Abstract
Leptin exerts control over energy metabolism, reproduction and bone mass accrual, raising the question does leptin act through a common neuronal circuit to mediate these effects? Historically, the hypothalamus has been viewed as the site for leptin signaling in the brain. Recent genetic studies, however, indicate that these physiological functions, notably the regulation of appetite and bone mass accrual by leptin, take place for the most part through inhibition of serotonin (5-hydroxytryptamine) synthesis and release by brainstem neurons. Here, we review how these findings have redefined the roadmap of leptin signaling in the brain. This has led to proof-of- principle studies showing that selective inhibition of the leptin–serotonin axis is a viable therapeutic approach to treat appetite disorders.
Questions raised by the discovery of leptin
The discovery of leptin has been a major advance of modern endocrinology. With its discovery came the appreciation that: (i) appetite is molecularly controlled; (ii) adipose tissue is an important endocrine organ; and (iii) despite being produced by adipocytes, leptin fulfills its function by acting in the brain [1–6]. Initially leptin functions were thought to be limited to inhibiting appetite and favoring energy expenditure and reproduction; the enthusiasm generated by leptin discovery overshadowed the surprising fact that leptin is specifically expressed in vertebrates [7–9]. This observation was revealing because appetite, energy expenditure and reproduction, the three functions initially described for leptin, are also present in invertebrates. Perhaps even more interesting is that leptin appeared in evolution at the same time as vertebrates, and then became restricted to bony vertebrates [8,9].
Another question that arose from the discovery of leptin was whether its function is indeed limited to energy metabolism and reproduction. Although not impossible, this would be unusual for two reasons. First, most hormones have a broad spectrum of action; second, energy metabolism and reproduction are dependent upon and affect many other aspects of whole-body physiology. A final question that arose in elucidating the mode of action of leptin was to define the precise road map of leptin signaling in the brain, a process that has been rather elusive. Here we review how answers to these seemingly independent questions are interlinked, at least in part, through a leptin–serotonin axis.
A broader view of leptin biology
Why does leptin appear specifically in bony vertebrates? Is this an irrelevant oddity of biology or it is significant? Moreover, why did a hormone that limits food intake arise during evolution at a time when food was scarce? Answers to evolutionary questions are always subject to speculation and difficult to prove. This limitation being acknowledged, two observations suggest a solution to this conundrum. Those observations emerged from testing the hypothesis that energy metabolism is coordinately regulated with bone mass accrual, or bone growth, to prevent vertebrates from growing when there is no food, in other words when an energy supply is unavailable. Genetic experiments have established that leptin is a powerful inhibitor of bone mass accrual: in the absence of leptin, mice and humans have increased bone mass [1,6]. This is an biological tour de force because mice and humans lacking leptin signaling are also hypogonadic, a condition that typically leads to bone loss rather than increased bone mass [3,4,10–14]. Is this an anecdotal finding that deserved to be ignored, despite the fact that during evolution leptin appeared at the same time as bone, or does it help us to understand leptin biology? One experiment among many indicates that the latter is true. After groundbreaking work deciphering the function of different types of phosphorylation of the leptin receptor (LepR), the group of M. Myers engineered a mouse strain harboring a Lepr mutation conferring partial gain of function in leptin signaling [15]. Because it is only a partial gain of function, this mutant mouse strain, known as l/l, allowed a hierarchy of leptin functions to be uncovered. Remarkably, l/l mice did not demonstrate, as one would expect, an increase in appetite when fed a normal diet, nor did they show an increase in fertility. In fact, l/l mice appeared normal when fed a normal diet except for one aspect: they demonstrated low bone mass – in other words osteoporosis affecting axial and peripheral skeleton alike [16]. This experiment showed that the threshold of leptin signaling that is necessary to inhibit bone mass accrual is lower than the one needed to inhibit appetite or to favor fertility. These genetic data supported a more complex view of leptin biology in which the overarching role of leptin would be to coordinate bone growth and food (i.e. energy) intake. This raised the possibility that the neuronal circuitry used by leptin to affect these two functions could overlap or even be identical.
Leptin signaling in the brain: the hypothalamus and much more
Although the l/l mouse model provided convincing evidence for the dual role of leptin in bone and energy metabolism, the question remained of how coordinated regulation takes place in the brain. The hypothalamus has been viewed as the focal point of leptin signaling [1,4,5,11,14,17,18], this is justified by many observations and in large remains true. The first evidence for this, of correlative nature, is that the hypothalamus is implicated in the control of many homeostatic functions. Because the control of appetite, reproduction and growth is a homeostatic function, it makes biological sense to examine the hypothalamus as a nexus for leptin signaling. Additional evidence suggesting that the hypothalamus plays a role in leptin signaling comes from experiments conducted in the 1940s in which physiologists used chemicals to destroy neurons of the arcuate or ventromedial hypothalamus (VMH) nuclei and observed that rats became hyperphagic with a decrease in energy expenditure [5,19–23], similar to that observed in leptin-deficient (ob/ob) mice [1,3,11,14,2]. Furthermore, chemical destruction of VMH neurons in mice resulted in a high bone mass phenotype that could not be corrected by intracerebroventricular infusion or injection of leptin [5]. A third line of evidence suggesting that leptin signaling takes place in the hypothalamus is that Lepr is more highly expressed in neurons of the arcuate and VMH nuclei than in most other parts of the brain [4]. Finally, intracerebroventricular (ICV) infusion of leptin, which includes the hypothalamus, corrects the phenotypes observed in ob/ob mice [1,5,24]. These seemingly strong data, however, were squarely contradicted by one landmark genetic experiment that ultimately changed the field.
In 2004, the groups of Elmquist and Lowell selectively inactivated the leptin receptor gene specifically in VMH or in arcuate neurons [25]. Although one can always argue that the Cre deleter they used to ablate this gene in these neurons did not cause 100% deletion, the results were as stunning as they were unexpected. Mice lacking leptin receptor in VMH and/or in arcuate neurons had a normal appetite when fed normal chow, were fertile, and had normal bone mass [25,26]. Were these genetic experiments contradicting the previous body of work, as was initially feared, or were they enriching it? The chemical lesion experiments demonstrated that leptin cannot regulate energy expenditure, reproduction and bone mass if specific neuronal populations and connections are destroyed or disrupted in the hypothalamus [5]. On the other hand, the genetic experiments showed that leptin does not need to signal in VMH and acruate neurons to regulate appetite, reproduction and bone mass accrual [25,26]. Therefore, the ‘old’/chemical and the ‘new’/genetic data complement each other, suggesting a new and testable hypothesis that leptin might signal elsewhere in the brain to favor or inhibit the synthesis of one or several neuropeptides, and these then act on VMH and/or arcuate neurons of the hypothalamus. Before going further in explaining the verification of this hypothesis, we should underscore the sobering fact that if the classical chemical lesion experiments had not been performed, the results from the genetic experiments would have been misleading.
Leptin: a brake on brain-derived serotonin signaling
As often happens, the clinic was able to provide an alternative viewpoint to this leptin conflict and suggested a key experiment. Serotonin reuptake inhibitors are a class of drugs widely used in psychiatry, that have side effects of osteoporosis and hyperphagia; this led clinicians to hypothesize a yet-to-be-defined relationship between brain-derived serotonin, bone mass accrual, and appetite [27–31]. Serotonin is a bioamine synthesized in neurons of the brainstem and in enterochomaffin cells of the duodenum by two distinct enzymes: tryptophan hydroxylase 2 (Tph2) in the brain and tryptophan hydroxylase 1 (Tph1) in the duodenum [26,32,33]. Moreover, and importantly, serotonin does not cross the brain–blood barrier [34]. In other words, inactivation of Tph2 in the mouse would result in a mutant mouse strain lacking serotonin in the brain but with a normal pool of peripheral serotonin. These Tph2-null mice demonstrate severe osteoporosis, a decrease in appetite and an increase in energy expenditure. As a result, they are markedly leaner than wild-type littermates [26,35,36]. Axon guidance experiments unambiguously demonstrated connections between serotonergic neurons of the brainstem and arcuate neurons of the hypothalamus where the ultimate control of appetite takes place (Figure 1). Arcuate neurons express two of the 14 serotonin receptors, Htr1a and Htr2b, and inactivation of each receptor gene selectively in these neurons also resulted in a decrease in appetite (Figures 1 and 2). Moreover, compound heterozygous mice lacking one copy of Tph2 and one copy of Htr1a or Htr2b in arcuate neurons were also hypophagic [26]. The role of Htr1a in the control of food intake is consistent with the pharmacological effects of its agonists or antagonists [37–40]. Taken together, these experiments established that, similar to its role in invertebrates [41,42], serotonin in vertebrates is an orexigenic molecule acting through two receptors, Htr1a and Htr2b. However, for the sake of clarity we should mention here that several studies have reported that other serotonergic receptors can also inhibit food intake [43–47]. These observations support the notion that serotonin regulates appetite positively or negatively depending on the receptor it binds to.
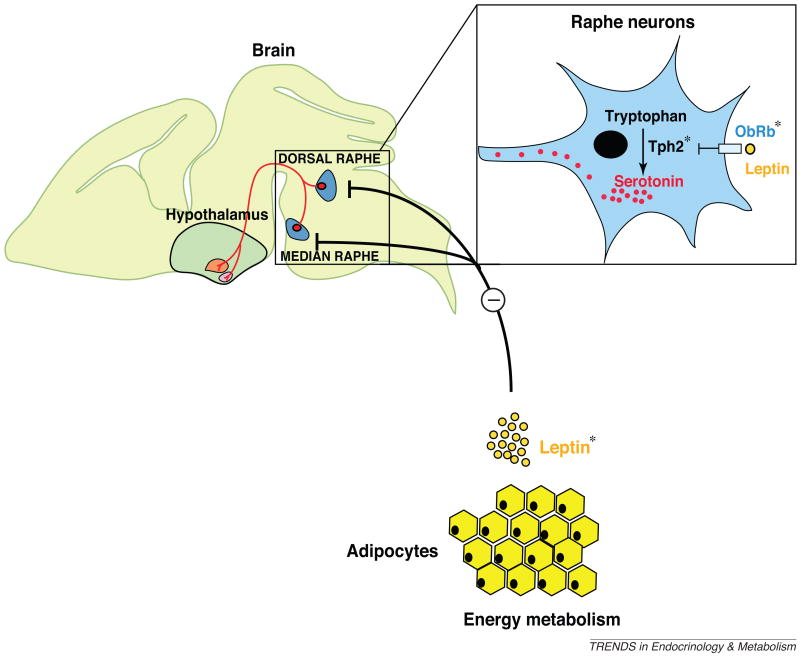
Figure 1.
Brain-derived serotonin synthesis is initiated by hydroxylation of tryptophan, a rate-limiting reaction performed by the enzyme tryptophan hydroxylase 2 (Tph2) in the neurons of the dorsal and median raphe nuclei in the brainstem. Leptin, an adipocyte-derived hormone, can directly inhibit serotonin production and release by the raphe nuclei neurons of the brainstem. The action of leptin is mediated by LepR, also known as the leptin/obese receptor ObRb, expressed on these neurons. The asterisks (*) represent the genes that were cell-specifically inactivated.
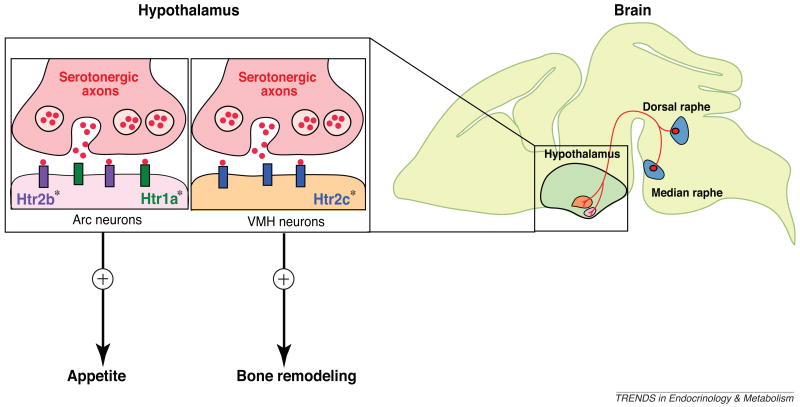
Figure 2.
The serotonergic neurons of the brainstem project to the ventromedial (VMH) and arcuate (Arc) neurons of the hypothalamus. Brain-derived serotonin regulates bone mass accrual positively after binding to Htr2c receptors in neurons of VMH, whereas serotonin binding to the Htr1a and Htr2b receptors on neurons of the arcuate nuclei (Arc) favors appetite. The inactivation of Htr1a receptor signaling in arcuate neurons by a selective antagonist can inhibit the positive regulation of appetite by serotonin. Asterisks (*) represent the genes that were cell-specifically inactivated.
The question remained, however, of what the orexigenic function of serotonin has to do with the anorexigenic function of leptin. One way to look at serotonin and leptin function is to hypothesize that leptin can inhibit appetite (and bone mass accrual, for that matter) by decreasing the synthesis and/or the release of serotonin by brainstem neurons [26]. Several experimental arguments demonstrated that this is the case in vivo [26,48]. These studies also provided the first indication of the biological and medical importance of leptin regulation of serotonin [36]. First, the signaling form of LepR was found to be expressed in serotonergic neurons of the brainstem; furthermore, following leptin ICV infusion, transcription factor Stat3 (signal transducer and activator of transcription 3), the predominant transcriptional mediator of leptin signaling [49–52], becomes phosphorylated in serotonergic neurons of wild-type mice, but not of mice lacking Lepr selectively in these neurons [26]. Second, peripheral injection of leptin into wild-type mice decreases Tph2 expression (i.e. brain serotonin synthesis) in a dose- and time-dependent manner [26]. Third, Tph2 expression and brainstem serotonin content increase over time in ob/ob mice [26]. Lastly, neurophysiology experiments using whole-cell patch recording performed on brain slices showed that leptin decreased action potential frequency in serotonergic neurons of wild-type mice but not of mice lacking the leptin receptor in neurons expressing Tph2 [26]. These results showed that leptin inhibits synthesis and release of serotonin by the brainstem neurons. Although suggestive, the results did not prove that mechanistically this is how leptin affects bone mass accrual and energy metabolism. This could only be achieved through gene deletion experiments performed in the mouse.
Perhaps the most definitive proof that leptin inhibits appetite and favors energy expenditure through inhibition of serotonin synthesis and release from the brainstem neurons came through genetic means (Figure 1). This was carried out in two complementary ways. First, removing one allele of Tph2 from ob/ob mice normalized brain-derived serotonin content and also appetite and energy expenditure [26]. Second, removing both Tph2 alleles from ob/ob mice resulted in a mouse model that was anorexic, despite having no leptin [26]. Instead, this was a novel leptin-deficient mouse that was unable to produce serotonin, demonstrating that the altered function of serotonergic neurons was responsible for the anorexic phenotype.
To explore the consequences for appetite and energy expenditure of inactivating leptin signaling only in serotonergic neurons, the converse genetic experiment was performed where the Lepr gene was deleted specifically in serotonergic neurons. A Cre-deletor mouse strain (tamoxifen- inducible Tph2–CreERT2 mice) was used that could delete Lepr both during development and after birth [36]. In the Tph2–CreERT2 mice, conditional CreERT2 was inserted at the Tph2 ATG in a bacterial artificial chromosome clone containing the entire mouse Tph2 gene [36]. Gene deletion in these mice is only achieved following treatment with tamoxifen (1 mg per 20 g body weight each day over 5 d). This treatment can be performed any time during the lifespan of the mice, and even in adults. Whereas deletion of Lepr in VMH or arcuate neurons did not, as shown previously by others [25], affect appetite or energy expenditure in mice fed a normal diet, its inactivation in serotonergic neurons resulted in a hyperphagic mouse with decreased energy expenditure, resembling the ob/ob phenotype [26]. At the cellular level, deletion of the leptin receptor in serotonergic neurons led to decreased diameter of Pomc-expressing neurons in the arcuate nuclei of the hypothalamus and reduced perikaryal synapse density of Pomc-expressing neurons, again similar to ob/ob mice [26]. Deletion of Lepr in serotonergic neurons resulted in a decrease in Mc4r and Pomc-1 and an increase in Npy and Agrp expression, also similar to the changes observed in ob/ob mice [28]. Even though deletion of the leptin receptor occurred during development in these mice, the experiments suggest a more important role than anticipated for leptin-mediated inhibition of serotonin synthesis in regulating bone mass accrual and energy metabolism. Indeed, the observations suggest that leptin does not itself act in the hypothalamus to regulate energy metabolism (or bone mass accrual), but instead it appears to act in the brainstem to inhibit the synthesis and the release of serotonin that acts on hypothalamic neurons to favor appetite (and bone mass accrual). This model was attractive conceptually because it provided an anatomic, cellular and molecular basis for explaining the coregulation exerted by leptin on both appetite and bone mass accrual. However, the model does not apply to leptin regulation of fertility.
The leptin–serotonin axis after birth; therapeutic implications
This model, although attractive and genetically verified, nevertheless raised legitimate questions that needed to be addressed to strengthen its case. For example, could these results be reproduced with an even more cell-specific Cre driver, for example in a Tph2–Cre mouse that only expresses Cre in serotonergic neurons? Also, is this mode of action of leptin at work in adult mice? In other words, was this merely a developmental defect? A third question – if this model holds true, is it possible to correct the orexigenic phenotype of ob/ob mice by inhibiting serotonin signaling?
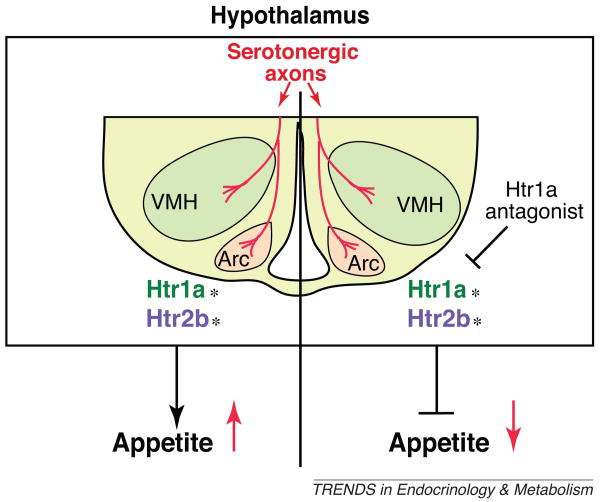
The answer to the first two questions came from the use of the same Cre driver mouse described above. Deletion of the leptin receptor from serotonergic neurons in six-week-old mice using the Tph2–CreERT2 mice resulted in a massive increase of appetite and a decrease in energy expenditure [26,36]. These results established that the leptin–serotonin axis operates in adult mice. Addressing the third question of whether inhibition of serotonin signaling could rescue the orexigenic phenotype of ob/ob mice was more delicate because serotonin acts to inhibit appetite through two receptors on arcuate neurons, Htr1a and Htr2b. On treating ob/ob mice with 20 mg/kg of an Htr1a inhibitor it was observed that food intake by the mutant mice was 20–25% lower than in those treated with vehicle (Figure 3). Moreover, when ob/ob mice were administered this compound daily for over one month, a 30% decrease of food intake and body weight was consistently reported [36]. Taken together, these data demonstrated firmly an important mechanism in which leptin inhibits appetite by decreasing brain-derived serotonin synthesis and/or release by the serotonergic neurons of the brainstem, which signals in arcuate neurons through Htr1a receptor (Figures 2 and 3).
Figure 3.
Concluding remarks: therapeutic implications
Do the data from this longstanding investigation into leptin signaling in the brain suggest that leptin exclusively signals via this pathway in the brain? Of course not, and these experiments do not pretend to suggest this. For example, it is known that leptin acts directly in hypothalamic neurons to control glucose metabolism [18,2], and there could be additional ways for leptin to regulate this process. What these genetic experiments clearly show is that, for the most part, leptin-mediated regulation of appetite and energy expenditure takes place by inhibiting serotonin synthesis and release in the brainstem neurons. From a therapeutic point of view, the results of the experiments using a small-molecule inhibitor of signaling through the Htr1a are obviously encouraging (Figure 2). The challenge facing chemists and biologists alike is that this receptor is broadly distributed in the brain and therefore its inhibition in other parts of the brain could cause unacceptable side effects. Another way to achieve the same effect could be to identify molecules whose expression are regulated by serotonin in the hypothalamus and which are more amenable to therapeutic intervention. Thus, from these observations there is still a long and difficult road ahead to the treatment of appetite disorders.
References
- 1.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Tartaglia LA, et al. Identification and expression cloning of a leptin receptor OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 5.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 6.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 7.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 8.Huising MO, et al. Increased leptin expression in common Carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology. 2006;147:5786–5797. doi: 10.1210/en.2006-0824. [DOI] [PubMed] [Google Scholar]
- 9.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 10.Clement K, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, et al. Identification of a developmentally regulated protein tyrosine phosphatase in embryonic stem cells that is a marker of pluripotential epiblast and early mesoderm. Mech Dev. 1996;59:153–164. doi: 10.1016/0925-4773(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 12.Strobel A, et al. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 13.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 15.Bjornholm M, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei H, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahima RS, et al. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 19.Hetherington AW, Ranson SW. Hypothalamic lesions and adipocity in the rat. Anat Rec. 1940;78:149. [Google Scholar]
- 20.Hetherington AW, Ranson SW. The relation of various hypothalamic lesions to adiposity in the rat. J Comp Neurol. 1942;76:475–499. [Google Scholar]
- 21.Brobeck JR. Mechanisms of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 22.Anand BK, Brobeck JR. Localization of a feeding center in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 23.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 24.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Yadav VK, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haney EM, et al. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med. 2007;167:1246–1251. doi: 10.1001/archinte.167.12.1246. [DOI] [PubMed] [Google Scholar]
- 28.Kaye W, et al. Serotonin neuronal function and selective serotonin reuptake inhibitor treatment in anorexia and bulimia nervosa. Biol Psychiatry. 1998;44:825–838. doi: 10.1016/s0006-3223(98)00195-4. [DOI] [PubMed] [Google Scholar]
- 29.Laekeman G, et al. Osteoporosis after combined use of a neuroleptic and antidepressants. Pharm World Sci. 2008;30:613–616. doi: 10.1007/s11096-008-9231-6. [DOI] [PubMed] [Google Scholar]
- 30.Richards JB, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 31.Ziere G, et al. Selective serotonin reuptake inhibiting antidepressants are associated with an increased risk of nonvertebral fractures. J Clin Psychopharmacol. 2008;28:411–417. doi: 10.1097/JCP.0b013e31817e0ecb. [DOI] [PubMed] [Google Scholar]
- 32.Heath MJ, Hen R. Serotonin receptors Genetic insights into serotonin function. Curr Biol. 1995;5:997–999. doi: 10.1016/s0960-9822(95)00199-0. [DOI] [PubMed] [Google Scholar]
- 33.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 34.Mann JJ, et al. Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and life-time psychopathology. Biol Psychiatry. 1992;32:243–257. doi: 10.1016/0006-3223(92)90106-a. [DOI] [PubMed] [Google Scholar]
- 35.Oury F, et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 2010;24:2330–2342. doi: 10.1101/gad.1977210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav VK, et al. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dourish CT, et al. Low doses of the putative serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) elicit feeding in the rat. Psychopharmacol (Berl) 1985;86:197–204. doi: 10.1007/BF00431709. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert F, et al. Relationship of increased food intake and plasma ACTH levels to 5-HT1A receptor activation in rats. Psychoneuroendocrinology. 1988;13:471–478. doi: 10.1016/0306-4530(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 39.Neill JC, Cooper SJ. MDL 72832, a selective 5-HT1A receptor ligand, stereospecifically increases food intake. Eur J Pharmacol. 1988;151:329–332. doi: 10.1016/0014-2999(88)90818-7. [DOI] [PubMed] [Google Scholar]
- 40.Moreau JL, et al. Behavioral profile of the 5HT1A receptor antagonist (S)-UH-301 in rodents and monkeys. Brain Res Bull. 1992;29:901–904. doi: 10.1016/0361-9230(92)90163-r. [DOI] [PubMed] [Google Scholar]
- 41.Nonogaki K, et al. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan S, et al. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 2008;7:533–544. doi: 10.1016/j.cmet.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halford JC, Blundell JE. Metergoline antagonizes fluoxetine-induced suppression of food intake but not changes in the behavioural satiety sequence. Pharmacol Biochem Behav. 1996;54:745–751. doi: 10.1016/0091-3057(95)02228-7. [DOI] [PubMed] [Google Scholar]
- 44.Lee MD, et al. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacol (Berl) 1998;136:304–307. doi: 10.1007/s002130050570. [DOI] [PubMed] [Google Scholar]
- 45.Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacol (Berl) 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 46.Kitchener SJ, Dourish CT. An examination of the behavioural specificity of hypophagia induced by 5-HT1B, 5-HT1C and 5-HT2 receptor agonists using the post-prandial satiety sequence in rats. Psychopharmacol (Berl) 1994;113:369–377. doi: 10.1007/BF02245211. [DOI] [PubMed] [Google Scholar]
- 47.Bonhaus DW, et al. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Galaz MC. Leptin accumulation in hypothalamic and dorsal raphe neurons is inversely correlated with brain serotonin content. Brain Res. 2010;1329:194–202. doi: 10.1016/j.brainres.2010.02.085. [DOI] [PubMed] [Google Scholar]
- 49.Bates SH, Myers MG. The role of leptin–STAT3 signaling in neuroendocrine function: an integrative perspective. J Mol Med. 2004;82:12–20. doi: 10.1007/s00109-003-0494-z. [DOI] [PubMed] [Google Scholar]
- 50.Stahl N, et al. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 51.White DW, et al. Leptin receptor (OB-R) signaling Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 52.Banks AS, et al. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]