Abstract
BACKGROUND & AIMS
Total pancreatectomy and islet autotransplant (TP/IAT) have been used to treat patients with painful chronic pancreatitis. Initial studies indicated that most patients experienced significant pain relief, but there were few validated measures of quality of life. We investigated whether health-related quality of life improved among pediatric patients undergoing TP/IAT.
METHODS
Nineteen consecutive children (ages 5–18 years) undergoing TP/IAT from December 2006 to December 2009 at the University of Minnesota completed the Medical Outcomes Study 36-item short form (SF-36) health questionnaire before and after surgery. Insulin requirements were recorded.
RESULTS
Before TP/IAT, patients had below average health-related quality of life, based on data from the SF-36; they had a mean physical component summary (PCS) score of 30 and mental component summary (MCS) score of 34 (2 and 1.5 standard deviations, respectively, below the mean for the U.S. population). By 1 year after surgery, PCS and MCS scores improved to 50 and 46 respectively (global effect, PCS p<0.001, MCS p=0.06). Mean scores improved for all 8 component subscales. More than 60% of IAT recipients were insulin independent or required minimal insulin. Patients with prior surgical drainage procedures (Puestow) had lower yields of islets (P=0.01) and greater incidence of insulin dependence (PCS=0.04).
CONCLUSIONS
Quality of life (physical and emotional components) significantly improve after TP/IAT in subsets of pediatric patients with severe chronic pancreatitis. Minimal or no insulin was required for most patients, although islet yield was reduced in patients with previous surgical drainage operations.
Keywords: pancreas, inflammation, therapy, clinical trial
BACKGROUND
Chronic pancreatitis (CP), though rare in childhood, can result in significant morbidity. In children, disease results most commonly from genetic mutations or unknown causes (1,2). Affected children generally present with abdominal pain, with or without elevation of serum amylase, lipase, or conventional imaging evidence of pancreatitis. The disease is usually progressive, with increasing pain and narcotic dependence, potential progression to exocrine and endocrine insufficiency, and an elevated lifetime risk for pancreatic adenocarcinoma (3). CP is associated with significant decrements in health-related quality of life (HRQOL) in adults (4–7), but the pediatric literature is sparse.
Treatment, directed at relieving pain and restoring quality of life, may include narcotic analgesics, pancreatic enzymes to reduce pancreatic stimulation, antioxidants, celiac plexus blocks, and endoscopic duct decompression (8–11). Patients who fail these medical and endoscopic interventions or remain narcotic dependent may be candidates for surgical intervention. Although partial resections (distal pancreatectomy or proximal [Whipple] pancreaticoduodenectomy), drainage operations such as pancreaticojejunostomy (Puestow), or variants (Frey, Beger) are considered standard surgical care, pain may not resolve or eventually relapse in up to 50% of patients, often with progression to exocrine and endocrine insufficiency (12,13). Drainage operations do not reduce the risk for developing adenocarcinoma in the residual pancreas, a lifetime risk which may exceed 40 percent with hereditary pancreatitis (3). Total pancreatectomy removes the entire pancreas and thus the cause of pain and presumably cancer risk, but by itself results in brittle surgical diabetes, and therefore is rarely performed for CP. A novel approach, first described in 1977, is to isolate the patient's own islets at the time of pancreatectomy and autotransplant the islets into the portal vein (14). They engraft in the liver and secrete insulin in response to glucose, without any need for immunosuppression (15).
Although total pancreatectomy (TP)and islet autotransplantation (IAT) has potential to relieve pain while preserving insulin secretion, few centers have experience with this technique, with only three worldwide reporting more than 50 cases (16). The bulk of experience with TP/IAT has been in adults. Overall, more than half of patients successfully wean off narcotic medications (17–21). At experienced institutions, insulin independence rates range from 26–41% (17,22–24). In addition, another one-third of patients require minimal insulin to maintain euglycemia (15,25). Data are limited for pediatric patients. Retrospective studies suggest that the majority have complete or significant pain relief, and half are insulin independent at one year (26). Objective measures of HRQOL are lacking.
The primary aim of the current study was to prospectively determine if HRQOL is improved in pediatric patients undergoing TP/IAT, using a standardized health status measure. The secondary aims were to prospectively follow narcotic requirements and islet function.
METHODS
Subjects
Nineteen consecutive pediatric patients (≤18 years old) scheduled for TP/IAT between December 2006 and December 2009 at the University of Minnesota (U of MN) Amplatz Children’s Hospital were enrolled in a prospective cohort evaluating HRQOL, narcotic use, and insulin requirements. All had a diagnosis of CP or acute relapsing pancreatitis confirmed by gastroenterologists with a specialty focus in pancreatic diseases, and had failed medical and/or endoscopic treatment. The diagnosis of CP was based on clinical history and imaging evidence (calcifications on CT, ductal abnormalities on MRCP or ERCP, and/ or endoscopic ultrasound findings), and in many cases was supported by positive genetic testing for hereditary pancreatitis (PRSS1 gene mutations). Since 2008, all patients were evaluated by a multi-disciplinary team, including surgeons, gastroenterologists, and endocrinologists; those meeting criteria in table 1 were considered candidates for surgery.
Table 1.
University Of Minnesota Criteria for TP/IAT*
Patient must fulfill criteria #1–5 below:
|
criteria were formally implemented in 2008
patients with C-peptide negative diabetes meeting criteria 1–4 are candidates for TP alone
One patient (#12) had pre-existing insulin-dependent diabetes secondary to CP, but was C-peptide positive (indicating functioning beta cells), and thus underwent an IAT to preserve residual beta cell mass. One (#10) did not receive the planned IAT because islet yield was insufficient.
The study protocol was approved by the U of MN Institutional Review Board. Informed consent and assent (where applicable) were obtained for all participants.
Surgical Procedure and Islet Isolation
The TP was done with a pylorus sparing segmental duodenectomy in most cases, with reconstruction via a duodenoenterostomy, and, usually, an adjacent choledochoenterostomy. Islet isolation and purification was performed in the U of MN Molecular and Cellular Therapeutics GMP facility, as previously described (15,27). Briefly, the pancreas was distended with cold enzyme solution (SERVA Electrophoresis GmbH, Heidelberg, Germany) (28) using a pressure-controlled pump system (29), and then digested using the semi-automated method of Ricordi (30). The islets were purified by continuous iodixanol (OptiPrep, Axis-Shield, Oslo, Norway) density gradient on a COBE 2991 cell separator (31) only if the total digest volume was large (>~20 mL). The number of islets were quantified as islet equivalents (IE), which is islet mass standardized to an islet size of 150 µm diameter.
The islet preparation was infused into the portal system after surgical enteric-biliary reconstruction and before closure of the laparotomy incision. In most cases, the splenic vein stump was cannulated proximal to its termination in the portal vein; alternatives include direct puncture of the portal vein or cannulation of the umbilical vein. If the portal pressures elevated to ≥25–30 cmH2O, the infusion was stopped. In 16 cases, all islets were infused intraportally. In 2 cases (#6 and 8), the majority were infused intraportally, with a portion infused into the peritoneal cavity due to elevated portal pressures (15).
HRQOL Assessments
Patients (with assistance of parents) were asked to complete comprehensive survey instruments before surgery and at 3, 6, 12 months, and annually after surgery. Baseline surveys were administered in the clinic at the pre-operative visit (within 1 week of surgery). Subsequent follow up surveys were mailed to patients and returned by mail or at follow up visits. All patients completed at least one follow up survey. Fifty surveys were available. The Medical Outcomes Study (MOS) 36-item Short Form (SF-36) Health Survey was used as a measure of generic HRQOL (32–33). The SF-36 gives a health status profile along 8 dimensions corresponding to the following scale scores: Physical Functioning, Role Limitations Attributed to Physical Health Problems, Bodily Pain, General Health, Social Functioning, Vitality, Role Limitations Attributed to Emotional Health Problems, and Mental Health. The scale scores range between 0 and 100 with higher values signifying more positive health attributes. These 8 scale scores are the basis of the Physical Component Summary (PCS) and the Mental Component Summary Scale (MCS) scores. These latter more global measures are standard normalized (mean of 50, standard deviation of 10) to a representative sample of the United States. Additional survey items included questions about pain symptoms, narcotic use, and insulin requirements.
Narcotic and insulin use
Narcotic and insulin requirements were determined from the medical records and self-reported survey data. For narcotic requirements, patients were asked to report their chronic (daily) and intermittent use (for flares). Those patients reported herein as narcotic-independent reported no narcotic use (daily or intermittent) at last follow up.
Fasting and stimulated glucose and C-peptide levels (before and after Boost HP, 6mL/kg to maximum of 360 mL) and hemoglobin A1c (HbA1c) levels were measured at 3 and 6 months and annually after surgery to assess islet function.
Postoperatively, all patients were placed on insulin therapy, weaned as tolerated to meet the following goals: fasting glucose<126 mg/dL, 2-hour post-prandial glucose<180 mg/dL, and HbA1c ≤6.5%. Patients able to maintain these goals off exogenous insulin were considered insulin independent. Patients were classified as having minimal insulin requirements if they required basal insulin alone or intermittent correction scale, with a total daily insulin dose<0.25 units/kg/day to maintain HbA1c ≤6.5%. Patients were classified as fully insulin dependent if they required a basal-bolus insulin regimen (multiple daily injections, >0.25 units/kg/day).
Statistical Analysis
For the analysis of SF-36 data, mixed model methods were used. Compared to least squares linear modeling techniques, mixed model methods has several advantages. First, this method can accommodate missing data, frequently encountered in panel and longitudinal studies. Second, the mixed method takes into account the dependence of replicate measures (34,35). With this approach, follow-up measures are more strongly associated with earlier scores for the subject and the within-subject-variability that is ignored in least squares regression is more fully accounted for. Thus, this method takes into account change within subject in testing statistical differences across the scale scores. Finally, the mixed approach allowed us to quantify the effects of the IAT in terms of a standard metric. An unstructured covariance was used in fitting the model; time was considered as a continuous variable. Post-hoc analysis was undertaken when p-value for global effect over time was <0.05. To account for error rate biases for making multiple comparisons over 8 subscales at 4 intervals, we applied a Bonferonni correction. Our minimally acceptable error was adjusted to p≤ 0.0016 (calculated as 0.05/32). Islet yield by surgical history was compared using Wilcoxon non-parametric tests. All analyses were performed using SAS® version 9.2.
RESULTS
Patient characteristics
Patient characteristics are summarized in table 2. Patient age at surgery ranged from 5 to 18 years (mean 14.5±3.6 years). Pancreatic disease was most commonly due to identified genetic mutations or idiopathic disease. All patients required narcotics daily (n=13) or intermittently (n=6), and all had recurrent hospitalizations for pain management. Four were dependent on jejunal tube feeds (n=2) or total parenteral nutrition (n=2). Seven had prior pancreatic surgery performed at outside institutions. All patients had 1–4 pancreatic biopsies (average 2 per patient) at the time of pancreatectomy; these were read by a pathologist at U of MN as showing features of CP (fibrosis and inflammation or acinar atrophy) in 18 cases, and minimal change CP (periductal fibrosis) in 1 case.
Table 2.
Baseline characteristics of pediatric patients undergoing total pancreatectomy and islet autotransplant at the University of Minnesota (n=19)
| Patient Characteristics | Mean ± SD |
|---|---|
| Age at surgery (years) | 14.5 ± 3.6 |
| Gender (n) | 13 F/ 6 M |
| Etiology of disease (n) | |
| Hereditary (PRSS1 gene or family history) | 9 |
| SPINK1 homozygote | 1 |
| Cystic Fibrosis | 2 |
| Idiopathic | 5 |
| Pancreas divisum | 1 |
| Goldston syndrome (cystic dysplasia) | 1 |
| Duration of disease (years) | 7.8 ± 3.6 |
| Prior Surgery (n) | |
| Puestow procedure | 3 |
| Puestow + distal pancreatectomy | 2 |
| Puestow + Whipple | 1 |
| Whipple | 1 |
| Baseline Metabolic testing | |
| Fasting plasma glucose (mg/dL) | 80 ± 11 |
| Fasting C-peptide (ng/mL) | 1.8 ± 0.7 |
| HbA1c level (%) | 5.2 ± 0.5 |
Average islet yield was 195,707 ±136,930 IE and 3,513 ±2,480 IE per kg body weight (IE/kg). Mean islet yield was substantially lower in patients with a prior Puestow procedure (n=6) with or without distal pancreatectomy (1,218 ±1,189 versus 4,457 ±2,145 IE/kg in those without surgery, p=0.01). Average duration of hospitalization post-operatively was 20.3±9.8 days. Major morbidities included reoperation in 3 patients (splenectomy, resection of necrotic bowel at duodenal anastamosis, and peritoneal lavage) and IR drainage of intra-abdominal abscess in an additional 1 patient.
HRQOL Outcomes
Prior to surgery, all patients had below average HRQOL, based on the SF-36, with a mean PCS score of 30 and a mean MCS score of 34. These component summary scale scores were 2 and 1.5 standard deviations lower than the standard normal US population, respectively. PCS improved significantly over time (p<0.001) and MCS showed a strong trend towards improvement (p=0.06) (figure 1). By 1 year after surgery, mean PCS was 50 and mean MCS was 46. Scale scores for the eight SF-36 health dimensions improved after surgery (table 3), with significant improvements after Bonferonni adjustment noted for Physical Functioning (3 and 12 months), Role Physical (12 and 24 months), Social Functioning (3, 12, and 24 months), and Bodily Pain (12 and 24 months).
Figure 1.
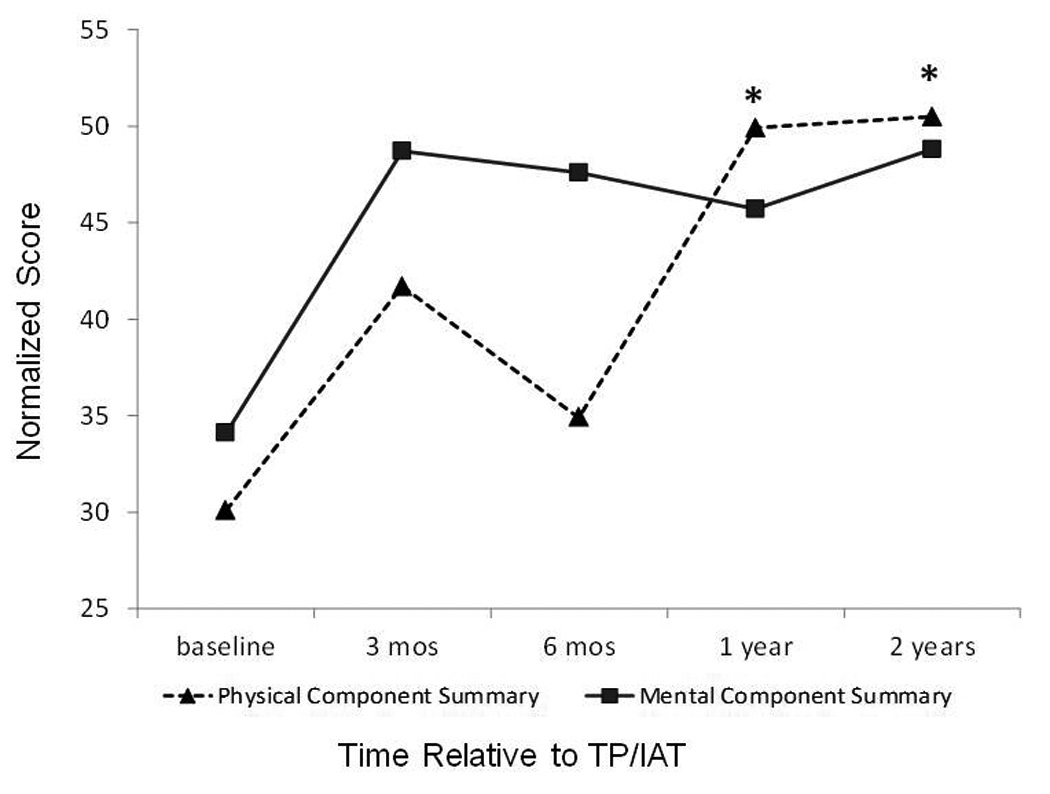
Change in physical component summary scores (triangles, dashed line, p<0.001) and mental component summary scores (squares, solid line, p=0.06) after TP/IAT. Asterix indicates statistically significant change from baseline applying Bonferonni adjustment (p<0.0016).
Table 3.
Change in the 8 subscale scores of the SF-36.
| baseline | 3 mos | 6 mos | 1 year | 2 year | p-value * | |
|---|---|---|---|---|---|---|
| Number of completed surveys | 16 | 13 | 6 | 10 | 5 | |
| Physical Functioning Scale | 43 | 79 | 66 | 89 | 88 | 0.003 |
| Role-Emotional Scale | 21 | 69 | 67 | 77 | 73 | 0.009 |
| Role-Physical Scale | 8 | 50 | 42 | 85 | 80 | <0.0001 |
| General Health Scale | 34 | 53 | 47 | 56 | 59 | 0.08 |
| Social Functioning Scale | 25 | 68 | 52 | 74 | 80 | 0.0002 |
| Bodily Pain Scale | 24 | 58 | 38 | 73 | 79 | <0.0001 |
| Mental Health Scale | 52 | 75 | 75 | 68 | 82 | 0.04 |
| Vitality Scale | 30 | 57 | 44 | 58 | 64 | 0.04 |
p-value for global effect over time. Using a Bonferonni adjustment, statistical differences were observed for Physical Functioning (3 and 12 months), Role Physical (12 and 24 months), Social Function (3, 12, and 24 months), and Bodily Pain (12 and 24 months) (p<0.0016).
Narcotic Requirements
After surgery, 14 patients discontinued narcotics entirely. Of the remaining 5 patients, 2 reported rare narcotic use (a few times a year), 1 used tramadol, and 2 used daily narcotics at a reduced dose (table 4).
Table 4.
Narcotic and insulin use in 19 nediatric natients after TP/IAT
| Case # |
Etiology of disease |
Current follow up (mos) |
Age at transplant |
Prior pancreatic surgery |
Narcotic use before surgery | Latest Known Narcotic Use |
Time to Narcotic D/C |
IE/kg | Latest Known Insulin Status |
Posttransplant HbA1c, range (most recent) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Genetic (SPINK1 ) | 27 | 18.4 | none | Fentanyl 100 mcg patch, morphine elixir PRN, oxycodone 5mg PRN |
none | 10 mos | 4013 | Basal-bolus† | 6.3–9.4% (7.5) |
| 2 | Hereditary (PRSS1) |
26 | 16.3 | none | Tramadol 50mg TID-QID, Oxycodone 5mg QD, proproxyphene 100mg TID |
Tramadol 50mg PRN | 2904 | Basal-bolus | 6.2–9.1% (9.1) | |
| 3 | Pancreas Divisum |
26 | 17.0 | Puestow; Whipple |
Fentanyl Lollipop 400 mcg QID | oxycodone 5mg several times per year |
1 year | 1808 | Basal-bolus | 5.8– 12.1%(12.1) |
| 4 | Hereditary (PRSS1) |
26 | 5.1 | none | Hydromorphone IV with hospitalizations |
none | 1 mos | 6362 | Independent | 5.5– 6.4% (5.7) |
| 5 | Hereditary (PRSS1) |
27 | 9.9 | none | Oxycodone 10mg QID | none | 9 mos | 3531 | Independent | 5.5– 5.7% (5.7) |
| 6 | Idiopathic | 26 | 17.5 | none | IV narcotics (various) with hospitalizations |
none | 1 mos | 3183 | Independent | 5.4– 5.7% (5.5) |
| 7 | Idiopathic | 14 | 13.3 | Puestow | Oxycodone q4h, morphine IV 5- 15mg PRN |
none | 1 mos | 280 | Basal-bolus | 6.4– 8% (6.3) |
| 8 | Idiopathic | 20 | 15.6 | none | Hydrocodone 10mg q4h | none | 3 mos | 4980 | Independent | 5.2– 5.4% (5.2) |
| 9 | CF (d508; 2789+ 5G2TOA) |
24 | 17.4 | none | Hydromorphone 6mg/hr IV (home infusion), Oxymorphone 20mg PO TID |
none | 13 mos | 4,287 | Independent | 5.4– 6.1% (5.6) |
| 10* | CF (d508; R117H) |
9 | 17.4 | none | Hydrocodone 10mg QID, IV during hospitalizations |
Hydromorphone 5mg 3 times per week |
637 | Basal-bolus | 8.1% (8.1) | |
| 11 | Hereditary (PRSS1) |
25 | 17.6 | none | Hydromorphone 16mg q4h, fentanyl 125 mcg patch |
Fentanyl 150mcg patch; Hydromorphone 8mg BID |
3,096 | Minimal (4u/d) | 5.8– 6.4% (6.4) | |
| 12** | Idiopathic | 3 | 16.2 | Puestow | Methadone 2.5mg TID; Oxycodone 5mg q4h PRN |
none | 3 mos | 0 | Basal-bolus | 9.3% (9.3) |
| 13 | Hereditary (PRSS1) |
20 | 12.4 | none | Morphine 30mg PO BID; Hydromorphone 1–2 mg PRN |
none | 1.5 mos | 1,926 | Independent | 5.3– 6% (5.3) |
| 14 | Idiopathic | 10 | 15.9 | Puestow | Fentanyl 25 mcg- 100 mcg patch | none | 2.5 mos | 1,937 | Minimal (6u/d) | 6–6.5% (6.0) |
| 15 | Hereditary (Family Hx+) |
14 | 12.9 | Puestow, DP |
Hydrocodone 5mg q4h PRN | none | 9 mos | 321 | Basal-Bolus | 7.6– 10.9% (10.9) |
| 16 | Hereditary (PRSS1) |
13 | 17.5 | none | Methadone 10mg BID; Hydromorphone 4mg BID or TID |
none | 4 mos | 6,147 | Minimal (11u/d) |
5.7– 5.9% (5.7) |
| 17 | Hereditary (PRSS1) |
12 | 11.7 | Puestow, DP |
Tramadol 25–50mg TID PRN | none | 3 mos | 2,936 | Basal-bolus | 5.6– 6.3% (6.3) |
| 18 | Goldston syndrome |
7 | 9.1 | Whipple | fentanyl 20 mcg IV PRN | IV fentanyl- 3 episodes in 6 months |
< 1 mos | 6,053 | Minimal (0–2 u/d) |
5.7% (5.7) |
| 19 | Hereditary (PRSS1) |
8 | 14.7 | none | Hydromorphone or oxycodone PRN; none daily |
none | 1 mos | 8,714 | Independent | 5.4– 5.9% (5.9) |
Time to narcotic discontinuation is month postoperatively when narcotic medications were stopped. IE/kg is the islet mass transplanted intraportally.
DP= distal pancreatectomy, CF= cystic fibrosis.
pre-operative diabetes, C-peptide positive but on insulin pump,
insufficient number of islets isolated for transplant,
patient received pancreas transplant at outside institution for exocrine and endocrine insufficiency; insulin use and HbA1c are before pancreas transplant
Insulin Requirements
Seven patients achieved and maintained insulin independence and 4 had minimal insulin requirements, all with HbA1c levels ≤6.5% (table 4), at a mean follow up of 18 ± 8 months posttransplant. HbA1c was more variable among the insulin dependent recipients, ranging from 5.8–12.1%. However, some islet function was present even among these recipients, as demonstrated by stimulated C-peptide values of 0.4–3.1 ng/mL.
Prior Puestow or other surgical drainage with (n=2) or without (n=4) distal pancreatectomy was associated with a higher likelihood of insulin dependence (p=0.04). None of the 6 patients a with prior drainage procedure achieved insulin independence.
CONCLUSIONS
Surgery is generally considered for patients with painful chronic pancreatitis that is refractory to medical and endoscopic therapies. Although partial resections or drainage operations are considered standard care at most specialized pancreatic centers, TP/IAT allows removal of the entire diseased gland, with amelioration or minimization of post-surgical diabetes. Post-operative narcotic use, insulin use, and standardized pain assessments have been previously reported by several groups; however, standardized HRQOL measurements have been lacking in the literature, especially in children (17,21,23,26,36,37). We report the first prospectively studied quality of life outcomes after TP/IAT, in 19 consecutive pediatric patients over a 2-year interval. HRQOL, as measured by the SF-36 questionnaire, significantly improved after surgery. Notably, both physical and emotional summary component scores, which were nearly 2 standard deviations below the population normal scores before surgery, completely normalized after TP/IAT in these patients.
Of the 8 health dimensions measured by the SF-36, each one improved, with the greatest improvements observed for role-physical limitation, physical functioning, bodily pain, and social functioning. Interestingly, there was a small decrease in the PCS and several of the subscales (role limitation-physical, bodily pain, social functioning, vitality, and physical functioning) at 6 months postoperatively compared to the 3 month assessment. This may represent a transition period, during which patients are weaning off narcotics and adjusting enzyme supplement therapy. Reassuringly, by 1 year improvement from baseline was noted, and stability was demonstrated through 2 years.
Consistent with prior reports, the majority of patients weaned off narcotic medications after surgery (26). Narcotic medications were completely discontinued in nearly 75% of patients, with several others rarely needing such medications.
Although published data are sparse for pediatric patients, adults with CP frequently have a low perceived HRQOL (4–6). Reports in adult patients have shown variable improvement in HRQOL after surgical procedures (38). Among one large cohort of adult patients undergoing medical or conventional surgical treatments (surgical drainage procedures or partial resections), average MCS and PCS scores did not change over a 2 year interval (6). This is in contrast to the significant improvements in HRQOL scores seen in our cohort after TP/IAT.
Insulin independence or minimal insulin use was observed in over 60% of patients. These patients maintained tight glycemic control at an average of 1.5 years posttransplant, with HbA1c levels consistently ≤6.5%, a threshold below which microvascular complications from diabetes are rare. A prior surgical drainage procedure was strongly associated with a lower islet yield and an increased risk of diabetes. Although disease severity or associated resection may play a confounding role, the lower islet yield is likely related to inability to use an intact ductal system to distribute collagenase throughout the gland and mechanically disrupt the exocrine pancreas, an integral part of the islet isolation process. Accordingly, the current data suggest that surgical drainage or resection procedures be used with caution in patients with diffuse pancreatic disease who may ultimately fail to respond and be considered for total pancreatectomy.
Eight patients in this series had a confirmed PRSS1 gene mutation resulting in hereditary pancreatitis. These mutations are associated with an approximately 40% lifetime risk of pancreatic cancer (3,39). It is likely that early TP minimizes this risk, by removing the exocrine pancreatic tissue before severe dysplasia has developed. Although there is theoretical concern that premalignant cells might be included with the transplanted islets, no cases of pancreatic cancer arising in the liver have been reported after TP/IAT, including 360 cases performed over 33 years at the U of MN. Nonetheless further formal investigation is required before this procedure can be considered for cancer prevention in premalignant conditions of the pancreas.
These data highlight the promising potential for TP/IAT to provide pain relief and improve physical and social/ emotional function for children affected by severe CP. These results, although optimistic, should be considered in the context of several limitations of the study. First, this is a small cohort with HRQOL follow up limited to 2 years. Second, although some follow up data is available for all 19 consecutive pediatric patients, return of the HR-QOL instruments was incomplete at each time point. Replicating these findings in a larger group of patients and for a prolonged period of time will be important in establishing the therapeutic value of this procedure in childhood. Third, etiology of disease was heterogenous, and differences by etiology of disease could not be assessed in this small cohort. Fourth, assessments were obtained shortly before surgery, which could bias baseline responses. Lastly, because of the wide range of patient ages (5–18 years), questionnaires were sometimes answered by parents and sometimes by the patients themselves. The SF-36 has been validated for patients age ≥14 years. However, because we did not want to exclude the youngest children, for whom medical providers are often reluctant to consider TP, those <14 years are included in the analysis.
These results may or may not be applicable to adults with CP. Adults and children differ in etiology of disease (particularly with respect to alcohol, smoking, and “minimal change” disease) and potential duration of narcotic dependence, which may be decades in adults. Additionally, it is possible that children have lower prevalence of associated gastrointestinal motility disorders, which may contribute to poor outcomes after interventions intended to relieve pain (40).
Due to the specialized nature of the procedure, TP/IAT should be performed at institutions with expertise in islet isolation and with a multidisciplinary team for preoperative evaluation and postoperative management. Currently at U of MN, patients are recommended for TP/IAT by a consensus of a multidisciplinary team, including pediatric and adult endocrinologists, gastroenterologists, interventional pancreaticobiliary endoscopists, and surgeons. Patients who meet the criteria in table 1 are considered candidates for surgery. Although optimal timing of surgery needs to be elucidated, for those who will go onto TP/IAT, earlier surgery may avoid progressive damage to the endocrine pancreas and the hyperalgesia associated with chronic narcotic use (41).
In conclusion, early results from this cohort of pediatric patients with severe chronic pancreatitis suggest dramatic improvement in HRQOL, including both physical and emotional functioning after TP/IAT. This procedure should be considered in children with CP when medical and endoscopic modalities have failed. Adoption of this procedure may imply a paradigm shift in the current management of CP, with avoidance of partial resections without islet autotransplantation and of surgical drainage procedures.
Acknowledgements
The authors would like to thank the National Pancreas Foundation for their contributions to this and related studies of outcomes after TP/IAT at the University of Minnesota. Dr. Bellin is supported by a K23 career development award from NIH (K23 DK084315-01A1). We also thank our nurses—Michelle James, Katherine Louise Berry, and Marie Cook—for their valuable contributions and excellent patient care.
Grant Support: This work was in part supported by the National Pancreas Foundation. Dr. Melena Bellin is supported by a K23 career development award from NIDDK.
Abbreviations
- TP
total pancreatectomy
- IAT
islet autotransplant
- CP
Chronic pancreatitis
- HRQOL
health-related quality of life
- U of MN
University of Minnesota
- SF-36
36 Item Short Form Medical Outcomes Survey
- PCS
physical component summary
- MCS
mental component summary
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author Contributions: MB, GB, TD, AM, and DS were responsible for study conception and design. MB, TD, GB, AB, DR were responsible for collection of data. DR performed the statistical analysis, and MB, MF, SS, SV, TD, GB, SC, BH, DR, AM, DS contributed to the interpretation of results. MB, MF, and DR drafted the first version of the manuscript, and SS, TD, GB, SV, SC, AB, BH, DR, AM, and DS provided critical revision of the manuscript. Funding was obtained by MB and DS. Technical and material support was provided by SV, BH, AB, and DS.
REFERENCES
- 1.Lowe ME. Pancreatitis in childhood. Curr Gastroenterol Rep. 2004;6:240–246. doi: 10.1007/s11894-004-0014-5. [DOI] [PubMed] [Google Scholar]
- 2.Kandula L, Whitcomb DC, Lowe ME. Genetic issues in pediatric pancreatitis. Curr Gastroenterol Rep. 2006;8:248–253. doi: 10.1007/s11894-006-0083-8. [DOI] [PubMed] [Google Scholar]
- 3.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 4.Wehler M, Reulbach U, Nichterlein R, et al. Health-related quality of life in chronic pancreatitis: a psychometric assessment. Scand J Gastroenterol. 2003;38:1083–1089. doi: 10.1080/00365520310005956. [DOI] [PubMed] [Google Scholar]
- 5.Wehler M, Nichterlein R, Fischer B, et al. Factors associated with health-related quality of life in chronic pancreatitis. Am J Gastroenterol. 2004;99:138–146. doi: 10.1111/j.1572-0241.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 6.Pezzilli R, Morselli Labate AM, Fantini L, Gullo L, Corinaldesi R. Quality of life and clinical indicators for chronic pancreatitis patients in a 2-year follow-up study. Pancreas. 2007;34:191–196. doi: 10.1097/mpa.0b013e31802e0301. [DOI] [PubMed] [Google Scholar]
- 7.Fitzsimmons D, Kahl S, Butturini G, vanWyk M, et al. Symptoms and Quality of Life in Chronic Pancreatitis Assessed by Structured Interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918–926. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 8.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 9.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 10.Choudari CP, Nickl NJ, Fogel E, Lehman GA, Sherman S. Hereditary pancreatitis: clinical presentation, ERCP findings, and outcome of endoscopic therapy. Gastrointest Endosc. 2002;56:66–71. doi: 10.1067/mge.2002.125103. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136 doi: 10.1053/j.gastro.2008.09.028. 149,159.e2. [DOI] [PubMed] [Google Scholar]
- 12.O'Neil SJ, Aranha GV. Lateral pancreaticojejunostomy for chronic pancreatitis. World J Surg. 2003;27:1196–1202. doi: 10.1007/s00268-003-7238-7. [DOI] [PubMed] [Google Scholar]
- 13.Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–684. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58:365–382. doi: 10.1016/s0039-6109(16)41489-1. [DOI] [PubMed] [Google Scholar]
- 15.Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007;87:1477–1501. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Ong SL, Gravante G, Pollard CA, Webb MA, Illouz S, Dennison AR. Total pancreatectomy with islet autotransplantation: an overview. HPB (Oxford) 2009;11:613–621. doi: 10.1111/j.1477-2574.2009.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 18.Wahoff DC, Papalois BE, Najarian JS, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222 doi: 10.1097/00000658-199522240-00013. 562,75; discussion 575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jie T, Hering BJ, Ansite JD, et al. Pancreatectomy and auto-islet transplant in patients with chronic pancreatitis. J Am Col Surg. 2005;201:S14. [Google Scholar]
- 20.Behrman SW, Mulloy M. Total pancreatectomy for the treatment of chronic pancreatitis: indications, outcomes, and recommendations. Am Surg. 2006;72:297–302. [PubMed] [Google Scholar]
- 21.Clayton HA, Davies JE, Pollard CA, White SA, Musto PP, Dennison AR. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: the first 40 patients at the leicester general hospital. Transplantation. 2003;76:92–98. doi: 10.1097/01.TP.0000054618.03927.70. [DOI] [PubMed] [Google Scholar]
- 22.Gruessner RW, Sutherland DE, Dunn DL, et al. Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg. 2004;198 doi: 10.1016/j.jamcollsurg.2003.11.024. 559,67; discussion 568-9. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez Rilo HL, Ahmad SA, D'Alessio D, et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978–989. doi: 10.1016/j.gassur.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37:282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr. 2008;47:37–44. doi: 10.1097/MPG.0b013e31815cbaf9. [DOI] [PubMed] [Google Scholar]
- 27.Bellin MD, Blondet JJ, Beilman GJ, et al. Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis. Pediatr Diabetes. 2010;11:227–234. doi: 10.1111/j.1399-5448.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anazawa T, Balamurugan AN, Bellin M, et al. Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes. Am J Transplant. 2009;9:2383–2391. doi: 10.1111/j.1600-6143.2009.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakey JR, Warnock GL, Shapiro AM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285–292. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 30.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38 Suppl 1:140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 31.Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 1989;38 Suppl 1:143–145. doi: 10.2337/diab.38.1.s143. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 33.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36) Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 34.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. System for mixed models. Cary, NC: SAS Institute, Inc.; 1996. [Google Scholar]
- 35.Murray DM. Design and analysis of group-randomized trials. volume 27. New York: Oxford University Press; 1998. [Google Scholar]
- 36.Dixon J, DeLegge M, Morgan KA, Adams DB. Impact of total pancreatectomy with islet cell transplant on chronic pancreatitis management at a disease-based center. Am Surg. 2008;74:735–748. doi: 10.1177/000313480807400812. [DOI] [PubMed] [Google Scholar]
- 37.Argo JL, Contreras JL, Wesley MM, Christein JD. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74 530,6; discussion 536-7. [PubMed] [Google Scholar]
- 38.Pezzilli R, Fantini L, Morselli-Labate AM. Pancreatectomy for pancreatic disease and quality of life. JOP. 2007 Jan 9;8(1 Suppl):118–131. [PubMed] [Google Scholar]
- 39.Howes N, Greenhalf W, Neoptolemos J. Screening for early pancreatic ductal adenocarcinoma in hereditary pancreatitis. Med Clin North Am. 2000;84:719–738. doi: 10.1016/s0025-7125(05)70254-6. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury RS, Forsmark CE, Davis RH, Toskes PP, Verne GN. Prevalence of gastroparesis in patients with small duct chronic pancreatitis. Pancreas. 2003;26:235–238. doi: 10.1097/00006676-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006 Mar;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]


