Abstract
The administration of progesterone as a neuroprotective agent following traumatic brain injury has recently entered phase III clinical trials. Previous work has demonstrated that therapeutic concentrations of progesterone decrease excitotoxicity through direct inhibition of voltage-gated calcium channels, an action independent of the nuclear progesterone receptor. Here we report using cultured rat striatal neurons that these same concentrations of progesterone also block voltage-gated potassium channels, sodium channels and GABAA currents. The actions of progesterone act at the surface membrane of neurons in a steroid specific, voltage-independent, concentration-dependent manner. Notably, these broad actions of progesterone on ion channel and neurotransmitter receptor function mirror those of dihydropyridines, and indicate potential shared mechanisms of action, the prospective of additional therapeutic applications, and possibly, untoward effects.
Keywords: traumatic brain injury, neuroprotection, steroid, striatum, side effects
1. Introduction
Progesterone is currently being tested as a therapeutic agent to combat neurotoxicity following various insults to the brain (Goss et al., 2003; Robertson et al., 2006; Sayeed et al., 2006; Wright et al., 2007; Xiao et al., 2008; Atif et al., 2009). However, in order to achieve therapeutic benefit, micromolar concentrations of the steroid have been required. This indicates a signaling mechanism distinct from the classically described actions of progesterone affecting reproduction-related behaviors via activation of the nuclear progesterone receptor. Indeed, previous work in striatal neurons has found that at micromolar concentrations, progesterone acts at the plasma membrane to directly block the conductance of voltage-gated calcium channels (Luoma et al., 2011).
We report here that progesterone has additional effects on various other ion channels and neurotransmitter receptors. Specifically, therapeutic concentrations of progesterone block voltage-gated potassium and sodium channels, as well as GABAA receptors. Interestingly, these widespread actions of progesterone may additionally account for the long-known anesthetic properties of high concentrations of progesterone (Selye, 1942; Gellersen et al., 2009), as well as more recent findings that high concentrations of progesterone affect uterine contractility and T-cell activity (Putnam et al., 1991; Ehring et al., 1998; Borna and Sahabi, 2008). These effects of progesterone on various ion channels are strikingly similar to data regarding the widespread actions of dihydropyridines (Yatani and Brown, 1985; Zhang et al., 1997; Zhang and Gold, 2009), which were originally characterized as specific blockers of L-type calcium channels. The similarities between progesterone and dihydropyridines on various ion channel functions offer the possibility of a shared mechanism of action, and for progesterone being used in additional therapeutic settings, such as for prevention of Parkinson’s disease (Ritz et al., 2010) and other CNS disorders (Judge et al., 2006). In addition, while preliminary results of the progesterone phase I clinical trial concluded no discernable harm of the hormone, extended surveillance on larger populations of patients within future trials is warranted, as progesterone therapy could result in some of the side effects observed with dihydropyridines.
2. Material and methods
2.1 Cell Culture
Striatal neurons were cultured from P0–P1 Sprague-Dawley rats as previously described (Mermelstein et al., 2000), using a protocol approved by the by the Animal Care and Use Committee at the University of Minnesota. The effects of progesterone reported here did not differ between cultures derived solely from males or females. Chemicals are from Sigma (St. Louis, MO) unless noted otherwise. Following decapitation, striatal neurons were isolated in ice-cold HBSS (pH 7.35, 300 mOsm) containing 4.2 mM NaHCO3, 1mM HEPES, and 20% fetal bovine serum (FBS; Hyclone, Logan, UT). Tissue was washed and digested for 5 min in trypsin solution (type XI, 10 mg/ml) containing (in mM): 137 NaCl, 5 KCl, 7 Na2HPO4, and 25 HEPES, with 1500 U of DNase, pH 7.2, 300 mOsm. Following washing in HBSS dissociation of the tissue was performed using Pasteur pipettes of decreasing diameters. The dissociated cells were pelleted twice to remove contaminants, plated on 10 mm coverslips (treated with Matrigel for adherence; BD Biosciences, San Jose, CA) and incubated at room temperature for 15 min. One milliliter of minimum essential medium (MEM; Invitrogen, Grand Island, NY) with 28 mM glucose, 2.4 mM NaHCO3, 0.0013 mM transferring (Calbiochem, La Jolla, CA), 2 mM glutamine, and 0.0042 mM insulin with 1% B-27 supplement (Invitrogen) and 10% FBS, pH 7.35, 300 mOsm was added to each coverslip containing well. To stunt glial growth, 1 mL of medium was added containing cytosine 1-β-D-arabinofuranoside (4 μM) and 5% FBS 24–48 h after plating. Four or five days later, 1 ml of medium was replaced with modified MEM containing 5% FBS.
2.2 Drugs
Drugs used are as follows: γ-aminobutyric acid (GABA; Tocris, Ellisville, MO); tetrodotoxin (TTX; Tocris, Ellisville, MO; Pregn-4-ene-3,20-dione (50 μM; progesterone; Sigma); 3β-Hydroxy-5α-pregnan-20-one acetate (allopregnanolone; Sigma, St. Louis, MO); (3a,5b)-3-Hydroxy-pregnan-20-one (pregnenolone; Tocris, Ellisville, MO); Nifedipine (Sigma, St. Louis, MO).
2.3 Electrophysiology
Whole-cell currents were recorded from cultured striatal neurons (7 to 14 d.i.v.) using standard voltage-clamp techniques. All experiments were conducted at room temperature. A minimum of five separate recordings were obtained per experimental condition with the median group size being seven. Recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA), controlled by a personal computer running pClamp software (version 10.2.0.14). All recordings were filtered at 2 kHz, and the series resistance was compensated 40–60%. Holding current and access resistance were monitored during recordings, and experiments with holding currents > 500 pA or unstable access resistance greater than 20 MΩ were excluded from analysis. Recording electrodes were pulled by a Model P-97 Flaming/Brown micropipette puller (Sutter Instrument Co., Novato, CA) to resistances between 2.5 and 5.5 MΩ. The perfusion system consisted of a convergent single-barrel that allowed for extracellular solution changes in ~1 s. Extracellular solution for recording voltage-gated potassium currents contained the following (mM): 140 Na-isethionate, 17.5 sucrose, 12 glucose, 10 HEPES, 5 KCl, 2 MgCl2, 0.4 CdCl2 and 0.0005 TTX. Extracellular solution for recording voltage-gated Na+ currents included the following (mM): 210 Sucrose, 20 TEA-Cl, 11 glucose, 10 HEPES, 10 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 0.5 CdCl2. Extracellular solution for GABA-mediated currents contained the following (mM): 130 NaCl, 5.4 KCl, 2.5 CaCl2, 1.5 MgCl2, 10 HEPES, 20 Glucose, 0.01 Glycine and 0.0005 TTX. The intracellular recording solution for potassium currents contained the following (in mM): 120 Potassium Gluconate, 40 HEPES, 5 BAPTA, 2 MgCl2 and 0.5 CaCl2. The intracellular recording solution for sodium currents contained the following (in mM): 110 NaCl, 20 CsCl, 10 HEPES, 10 EGTA, 4 ATP, 1 MgCl2, 0.5 CaCl2, and 0.4 GTP. The intracellular recording solution for GABA-mediate currents contained the following (in mM): 145 K-gluconate, 10 HEPES, 8 NaCl, 2 ATP, 3 GTP and 0.2 EGTA. Drugs activating ligand-gated channels were applied via an adjacent (~200 microns from the soma) pipette via a Picospritzer (Parker-Hannifin, Cleveland, OH) at 10 psi for a duration 200 ms. The Picospritzer was activated by a trigger from the acquisition software. Membrane potential was held at −70 mV for GABA experiments. In voltage-gated ion channel experiments whole-cell currents were activated by a step from −80 mV to 0mV for a period of 100 ms. Data was analyzed in Clampfit 10.2 and Microsoft Excel (Redmond, WA) then plotted using GraphPad Prism 4.0 (La Jolla, CA).
3. Results
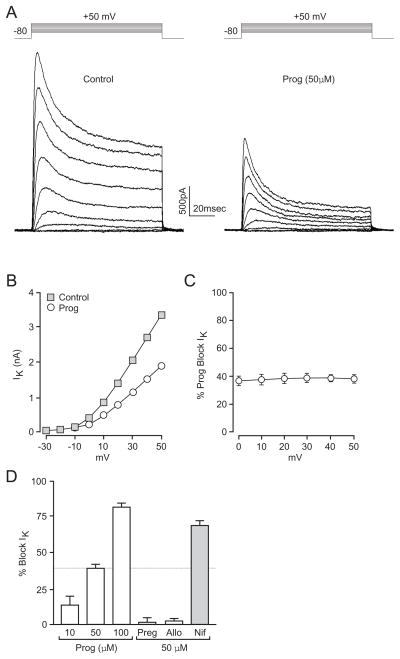
Outward potassium currents in cultured striatal neurons were evoked via voltage steps from −80 mV to membrane potentials ranging from −20 mV to +50 mV. Progesterone (50μM) blocked these voltage-gated potassium currents. Figure 1A provides data obtained from a typical neuron. The actions of progesterone were voltage-independent as demonstrated by plotting the percent inhibition of these currents by progesterone as a function of the membrane potential (n=6) (Figure 1C). Current block occurred within seconds of progesterone application, fully effective within approximately one minute of hormone exposure, and reversed within 1–2 min upon wash. Additionally, progesterone decreased voltage-dependent potassium currents in a concentration-dependent manner, resulting in a 14 ± 6 (n=6), 38 ± 3 (n=12) and 82 ± 3% (n=6) block when using 10, 50 and 100μM progesterone (Figure 1D). Pregnenolone (n=6) and allopregnanolone (n=6), the progesterone precursor and metabolite, had no effect, demonstrating high hormone specificity (Figure 1D).
Figure 1.
Progesterone inhibits voltage-gated potassium currents. (A) Representative traces of whole-cell voltage clamp recordings of potassium currents evoked by a series of 100 ms steps in 10 mV increments from −20 to +50 mV (from a resting potential of −80 mV) in the absence (Left) or presence (Right) of 50 μM progesterone. (B) Current/voltage plot of whole-cell potassium currents in the presence or absence of progesterone. (C) Plot of the fractional block (%) by progesterone as a function of voltage, indicating the effects of progesterone are voltage-independent. (D) The effects of progesterone were concentration-dependent. Pregnenolone and allopregnanolone did not block potassium currents, whereas the nifedipine exhibited significant block.
To test whether progesterone was acting at the extracellular face or passing through the neuronal membrane to block voltage-gated potassium channels, we performed experiments in which the internal pipette solution contained 50μM progesterone. In cells dialyzed with progesterone, extracellular application of 50μM progesterone still resulted in a reduction of current by 52 ± 4% (n=5). These data demonstrate that like the blockade observed for calcium channels (Luoma et al., 2011), progesterone inhibits potassium channels by acting on the outer membrane of neurons. These findings of progesterone parallel in many respects the notable widespread actions of dihydropyridines. Consistent with this idea, 50μM nifedipine was found to inhibit potassium currents by 69 ± 3% (n=7) (Figure 1D), consistent with results from Dorsal Root Ganglion neurons (Zhang and Gold, 2009).
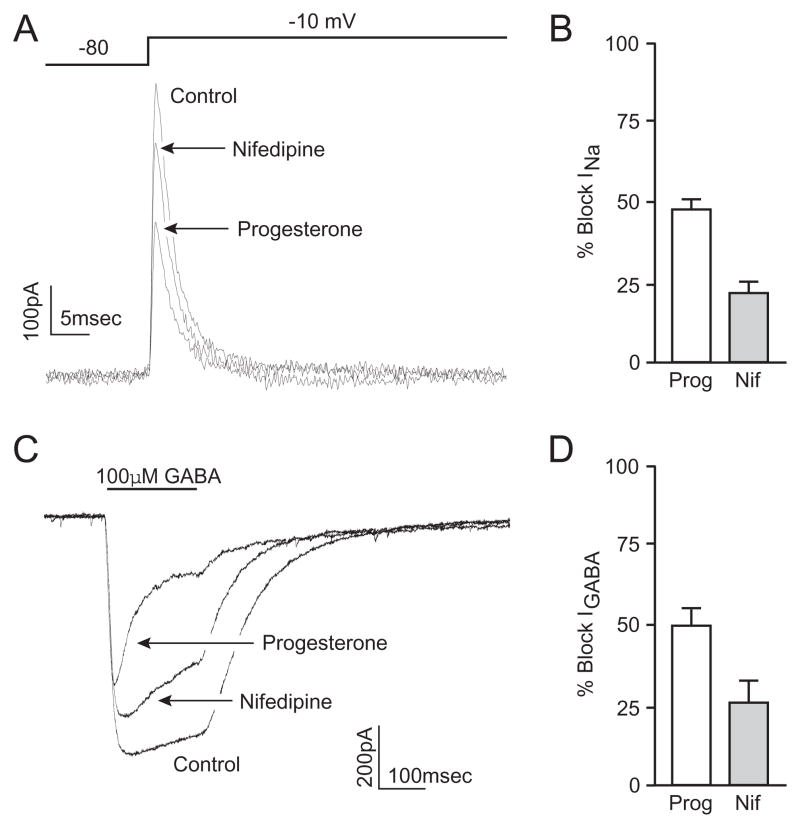
Our observations of potassium channel blockade suggest the effects of progesterone on cellular physiology are far more ubiquitous than inhibition of voltage-gated calcium channels. Indeed, we also observed that progesterone reduced the whole-cell currents associated with voltage-gated sodium channels (Figure 2A). Currents were evoked by a voltage step from −80 mV to 0 mV. Pipette and bath solutions were manipulated to create an outward sodium current, preventing large regenerative currents. Progesterone (50μM) inhibited the voltage-gated sodium current by 47 ± 4% (n=12) (Figure 2B). Again, progesterone block rapidly reversed following washout. Similar to the experiments measuring potassium currents, experiments were also performed with progesterone (50μM) in the internal pipette solution. Standard bath application of progesterone again resulted in a 65 ± 4% inhibition (n=6) of the sodium current in cells dialyzed with progesterone. To test for an effect of dihydropyridines, application of 50 μM nifedipine was found to reduce peak currents by 20 ± 4% (n=6) (Figure 2A,B).
Figure 2.
Progesterone inhibits voltage-gated sodium channels and GABAA-mediated whole-cell currents. (A) Representative traces of whole cell sodium currents evoked by a voltage step from −80 to 0 mV under control conditions, and in the presence of progesterone or nifedipine. (B) Bar graphs summarizing the inhibition of sodium current by progesterone (47 ± 4%) and nifedipine (20 ± 4%) (C) Representative traces of whole-cell voltage clamp recordings during a 200 ms application of GABA (100 μM) under control conditions, and in the presence of progesterone or nifedipine. (D) Bar graphs summarizing the inhibition of GABAA-mediated peak current by progesterone (49 ± 6%) and nifedipine (25 ± 7%).
The effects of progesterone were not limited to voltage-gated ion channels. GABAA receptors were also reversibly inhibited by progesterone. GABA (100 μM) was applied via picospritzer onto neurons under voltage-clamp conditions (Figure 2C,D). In the presence of progesterone, the peak GABA-mediated current was inhibited 49 ± 9% (n=14) (Figure 2D). Similarly, bath application of nifedipine resulted in a reduction in GABA-mediated currents by 25 ± 7 % (n=8) (Figure 2C,D).
4. Discussion
The data presented here demonstrates that progesterone inhibits voltage-gated potassium channels in a concentration-dependent, voltage-independent manner. This action is specific to progesterone, as both the steroid precursor and metabolite were without effect. In addition, progesterone inhibited voltage-gated sodium channels and GABAA receptor-mediated currents, demonstrating widespread effects of the hormone. This is in addition to the previous finding that progesterone blocks voltage-gated calcium channels (Luoma et al., 2011).
The concentrations required for progesterone to modulate ion channels are far beyond those necessary for the activation of classical progesterone receptors (Hurd and Moudgil, 1988) and are strikingly similar to studies examining progesterone receptor-independent effects in non-neuronal cells (Ehring et al., 1998; Zhang et al., 2002; Bielefeldt et al., 1996). Furthermore, the persistent inhibition of both potassium and sodium currents by extracellular progesterone administration when the cell is dialyzed with progesterone demonstrates that the block occurs at the extracellular surface. The lack of voltage-dependence of potassium channel block indicates progesterone does not occlude the channel pore in a way that is overcome by increasing the driving force. Additionally, the specificity of progesterone as compared to pregnenolone and allopregnanolone gives some insight into a possible structure-function relationship. The presence of the ketone at the 3-position of the A-ring is a notable difference between these compounds. The rapid onset and reversible nature of these effects of progesterone further differentiate the results here from a classical model of steroid hormone receptor signaling. Clearly, therapeutic concentrations of progesterone can impact a variety of cellular functions independent of nuclear progesterone receptors
The effects of progesterone presented here are in many ways reminiscent of dihydropyridine action, suggesting several implications regarding its clinical use. Indeed, both dihydropyridines and progesterone have been independently examined as therapeutics to delay preterm labor (King et al., 2003; Borna and Sahabi, 2008; Lyell et al., 2008; Anderson et al., 2009). Dihydropyridines have been characterized for decades as “specific” blockers to L-type voltage-gated calcium channels, and have been tested for their neuroprotective properties, based on the idea that a dihydropyridine-mediated reduction in calcium influx would reduce the cell death associated with excitotoxicity (Kittaka et al., 1997; Ginsberg, 2008). As an example, nimodipine has been used as a neurological therapeutic to reduce vasospasm associated with subarachnoid hemorrhage (Allen et al., 1983). Through blockade of calcium channels, dihydropyridines have primarily been used to reduce cardiovascular symptoms of hypertension and angina, treat bipolar disorder and alleviate migraine (Gelmers, 1985; Goodnick, 2000; Rosendorff, 2007). In addition, recent studies reviewing the incidence of Parkinson’s disease revealed a risk reduction for individuals taking centrally acting L-type calcium channel blockers (Becker et al 2008, Ritz et al 2010). This is consistent with data from a mouse model of Parkinson’s disease, where dihydropyridine-mediated neuroprotection occurred following administration of isradipine at concentrations that specifically target one class (i.e. CaV1.3) of L-type calcium channel (Ilijic et al 2011).
Interestingly, at marginally higher concentrations than generally used to investigate L-type calcium channel activity, many dihydropyridines block other voltage-gated potassium and sodium channels as well as inhibit GABAA receptors (data reported here and: Yatani and Brown, 1985; Zhang et al., 1997; Das et al., 2004; Zhang and Gold, 2009). In the clinical setting, steady state serum concentrations following therapeutic application of dihydropyridines (30–180 mg/day) are typically in the nanomolar range, below the acute concentrations used here. However, concentrations of even a low dose of an administered dihydropyridine (e.g. 10–20 mg) can approach micromolar levels with any one of several contributing factors, such as advanced age, use of H2-receptor antagonists and/or being of non-caucasian lineage (Renwick et al, 1988; Chien et al, 2004; Ahmad et al, 2009; Robertson et al, 1988; Kahn et al, 1991). As such, it has been postulated that dihydropyridines may act within the CNS of patients to affect these additional channels and receptors (Das et al., 2004). These investigators postulate that the actions of dihydropyridines outside of L-type calcium channel block may contribute to the therapeutic effectiveness of the drug. In general, potassium channels are garnering more attention as therapeutic targets in multiple CNS disorders including Multiple Sclerosis, Parkinson’s disease and Alzheimer’s disease (Wang et al, 2008; Judge et al, 2006). Hence, the spectrum of disorders that may be treated by dihydropyridines may be expanded. That said, dihydropyridines are often not favored by doctors or patients, due to various side-effects including dizziness, peripheral edema, hypotension, reflex tachycardia and headache (Myers, 1994; Pedrinelli et al., 2001). The underlying causes of these side-effects may be due to a variety of contributing factors.
Initial in vivo studies demonstrating neuroprotective properties of progesterone used micromolar concentrations of the hormone. Subsequent in vitro studies utilized similar concentrations (Shear 2002, Wright 2001, Atif 2009). Additionally, a study investigating the hypnotic properties of progesterone reported peak levels within 15 minutes of administration, with elevated brain concentrations compared to serum suggesting a preferential distribution of progesterone to the CNS (Lancel 1996). Current clinical trial dosage regimens result in a relatively lower steady state serum concentration (337 ± 135 ng/ml) than the levels we and others have used to affect neuronal function (Wright 2007, BHR Pharma, LLC 2010). Earlier clinical trials indicated considerable variation in the volume of distribution between patients (Wright 2005). The study focused on safety and pharmacokinetics rather than efficacy and authors noted that increases in sustained doses and future dose-effect studies to determine proper effective administration were likely in order. Further, clinical applications of progesterone have been proposed where dosages are increased ten-fold (Hoffman 2008). The variable pharmacokinetic states of traumatic brain injury patients, as well as the undefined dose-effect properties of progesterone in humans demand attention. Ultimately, understanding the actions of progesterone on various ion channels and neurotransmitter receptors following clinical intervention will identify both mechanisms of action, as well as potential therapeutic roles and possible side effects of drug administration.
Acknowledgments
This research was supported by the National Institutes of Health NS41302 (PGM) and an IRACDA Post-Doctoral Fellowship GM074628 (BGK). We thank Dr. John Meitzen for his comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad A, Ahmad T, Sultan RA, Murtaza G. Pharmacokinetic Study of Nifedipine in Healthy Adult Male Human Volunteers. Tropical J of Pharm Res. 2009;8:385–91. [Google Scholar]
- Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, Chou SN, Kelly DL, Weir BK, Crabbe RA, Lavik PJ, Rosenbloom SB, Dorsey FC, Ingram CR, Mellits DE, Bertsch LA, Boisvert DP, Hundley MB, Johnson RK, Strom JA, Transou CR. Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308:619–24. doi: 10.1056/NEJM198303173081103. [DOI] [PubMed] [Google Scholar]
- Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy. Reprod Sci. 2009;16:1052–61. doi: 10.1177/1933719109340926. [DOI] [PubMed] [Google Scholar]
- Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol Med. 2009;15:328–36. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Jick SS, Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurol. 2008;70:1438–44. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- BHR Pharma. LLC Efficacy and Safety Study of Intravenous Progesterone in Patients With Severe Traumatic Brain Injury (SyNAPSe) 2010–2012 Available from: http://clinicaltrials.gov/ct2/show/NCT01143064. NLM Identifier: NCT0114306.
- Bielefeldt K, Abboud M, Conklin JL. Nongenomic effects of progesterone intestinal smooth muscle cells. Am J Physiol. 1996;271:370–376. doi: 10.1152/ajpgi.1996.271.2.G370. [DOI] [PubMed] [Google Scholar]
- Borna S, Sahabi N. Progesterone for maintenance tocolytic therapy after threatened preterm labour: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2008;48:58–63. doi: 10.1111/j.1479-828X.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- Chien SC, Uang YS, Lin HY, Hsu KY. Pharmacokinetics of nifedipine in Taiwanese. Biopharm Drug Dispos. 2004;25:77–84. doi: 10.1002/bdd.386. [DOI] [PubMed] [Google Scholar]
- Das P, Bell-Horner CL, Huang RQ, Raut A, Gonzales EB, Chen ZL, Covey DF, Dillon GH. Inhibition of type A GABA receptors by L-type calcium channel blockers. Neurosci. 2004;124:195–206. doi: 10.1016/j.neuroscience.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Ehring GR, Kerschbaum HH, Eder C, Neben AL, Fanger CM, Khoury RM, Negulescu PA, Cahalan MD. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med. 1998;188:1593–602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–38. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- Gelmers HJ. Calcium-channel blockers in the treatment of migraine. Am J Cardiol. 1985;55:139–143. doi: 10.1016/0002-9149(85)90622-8. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharm. 2008;55:363–89. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnick PJ. The use of nimodipine in the treatment of mood disorders. Bipolar Disord. 2000;2:165–73. doi: 10.1034/j.1399-5618.2000.020303.x. [DOI] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharm Biochem Behav. 2003;76:231–42. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Hoffman SW, Kellermann AL, Stein DG, Wright DW, Lowery-North DW. Methods for the treatment of a traumatic central nervous injury. 7915244 US Patent. 2008
- Hurd C, Moudgil VK. Characterization of R5020 and RU486 binding to progesterone receptor from calf uterus. Biochem. 1988;27:3618–23. doi: 10.1021/bi00410a014. [DOI] [PubMed] [Google Scholar]
- Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 2011;43:364–71. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SI, Lee JM, Bever CT, Jr, Hoffman PM. Voltage-gated potassium channels in multiple sclerosis: Overview and new implications for treatment of central nervous system inflammation and degeneration. J Rehabil Res Dev. 2006;43:111–22. doi: 10.1682/jrrd.2004.09.0116. [DOI] [PubMed] [Google Scholar]
- Khan A, Langley SJ, Mullins FG, Dixon JS, Toon S. The pharmacokinetics and pharmacodynamics of nifedipine at steady state during concomitant administration of cimetidine or high dose ranitidine. Br J Clin Pharmacol. 1991;32:519–22. doi: 10.1111/j.1365-2125.1991.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JF, Flenady VJ, Papatsonis DN, Dekker GA, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev. 2003;1:CD002255. doi: 10.1002/14651858.CD002255. [DOI] [PubMed] [Google Scholar]
- Kittaka M, Giannotta SL, Zelman V, Correale JD, DeGiorgio CM, Weiss MH, Zlokovic BV. Attenuation of brain injury and reduction of neuron-specific enolase by nicardipine in systemic circulation following focal ischemia and reperfusion in a rat model. J Neurosurg. 1997;87:731–7. doi: 10.3171/jns.1997.87.5.0731. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271:763–72. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- Luoma JI, Kelley BG, Mermelstein PG. Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids. 2011 doi: 10.1016/j.steroids.2011.02.013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyell DJ, Pullen KM, Mannan J, Chitkara U, Druzin ML, Caughey AB, El-Sayed YY. Maintenance nifedipine tocolysis compared with placebo: a randomized controlled trial. Obstet Gynecol. 2008;112:1221–6. doi: 10.1097/AOG.0b013e31818d8386. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–73. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG. Dihydropyridine calcium antagonists and the trough: peak ratio: focus on adverse effects. J Hypertens Suppl. 1994;12:S73–6. [PubMed] [Google Scholar]
- Pedrinelli R, Dell’Omo G, Mariani M. Calcium channel blockers, postural vasoconstriction and dependent oedema in essential hypertension. J Hum Hypertens. 2001;15:455–61. doi: 10.1038/sj.jhh.1001201. [DOI] [PubMed] [Google Scholar]
- Putnam CD, Brann DW, Kolbeck RC, Mahesh VB. Inhibition of uterine contractility by progesterone and progesterone metabolites: mediation by progesterone and gamma amino butyric acidA receptor systems. Biol Repro. 1991;45:266–72. doi: 10.1095/biolreprod45.2.266. [DOI] [PubMed] [Google Scholar]
- Renwick AG, Robertson DR, Macklin B, Challenor V, Waller DG, George CF. The pharmacokinetics of oral nifedipine--a population study. Br J Clin Pharmacol. 1988;25:701–8. doi: 10.1111/j.1365-2125.1988.tb05256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol. 2010;67:600–6. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exper Neurol. 2006;197:235–43. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Robertson DR, Waller DG, Renwick AG, George CF. Age-related changes in the pharmacokinetics and pharmacodynamics of nifedipine. Br J Clin Pharmacol. 1988;25:297–305. doi: 10.1111/j.1365-2125.1988.tb03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendorff C. Hypertension and coronary artery disease: a summary of the American Heart Association scientific treatment. J Clin Hypertens. 2007;9:790–5. doi: 10.1111/j.1751-7176.2007.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emer Med. 2006;47:381–9. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Selye H. Correlations between the chemical structure and the pharmacological actions of the steroids. Endo. 1942;30:437–453. [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang PL, Tang JF, Lin JF, Cai XH, Wang XT, Zheng GQ. Potassium channels: possible new therapeutic targets in Parkinson’s disease. Med Hypotheses. 2008;71:546–50. doi: 10.1016/j.mehy.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emer Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Wright DW, Ritchie JC, Mullins RE, Kellermann AL, Denson DD. Steady-state serum concentrations of progesterone following continuous intravenous infusion in patients with acute moderate to severe traumatic brain injury. J Clin Pharmacol. 2005;45:460–8. doi: 10.1177/0091270005276201. [DOI] [PubMed] [Google Scholar]
- Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A, Brown AM. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Cir Res. 1985;56:868–75. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]
- Zhang M, Benishin CG, Pang PKT. Rapid inhibition of the contraction of rat tail artery by progesterone is mediated by inhibition of calcium currents. J Pharmacy Pharm. 2002;54:1667–74. doi: 10.1211/002235702405. [DOI] [PubMed] [Google Scholar]
- Zhang X, Anderson JW, Fedida D. Characterization of nifedipine block of the human heart delayed rectifier, hKv1.5. J Pharm Exper Ther. 1997;281:1247–56. [PubMed] [Google Scholar]
- Zhang X, Gold MS. Dihydropyridine block of voltage-dependent K+ currents in rat dorsal root ganglion neurons. Neurosci. 2009;161:184–94. doi: 10.1016/j.neuroscience.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]