Abstract
The global obesity epidemic is associated with a series of health threatening diseases including type 2 diabetes. Accumulating evidence suggest that the physiology and homeostasis of the endoplasmic reticulum (ER) is intimately involved in the underlying mechanisms linking obesity and diabetes. Specifically, recent studies indicate a critical role for the inositol-requiring enzyme 1α (IRE1α)/X-box binding protein 1 (XBP1) pathway, the most conserved branch of the unfolded protein response (UPR), in glucose and lipid metabolism as well as insulin function. Focusing on the IRE1α-XBP1 pathway, here we review recent advances in our understanding of the role of the UPR in obesity and obesity-associated metabolic disorders.
Obesity and ER stress
The prevalence of obesity, likely due to a global shift towards consumption of energy-dense foods and a sedentary lifestyle, is now considered to be a major worldwide health threat associated with increased risk of cardiovascular disease, type 2 diabetes, musculoskeletal disorders, and cancers [1]. Addressing the mechanisms underlying these pathologies will be central in the development of new therapeutic approaches for the treatment of these conditions.
Extensive research has greatly increased our understanding of the mechanisms linking obesity to these pathologies. One emerging mechanism involves the endoplasmic reticulum (ER), a vast membranous network that maintains Ca2+ homeostasis and is responsible for the folding, modification, assembly, and trafficking of membrane and secretory proteins [2]. A number of physiological and pathological conditions including folding-defective mutations, viral infection, energy depletion and hypoxia may interfere with protein maturation and trafficking processes within the ER lumen and lead to accumulation of unfolded or misfolded proteins, a cellular condition defined as ER stress [2, 3]. However, the exact nature of the stimuli culminating in ER stress in obesity and type 2 diabetes remains vague.
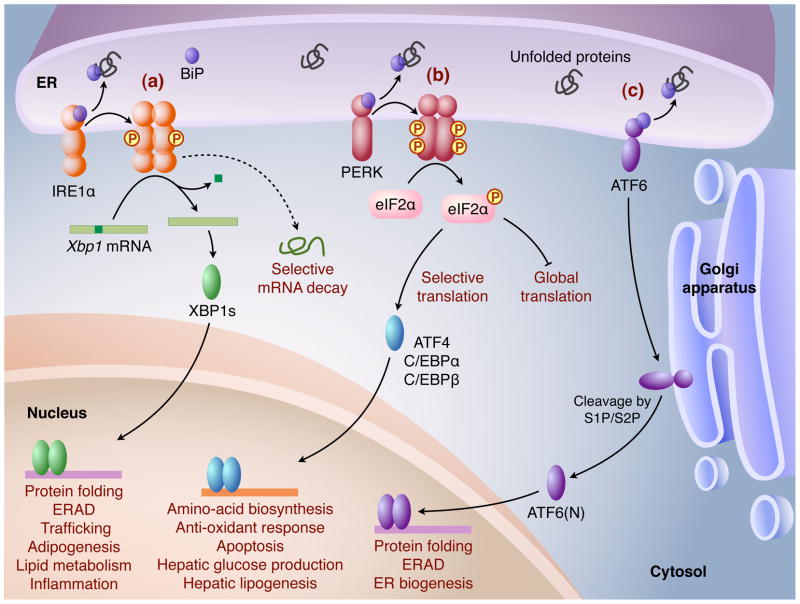
To alleviate ER stress and ensure protein folding fidelity, the ER elicits an elaborate adaptive response by initiating several signal transduction cascades collectively known as the unfolded protein response (UPR). First characterized in yeast, UPR is mediated by a single signaling pathway initiated by a type I transmembrane ER protein IRE1p [3]. However, mammalian UPR has evolved to be governed by three major signaling cascades initiated by three ER transmembrane protein sensors, IRE1α, PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Figure 1). The overall outcome of UPR is reduced protein load, increased ER folding capacity and enhanced ER-associated protein degradation (ERAD) to restore ER homeostasis. However, if these mechanisms of adaptation and survival are insufficient to relieve ER stress, UPR induces cellular apoptosis [3].
Figure 1.
Three major UPR pathways in mammals are shown. Homeostatic alterations in the ER result in accumulation of unfolded or misfolded proteins, which induce dissociation of BiP and subsequently allows for activation of three ER membrane sensors: (a) IRE1α, (b) PERK and (c) ATF6. (a) IRE1α undergoes dimerization or oligomerization, and trans-autophosphorylation, activating its cytosolic endoribonuclease activity. IRE1α then removes a 26-base intron from Xbp1 mRNA to generate a potent transcription factor XBP1s (XBP1 spliced) that translocates into the nucleus and regulates a diverse array of genes. In addition, activated IRE1α may selectively degrade certain mRNAs. (b) Activated PERK phosphorylates Ser51 on the translation initiation factor eIF2α to attenuate global translation, but also preferentially upregulates the translation of selected mRNAs including ATF4, C/EBPα, and C/EBPβ. ATF4 activates the expression of UPR target genes involved in amino acid biosynthesis, the anti-oxidant response, and apoptosis, whereas C/EBPα and C/EBPβ activates genes regulating glucose production and lipogenesis in the liver. (c) Activated ATF6 translocates to the Golgi, where it is cleaved by the proteases S1P/S2P, yielding the mature transcription factor ATF6(N), which activates the transcription of UPR target genes.
Under stress-free conditions, IRE1α is associated with heat shock protein 5 (Hspa5/BiP/Grp78, hereafter GRP78) and maintained in a repressed state. Upon ER stress, GRP78 dissociation allows for IRE1α activation via dimerization and trans-autophosphorylation. Activated IRE1α possesses site-specific endoribonuclease (RNase) activity at the carboxyl end of its cytoplasmic domain, which splices 26 nucleotides from the mRNA encoding X-box-binding protein 1 (Xbp1). This unique non-conventional splicing event occurs outside of the nucleus, resulting in a translational frameshift and the generation of a 371 amino-acid isoform, XBP1s (XBP1 spliced) with potent activity as a basic region leucine zipper (bZIP) transcription factor [3]. The XBP1s protein functions by translocating into the nucleus to initiate transcriptional programs that upregulate a broad spectrum of UPR-associated genes involved in protein entry into the ER, protein folding, ERAD, and ER biogenesis, as well as non-UPR related genes associated with adipogenesis, lipid metabolism, and inflammation (Figure 1).
The link between ER stress and metabolism has been suggested to underlie the pathogenesis of obesity-related complications, particularly insulin resistance and type 2 diabetes [2]. Indeed, recent studies suggest that the IRE1α-XBP1 pathway may influence metabolism directly by regulating metabolic gene expression or indirectly via cross-talk with inflammatory and stress signaling pathways [2]. As its role in pancreatic βcells and type 1 diabetes has been reviewed recently [4, 5], here we focus on the role of the IRE1α-XBP1 pathway in obesity and metabolic disorders, an increasingly complex but exciting field.
ER homeostasis and metabolic diseases
Several lines of evidence support an intimate relationship between disruption of ER homeostasis, UPR activation, and obesity-associated metabolic diseases [2]. First, Xbp1+/− mice exhibit increased ER stress coupled with impaired glucose and insulin tolerance upon high fat diet (HFD)-induced obesity [6]. Accordingly, reducing ER stress by administration of chemical chaperones improves insulin sensitivity of obese mice [7]. Moreover, manipulations of ER chaperones affect glucose homeostasis. For example, overexpression of oxygen-regulated protein 150 (ORP150), an ER chaperone activated during UPR, improves glucose tolerance and enhances insulin receptor signaling in db/db mice, whereas haploinsufficiency or liver-specific knockdown of ORP150 results in impaired glucose tolerance and decreased insulin signaling [8, 9]. Similarly, overexpression of the ER chaperone GRP78 in the liver of obese mice inhibits the activation of sterol regulatory element binding protein (SREBP)-1c, an important lipogenic factor, suppresses hepatic steatosis, and increases insulin signaling [10]. Surprisingly, Grp78+/− mice on HFD also show improved insulin sensitivity, likely due to a compensatory increase in other ER chaperones that can enhance overall ER homeostasis and improve metabolic parameters [11]. In addition to the IRE1α-XBP1 pathway, phosphorylation of eIF2α on serine 51, a key downstream event of the PERK pathway, upregulates protein translation of CCAAT enhancer binding protein α (C/EBPα) and C/EBPβ, and contributes to liver glucose production and hepatic steatosis in obesity and diabetes [12]. In the central nervous system, elevated ER stress contributes to insulin and leptin resistance in the hypothalamus of obese mice [13, 14]. Finally, UPR signaling has been linked to autophagy, which may promote cell survival by eliminating aggregating proteins and damaged organelles in stressed cells [15–17]. Indeed, defective autophagy in obesity elevates ER stress and promotes insulin resistance [18]. Taken together, these studies provide evidence linking ER dysfunction with the pathogenesis of metabolic disorders.
However, this paradigm likely represents an oversimplification. First, in ER stress transgenic reporter mice on HFD, increased Xbp1 mRNA splicing is detected in the liver, but not in adipose tissue or muscle [19], pointing to a tissue-specific effect. Moreover, protein levels of both full length and active forms of ATF6 are reduced in hepatocytes of ob/ob mice, suggesting differential regulation of this pathway, although the authors suggest that this effect is likely due to persistent and chronic ER stress in obesity [20]. Finally, it remains unclear how XBP1 haploinsufficiency in Xbp1+/− cells or mice causes ER stress [6] as Xbp1−/− hepatocytes do not exhibit overt ER stress activation or altered ER ultrastructure [21]. Together, these data imply that during obesity, UPR activation is differentially regulated in a tissue-specific manner, which may provide an explanation for some of the inconsistencies in this field.
Regulation of the IRE1α-XBP1 pathway
To fully understand the function of the IRE1α-XBP1 pathway in metabolic diseases and harness components of this pathway as potential targets, it is essential to understand the underlying mechanisms regulating this pathway. Indeed, recent studies have shown that the IRE1α-XBP1 pathway can be regulated at multiple levels.
One level of regulation occurs at the level of the sensor IRE1α. It is generally accepted that Xbp1 mRNA is the only specific substrate for IRE1α in vivo [3, 22]. However, several recent studies showed that the endoribonuclease activity of IRE1α may also promote the degradation of mRNAs encoding a subset of ER or secretory proteins prone to misfolding [23–26] (Figure 1). Although the precise molecular mechanism is unclear, a recent in vitro study identified a consensus RNA sequence critical for IRE1α-mediated cleavage [25], providing a possible mechanism for this mRNA decay. Interestingly, while the splicing of Xbp1 mRNA can be induced merely by artificial dimerization of IRE1α, IRE1α-dependent mRNA decay requires ER stress-dependent signals [26], suggesting that distinct mechanisms underlie these two functions of IRE1α. In light of our recent findings that IRE1α is constitutively active albeit at low levels in various cell and tissue types [27], an interesting and important question is whether IRE1α activity under physiological conditions is sufficient to mediate mRNA decay. Addressing this question will address the physiological relevance of these events (Box 1).
BOX 1. Outstanding Questions.
1. What activates ER stress under physiological or pathological conditions?
Bioactive lipids perturb ER homeostasis and activate UPR in various cell types, including macrophages [71, 72]. It remains unclear whether lipids may serve as activating signals for ER stress in vivo in obesity. If so, it will be interesting to identify the target cells or tissues and to delineate their downstream signaling cascade. The prevailing notion of extra-ER activation of UPR sensors requires more direct evidence examining the effect of these nutrients on the ultrastructure, microenvironment, and folding capacity of ER.
2. Which disease conditions are associated with ER stress and IRE1α activation?
ER stress has been implicated in over 50 human diseases. Whether ER stress is truly associated with all these diseases and what role ER stress plays in the development of these diseases are important questions. Several studies have suggested that XBP1s-mediated ER expansion acts as a “pre-emptive strike” to counter future possible disruptions in ER homeostasis. We believe this may represent a more general adaptation strategy for cells to avoid the induction of ER stress response. On the other hand, cells that cope with ER stress in the development of certain diseases, especially chronic ones such as obesity and the metabolic syndrome, may have adapted to ER stress while those that fail to adapt may have been cleared from the system via apoptosis. Therefore, employing and developing more sensitive assays to quantitate and visualize ER stress during the development of these diseases may answer this question (Box 1).
3. Can IRE1α be selectively activated by ER stress-independent mechanisms in vivo?
This selective activating mechanism is very interesting and should be tested more extensively in other cell types and under more physiological stimuli. If successful, this may allow the development of IRE1α- and XBP-1 specific agonists or antagonists.
4. What is the physiological relevance of IRE1α-mediated mRNA decay?
Although IRE1α has been shown to splice mRNAs other than Xbp1, the question remains whether these splicing events are directly mediated by IRE1α or by IRE1α-interacting RNase. More importantly, what is the physiological relevance and biological significance of these events? Answers to these questions will provide key insights into IRE1α function.
5. What is the biological significance of IRE1α- and XBP1s-interacting proteins?
Although several proteins are recognized as part of the IRE1α interactome”, their physiological relevance has not yet been elucidated in vivo. The identification of additional proteins and components in the IRE1α-XBP1 signaling axis may reveal novel functions for IRE1α and XBP1s and provide insights into the regulatory mechanisms underlying IRE1α-XBP1 activation.
6. What are the tissue- and cell type-specific functions of IRE1α-XBP1s in the pathogenesis of obesity and diabetes?
Recent studies in mouse models have provided key insights into the function of IRE1α-XBP1 in hepatic lipid metabolism, glucose production, and macrophage inflammation. Its physiological role in other tissues, such as adipose tissue and muscle, remains unclear.
The resolution of these issues will be of paramount importance to our understanding of how ER stress and its related pathways contribute to the pathogenesis of metabolic syndrome and other diseases.
In addition to regulation at the level of RNase activity, a number of IRE1α-interacting proteins, collectively termed the “IRE1α interactome”, can modulate IRE1α function [28]. This interactome includes the proapoptotic B-cell lymphoma 2 (BCL2) family members BCL2-associated X protein (BAX) and BCL2-antagonist/killer (BAK), apoptosis signal-regulating kinase 1 (ASK1)-interacting protein 1 (AIP1), tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2), BAX inhibitor-1 (BI-1), and receptor for activated C-kinase 1 (RACK1), that differentially regulate IRE1α phosphorylation and activity. BAX/BAK and AIP1 are required for the optimal activation of IRE1α [29] (Figure 2). Conversely, the ER transmembrane protein BI-1 negatively regulates IRE1α activation and downstream Xbp1 mRNA splicing [30, 31], whereas RACK1 inhibits glucose-stimulated IRE1α activation in pancreatic β cells by recruiting protein phosphatase 2 (PP2A) to de-phosphorylate IRE1α [32]. Given the complexity of IRE1α regulation, we speculate that additional interacting proteins will be identified and that the “interactome” will collectively mediate IRE1α function in a cell type-specific manner.
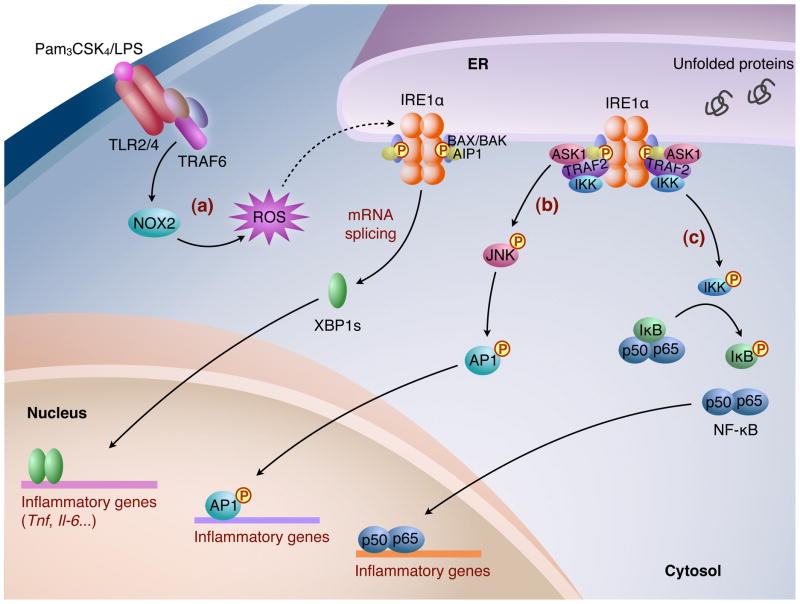
Figure 2.
The IRE1α-XBP1 pathway in inflammation. (a) In macrophages, upon ligand activation, TLR2/TLR4 recruits adaptor proteins including TRAF6 and induces NOX2-dependent production of ROS, which leads to IRE1α activation. Subsequently, XBP1s induces transcription of inflammatory cytokines, such as Tnf and Il-6. The recruitment of adapter proteins to IRE1α, such as BAX/BAK and AIP1, seems to be important for optimal activation of IRE1α, and for Xbp1 mRNA splicing and JNK activation. (b) In response to ER stress, the cytoplasmic domain of IRE1α forms a complex containing TRAF2 and triggers the activation of ASK1/JNK, which subsequently phosphorylates AP1. (c) The IRE1α-TRAF2 complex interacts with and activates IKK, which IKK initiates the degradation of IκB by phosphorylation, leading to nuclear entry of NF-κB. Activated AP1 and NF-κB then induce the transcription of genes involved in inflammation.
A second level of regulation occurs at the effector level, which encompasses gene expression, posttranslational modifications, and/or intracellular localization of XBP1. Most notably, transcriptional induction of Xbp1 mRNA has been suggested to be an important mechanism in regulating ER homeostasis in multiple biological processes including B cell differentiation [33–35], adipogenesis [36], myogenesis [35, 37], and thyroglobulin production [38]. Due to the basal activity of IRE1α [27], Xbp1 mRNA induction translates to elevations in XBP1s protein levels, which may serve to fine-tune ER homeostasis. A similar phenomenon has been observed in yeast and termed as “Super-UPR” [39]. Thus, we speculate that the cell has evolutionarily adapted this mechanism to accommodate various cellular differentiation processes, without activation of ER stress. This model may represent a fundamental mechanism for the cell to maintain ER homeostasis and prepare for fluxes in protein load while avoiding elicitation of an overt UPR.
In addition, XBP1s activity can be modulated by posttranslational modifications including small ubiquitin-like modifier (SUMO) [40] and acetylation/deacetylation [41, 42]. Two SUMOylation events occur at the transactivation domain of XBP1s to reduce its transcriptional activity while acetylation of XBP1s by p300 has the opposite effect. As most studies were carried out in cell culture models, the physiological relevance of these modifications in metabolism remains to be elucidated. Finally, the activity of XBP1s may be regulated at the level of nuclear entry by association with p85α and p85β, two regulatory subunits of phosphoinositide 3-kinase (PI3K) [43, 44]. These recent developments are certainly exciting as they shed light on potential therapeutic approaches that can target the IRE1α-XBP1 pathway. Nonetheless, the physiological and pathological relevance of these regulatory events in the context of obesity and metabolic diseases warrants further studies (Box 1).
The IRE1α-XBP1 pathway in inflammation
Obesity is a chronic low-grade inflammatory disorder [2]. UPR signaling, in addition to its canonical role in alleviating ER stress, has recently been shown to be involved in inflammation. Activation of inflammatory signaling cascades, such as the c-Jun N-terminal kinase (JNK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, is suggested to play a causal role in the development and pathogenesis of obesity-associated insulin resistance [45–47].
It has been proposed that IRE1α-dependent activation of JNK and NF-κB integrates UPR with inflammation (Figure 2). In response to ER stress, activated IRE1α recruits adaptor protein TRAF2, leading to activation of the JNK pathway [48, 49]. Activated JNK is responsible for the transcriptional induction of many inflammatory genes through phosphorylation and activation of the transcription factor activator protein 1 (AP1) [50]. In addition, the IRE1α-TRAF2 complex may also recruit and activate Iκ B kinase (IKK), which phosphorylates IκB, leading to its degradation and the nuclear translocation and activation of NF-κB [51]. ER stress-induced NF-κB activation and production of the inflammatory cytokine TNF-α are impaired in IRE1α-deficient mouse embryonic fibroblasts (MEFs) [51]. Thus, IRE1α may regulate the status of inflammation in response to ER stress. It should be noted, however, that the physiological relevance and importance of these signaling cascades have not been well characterized in vivo.
ER stress may also be linked to inflammation and metabolic disorders through activation of Toll-like receptors (TLR) in obesity, possibly by excess fatty acids [52, 53]. Activation of TLR2 and TLR4 engages the IRE1α-XBP1 pathway in macrophages, which is required for mounting an optimal response to bacterial infections and sustained expression of a subset of inflammatory cytokines including interleukin-6 (IL-6) and TNFα [54] (Figure 2). IRE1α activation by TLR agonists is distinct from canonical ER stress, as the PERK and ATF6 pathways are unaffected. The signaling from TLRs to IRE1α requires adaptor proteins including TRAF6 and the activity of an NADPH oxidase NOX2, an enzyme known to mediate TLR-induced reactive oxygen species (ROS) production in macrophages [55]. Intriguingly, TLR2/4 mediated activation of the IRE1α-XBP1 pathway does not induce classic UPR genes such as Erdj4 or Pdi [54], suggesting a signaling-dependent and cell type-specific role of XBP1. In addition, a C. elegans study instead points to an important and conserved role of XBP1 in host protection during development as XBP1 deficiency leads to disruption of ER morphology and larval lethality in response to bacterial infection [56]. Given the role of inflammation in the development of metabolic diseases [53], further studies investigating the impact of TLR-mediated IRE1α-XBP1 activation in the context of obesity-associated disorders are warranted.
The IRE1α-XBP1 pathway in glucose homeostasis
Our current understanding of the IRE1α-XBP1 pathway as a link between obesity, insulin resistance, and type 2 diabetes centers on the IRE1α-JNK signaling axis [48, 57] (Figure 3). IRE1α phosphorylation and JNK activity are significantly increased in both the liver and adipose tissue of obese mice [7, 57]. Subsequently, the IRE1α-JNK signaling axis attenuates insulin signaling by mediating serine phosphorylation of IRS-1 at residue 307 (Ser307) [57, 58]. Supporting the role of IRE1α activation in elevating JNK activity and attenuating insulin signaling, XBP1s overexpression in MEF cells suppresses ER-stress-induced JNK activation and increases activating tyrosine phosphorylation of insulin-receptor substrate 1 (IRS-1); as expected, XBP1 deficient cells show opposite effects [57]. Furthermore, Xbp1+/− mice exhibit increased ER stress and more severe insulin resistance upon HFD-induced obesity [57], presumably due to IRE1α-JNK activation. Thus, this current model links the IRE1α-XBP1 pathway to insulin resistance via the JNK-IRS1 signaling axis.
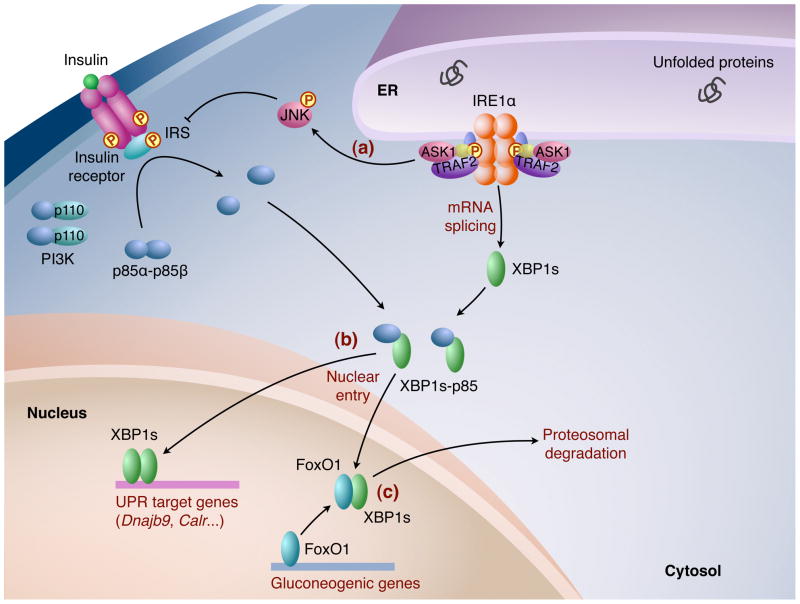
Figure 3.
The IRE1α-XBP1 pathway in glucose homeostasis. (a) ER stress triggers IRE1α-dependent JNK activation, which leads to Ser307 phosphorylation of IRS protein to promote insulin resistance. (b) In hepatocytes, the regulatory subunits of PI3K, p85α and p85κ, form heterodimers that dissociate upon insulin stimulation and interact with XBP1s to facilitate its nuclear translocation. Nuclear XBP1s then induces the expression of UPR target genes including chaperones and ERAD components, and improves insulin sensitivity. (c) In addition, nuclear XBP1s promotes glucose tolerance in the liver by interacting with FoxO1, a transcription factor governing gluconeogenesis, to induce its proteosomal degradation.
Nonetheless, the IRE1α-JNK signaling axis may not be all-inclusive. First, although whole-body JNK1 deficiency in mice results in improved insulin sensitivity and enhanced insulin receptor signaling in obese mice [45], hepatic JNK1 is required to maintain insulin sensitivity and prevent hepatic steatosis [59], and JNK1 in hematopoietic cells of adipose tissue promotes insulin resistance [60, 61]. These findings indicate tissue- and cell type-specific functions of JNK1 in obesity and insulin resistance. Furthermore, a recent study demonstrates that IRS-1 Ser307Ala knock-in mice exhibit increased insulin resistance upon HFD-induced obesity, suggesting that p-Ser307 of IRS-1 does not attenuate, but rather improves insulin sensitivity [62]. Thus, the molecular mechanisms underlying IRE1α signaling in obesity and insulin resistance remain elusive and warrant further studies.
Intriguingly, a recent study suggests that XBP1s affects glucose homeostasis in obese mice by regulating a gluconeogenic transcription factor FoxO1 [66]. XBP1s overexpression promotes the ubiquitination and degradation of FoxO1 in hepatocytes of ob/ob mice, which may account for improved glucose homeostasis independent of insulin signaling. In addition, XBP1s deficiency in the liver leads to FoxO1 accumulation and glucose intolerance in HFD mice [66]. This study unveils an unexpected function of hepatic XBP1s in improving glucose homeostasis in obese mice independent of its canonical UPR role and hence, raises questions as to the roles served by XBP1s in other important metabolic organs, such as adipose tissue and central neuron system.
Insulin signaling has also been implicated in modulating UPR by regulating the nuclear translocation of XBP1s [43, 44] (Figure 3). Insulin stimulation disrupts the heterodimer formed by p85α and p85β, which subsequently interacts with XBP1s to facilitate its nuclear translocation independent of PI3K catalytic activity [43]. Accordingly, p85α deficiency in fibroblasts or mouse liver leads to decreased nuclear XBP1s levels and blunts UPR induction upon ER stress [44]. Interestingly, the interaction between p85 and XBP1s is disrupted in insulin resistant ob/ob mice, leading to defects in the nuclear entry of XBP1s and elevated ER stress during feeding [43]. Overexpression of p85α or p85β in the liver significantly increases nuclear translocation of XBP1s and improves glucose tolerance in ob/ob mice [43]. Thus, insulin resistance and ER stress may participate in a vicious cycle in obesity that contributes to the development of metabolic disorders. However, the purported link between XBP1-p85 and insulin sensitivity seems inconsistent with previous reports showing that liver-specific disruption of p85α improves systemic glucose tolerance and insulin sensitivity in both lean and HFD-induced obese mice [63, 64]. Moreover, the liver of ob/ob mice expressed increased nuclear XBP1s protein levels, conflicting with the model that nuclear entry of XBP1s is defective in obesity [10]. Future studies are required to delineate the interplay between the IRE1α-XBP1 pathway and insulin signaling.
The IRE1α-XBP1 pathway in lipid metabolism
The IRE1α-XBP1 pathway also integrates with signaling networks that control lipid metabolism. Indeed, XBP1s regulates phospholipid biosynthesis and ER biogenesis, and thus controls ER expansion and the secretory capacity of cells [34, 65].
In hepatocytes, XBP1s regulates hepatic lipogenesis by directly binding to the promoters and activating the expression of a subset of lipogenic genes including stearoyl coenzyme A (CoA) desaturase 1 (SCD1), diacylglycerol acetyltransferase 2 (DGAT2), and acetyl CoA carboxylase 2 (ACC2) [21]. Consequently, XBP1 deficiency specifically in the postnatal liver of mice results in reduced de novo lipid biosynthesis [21]. Accordingly, mice with a hepatocyte-specific deletion of Ire1α exhibit downregulation of many genes involved in lipid metabolism in the liver, such as peroxisome proliferator-activated receptor α (PPARα), PPAR-coactivator α (PGC-1α), and C/EBPα, when exposed to ER stress [66]. Given the close relationship between lipid metabolism and hepatic steatosis, the role of IRE1α-XBP1 in the liver under other disease settings such as obesity and type 2 diabetes requires further study.
Interestingly, hepatic lipid metabolism and ER stress signaling may intersect with circadian regulation. A recent study suggests that the IRE1α-XBP1 pathway is activated rhythmically every 12 h in the liver, and considered as a secondary clock controlled by the canonical circadian clock [67]. Perturbation of this rhythm is associated with dysregulated hepatic lipid metabolism [67]. However, it remains unclear whether the circadian clock controls genomic recruitment of XBP1s to its target genes. In light of a recent study showing the circadian-dependent involvement of histone deacetylase 3 (HDAC3) in regulating hepatic lipid metabolism [68], it will be interesting to delineate whether XBP1s and HDAC3 are functionally related.
We recently showed that XBP1-deficient pre-adipocytes and MEF cells exhibit profound defects in adipocyte differentiation [36]. During adipogenesis, Xbp1 expression is induced by the upstream adipogenic transcription factor, CCAAT enhancer binding protein β (C/EBPβ). Through the basal RNase activity of IRE1α, XBP1s subsequently binds to the promoter of and activates a master adipogenic factor C/EBPα [36]. Thus, the IRE1α-XBP1 pathway exerts a crucial role in regulating in vitro adipogenesis by integrating XBP1 into the adipogenic transcriptional cascade. Consistent with our finding, a study showed that the fat depot is absent in XBP1-deficient neonates rescued with hepatic XBP1 overexpression [69]. Interestingly, although several known XBP1 target genes, such Erdj4 and P58ipk, are upregulated during adipogenesis, their expression is XBP1-independent [36].
Taken together, these studies suggest that the IRE1α-XBP1 pathway is intimately involved in lipid metabolism in the liver and adipose tissue. More genomic studies will be essential in understanding the mechanism by which XBP1s regulates gene expression required for lipid metabolism and ER homeostasis under different conditions.
Concluding remarks
The recognition that obesity is associated with ER stress and that activation of UPR, particularly the IRE1α-XBP1 pathway, regulates the pathogenesis of obesity-associated insulin resistance and type 2 diabetes has extended our appreciation of the importance of ER homeostasis as well as the etiology of metabolic syndrome. Our current understanding of the mechanism by which the IRE1α-XBP1 pathway regulates glucose homeostasis may allow for the development of therapies targeting this pathway for obesity and diabetes. For example, given the glucose-lowering and insulin-sensitizing effects of XBP1s under obese conditions, small molecules targeting IRE1α RNase activity, Xbp1 gene transcription or XBP1s transcriptional activity may have therapeutic implications in treating these diseases. Future discoveries on the novel molecular mechanisms underlying UPR-related energy homeostasis may facilitate the design of more efficacious therapeutic strategies (Box 1). However, the key to this endeavor will be the development of improved and more sensitive tools and methods to detect sensor activation and to quantitate the levels of ER stress (Box 2), which will allow for an accurate assessment of drug efficacy in different tissues.
BOX 2. Visualizing and Quantitating Physiological ER Stress.
Although there is little doubt about the role and significance of ER stress in the field of β cell biology, the area of ER stress in health and diseases has become progressively more complex since its discovery two decades ago. The traditional methods of inducing and measuring ER stress have several intrinsic problems including but not limited to integration of non-ER stress pathways into the classical ER stress pathways, the mild nature of physiological ER stress relative to the strong UPR induced pharmacologically, and a cell s high adaptability to ER stress and its propensity to maintain ER homeostasis. These inherent problems may necessitate careful reinterpretation of results from earlier studies linking ER stress to certain diseases and may address some of the inconsistencies observed from various studies, some described herein.
We recently optimized a Phos-tag-based Western blot system that has significantly improved the detection, visualization and quantitation of the activation of IRE1α and PERK [27, 36, 70]. This method allows for a quantitative assessment of the levels of ER stress by quantitating the percent of phosphorylated IRE1α protein levels out of total IRE1α. Indeed, we have used this method to detect and quantitate IRE1α activation under different physiological conditions including adipogenesis [36], accumulation of misfolded proteins, ad libitum feeding conditions in various mouse tissues, and the fasting-refeeding cycle in the pancreas [27]. Recently, this method was also used to visualize IRE1α activation in macrophages following TLR agonist stimulation such as LPS [54]. As this Phos-tag-based method is direct, sensitive, and provides a complete view of the phosphorylation status of two UPR sensors, we believe that it has potential applications in the analyses of physiological and pathological UPR and diagnosis of ER-associated diseases.
Since its initial characterization as a key UPR signaling pathway, many novel roles of the IRE1α-XBP1 pathway in inflammation and lipid metabolism, unrelated to their canonical functions in UPR, have been described and more are likely to be discovered. How these functions modulate insulin action and energy metabolism in vivo remains to be explored. Currently, the IRE1α-XBP1 pathway can be manipulated at multiple levels including IRE1α activation by its interacting proteins, transcriptional Xbp1 induction, XBP1s nuclear translocation, and XBP1s post-translational modifications. Whether and how these regulations contribute to the function of the IRE1α-XBP1 pathway in regulating insulin signaling is worth future investigations.
Acknowledgments
We apologize for being unable to cite all relevant contributions due to space limitations. We thank the members of Qi laboratory for comments and suggestions. This work is supported by the American Federation for Aging Research (AFAR), the American Diabetes Association (ADA), and NIH NIDDK R01DK082582.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Obesity and overweight. Fact sheet number 311. 2006 http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 4.Eizirik DL, Cnop M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci Signal. 2010;3:pe7. doi: 10.1126/scisignal.3110pe7. [DOI] [PubMed] [Google Scholar]
- 5.Oslowski CM, Urano F. The binary switch that controls the life and death decisions of ER stressed beta cells. Curr Opin Cell Biol. 2011;23:207–215. doi: 10.1016/j.ceb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 7.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakatani Y, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa K, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 10.Kammoun HL, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye R, et al. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyadomari S, et al. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogata M, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetz C, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshiuchi K, et al. Direct monitoring of in vivo ER stress during the development of insulin resistance with ER stress-activated indicator transgenic mice. Biochem Biophys Res Commun. 2008;366:545–550. doi: 10.1016/j.bbrc.2007.11.182. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AH, et al. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nekrutenko A, He J. Functionality of unspliced XBP1 is required to explain evolution of overlapping reading frames. Trends in genetics : TIG. 2006;22:645–648. doi: 10.1016/j.tig.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 24.Han D, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikawa D, et al. Identification of a consensus element recognized and cleaved by IRE1 alpha. Nucleic Acids Res. 2010;38:6265–6273. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, et al. A Phos-tag-based approach reveals the extent of physiological endoplasmic reticulum stress. PLoS One. 2010;5:e11621. doi: 10.1371/journal.pone.0011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetz C, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 30.Lisbona F, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailly-Maitre B, et al. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285:6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Y, et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Science Signaling. 2010;3:ra7. doi: 10.1126/scisignal.2000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimold AM, et al. Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. J Exp Med. 1996;183:393–401. doi: 10.1084/jem.183.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaffer AL, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 35.He Y, et al. Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr. 2010;15:13–25. doi: 10.3727/105221610x12819686555051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sha H, et al. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blais A, et al. An initial blueprint for myogenic differentiation. Genes Dev. 2005;19:553–569. doi: 10.1101/gad.1281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christis C, et al. Regulated increase in folding capacity prevents unfolded protein stress in the ER. J Cell Sci. 2010;123:787–794. doi: 10.1242/jcs.041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leber JH, et al. IRE1-independent gain control of the unfolded protein response. PLoS Biol. 2004;2:E235. doi: 10.1371/journal.pbio.0020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Qi L. SUMO modification regulates the transcriptional activity of XBP1. Biochem J. 2010;429:95–102. doi: 10.1042/BJ20100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang FM, et al. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem J. 2010;433:245–252. doi: 10.1042/BJ20101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng L, et al. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SW, et al. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winnay JN, et al. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 46.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 47.Chiang SH, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 49.Nishitoh H, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 51.Hu P, et al. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Martinon F, et al. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grandvaux N, et al. Innate host defense: Nox and Duox on phox's tail. Biochimie. 2007;89:1113–1122. doi: 10.1016/j.biochi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Richardson CE, et al. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463:1092–1095. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 58.Aguirre V, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 59.Sabio G, et al. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Copps KD, et al. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11:84–92. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taniguchi CM, et al. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc Natl Acad Sci USA. 2006;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taniguchi CM, et al. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Molecular and Cellular Biology. 2007;27:2830–2840. doi: 10.1128/MCB.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sriburi R, et al. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutkowski DT, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cretenet G, et al. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee A-H, et al. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi L, et al. Detecting and quantitating physiological endoplasmic reticulum stress. Methods Enzymol. 2011;490:137–146. doi: 10.1016/B978-0-12-385114-7.00008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erbay E, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lichtenstein L, et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell metab. 2010;12:580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]