Summary
Immune function declines progressively with age, resulting in increased susceptibility of the elderly to infection and impaired responses to vaccines. A diverse repertoire of T cells is essential for a vigorous immune response, and an important manifestation of immune aging is the progressive loss of repertoire diversity, predominantly among CD8 T cells in both mice and humans. Importantly, perturbations in the peripheral T cell repertoire, including reduction of the CD4:CD8 ratio and cytomegalovirus-driven T cell clonal expansions, make a major contribution to the “immune risk phenotype” defined for humans, which predicts two-year mortality in very old individuals.
Introduction
A diverse repertoire of naïve T cells is essential for a vigorous response to new infections and vaccination. Age-associated narrowing of the T cell repertoire, largely in the CD8 T cell pool, is thought to have profound effects on host immunity [1–3]. T cells mature in the thymus and are exported to the periphery, where they comprise the pool of naïve T cells. The naïve T cell repertoire in young individuals is very diverse. Estimates are in the range of ~2 × 106 clones in mice [4] and ~2.5 × 107 in humans [5]. Upon encounter of a naïve host with antigen, antigen-specific cells undergo expansion, followed by a contraction phase, and stabilization at a higher precursor frequency than before encounter with antigen. After clearance of antigen, a population of memory cells persists, with an enhanced precursor frequency and lower activation threshold. Both naïve and memory CD8 T cell pools are maintained by homeostatic proliferation. Aging has several specific effects on the naïve and memory T cell repertoires.
The diversity of naïve and memory T cells is reduced with age
Aging is associated with a decline in the proportion of naïve to memory T cells and reduced T cell repertoire diversity among both populations. T cells mature in the thymus and naive T cells are continuously exported as “recent thymic emigrants” or RTEs. The maintenance of a diverse peripheral T cell repertoire is dependent on continuous replenishment by RTEs. However, as the thymus progressively involutes with age, the diversity of the naïve repertoire is compromised. In the absence of adequate export of new naïve T cells from the aging thymus, homeostatic proliferation of the existing naïve cells increases, sometimes resulting in their phenotypic conversion to memory cells in the absence of antigen encounter and further depleting the naïve repertoire [6–8].
To formally demonstrate changes in naïve repertoire diversity with age, peripheral T cells in young and aged mice were analyzed for T cell receptor Vβ diversity, using a panel of monoclonal antibodies, as well as DNA spectratype and sequence analysis. The data confirmed reduced naïve CD8 T cell repertoire diversity in aged compared with young mice. Interestingly, small clonal expansions among T cells with a naïve phenotype were observed in some aged mice, suggesting dysregulation in the normal homeostatic proliferative mechanisms that operate in young mice to maintain diversity in the repertoire of naïve T cells [9].
In addition, the memory pool expands with the accumulating antigen experience of the host over time [10–12]. The result is a dramatic skewing of the ratio of memory to naïve CD8 T cells in the periphery. Finally, large populations of clonally-expanded T cells (TCEs) develop among memory cells with age [13,14]. These clones can dominate the memory pool over time, greatly restricting repertoire diversity [15,16].
Together, decline in thymic export, accumulating antigen experience and dysregulation in homeostatic maintenance mechanisms in both the naïve and memory pools contribute to the overall decline in diversity of the repertoire with age.
Reduced repertoire diversity leads to impaired immune responses
New antigens are predominantly recognized by naïve CD8 T cells. Consequently, maintenance of a diverse repertoire of naïve cells is important for a vigorous and effective response to new antigens and vaccination. With age, the number and diversity of naïve cells decreases. This loss of repertoire diversity has been correlated with impaired immune responses [17–19]. We have shown that this can be so severe as to lead to “holes in the repertoire”, defined by an inability to respond to individual epitopes. We examined the ability of young and aged mice to respond to de novo infection with influenza virus [19]. As expected, young mice responded vigorously to an immunodominant influenza nucleoprotein epitope. However, there was a preferential loss of the ability to respond to this epitope in some, but not all, aged mice, indicating the development of a “hole” in the repertoire. A hole in the repertoire in response to primary infection correlated closely with impaired responses to heterosubtypic challenge. Aged mice that were still able to generate a response to the NP epitope used a repertoire of T cells that differed significantly from that of young mice [19]. The decline in responsiveness was accelerated in thymectomized mice. Importantly, precursor frequency analysis in young naïve mice showed that the precursor frequency of the nucleoprotein epitope was substantially lower than the other influenza virus epitopes examined. The preferential loss of the ability to respond to this epitope, despite the fact that it is normally immunodominant, is consistent with the loss of precursor cells of the lowest frequency as the numbers and diversity of the naïve repertoire is reduced by age. These data directly confirm that age-associated reductions in the CD8 T cell repertoire play a major role in declining cellular immune function in the elderly.
Do responses by fortuitously cross-reactive memory cells compensate for the decline in naïve T cells?
Although naïve T cells are the major responders to new antigens, declining numbers and diversity of naïve T cells with age raise the possibility that memory cells generated in response to unrelated antigens increasingly participate in the response to new antigens. In this regard, it has been shown that recognition of antigen by T cells is degenerate [3,20–23], and that memory T cells are more readily triggered than naïve cells [24]. In addition, numerous examples of memory CD8 T cells recognizing cross-reactive epitopes from closely related or totally unrelated pathogens have been reported [20,22,25,26]. This is referred to as “heterologous memory” [27] or “fortuitously cross reactive memory.” We have hypothesized that fortuitously cross reactive memory cells may dominate in the response to de novo infections in aged mice because of the decline in the naïve repertoire [28]. We have tested the ability of fortuitously cross-reactive memory cells to respond to influenza virus using adoptive transfer experiments in which CD8 memory (CD44high) T cells from influenza virus naïve mice were transferred into T cell-deficient recipients. These mice were then infected with influenza virus and the epitope-specific response was measured. The data show a response to influenza virus-specific epitopes from the adoptively transferred aged memory cells in ~40% of mice, confirming the ability of these cells to respond to influenza virus (Blackman, unpublished data). We predict that the contribution of fortuitously cross-reactive memory T cells to the response to new antigens in aged mice may frequently lead to suboptimal or even pathologic responses. We also predict that the presence of TCE in the memory pool will reduce the repertoire diversity of the memory pool and limit the ability of irrelevant memory cells to cross react with a new antigen.
It has recently been directly shown in both mouse and human viral infections that cross-reactive CD8 T cells contribute to immunity to different viruses, for example influenza A and Epstein Barr virus (EBV) in humans and lymphocytic choriomeningitis virus (LCMV) and vaccinia virus in mice [29]. The cross-reactivity network is unique in individuals, in part determined by the history of prior infections and epitope specificities of their T cell memory repertoires. Variation of the efficacy of the cross-reactive T cells in controlling heterologous infection depended partly on the avidity of the T cell for its ligand and the profile of cytokines elicited. Cross-reactive responses in an individual cannot be predicted, and in some cases have been shown to be pathological. The contribution of these fortuitously cross-reactive T cells to immunity in the elderly has not been directly examined, and is likely to vary in each individual, depending on their repertoire of T cells and their immunological history.
Clonally-expanded T cells develop in the memory pool with age
Clonally-expanded CD8 T cells (TCEs), which consist of a single clone of T cells bearing a single T cell receptor, develop with aging in both humans and mice. TCEs can occupy >50% of the memory pool, with frequencies reaching as high as 90% in aged mice [13,14,30]. Although TCEs do not lead to generalized immunosuppression, they have been correlated with a reduced repertoire of responding T cells resulting in impaired responses to vaccination [18,31] and infection [32].
There is considerable heterogeneity in the phenotype and function of TCEs, not only within the mouse, but between mouse and human. Human TCEs are largely CMV-specific. In the mouse, two major types of TCEs have been identified that differ in many characteristics, including in vivo stability and growth, anatomical location, in vitro proliferative capacity, cell surface phenotype, and the age of initial detection [33–35]. Whereas in the human, TCEs are largely driven by chronic cytomegalovirus infection (CMV, see below), most studies in the mouse are carried out in specific pathogen free mice and are detected non-specifically using TCR Vα and Vβ antibodies. Thus, the antigen specificity of the clonally expanded cells is unknown. However, recently, TCE specific for acute respiratory viruses have been shown to develop in aged mice that have previously recovered from the infection [36]. Most of the mice developed antigen-specific TCEs after two years, suggesting that these TCEs arise during long-term homeostatic proliferation of the memory pool. Interestingly, the individual TCEs were found to vary in their ability to mediate recall responses to infection [37]. In the majority of cases these TCEs had impaired proliferation compared with non-clonally-expanded memory cells specific for the same antigen when directly stimulated in vivo in a dual adoptive transfer model, but in some cases the response of the TCEs was equivalent or better to the non-expanded memory cells, consistent with the possibility that there is a range of affinities for antigen in cells that clonally expand.
The mechanisms driving clonal expansions to chronic CMV infection in humans appear to be different from the mechanisms underlying the clonal expansions elicited in response to acute infections that are completely cleared. In the case of CMV it is likely that reactivating virus continually stimulates antigen-specific CD8 T cells to expand. In the case where antigen has been cleared, it is likely that TCEs arise gradually over time due to slight differences in rates of homeostatic proliferation.
Persistent CMV infection generates TCEs in humans and is strongly associated with the immune risk phenotype
It is emerging that CMV infection plays an important role in human aging, not just because of its association with the development of large TCEs, but because it drives the development of dysfunctional CD8 T cells leading to development of immune senescence. CMV infection is strongly associated with an “immune risk phenotype,” that predicts 2-year mortality in very old individuals [38,39]. Most adults are chronically infected with CMV, either at birth in some geographical areas, or later in life. CMV frequently undergoes subclinical reactivation, resulting in chronic stimulation of CD8 T cells. This leads to skewing of the repertoire of the CD8 memory pool, induces the development of large TCEs, and ultimately drives the cells toward immune senescence. CMV-specific CD8 T cells represent 5–10% of the CD8 T cell pool, and infection early in life is thought to completely and permanently remodel the immune repertoire. The memory T cell response continues to increase with age (to as much as 20–50% of the CD8 T cell repertoire in individuals over 85 years of age) and has been directly correlated with impaired immunity to other infections and vaccination. Mechanisms underlying the importance of CMV in driving the pathogenesis of senescence are currently under intense investigation.
Conclusions and implications for therapy
The well-documented associations between aging, repertoire diversity and immune function have implications for therapeutic approaches to prevent or repair the loss of repertoire diversity. Three key approaches are considered here.
First, efforts to improve vaccination efficacy for the elderly, particular influenza virus vaccines, have been underway for quite some time. Recently, TLR adjuvants have been suggested to enhance the magnitude or functional avidity of responding T cells [40–42]. Recently, a link was made between Granzyme B production, which correlates with cytolytic activity of CD8 T cells, and outcomes of influenza virus infection in older people [43,44]. Pre-clinical testing of different vaccine/TLR combinations in stimulating T cell responses is underway, to discover how different adjuvants enhance T cell responses, which can then be used to guide the development of new vaccine/adjuvant formulations with enhanced efficacy in the elderly. Such an approach allows a direct translational link between adjuvant studies in aged mice and vaccination trials in humans. It should be emphasized that it will be important to vaccinate the elderly prior to extreme losses in repertoire diversity.
Second, given the dominant role of CMV in clonal expansions and the immune risk phenotype in humans, another strategy that has been considered is to vaccinate against CMV [38,39]. Vaccine development is already underway to reduce congenital infection. However, protection against this herpesvirus that has multiple mechanisms to evade immune control may be difficult to achieve, and is controversial since herpesvirus infections have been linked to enhanced protection against bacterial infections [45,46]. Perhaps therapeutic vaccination approaches during middle age could be developed, which would allow the putative beneficial effects of the infection during the early years, yet not allow manifestation of the disastrous consequences associated with CMV infection later in life.
Third, another possible approach taking into account the impact of age on loss of repertoire diversity, is restoration of thymic function. Importantly, the thymus retains plasticity and can be therapeutically regenerated by several approaches, including modulation of sex steroids and administration of growth hormone, IL-7, and keratinocyte growth factor [47–49]. These approaches show promise, and further experimentation is warranted.
It is clear that an increased understanding of mechanisms underlying repertoire changes and loss of immune function in the elderly is necessary to therapeutically restore immune function and enhance vaccination efficacy in this rapidly growing segment of the population.
Highlights.
Repertoire diversity declines with age
clonally-expanded T cells develop in the memory pool with age
Reduced repertoire diversity leads to impaired immunity
Fortuitously cross-reactive memory cells may recognize new antigens
persistent CMV infection strongly correlates with the immune risk phenotype
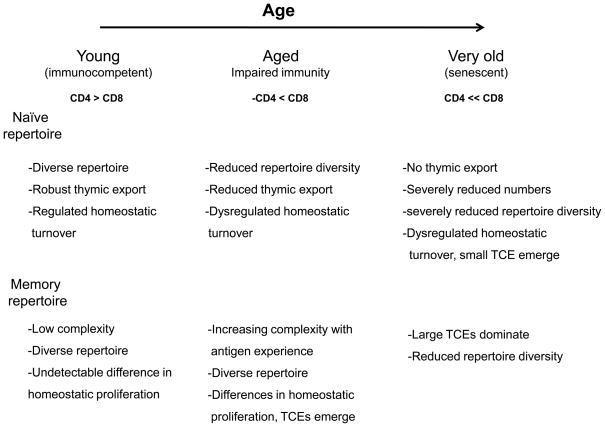
Figure 1. Progressive impact of age on the T cell repertoire.
Prior to the onset of age-associated changes in CD8 T cell immunity, there is robust thymic export. The diversity of the naïve repertoire is maintained by new naïve T cells and homeostatic proliferation. The memory repertoire is diverse. It starts out with low complexity, which gradually increases with antigen experience. Memory T cells homeostatically proliferate at slightly different rates, but this is not manifest as TCEs until later. With age, the thymus involutes, and fewer naïve T cells are exported. With increasing antigen experience, many naïve cells convert to the memory pool. Together, this leads to reduced numbers of naïve T cells and dysregulated homeostatic proliferation, driving more naïve cells into the memory pool. The diversity of the naïve T cell pool becomes extremely limited, resulting in a greater dependence on fortuitously cross reactive memory cells to respond to new antigens, and impairing the quality of the response. The memory repertoire becomes increasing dysregulated with the appearance of TCEs, which limits repertoire diversity. Chronic infection with CMV further drives the development of very large clonal expansions and immune senescence.
Acknowledgments
We thank Dr. Laura Haynes for on-going discussions and critical reading of the manuscript. We acknowledge financial support from the NIH (AG 021600 to M.A.B. and D.L.W., and AG032128 and AG022175 to M.A.B.), and the Trudeau Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marcia A. Blackman, Email: mblackman@trudeauinstitute.org.
David L. Woodland, Email: dwoodland@trudeauinstitute.org.
References and recommended reading
- 1.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mMHCpolymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 2.Nikolich-Zugich J, Fremont DH, Miley MJ, Messaoudi I. The role of MHC polymorphism in antimicrobial resistance. Microbes Infect. 2004;6:501–512. doi: 10.1016/j.micinf.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 4.Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 5.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- **6.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. This report shows that naïve cells can homeostatically proliferate and convert to a memory phenotype in the absence of antigen, which has implications for homeostasis of naïve T cells under lymphopenic conditions in the elderly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. This report shows the presence of small TCE in the naïve T cell pool of some aged mice, consistent with dysregulation with age in homeostatic proliferation mechanisms that maintain the naïve T cell pool. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 11.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 12.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- 13.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 14.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 16.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 17.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 21.Shimojo N, Cowan EP, Engelhard VH, Maloy WL, Coligan JE, Biddison WE. A single amino acid substitution in HLA-A2 can alter the selection of the cytotoxic T lymphocyte repertoire that responds to influenza virus matrix peptide 55–73. J Immunol. 1989;143:558–564. [PubMed] [Google Scholar]
- 22.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–743. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 23.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 24.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 25.Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. 2004;16:335–347. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 27.Selin LK, Welsh RM. Plasticity of T cell memory responses to viruses. Immunity. 2004;20:5–16. doi: 10.1016/S1074-7613(03)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim SK, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J Immunol. 2010;184:2825–2838. doi: 10.4049/jimmunol.0902168. This study describes cross-reactive networks operating in humans during EBV infection and in mice during vaccinia virus infection, and directly demonstrates that cross-reactive T cells generated during one infection can mediate protective immunity against a second, unrelated infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hingorani R, Choi IH, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993;151:5762–5769. [PubMed] [Google Scholar]
- 31.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 32.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 34.Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Exp Gerontol. 2007;42:407–411. doi: 10.1016/j.exger.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, Woodland DL. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- **37.Kohlmeier JE, Connor LM, Roberts AD, Cookenham T, Martin K, Woodland DL. Nonmalignant clonal expansions of memory CD8+ T cells that arise with age vary in their capacity to mount recall responses to infection. J Immunol. 2010;185:3456–3462. doi: 10.4049/jimmunol.1001745. This study in mice shows that antigen-specific TCEs develop in most mice within two years following infection with acute respiratory viruses. In addition, most but not all TCEs were poorly responsive to secondary viral infection compared with young memory mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **38.Moss P. The emerging role of cytomegalovirus in driving immune senescence: a novel therapeutic opportunity for improving health in the elderly. Curr Opin Immunol. 2010;22:529–534. doi: 10.1016/j.coi.2010.07.001. Together with reference 39, this review summarizes the emerging concept that persistent viral infections, particulary CMV, make a previously unappreciated contribution to immune senescence associated with aging. [DOI] [PubMed] [Google Scholar]
- **39.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.08.003. Together with reference 38, this review summarizes the emerging concept that persistent viral infections, particulary CMV, make a previously unappreciated contribution to immune senescence associated with aging. [DOI] [PubMed] [Google Scholar]
- 40.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, DeVos J, Hancock K, Katz JM, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5:e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElhaney JE. Prevention of infectious diseases in older adults through immunization: the challenge of the senescent immune response. Expert Rev Vaccines. 2009;8:593–606. doi: 10.1586/erv.09.12. [DOI] [PubMed] [Google Scholar]
- **44.McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, Barry MB, Kleppinger A, Wang Y, Bleackley RC, Granzyme B. Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27:2418–2425. doi: 10.1016/j.vaccine.2009.01.136. This study shows that granzyme B levels are a better correlate of immune protection than antibody titers following stimulation of peripheral blood mononuclear cells in aged individuals folowing vaccination with influenza virus, and can be used to predict vaccine efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- **46.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. This review article summarizes the immunological imprint of chronic viral infections, and discusses the beneficial, neutral and dangerous consequences of the equilibrium between the virus and the host. It is important to bear the beneficial effects of CMV infection in mind when considering therapeutic strategies for immune senescence in the elderly. [DOI] [PubMed] [Google Scholar]
- 47.Holland AM, van den Brink MR. Rejuvenation of the aging T cell compartment. Curr Opin Immunol. 2009;21:454–459. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: Treatments, delivery, and side effects. Exp Gerontol. 2008 doi: 10.1016/j.exger.2008.04.014. [DOI] [PubMed] [Google Scholar]