, and for females, this can result in physical and cognitive masculinization20,21. Although no research has directly studied the effect of prenatal testosterone exposure on SOR, past findings indicate that the relationship may be plausible. For example, masculinized finger-length ratios, which serve as a proxy for prenatal testosterone exposure, show positive associations with ADHD symptoms22. Since ADHD is also associated with SOR symptoms, we investigated whether the presence of a male co-twin was a risk factor for tactile SOR in female co-twins1.
Genetic and Environmental Etiology
The etiology of SPD is poorly understood. Our previous study utilized the twin method to identify potential genetic contributions to SOR symptoms, suggesting that SOR is moderately heritable13. For auditory defensiveness, 38% of the variance was explained by genetic, 33% by shared environmental, and 28% by unique environmental influences. Similarly, genetic influences accounted for 52% of the variance in tactile defensiveness, with the shared (17%) and nonshared (31%) environment accounting for the remainder of the variance. The covariance between auditory and tactile SOR was due to the environment rather than overlap in genetic influences. To date, no study has attempted to determine the underlying sources of the covariance between SOR symptoms and temperament domains, which may provide evidence for the distinctiveness of SPD from temperamental domains.
Current Study
This study expands on our previous work by using genetic modeling to investigate the etiological relationship between SOR and temperament domains and by studying the relationship between prenatal complications and the development of SOR. Two-year-old twins were studied to allow more sensitive detection of prenatal influence, as well as to detect temperamental influences prior to the clinical diagnosis of behavior disorders. Specifically, auditory and tactile—the two most common modalities—were examined in relation to temperament and birth and prenatal events. The goals of this study were to examine (a) the association between SOR and temperament traits of negative affectivity, effortful control and fear; (b) the association of obstetrical and neonatal events with SOR symptoms at age 2; (c) the potential role of testosterone exposure in increasing the prevalence of SOR symptoms in girls with boy co-twins; and (d) common and unique sources of genetic and environmental variance on SOR, and on the association between SOR and temperament.
METHOD
Participants
Participants were from the Wisconsin Twin Panel, a statewide, birth-record based sample of twins.23 Families of twin born between 1998 and 2002 were invited via mail to join the study with approximately 76.7% of families responding, 90% of whom agreed to be enrolled. We used data from a telephone interview with the primary caregiver that included questions concerning temperament, medical history, zygosity, and demographics. The response rate for the interviews was 61.5% of enrolled families; of those families that participated in the interview, 62.0% also completed a mailed packet of questionnaires. Medical and behavioral conditions such as autism and Fragile X were excluded from the study due to their association with SOR. Fifty-eight families (5.4%) were omitted from the final sample, due to at least one twin with autism (43 families), medical conditions such as cerebral palsy, spina bifida and Down’s Syndrome (12 families) and unclear genetic relationship between the twins (3 families). The final sample included 2,052 individual twins (n = 1,018 female), with an average age at assessment of 2 years and 2 months (SD = 3 months). The age variable was not corrected for prematurity in the analyses because we directly examined gestational age as a predictor. There were 348 (33.9%) monozygotic (MZ), 358 (34.9%) dizygotic same-sex (DZ-SS), and 320 (31.1%) dizygotic opposite sex (DZ-OS) twin pairs. The race of the twins was reported as 94.4% Caucasian. Average education of mothers was 14.9 years (SD = 2.30 years), and 75.6% of families reported an annual income of $41,000 or more. SES was computed as the mean of the z-score of mother years of education, father years of education, and family income.
As part of a follow-up study on a random subsample, medical records concerning the twin pregnancy and birth were accessed for 372 twins, who were the only families used in the analyses of prenatal and birth complications. The subsample was representative of the larger sample, in that the means for auditory and tactile SOR of the groups with and without birth record data only differed by 14% and 13% of a standard deviation, respectively, which we did not regard as biasing. Using pregnancy-related variables transcribed directly from hospital-obtained medical records avoided maternal recall bias that is inherent in studies based on retrospective report. However, for all participating twins (n=2,052), gestational age and birth weight were reported by mothers. These assessments were reliable as the correlations between maternally reported and birth record obtained variables was r = 0.89, p < .001 for gestational age and r = 0.97, p < .001 for birth weight. Therefore, maternal report of gestational age and birth weight were used for all twins to increase sample size for some birth-related analyses.
Measures
Sensory Over-Responsivity
SOR was assessed at age two with items from two instruments, the Infant-Toddler Social and Emotional Assessment (ITSEA) and a revised version of the Toddler Behavior Assessment Questionnaire (TBAQ). Items from both instruments have been used in prior studies as measures of SOR13,26, and thus we combined the ITSEA and TBAQ items that measured tactile and auditory SOR to increase reliability (with a larger number of items) and to reduce method-specific variance. Table 1 shows the loadings for 13 TBAQ and ITSEA items on two common (principal axis) factors, interpreted as tactile and auditory domains. The principal axis loadings in Table 1 were replicated using confirmatory factor analysis. The χ2 value for the confirmatory factor analysis was 395.73, df = 64, RMSEA =0.043 and CFI = 0.89. Each participant in the study had a TBAQ score (inclusion criterion), although only a portion of the sample (N = 907) had both TBAQ and ITSEA scores. There were no mean differences in auditory (t = 1.59, p = .11) or tactile SOR scores (t = “−1.42, p = .16) between the subsample with both TBAQ and ITSEA scores and the sample with TBAQ scores alone. The results of this factor analysis were used to create unit mean composite scores, which were used instead of factor scores. The auditory and tactile SOR scales were moderately internally consistent (α’s = 0.70, and 0.72, respectively).
Table 1.
Varimax Rotated Principal Axis Loadings of Sensory Over-Responsivity Symptoms
| Items | Factors | |
|---|---|---|
| Auditory | Tactile | |
| How often did your child seem overly sensitive to, or irritated by, certain sounds, voices or music?* | .69 | .15 |
| How often did your child ask or gesture for the volume of loud music, radio, or TV to be lowered?* | .54 | .03 |
| How often did you child seem to be alarmed when s/he heard sirens in the distance?* | .50 | .15 |
| Is bothered by loud noises or bright lights† | .50 | .17 |
| How often was your child distracted by background sounds that do not bother most other people?* | .49 | .25 |
| Is easily startled† | .37 | .19 |
| How often does your child object to scratchy clothing fabrics such as wool?* | .15 | .62 |
| Is bothered by how some things feel on his/her skin† | .06 | .60 |
| Won’t touch some objects because of how they feel† | .23 | .55 |
| How often did your child object to changes in articles of clothing that fit snuggly or tightly?* | .10 | .50 |
| Dislikes some foods because of how they feel† | .12 | .45 |
| How often did your child refuse to touch a sticky or gooey substance?* | .19 | .38 |
| When touching a new object, how often did your child seem concerned by how smooth or rough the texture was?* | .25 | .38 |
Notes: N = 907.
Item is taken from Toddler Behavior Assessment Questionnaire (TBAQ) Perceptual Sensitivity Scale,
Item is taken from the Infant-Toddler Social and Emotional Assessment (ITSEA) Sensory Sensitivity Scale
Zygosity
Zygosity was classified during the age two assessment using the Zygosity Questionnaire for Young Twins27, which has demonstrated over 95% agreement with genotypic zygosity determination28. Cases of ambiguous zygosity were resolved via hospital placenta(e) reports (an unambiguous monochorionic placenta indicating monozygosity) and follow-up zygosity questionnaires. If this information was not definitive, photographs, video images, and genotyping were utilized. Seven pairs of twins (0.6%) with unknown or ambiguous zygosity who were not genotyped were excluded from genetic analyses.
Temperament Assessment
Toddler Behavior Assessment Questionnaire (TBAQ). The TBAQ assessed 10 temperament dimensions, including the soothability, object fear and social fear of toddlers25. Factor analysis of the 10 scales yielded three factors labeled Negative Affect, Effortful Control, and Fear. The Negative Affect factor, which involves distress reactions, was defined by the anger (factor loading (l) = 0.82), sadness (l = 0.65), activity level (l = 0.55), and soothability (l = −0.45) scales. Effortful Control signifies the ability of the child to stay interested and allocate attention to relevant activities, and to stop an activity when asked to do so. This factor had primary loadings for TBAQ appropriate attention allocation (l = 0.89), interest (l = 0.80) and inhibitory control (l = 0.63) scales. Finally, Fear was a bipolar factor contrasting being fearful of objects and social situations versus finding many situations pleasurable. This factor comprised the social fear (l = 0.74), object fear (l = 0.50) and pleasure (l = −0.50) scales (Complete factor analysis results are available in online supplement 1). These results were used to make mean unit composite scores for each of the three factors. Reliability estimates (α’s) for the three factor-derived scales were 0.85, 0.91, and 0.86 for Negative Affect, Effortful Control, and Fear, respectively.
Assessment of Pregnancy and Birth Events
We obtained obstetrical and prenatal data through medical record transcription for obstetrical complications, neonatal complications, and neonatal morbidity variables; these records came from a total of 113 different Wisconsin hospitals. Two independent researchers coded all records; the rare discrepancies were resolved via consensus. For each scale, composite scores indicated relative risk. A more extensive review of the scales and coding procedures can be found elsewhere29.
Obstetrical Complications Scale (OCS)
The OCS included 38 obstetrical variables (11 maternal variables and 27 pregnancy-specific variables), as well as 12 twin-specific (fetal) items30. Maternal variables included mother’s age, gravidity, and chronic medical conditions. Pregnancy variables included maternal substance use, bleeding, infections, preterm labor and placental configuration. Twin-specific items included problems during birth, such as malpresentation or nuchal cord. These variables were generally coded as 0 (not present) or 1 (present). Variables with multiple potential outcomes, such as fetal presentation, were scored on a point scale relative to risk. The maximum score of the total risk index was 85. Given the literature illustrating the role of prenatal complications, the OCS was divided into two scales, a prenatal scale (30 items) and a birth complications scale (8 items).
Neonatal Complications Scale (NCS)
The NCS included 20 neonatal variables related to the first 24 hours of life32. Variables included gestational age, birth weight, infectious and non-infectious diseases, and feeding type. Gestational age, birth weight and length of hospitalization were removed from the risk index and analyzed separately. Without these variables, the maximum NCS score was 19.
Neonatal Morbidity Scale (NMS)
The NMS involved 7 relatively common neonatal complications, including bradycardia, tachypnea, and feeding restrictions during the hospital stay31. Treatment type and duration for conditions such as apnea, respiratory distress syndrome and hyperbilirubinemia required these variables to be scored based on relative risk, yielding a maximum score of 15.
Statistical Analysis
Most scores were z-transformed prior to analyses. We used correlational and linear regression approaches to examine associations of temperament and birth-related risk factors with SOR. Then, we used hierarchical linear modeling to estimate the predictive power of temperament and obstetrical/neonatal variables while taking twin dependency into account. Mx software was used for behavior-genetic modeling32. We fit bivariate Cholesky models33 using maximum likelihood to the raw twin data to test for genetic and environmental contributions to the covariation between temperament variables and auditory or tactile symptoms.
RESULTS
Means and standard deviations for relevant variables were as follows: prenatal complications (M = 8.91, SD = 4.27), birth weight (M = 5.58 lbs, SD = 1.29), and Neonatal Intensive Care Unit (NICU) stay (M = 15.71 days, SD = 18.30). The mean gestational age was 35.7 weeks, with approximately half of twins born <37 weeks and 12% born <33 weeks gestation; 44.1% were low birth weight (<5.5lbs), but only 6.3% were very low birth weight (<3.3lbs). The SOR and temperament variables were standardized prior to analyses.
Zero-Order Correlations
Table 2 provides correlations among the dependent and independent variables. Gender was not significantly correlated with SOR and thus was not included in subsequent analyses. Findings to note are the moderate correlations of the SOR variables with negative affect and fear, and the relatively low correlations with effortful control. Gestational age was very weakly correlated with both domains of SOR, although this association was insignificant when the sample was reduced to the subsample with birth record data. Prenatal complications were correlated with tactile and not auditory SOR, while birth weight and NICU stay were not associated with either SOR domain. Omitted from Table 2 are birth complications, neonatal complications, and neonatal morbidity, which were not associated with either SOR variable and thus dropped from subsequent analyses.
Table 2.
Correlations among Outcome and Predictor Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Auditory SOR | -- | .34** | .00 | .12** | .04† | −.15** | .26** | −.08** | .28** | .04 | −.08** | −.02 | −.07 |
| 2. Tactile SOR | -- | .04† | .09** | .03 | −.13** | .21** | −.02 | .20** | .11* | −.06* | −.03 | −.04 | |
|
| |||||||||||||
| 3. Gender (1 = female; 2 = male) | -- | .05* | .03 | .02 | .02 | −.11** | −.10** | .01 | −.02 | .03 | .21* | ||
| 4. Age (months) | -- | .05* | −.07* | .07** | .14** | −.01 | .03 | .00 | −.01 | .14 | |||
| 5. Zygosity (0 = DZ; 1 = MZ) | -- | .07* | .00 | .08** | −.02 | .12* | .07** | .10** | −.24* | ||||
| 6. Socioeconomic Status | -- | −.07* | .05* | −.02 | −.01 | .06* | −.07* | −.10 | |||||
| 7. Negative Affect | -- | −.45** | .23** | .01 | −.03 | .00 | −.14 | ||||||
| 8. Effortful Control | -- | −.16** | .05 | .06* | .01 | .07 | |||||||
| 9. Fear | -- | .03 | −.05* | −.01 | −.11 | ||||||||
| 10. Prenatal Complications | -- | −.10* | −.07 | −.15† | |||||||||
| 11. Gestational Age (weeks) | -- | .72** | −.77** | ||||||||||
| 12. Birth Weight (lbs) | -- | −.78** | |||||||||||
| 13. NICU stay (days) | -- | ||||||||||||
Notes: Zygosity is coded as dizygotic = DZ (same and opposite sex), and monozygotic = MZ. N = 2052 individual twins for all correlations except the following: prenatal complications and NICU stay, N = 371, gestational age and birth weight N = 2035.
Correlation shows a trend with p ≤ .10 (2-tailed).
Correlation is significant at p ≤ .05 (2-tailed).
Correlation is significant at p ≤ .001 (2-tailed).
Examination of Risk Factors for SOR: Linear and Hierarchical Regression Approaches
Regression equations were computed to predict auditory and tactile SOR using age, SES, negative affect, fear and prenatal complications as simultaneous predictors. Effortful control was omitted from the analyses due to low correlations with the outcome variables, and high colinearity with negative affect. R2 values are reported for each of the models along with unstandardized betas in Table 3 (Panel A). SES, negative affect and fear were significant, independent predictors for both sensory domains. Prenatal complications significantly predicted tactile but not auditory scores.
Table 3.
Regression and Hierarchial Linear Modeling Results with Prenatal Complications, Temperament and Male Co-twin as Predictors
| PANEL A | B | S.E. | p | PANEL B | Estimate | S.E. | p | PANEL C | Estimate | S.E. | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Auditory SOR | R2 = 0.13 | Auditory SOR | Auditory SOR | ||||||||
|
| |||||||||||
| Age | 0.04 | 0.01 | .001 | Level 1 | Level 1 | ||||||
| Socioeconomic Status | −0.09 | 0.04 | .031 | Negative Affect | 0.21 | 0.04 | ≤ .001 | Negative Affect | 0.15 | 0.06 | .01 |
| Negative Affect | 0.15 | 0.04 | ≤ .001 | Fear | 0.24 | 0.03 | ≤ .001 | Fear | 0.16 | 0.06 | .01 |
| Fear | 0.16 | 0.04 | ≤ .001 | Level 2 | Level 2 | ||||||
| Prenatal Complications | 0.21 | 0.43 | .62 | Age | 0.02 | 0.01 | .09 | Age | 0.03 | 0.02 | .07 |
| Socioeconomic Status | −0.07 | 0.03 | .028 | Socioeconomic Status | −0.12 | 0.06 | .05 | ||||
| Female v. male co-twin | 0.02 | 0.03 | .41 | Prenatal Complications | 0.77 | 0.78 | .32 | ||||
|
| |||||||||||
| Tactile SOR | R2 = 0.09 | Tactile SOR | Tactile SOR | ||||||||
|
| |||||||||||
| Age | 0.02 | 0.01 | .11 | Level 1 | Level 1 | ||||||
| Socioeconomic Status | −0.13 | 0.04 | .004 | Negative Affect | 0.11 | 0.04 | .003 | Negative Affect | 0.17 | 0.07 | .02 |
| Negative Affect | 0.11 | 0.04 | .009 | Fear | 0.18 | 0.03 | ≤ .001 | Fear | 0.14 | 0.08 | .06 |
| Fear | 0.13 | 0.04 | .003 | Level 2 | Level 2 | ||||||
| Prenatal Complications | 0.92 | 0.46 | .045 | Age | 0.01 | 0.01 | .26 | Age | 0.00 | 0.02 | .99 |
| Socioeconomic Status | −0.09 | 0.03 | .01 | Socioeconomic Status | −0.15 | 0.06 | .01 | ||||
| Female v. male co-twin | 0.17 | 0.06 | .01 | Prenatal complications | 1.69 | 0.59 | .01 | ||||
Notes: N = 371 individual twins for Panel A, which represents basic linear regression for participants with birth record data. N = 488 individual twins for Panel B, which includes only female dizygotic twins drawn from the full sample. Female vs. male co-twin is coded as female twin = 0, male twin = 1. For Panel C, N = 125 individual twins, as it uses the same sample as panel A, with the exception that opposite sex twins are excluded.
The interpretation of these linear regressions is constrained by two main factors: (1) the dependency between certain values for co-twins within a pair; and (2) the potential confounding effect of a male co-twin on females from DZ-OS pairs. We addressed the first limitation by using hierarchical linear modeling (HLM) to account for the twin dependency; that is, HLM takes into account that both twins in a pair will share SES, age and prenatal complications. The effect of a male co-twin was also assessed by adding the presence vs. absence of a male co-twin as a predictor in additional HLM analyses that included only females from DZ twin pairs (Table 3, Panel B). Because the presence of a male twin significantly increased the tactile scores of their female co-twin, OS twins were dropped from the final HLM analysis of the full sample to prevent confounding the results by an effect that was specific to OS pairs (Table 3, Panel C). When OS twins were dropped from the analyses, negative affect, fear and SES were predictors for auditory SOR. On the other hand, negative affect, SES and prenatal complications significantly predicted tactile SOR.
Univariate Genetic Analyses
Twin intraclass correlations were computed for auditory and tactile SOR and the three temperament domains (Table 4). As MZ twins share all of their segregating genes while DZ twins share only half of their segregating genes, rMZ > rDZ is indicative of additive genetic influences (A). Similarly rMZ < 1.0 signifies the presence of non-shared environmental (E) factors (i.e., factors specific to each individual and measurement error). Finally shared family environmental (C) factors are indicated when rDZ > .5(rMZ) and dominant genetic (D) factors are indicated if the rDZ < .5(rMZ) value. The classical twin method decomposes the variance of a trait into variance in underlying genetic and environmental influences. Each univariate model included A and E, and either C or D depending on the pattern of intraclass twin correlations. We then tested whether each parameter could be dropped without a significant decrement in fit of the overall model. The best fitting model (see Table 4) adequately explained the data with the fewest parameters (based on AIC and chi-square difference score). All variables had a significant additive genetic component. Tactile SOR, auditory SOR and negative affect also had modest shared environment components. Although twin intraclass correlations suggested that temperamental effortful control and fear might be influenced by dominance effects, both were best described by a more parsimonious AE model. Examining the models descriptively, we note that auditory SOR was more heritable (62% in the best-fitting model) than tactile SOR (44% in the best-fitting model).
Table 4.
Twin Correlations and Univariate Genetic Models for Sensory Over-Responsivity (SOR) and Temperament Variables
| Twin Correlations | Variance Components | Model Fit | Model Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rMZ | rDZ | h2 | c2 | d2 | e2 | AIC | BIC | Δ χ2 | Δdf | p | ||
| Auditory SOR | 0.70 | 0.47 | ACE | 0.62 | 0.12 | -- | 0.26 | 1775.83 | −4727.65 | |||
| AE | 0.75 | -- | -- | 0.25 | 1778.15 | −4729.01 | 4.31 | 1 | 0.038 | |||
|
| ||||||||||||
| Tactile SOR | 0.67 | 0.45 | ACE | 0.44 | 0.20 | -- | 0.36 | 1810.46 | −4701.81 | |||
| AE | 0.66 | -- | -- | 0.34 | 1818.34 | −4700.38 | 9.88 | 1 | 0.002 | |||
|
| ||||||||||||
| Negative Affect | 0.73 | 0.44 | ACE | 0.59 | 0.15 | -- | 0.27 | 22.93 | −5048.28 | |||
| AE | 0.74 | -- | -- | 0.26 | 26.32 | −5049.06 | 5.39 | 1 | 0.02 | |||
|
| ||||||||||||
| Effortful Control | 0.75 | 0.30 | ADE | 0.75 | -- | 0.00 | 0.25 | 692.78 | −4715.83 | |||
| AE | 0.75 | -- | -- | 0.25 | 690.78 | −4719.30 | 0.00 | 1 | 1 | |||
|
| ||||||||||||
| Fear | 0.70 | 0.26 | ADE | 0.69 | -- | 0.00 | 0.31 | 188.76 | −4967.84 | |||
| AE | 0.69 | -- | -- | 0.31 | 186.76 | −4971.31 | 0.00 | 1 | 1 | |||
Notes: Twin correlations are intraclass correlations, with N = 349 for monozygotic (MZ) twin pairs and N = 679 for same-sex and opposite-sex dizygotic (DZ) pairs. For all correlations, p ≤ .001. N = 1033 twin pairs for the univariate analyses. A and h2 denote additive genetic effects, D and d2 denote dominant (non-additive) genetic effects, C and c2 denote shared environmental effects, and E and e2 denote unique environment effects.
Bivariate Genetic Analyses
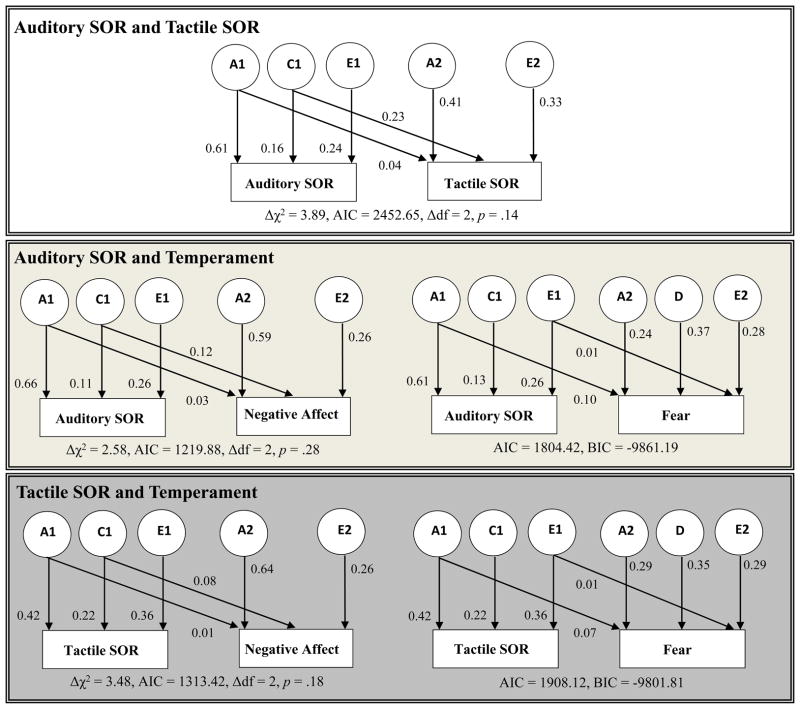
We used bivariate Cholesky decompositions to investigate the covariance between auditory and tactile SOR, as well as the relationship of each SOR domain with negative affect and fear (Figure 1; results available in table form in online supplement 2). The bivariate model is an extension of the classical twin model wherein the covariation between two traits is decomposed into covariation between underlying genetic or environmental factors influencing each trait. Effortful control was not included in these analyses due to its low phenotypic correlations with the SOR variables. Standardized parameter estimates are presented in Figure 1. Paths from A1, C1, and E1 to the first trait represent the proportions of phenotypic variance accounted for by the latent A1, C1, and E1 factors. Paths from A1 or C1 to the second trait indicate the portion of variance in the second trait accounted for by latent genetic or shared environment factors that also influence the first trait. Paths from A2, C2, and E2 to the second trait represent the residual variance in the second trait that is accounted for by the latent variables A2, C2, and E2. Note that while some of the path coefficients are small, they are significant (based on the smallest AIC and non-significant chi-squared difference score) due to the large sample size. The relationship between auditory and tactile SOR, which had a phenotypic correlation of .35, had significant common genetic (rA = 0.29) and shared environmental (rC = 1.0) correlations. Note that even when total genetic variation in two traits is low, the amount of overlapping genetic influences (i.e. genetic correlation) may be high. Both auditory SOR and tactile SOR had modest genetic correlations with negative affect (rA = 0.21 and .14 respectively) and wholly overlapping shared environmental influences (rC = 1.0) with negative affect. The genetic and environmental underpinnings of the relationship of each SOR domain with fear were similar, with significant genetic (rA = 0.53 and 0.43 for auditory and tactile SOR, respectively) as well as unique environmental correlations (rE = 0.17 for both domains).
Figure 1.
Best-fitting Bivariate Cholesky Models of Sensory Over-Responsivity (SOR) variables and temperament domains (Standardized Results).
Notes: N = 1056 twin pairs. A denotes additive genetic effects, D denotes dominant (non-additive) genetic effects, C denotes shared environmental effects, and E denotes unique environment effects. 1 refers to factors that influence the first variable and 2 refers to factors that only influence the second variable. Pathways from A1, C1, or E1 to the second variable reflect covariance due to genetic, shared environment or non-shared environment factors, respectively, common to both variables.
Model fit statistics: Panel A1. Full model AIC = 3518.14; reduced model AIC = 3516.48, Δχ2 = 2.34, Δdf = 2, p = .31.
Panel B1. Full model AIC = 1818.09; reduced model AIC = 1817.33, Δχ2 = 3.26, Δdf = 2, p = .20.
Panel B2. Full model AIC = 1964.37; no reduced model.
Panel C1. Full model AIC = 1813.48; reduced model AIC = 1814.32, Δχ2 = 6.84, Δdf = 3, p = .08.
Panel C2. Full model AIC = 1990.10; no reduced model.
DISCUSSION
Interpretation of Key Findings
We sought to understand the association of SOR with temperamental negative affect, effortful control, and fear. In general, both correlational and regression analyses showed that negative affect and fear were moderately related to both auditory and tactile SOR. These correlations were low enough to counter the suggestion that SOR are “just” signs of toddler temperament but high enough and clearly significant so that the association of SOR with temperament appears real and in need of an explanation, part of which was provided by the bivariate genetic analyses. The lack of association of SOR with effortful control may also be noteworthy because both of these behavioral patterns are associated with ADHD1,17.
We also sought to identify associations between obstetrical, birth, or neonatal complications and SOR. Generally, birth weight, gestational age, duration of NICU stay, birth complications, neonatal complications and neonatal morbidity were not significant independent predictors of SOR symptoms (Table 3), although some of these variables did show zero-order associations with auditory and tactile SOR (Table 2). This difference may help clarify previous literature. The only prenatal or perinatal variable that showed a significant independent relationship was prenatal complications, which predicted tactile SOR, even after controlling for negative affect, fear, age, and SES. This finding appears compatible with research in rhesus monkeys suggesting that prenatal maternal stress such as alcohol or cortisol exposure increased tactile sensitivity of the offspring18. These results highlight another empirical difference in the correlates of auditory versus tactile SOR. They also suggest a need for more research investigating the relationship between specific prenatal events and SOR symptoms.
Our third research question concerned any potential risk for SOR associated with in utero testosterone exposure in females with a male co-twin. The presence of a male co-twin and potential in utero testosterone exposure predicted tactile, but not auditory, SOR. Notably, the presence of a male co-twin did not influence either SOR domain in male twins (results not shown). Although our study design did not allow us to measure testosterone as a risk factor per se, the finding that females with a male co-twin have higher tactile SOR than females with a female co-twin supports the need for more research into the topic. Since past research has shown that testosterone exposure can occur between opposite sex twins in utero and that this hormonal transfer can have significant, measurable effects in female twins, the ability of testosterone exposure to potentiate risk of SOR symptoms seems plausible20,21. Again, we note that the male co-twin effect held only for tactile, and not auditory, SOR.
The genetic analyses lead to several conclusions. First, univariate analyses showed that both auditory and tactile SOR were heritable, supporting our past findings13. The shared environmental effects that we detected for tactile SOR adds to our previous findings that prenatal effects, shared by twins, contribute to tactile SOR. Second, bivariate analyses showed that auditory and tactile SOR share some degree of both genetic and shared environmental variances. Bivariate genetic analyses of each SOR with the temperamental variables revealed that auditory and tactile SOR had similar genetic and environmental underpinnings of their associations with temperament. That is, both tactile and auditory SOR showed modest common genetic overlap with fear and negative affect, and fear showed modest common non-shared environmental overlap with the two types of SOR whereas negative affect showed strong common shared environmental overlap with the two types of SOR. Future analyses will need to elucidate the nature of these environmental influences on SOR and temperament by investigating such variables as caregiver responsivity and exposure to noxious environmental stimuli.
Limitations
Several limitations attend our study. First, while our epidemiologically defined community sample included children expected to develop a wide range of behavioral problems (except autism, an exclusion criterion), the results should not be generalized to individuals with specific medical conditions who report SOR symptoms. Another limitation is that temperament assessment was obtained via caregiver report, the same methodology that supplied the SOR symptom data. Thus, compared with objectively observed temperament and SOR data, which would be difficult to collect in such a large sample, the associations between temperament traits and SOR might be biased upwards. In addition, it would be inappropriate to generalize our results to ethnic and racial minorities, who are not highly represented in Wisconsin state birth records. Lastly, SOR symptoms likely change during development, and our analyses are confined to the toddler period.
Conclusions
The results contribute to emerging knowledge about sensory responsivity as a condition of early childhood. Individual differences in SOR are heritable and modestly associated with temperamental fear (versus pleasure) and negative affect. Prenatal complications were an independent predictor of tactile SOR. Several lines of evidence supported the distinctiveness of auditory versus tactile SOR.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institute of Mental Health (R01 MH59785 and R37 MH50560 to Goldsmith), the Wisconsin Center for Affective Science (P50 MH069315), the Wallace Research Foundation and the Hilldale Faculty/Undergraduate UW Research Fellowship. Infrastructure support was provided by the Waisman Center via a core grant from the National Institute of Child Health and Human Development (P30 HD03352).
References
- 1.Mangeot SD, Miller LJ, McIntosh DN, et al. Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2001;43:399–406. doi: 10.1017/s0012162201000743. [DOI] [PubMed] [Google Scholar]
- 2.Schoen SA, Miller LJ, Brett-Green BA, et al. Physiological and behavioral differences in sensory processing: A comparison of children with Autism Spectrum Disorder and Sensory Modulation Disorder. Front Integr Neurosci. 2009;3:1–11. doi: 10.3389/neuro.07.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller LJ, McIntosh DN, McGrath J, et al. Electrodermal responses to sensory stimuli in individuals with Fragile X Syndrome. Am J Med Genet. 1999;83:268–79. [PubMed] [Google Scholar]
- 4.Miller LJ, Anzalone ME, Lane SJ, et al. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am J Occup Ther. 2007;61:135–40. doi: 10.5014/ajot.61.2.135. [DOI] [PubMed] [Google Scholar]
- 5.DeGangi GA, Porges SW, Sickel RZ, et al. Four-year follow-up of a sample of regulatory disordered infants. Infant Ment Health J. 1993;14:330–43. [Google Scholar]
- 6.Miller LJ. Sensational kids: Hope and help for children with Sensory Processing Disorder. New York: Penguin Group; 2006. [Google Scholar]
- 7.Zero to Three. Diagnostic classification of mental health and developmental disorders of infancy and early childhood, revised (DC:0–3R) Arlington, VA: National Center for Clinical Infant Programs; 2005. [Google Scholar]
- 8.Interdisciplinary Council on Developmental and Learning Disorders. Diagnostic manual for infancy and early childhood: Mental health, developmental, regulatory-sensory processing and language disorders and learning challenges (ICDL-DMIC) Bethesda, MD: Author; 2005. [Google Scholar]
- 9.PDM Task Force. Psychodynamic diagnostic manual. Silver Spring, MD: Alliance of Psychoanalytic Organizations; 2006. [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) Washington, DC: Author; 2000. [Google Scholar]
- 11.Ahn RR, Miller LJ, Milberger S, et al. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. Am J Occup Ther. 2004;58:287–93. doi: 10.5014/ajot.58.3.287. [DOI] [PubMed] [Google Scholar]
- 12.Case-Smith J, Butcher L, Reed D. Parents’ report of sensory responsiveness and temperament in preterm infants. Am J Occup Ther. 1998;52:547–55. doi: 10.5014/ajot.52.7.547. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith HH, Van Hulle CA, Arneson CL, et al. A population-based twin study of parentally reported tactile and auditory defensiveness in young children. J Abnorm Child Psych. 2006;34:393–407. doi: 10.1007/s10802-006-9024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGangi GA, Craft P, Castellan J. Treatment of sensory, emotional, and attentional problems in regulatory disordered infants. Infant Young Child. 1991;3:9–19. [Google Scholar]
- 15.Hofmann SD, Bitran S. Sensory-processing sensitivity in social anxiety disorder: Relationship to harm avoidance and diagnostic subtypes. J Anxiety Disord. 2007;21:944–54. doi: 10.1016/j.janxdis.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerin DW, Gottfried AW, Thomas CW. Difficult temperament and behavior problems: A longitudinal study from 1.5 to 12 years. Int J Behav Dev. 1997;21:71–90. [Google Scholar]
- 17.Caspi A, Henry B, McGee RO, et al. Temperamental origins of child and adolescent behavior problems: From age three to age fifteen. Child Dev. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 18.Schneider ML, Moore CF, Gajewski LL, et al. Sensory processing disorder in a primate model: Evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Dev. 2008;79:100–13. doi: 10.1111/j.1467-8624.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May-Benson TA, Doomar JA, Teasdale A. Incidence of pre-, peri-, and post-natal birth and developmental problems of children with sensory processing disorder and children with autism spectrum disorder. Front Integr Neurosci. 2009;3:1–12. doi: 10.3389/neuro.07.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Anders SM, Vernon PA, Wilbur CJ. Finger-length ratios show evidence of prenatal hormone-transfer between opposite-sex twins. Horm Behav. 2006;49:315–9. doi: 10.1016/j.yhbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Vuoksimaa E, Kaprio J, Kremen WS, et al. Having a male co-twin masculinizes mental rotation performance in females. APS. doi: 10.1177/095679610376075. Epub 2010 June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martel MM. Conscientiousness as a mediator of the association between masculinized finger-length ratios and attention-deficit/hyperactivity disorder (ADHD) J Child Psychol Psyc. 2009;50:790–8. doi: 10.1111/j.1469-7610.2009.02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith HH, Lemery-Chalfant K, Schmidt NL, et al. Longitudinal analyses of affect, temperament, and childhood psychopathology. Twin Res Hum Genet. 2007;10:118–26. doi: 10.1375/twin.10.1.118. [DOI] [PubMed] [Google Scholar]
- 24.Carter AS, Briggs-Gowan MJ, Jones SM, et al. The Infant-Toddler Social and Emotional Assessment (ITSEA): Factor structure, reliability and validity. J Abn Child Psych. 2003;31:495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Dev. 1996;67:218–35. [PubMed] [Google Scholar]
- 26.Ben-Sasson A, Cermak SA, Orsmond GI, et al. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. Am J Occup Ther. 2007;61:584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith HH. A zygosity questionnaire for young twins: A research note. Behav Genet. 1991;21:257–69. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- 28.Forget-Dubois N, Pérusse D, Girard A, et al. Diagnosing zygosity in infant twins: Physical similarity, genotyping and chorionicity. Twin Res. 2003;6:97–102. doi: 10.1375/136905203322686464. [DOI] [PubMed] [Google Scholar]
- 29.Wagner AI, Schmidt NL, Lemery-Chalfant K, et al. The limited effects of obstetrical and neonatal complications on conduct and attention-deficit hyperactivity disorder symptoms in middle childhood. J Dev Behav Pediatr. 2009;30:217–25. doi: 10.1097/DBP.0b013e3181a7ee98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littman B, Parmalee AH. Manual for obstetrical complications. Los Angeles, CA: Department of Pediatrics, University of California at Los Angeles; 1974. [Google Scholar]
- 31.Pleasure J, Gennaro S, Cnaan A, et al. An expanded neonatal morbidity scale for premature infants. J Nurse Meas. 1997;5:119–38. [PubMed] [Google Scholar]
- 32.Neale MC, Boker SM, Xie G, et al. Mx: Statistical modeling. 5. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2001. [Google Scholar]
- 33.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Boston: Kluwer Academic Publishers; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.