Abstract
Heavy metal and pesticide contamination has previously been reported in Chinese Herbal Medicines (CHMs), in some cases at potentially toxic levels. This study was conducted to determine general patterns and toxicological significance of heavy metal and pesticide contamination in a broad sample of raw CHMs. Three-hundred-thirty-four samples representing 126 species of CHMs were collected throughout China and examined for arsenic, cadmium, chromium, lead, and mercury. Of the total, 294 samples representing 112 species were also tested for 162 pesticides. At least 1 metal was detected in all 334 samples (100%) and 115 samples (34%) had detectable levels of all metals. Forty-two different pesticides were detected in 108 samples (36.7%), with 1 to 9 pesticides per sample. Contaminant levels were compared to toxicological reference values in the context of different exposure scenarios. According to a likely scenario of CHM consumption, only 3 samples (1%) with heavy metals and 14 samples (5%) with pesticides were found with concentrations that could contribute to elevated background levels of contaminant exposure. According to the most conservative scenario of CHM consumption, 231 samples (69%) with heavy metals and 81 samples (28%) with pesticides had contaminants that could contribute to elevated levels of exposure. Wild collected plants had higher contaminant levels than cultivated samples. Cadmium, chromium, lead, and chlorpyrifos contamination showed weak correlations with geographic location. Based on our assumptions of the likely mode of consumption of raw CHMs, the vast majority (95%) of the 334 samples in this study contained levels of heavy metals or pesticides that would be of negligible concern. However, given the number of samples with detectable contaminants and the range between the more likely and more conservative scenarios of contaminant exposure, more research and monitoring of heavy metals (especially cadmium and chromium) and pesticide residues (especially chlorpyrifos) in raw CHMs are advised.
Keywords: Traditional Chinese Medicine (TCM), heavy metals, pesticide residues, herbal products, exposure assessment
1. Introduction
Chinese Herbal Medicines (CHMs) are used throughout the world and their use is growing (Li et al., 2009). In the United States, a recent national survey indicated that approximately 14.8 billion USD were spent in 2007 on non-mineral, non-vitamin natural products, most of which consisted of herbal medicines (Nahin et al., 2009), up from an estimated total of 6.6 billion USD spent ten years previously (Eisenberg et al., 1998). While the herbal supplement market in the United States continues to expand (Cavaliere et al., 2010), the United States remains a modest market for Chinese herbs. For example, about 3 of the 10 most commonly used herbs in the United States are from Chinese Medicine (Cavaliere et al., 2010).
Several studies have shown that CHMs and other botanical supplements may be contaminated with heavy metals, and in some cases at toxic levels (Ernst, 2002; Ernst and Coon, 2001; Lin et al., 2010). Much of what has been reported regarding potentially worrisome contamination in herbal medicines relates to patent or proprietary medicines (Ang et al., 2003; Au et al., 2000; Cooper et al., 2007; Dolan et al., 2003; Ernst, 2002; Ko, 1998; Koh and Woo, 2000; Martena et al., 2010; Raman et al., 2004; Saper et al., 2004), which differ from raw herbs in that they are frequently a mix of different substances (e.g., plant, mineral, animal) in either pill or extract form (Yee et al., 2005). Patent herbal medicines may contain heavy metals that were intentionally added, such as arsenic (Liu et al., 2008a), mercury (Liu et al., 2008b), and lead (Saper et al., 2008). Heavy metals have also been found in raw CHMs (e.g., Han et al., 2008; Lu et al., 2009; Rai et al., 2001; Wong et al., 1993; Wu et al., 2008), and some CHM plant species are known to be heavy metal hyper-accumulators (e.g., Imahara et al., 1992; Lai and Chen, 2005; Pollard et al., 2002; Turan and Bringue, 2007; Wei et al., 2008).
Pesticides have also been reported in CHMs (Leung et al., 2005; Wong et al., 2007; Xue et al., 2008; Zuin and Vilegas, 2000). A recent study of over-the-counter herbal dietary supplements sold in the United States found detectable levels of heavy metals and pesticide residues in some samples, although the FDA and EPA officials who reviewed the data did not express concern about immediate negative health consequences (US GAO, 2010).
Most CHMs in the United States are typically covered under the Dietary Supplements Health and Education Act (DSHEA) of 1994 (Frankos et al., 2010), and as such, lack official limits for regulation of heavy metals or pesticides. Some guidelines for maximum levels of heavy metals and pesticides in raw CHMs have been published at the international (e.g., WHO, 1998) or national level (e.g., CPC, 2005), but considerable variation exists in the proposed standards (Zhao et al., 2007), and in most cases regulated limits apply to only a small subset of contaminants or CHMs.
This study was conducted as part of a larger project initiated to evaluate more than 200 species of commonly used raw CHMs for their bioactivity and potential for drug discovery (Eisenberg et al., 2011). A unique aspect of the study was that a large number of CHMs were collected and each collection was from a single sampling location, as opposed to being a mixture from different collection sites, which is what is usually available on the market. Moreover, each sample was processed using traditional methods, authenticated according to visual, microscopic, and chemical characteristics, and was accompanied by detailed collection information, including photographs, video, and GPS coordinates. As part of the project, all samples were examined for heavy metals and pesticide residues.
It was hypothesized that because raw CHMs are typically free of substances that are added in patent medicines, they would contain relatively low levels of heavy metals. Furthermore, because roughly two-thirds of medicinal plants are collected from the wild and cultivation is typically on a small scale (Canter et al., 2005), it would be expected that the pesticide content in raw CHMs, particularly those collected from the wild, would be low. The goals of this study were to: summarize patterns of heavy metal and pesticide content observed in a large sample of CHMs collected throughout China and compare observed levels to established limits; examine the association between heavy metal and pesticide contamination, collection location in China, and cultivation status; assess the contribution of contaminant levels found in the CHMs to background levels of exposure; and discuss implications of these findings for policy makers and researchers in the field of herbal medicine.
2. Methods
2.1. Sample Collection
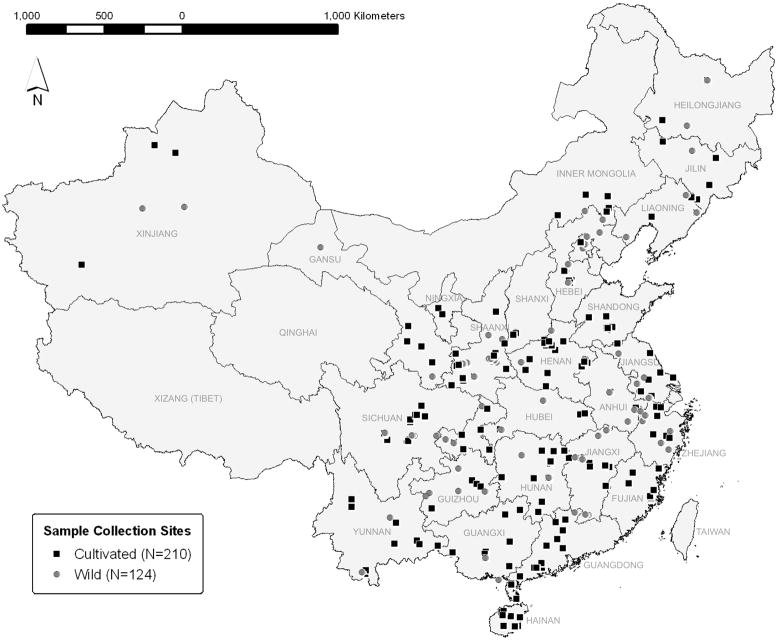
A total of 334 samples representing 126 species of commonly used CHMs were collected for examination of heavy metal content (Fig. 1). Of those, 210 samples (62.9%) were collected from cultivated locations and 124 samples (37.1%) were collected from the wild. Of the total samples, 294 samples representing 112 species were also examined for 162 pesticide residues. Species of CHMs used in this study were chosen only if they were listed in the 2005 edition of the Chinese Pharmacopoeia (CPC, 2005) and were not endangered (CITES, 2010). Each species was collected between 2006 and 2009 from 1 to 3 different sampling locations (species listed in Supplementary Data, Table S1). Sampling locations were chosen according to areas of traditional production of the CHM, without regard to possible pollution sources. Collection site information, including photographs, video, and GPS data, was obtained for all samples. Each collection consisted of a bulk sample of 10 kg dry weight of the medicinal part of the plant (e.g., root) and an accompanying voucher sample for botanical authentication. Each bulk sample was harvested, cleaned, and processed according to traditional methods, then authenticated by Chinese medicine experts according to visual, microscopic, and chemical characteristics (Eisenberg et al., 2011). As part of the authentication process, all samples were tested for purity according to total water and ash content (CPC, 2005). The test of ash content provided information on the presence of impurities such as soil. Samples that did not meet the Chinese Pharmacopoeia standards of any authentication test were removed from the study. Following authentication, 250 g were separated for heavy metal and pesticide testing. The 250 g sample was first ground into a powder using an IKA grinder (Model A11 B21; IKA Works, Inc., Wilmington, NC). Sample preparation time varied according to the quality and consistency of the samples. For example, leaf material required less grinding than root material.
Fig. 1.
Map of all sample locations according to cultivation status (N=334).
2.2. Examination of Heavy Metals
Samples were tested for the total concentration of arsenic, cadmium, chromium, lead, and mercury. These five metals were chosen since they are most commonly associated with possible toxicity in dietary supplements (WHO, 1998) and/or are specified in the NSF International (NSF) and American National Standards Institute (ANSI) Standard 173 for dietary supplements (NSF International, 2008). Metal concentrations were measured as the total, unspeciated amounts. Approximately 0.5 g of ground sample was first microwave digested for 40 min. in concentrated nitric acid (HNO3) and 30% hydrogen peroxide (H2O2) in a quartz vessel. The resulting digestate was analyzed using a Thermo X-Series Inductively-Coupled Plasma Mass Spectrometer (ICP-MS) (Thermo Fisher Scientific, Waltham, MA). Heavy metal results from the ICP-MS were quantified against standard curves generated from 1 blank and at least 4 standard reference solutions (High-Purity Standards, Charleston, SC) run separately. Quality control was assessed by running a laboratory reagent blank after every 10 samples. The limit of detection achieved for each metal was 0.08 ppm for arsenic, 0.02 ppm for cadmium, 0.01 ppm for chromium, 0.04 ppm for lead, and 0.01 ppm for mercury. The detection limit was based on consideration of the blank runs, concentration of the low standard in the calibration curve, and the sample preparation procedure. Based on this method, the limit of detection was considered equivalent to the limit of quantitation.
2.3. Examination of Pesticides
Samples were tested for the presence of 162 different pesticide residues. Information about pesticides applied to the CHMs or to neighboring areas of cultivation in this study was not always known. In addition, there are no international recommendations for specific pesticides that should be screened in medicinal plants (WHO, 1998), so for this project a broad, comprehensive test was employed. A modified QuEChERS method (“Quick, easy, cheap, effective, rugged, safe”; Anastassiades et al., 2003) was used for preparation of the samples for pesticide analysis. Approximately 2 g of the ground sample was first extracted in acetonitrile. If required, some samples were cleaned with dispersive solid-phase extraction clean-up (dSPE, Supelclean ENVI-Carb II/PSA SPE Tube; Supelco, Bellefonte, PA). A portion of the dSPE eluted solution or original extract was then dried under nitrogen gas flow at 60°C and redissolved in methanol. Depending on the analyte, the re-dissolved samples were analyzed using either gas chromatography coupled with triple quadrupole mass spectrometry (GC-MS/MS; Waters Quattro Micro GC system, GC/Triple Quadrupole Mass Spectrometer system; Milford, MA) or liquid chromatography coupled with triple quadrupole mass spectrometry (LC-MS/MS; Waters Acquity UPLC system, Quattro Micro Triple Quadrupole Mass Spectrometer; Milford, MA) following published methods (Lehotay et al., 2005). The results of pesticide analysis were quantified using pesticide standard solutions run separately. Quality control was assessed using spiked samples containing mixtures of authentic references for the 162 pesticide residues (AccuStandard, New Haven, CT). A list of all pesticides is provided in Supplementary Data, Table S2. The limit of detection for each individual pesticide varied according to the standard curve used, but typically was within the range of 10–50 ppb. As with the analysis of heavy metals, the limit of detection for the pesticide residues was equivalent to the limit of quantitation.
2.4. Analyses
All samples were run in duplicate. The resulting values for all samples were calculated by determining the average of the duplicate measurements. When determining the average of a non-detect (ND) and detect value, the limit of detection (LOD) was used for the ND value. The full value of the LOD was used since this was the most conservative method. All calculations were also done using half of the LOD (0.5*LOD) for ND values, but this did not affect the results.
2.5. Calculation of Risk
Three methods were used to interpret the significance of detected levels of contaminants. The first method compared detected values with established maximum acceptable concentration limits, using heavy metal limits proposed for dietary supplements by the NSF/ANSI Standard 173 (NSF International, 2008), and pesticide residue limits published by the European Pharmacopoeia for dried herbal infusions (EUP, 2010). In the case of pesticides, action limits proposed for certain pesticides banned in the United States were also used (US FDA, 2009).
The second method of interpretation was based on calculating the levels of contamination as a percent of a reference dose (RfD) for heavy metals, or population adjusted dose (PAD) for pesticides. All calculations of %RfD and %PAD used the maximum daily dose of the herb as specified in the Chinese Pharmacopoeia (CPC, 2005) and an assumed body weight of 70 kg. The RfD values for chronic oral exposure to inorganic arsenic, cadmium in food, and chromium VI were derived from the EPA Integrated Risk Information System (IRIS) (US EPA, 2010). Reference dose values for lead and mercury were extrapolated from the Joint FAO/WHO Expert Committee On Food Additives, Provisional Tolerable Weekly Intake (JEFCA PTWI) for lead (JECFA, 2000) and inorganic mercury (JECFA, 2010). The PAD values for pesticides were from the United States Environmental Protection Agency (EPA) Office of Pesticide Programs ISTEP Toxicity Endpoint Selection Database (Miller DJ, Chief, Chemistry & Exposure Branch, Office of Pesticide Programs, EPA, email communication, April 2010).
The third method of interpreting contaminant results was based on minimal risk levels (MRLs) provided by the Agency for Toxic Substances and Disease Registry (ATSDR, 2010). Contamination of each sample was calculated as a %MRL based on the maximum daily dose of the herb (CPC, 2005) and an assumed body weight of 70 kg. All reference values (e.g., RfD, MRL, PAD) used for the analyses are provided in Supplementary Data, Table S3.
A cut-off limit for determination of an elevated level of daily intake using the %RfD, %PAD, and %MRL values was calculated assuming that the amount of contaminants in individual CHMs should not exceed 10% of the total allowable daily intake for heavy metals, and 1% of the total daily intake of pesticides. The limit of 10% of allowable daily intake was suggested for heavy metal content in dietary supplements in the NSF/ANSI Std. 173 (NSF International, 2008). The value of 1% of the total pesticide daily intake was recommended by the WHO (1998). Note that 1% of the total pesticide daily intake (i.e., 1% of PAD or MRL) or 10% of the total heavy metal intake (i.e., 10% of RfD or MRL) is equivalent to roughly 1/104–105 of the no observed adverse effect level (NOAEL). Therefore, samples with contaminant levels above the cut-off limits proposed in this paper represent samples that have the potential to contribute to elevated background exposure levels, depending on exposure to the contaminants through other pathways, such as in food and drinking water.
2.6. Assumptions for Preparation of Raw Herbs
In order to provide context for interpreting the %RfD, %PAD, or %MRL it was necessary to make assumptions about the most common mode of consumption of raw CHMs. All analyses based on the RfD, PAD, or MRL reference values were calculated assuming that either 100% or 10% of the detected levels of the contaminants were actually ingested. The assumption of 10% ingestion of contaminants accounts for the fact that Chinese herbs are most often prepared by boiling as a soup or tea (Scheid et al., 2009). Using 10% of the detected value assumes a low, but realistic, leaching efficiency of the contaminant into the broth that is actually consumed, as noted by previous studies (Jaggi et al., 2001; Nookabkaew et al., 2006). In addition, both consumption scenarios were calculated assuming either acute or chronic exposure, using the corresponding acute or chronic PAD or MRL values. Only chronic exposure values for EPA IRIS RfD values were available, so the acute exposure scenario was not calculated in that case. Based on considerations of the mode of ingestion of CHMs and leaching behavior of contaminants in teas and soups, and because it is doubtful that all of the contaminants are in their most toxic forms (e.g., Cr VI vs. Cr III), it is our opinion that the lower-exposure scenario (i.e., 10% leaching; acute exposure) is most appropriate for interpreting our results. Below we refer to the different scenarios by comparing the “more-likely” assessment (i.e., 10% leaching of contaminants; acute exposure) versus the “most-conservative” assessment (i.e., 100% of contaminants ingested; chronic exposure). In any cases of disagreement between the scenarios provided by different toxicological reference values (i.e., EPA RfD or PAD vs. ASTDR MRL), the more stringent method was always used. For example, if the chronic RfD comparison indicated that five samples were above the cut-off limit whereas the chronic MRL comparison indicated three samples were above the cut-off limit, then the chronic RfD value would be used since it was more stringent.
2.7. Geographic Distribution
Results of contaminant concentration were mapped in ArcGIS (ESRI, 2009) using GPS coordinates of the collection site. The samples were tested for spatial autocorrelation in GeoDa (Anselin et al., 2006) using the Moran’s I statistic (Anselin, 1995). Data were first log-transformed, and the Moran’s I statistic was calculated using both a neighbor and distance-based approach. Statistical significance of spatial autocorrelation was determined by comparison with a reference distribution based on 999 random permutations of the data.
2.8. Statistics
Levels of contamination were quantified using descriptive statistics. The relative levels of heavy metal and pesticide contamination in wild versus cultivated samples were compared using a non-parametric Wilcoxon rank sums test. The alpha level to achieve significance was set at 0.05.
3. Results
Results of toxicological significance are summarized below only in terms of the %RfD, %PAD, and %MRL comparisons. The results of the comparison with fixed limits (i.e., NSF/ANSI Std. 178; EU Pesticides Database; FDA Action Limits) are not provided in the text but are provided in the corresponding tables and figures. The fixed limits do not account for differences in mode of ingestion of CHMs, daily dose amounts, or acute versus chronic exposure scenarios. For that reason, although the fixed limits are included for reference purposes, we believe that the %RfD, %PAD, and %MRL are better for estimating the possible toxicological significance of contaminant levels and therefore focus on those results in the text.
3.1. Heavy Metals
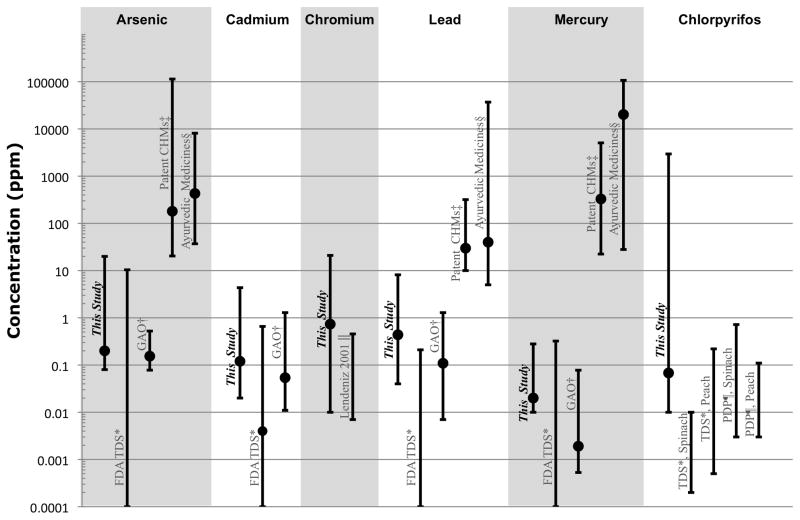
At least one heavy metal was detected in all samples examined, and 115 samples (34.4%) had detectable levels of all five metals. The number and percent of samples with detectable levels of each metal are summarized in Table 1, and shown in comparison with other studies (Egan et al., 2007; Ko, 1998; Lendinez et al., 2001; Saper et al., 2004; USDA, 2009; US FDA, 2007; US GAO, 2010) in Fig. 2.
Table 1.
Detected levels of heavy metals and chlorpyrifos in Chinese Herbal Medicines (CHMs).
| Limit of Detection (ppm) | # Samples with Detectable Contaminant (% of total) | Median (ppm) | Min (ppm) | Max (ppm) | |
|---|---|---|---|---|---|
| Arsenic | 0.08 | 221 (66.2%) | 0.2 | 0.08 | 20 |
| Cadmium | 0.02 | 279 (83.5%) | 0.12 | 0.02 | 4.35 |
| Chromium | 0.01 | 333 (99.7%) | 0.735 | 0.01 | 21 |
| Lead | 0.04 | 320 (95.8%) | 0.4375 | 0.04 | 8.15 |
| Mercury | 0.01 | 143 (42.8%) | 0.02 | 0.01 | 0.28 |
| Chlorpyrifos | 0.01–0.05 | 76 (25.9%) | 0.068 | 0.01 | 2955 |
Fig. 2.
Range and median (indicated by circle, if available) of detected values of contaminants, as compared with other studies. The median value of arsenic, lead, and mercury in the FDA Total Diet Study was 0 ppm.
*Data from United Slates FDA Total Diet Study (Egan et al.,2007: US FDA, 2007) Ranges for heavy metals are a summary of all foods in the study. Ranges for chlorpyrifos are from values detected in peach and spinach.
†Values detected in herbal dietary supplements reported by United States Government Accountability Office (US GAO. 2010).
‡Report on Chinese patent medicines purchased in San Francisco, United States (Ko, 1998).
§Report on Ayurvedic patent medicines purchased in Boston. United States (Saper et al., 2004).
||Data on fruits and vegetables from Spain (Lendinez et al., 2001).
¶Values detected in peach and spinach by United States EPA Pesticide Data Program (USDA, 2009).
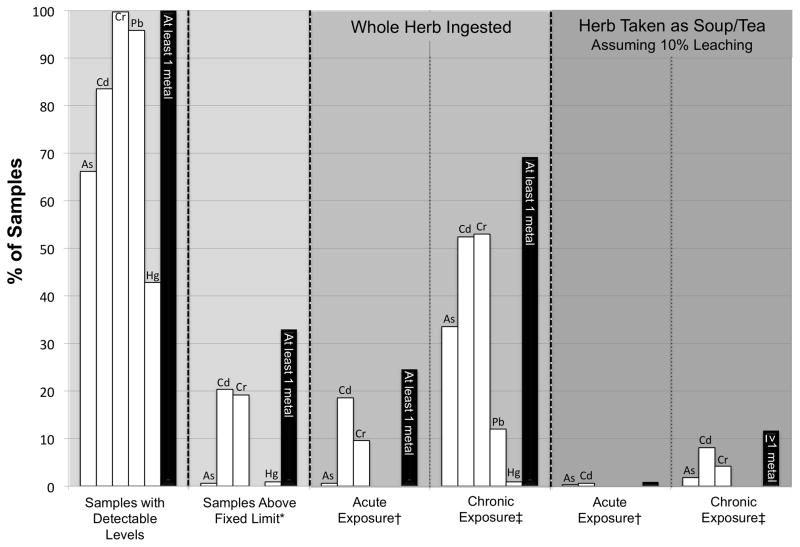
Three samples (0.9%) had levels of heavy metals that could contribute to an elevated level of background exposure in the more likely assessment of consumption of raw CHMs (10% leaching; acute exposure), whereas 231 samples (69.2%) had levels of heavy metals that could contribute to elevated background exposure in the more conservative assessment (100% ingested; chronic exposure). The more likely assessment is relatively robust to changes in the assumption of exposure, with only 39 samples (12%) with heavy metals that could contribute to elevated level of background exposure if it is assumed that CHMs are consumed as a soup or tea, and exposure is chronic. In terms of individual metals, 0.3% of samples could contribute to high background exposure levels of arsenic, 0.6% of samples could contribute to high background exposures of cadmium, and no samples were of concern in terms of chromium, lead, and mercury contamination in the more likely assessment. According to the more conservative method, the percent of samples with the potential for contributing to elevated background levels of each metal were: 34% for arsenic; 52% for cadmium; 53% for chromium; 12% for lead, and 1% for mercury. The percent of samples that could contribute to elevated background levels of heavy metals is shown in Table 2 and summarized in Fig. 3.
Table 2.
Summary of heavy metal and chlorpyrifos content in comparison with toxicological reference limits. For additional explanation see Sections 2.5 and 2.6.
| Fixed Cut-off Limits (ppm) (NSF International, 2008; EUP, 2010) | # Samples > Limit (% all samples in parentheses) | # Samples > Chronic RfD or Chronic PAD Cut-off (% all samples in parentheses) (US EPA, 2010; JECFA, 2000, 2010) | # Samples > MRL Cut-off (% all samples in parentheses) (ATSDR, 2010) | |||||
|---|---|---|---|---|---|---|---|---|
| Herb Taken as Soup/Tea (10% Leaching) | Whole Herb Ingested | Herb Taken as Soup/Tea (Assuming 10% Leaching) | Whole Herb Ingested | |||||
| Acute Exposure | Chronic Exposure | Acute Exposure | Chronic Exposure | |||||
| Arsenic | 5 | 2 (0.6%) | 6 (1.8%) | 112 (33.5%) | 1 (0.3%) | 6 (1.8%) | 2 (0.6%) | 112 (33.5%) |
|
| ||||||||
| Cadmium | 0.3 | 68 (20.4%) | 0 (0%) | 27 (8.1%) | 2 (0.6%) | 27 (8.1%) | 62 (18.6%) | 175 (52.4%) |
|
| ||||||||
| Chromium | 2 | 64 (19.2%) | 1 (0.3%) | 63 (18.9%) | 0 (0%) | 14 (4.2%) | 32 (9.6%) | 177 (53.0%) |
|
| ||||||||
| Lead | 10 | 0 (0%) | 0 (0%) | 40 (12.0%) | n/a | n/a | n/a | n/a |
|
| ||||||||
| Mercury | 0.2 | 3 (0.9%) | 0 (0%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (0.9%) |
|
| ||||||||
| Chlorpyrifos | 0.5 | 13 (4.4%) | 66 (22.4%) | 75 (25.5%) | 5 (1.7%) | 13 (4.4%) | 18 (6.1%) | 37 (12.6%) |
Fig. 3.
Percent of samples with heavy metals at detectable levels or above a limit of elevated background exposure according to different exposure scenarios (for explanation see Sections 2.5 and 2.6). White bars indicate individual metals as labeled, black bars indicate at least one metal above limit. Lead values are only shown for the chronic exposure scenarios because an acute RfD or MRL for lead was not available.
*Fixed limit derived from NSF/ANSI Standard 173 (NSF International, 2008).
†Acute exposure for all metals based on the ASTDR MRL reference values (ATSDR, 2010).
‡Chronic exposure for As, Cd, Cr, and Hg based on the ASTDR MRL reference values; Pb was calculated using the JECFA PTWI (JECFA, 2000).
3.2. Pesticides
Forty-two different pesticides were detected in 108 plant samples (36.7%). Contamination ranged from 1 to 9 detectable pesticides per sample. Most of the pesticide residues were detected in low amounts, but half of the detected pesticides (N=21) are not registered for use in the United States. Of those, 10 pesticides have FDA determined action limits as unavoidable pesticides (US FDA, 2009), such as organochlorine residues that persist in the environment (e.g., DDT). No single pesticide was detected in more than 6 (<2%) samples, with the exception of chlorpyrifos. Because it was the most common pesticide detected (in 26% of samples), chlorpyrifos levels are summarized in Table 1 and Fig. 2. Details for all detected pesticides are provided online in Supplementary Data, Table S4.
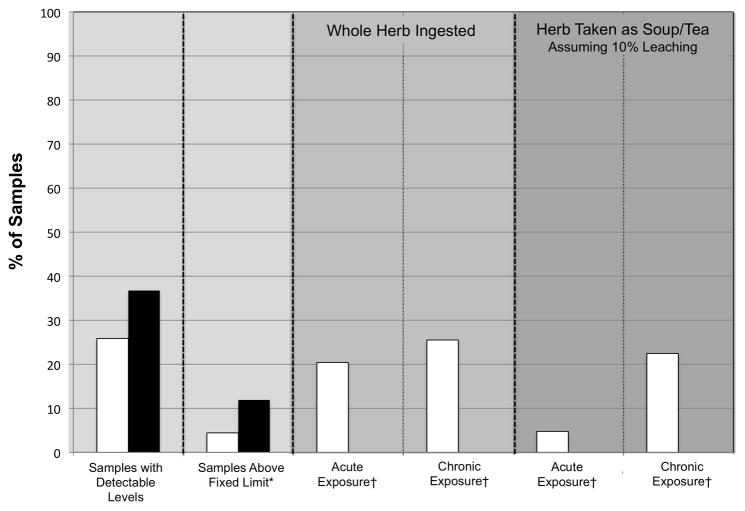
Fourteen samples (4.8%) had levels of pesticides that could contribute to an elevated background exposure in the more likely assessment of consumption of raw CHMs (i.e., herb consumed as soup/tea with 10% leaching of contaminants; acute exposure). If chronic exposure is assumed in the more likely scenario, then 69 samples (23.5%) would have levels of pesticides that could contributed to elevated background exposure. Eighty-one samples (27.5%) had levels of pesticides that could contribute to an elevated background exposure in the more conservative assessment. In terms of individual pesticides, with the exception of 14 samples (4.8%) that contained chlorpyrifos, all other samples had pesticides at levels of negligible concern in the more likely assessment (i.e., 10% ingested; acute exposure). In the more conservative assessment, the percent of samples with levels of pesticides that could contribute to an elevated level of background exposure was: 26% with chlorpyrifos; 0.3% with esfenvalerate; 0.3% with fenvalerate; 0.3% with fipronil; 0.3% with lindane; 1.4% with methyl-parathion; and, 0.3% with quintozene. Because chlorpyrifos was the only pesticide frequently detected, patterns of contamination in chlorpyrifos are summarized in Table 2 and Fig. 4.
Fig. 4.
Percent of samples with pesticides at detectable levels or above a limit of elevated background exposure according to different exposure scenarios (for explanation see Sections 2.5 and 2.6). White bars indicate samples with chlorpyrifos, black bars indicate at least one pesticide above limit. Only chlorpyrifos values are shown for acute and chronic exposure scenarios because all other pesticides were detected in fewer than 2% of samples and many lacked toxicological reference values.
*Fixed limit derived from EU Pesticides Database (EUP, 2010) or FDA action levels for unavoidable pesticides (US FDA, 2009).
†Acute and chronic exposure scenarios for chlorpyrifos are based on the EPA PAD acute and chronic reference values, respectively.
3.3. Collection Location
Arsenic and mercury concentrations were not significantly associated with geographic location according to Moran’s I (Anselin et al., 2006). However, lead and cadmium appear to have clusters of high values in Southwest China, chromium has clusters of high values in Northeast China, and chlorpyrifos has clusters of high values in Southeast China. In each case, the correlation explains only a small part of the data (coefficient of determination, r2<0.1).
3.4. Cultivation Status
Levels of contamination were higher in wild samples as compared to cultivated samples. Specifically, levels of arsenic, cadmium, lead, chromium, and chlorpyrifos were significantly higher in wild samples (Table 3).
Table 3.
Correlations between cultivation status and heavy metal or chlorpyrifos content.
| Contaminant | Group | N | Median (ppm) | Minimum (ppm) | Maximum (ppm) | P value | Interpretation |
|---|---|---|---|---|---|---|---|
| Arsenic | Cultivated | 210 | 0.115 | 0.08 | 6.95 | 0.02 | wild > cultivated |
| Wild | 124 | 0.1475 | 0.08 | 20 | |||
|
| |||||||
| Cadmium | Cultivated | 210 | 0.06 | 0.02 | 1.3 | 0.003 | wild > cultivated |
| Wild | 124 | 0.1225 | 0.02 | 4.35 | |||
|
| |||||||
| Chromium | Cultivated | 210 | 0.6 | 0.01 | 10.7 | 0.02 | wild > cultivated |
| Wild | 124 | 0.875 | 0.01 | 21 | |||
|
| |||||||
| Lead | Cultivated | 210 | 0.2825 | 0.04 | 7.4 | <.001 | wild > cultivated |
| Wild | 124 | 0.6425 | 0.04 | 8.15 | |||
|
| |||||||
| Mercury | Cultivated | 210 | 0.01 | 0.01 | 0.28 | 0.07 | wild = cultivated |
| Wild | 124 | 0.01 | 0.01 | 0.21 | |||
|
| |||||||
| Chlorpyrifos | Cultivated | 190 | 0.01 | 0.01 | 13.275 | <.001 | wild > cultivated |
| Wild | 104 | 0.01 | 0.01 | 2955 | |||
4. Discussion
The contaminant levels found in the 334 raw CHMs collected for this study are within the range of values found by the recent United States GAO survey of over-the-counter herbal dietary supplements (US GAO, 2010), but much lower than previously reported for patent HMs (Ko, 1998; Saper et al., 2004) (Fig. 2). The contaminant levels are also within the range of values found in a recent FDA total diet study (Egan et al., 2007; US FDA, 2007), but the median values in the FDA study were lower. For example, maximum levels of heavy metals found in the FDA total diet study for spinach were: arsenic, 0.043 ppm; cadmium, 0.524 ppm; lead, 0.062 ppm; and, mercury, 0.018 ppm (US FDA, 2007). By comparison, the maximum values found in this study were: arsenic, 20 ppm; cadmium, 4.35 ppm; lead, 8.15 ppm; and mercury, 0. 28 ppm. These values are 100 to 1000 times lower than those found in other studies on patent CHMs (Ko, 1998) or commonly available Indian (Ayurvedic) medicine (Saper et al., 2004). For example, in a survey of Chinese patent medicines sold in California, levels of arsenic, lead, and mercury reached levels as high as 14533 ppm, 319 ppm, and 1046 ppm, respectively (Ko, 1998). Since these 3 metals are frequently added to HMs in the preparation of patent medicines (e.g., Liu et al., 2008a, 2008b), it is not surprising that the observed levels in individual raw CHMs are much lower.
Some conclusions can be made regarding certain heavy metal or pesticide contaminants. First, mercury does not appear at unacceptable levels by any measure. Therefore the individual CHMs in this study would pose little risk for mercury toxicity. Second, it is known that chromium VI is considerably more toxic than chromium III, and inorganic arsenic is more toxic than organic arsenic. All reference values used in this paper to interpret our results of total metal concentration (i.e., all ionic forms) are based on values for the more toxic form. We may thus assume that our interpretations of the percent of samples with significant arsenic and chromium contamination represent an over-estimate. In addition, with the exception of NSF/ANSI Std. 173 (NSF International, 2008), chromium limits are not generally included in herbal pharmacopoeias (CPC, 2005). Since chromium was detected in almost all samples, more research and development of reference levels for this metal are recommended. Third, since cadmium was observed at high levels as compared to published standards in at least some of the individual CHMs in this study, many previous studies on CHMs do not test for this metal (e.g., Ko, 1998), and China is the world’s largest producer of cadmium (Hetherington et al., 2008), further research on cadmium levels in CHMs is warranted. Finally, chlorpyrifos was detected in roughly 1 in 4 samples and occurred at high concentrations in two samples (0.1 and 3.0 parts per thousand). Chlorpyrifos is not currently monitored in the Chinese Pharmacopoeia (CPC, 2005). Therefore, further monitoring of chlorpyrifos and development of allowable limits for chlorpyrifos in HMs is advised.
Surprisingly, samples collected from the wild tended to have higher levels of arsenic, cadmium, lead, and chlorpyrifos than cultivated samples. The reason for this difference is unclear, but some possible explanations for this are that: i) many CHMs that are collected from the “wild” are actually located very close to cultivation sites where pesticides are applied; ii) wild CHMs are near industrial sites that are sources of heavy metals; and/or iii) the heavy metals in soils where CHMs are cultivated have been absorbed from the soil by previous crops. The potential influence of local sources of industrial or agricultural pollution may also explain the weak spatial patterning of contamination of cadmium, chromium, lead, and chlorpyrifos, but this clustering effect needs to be examined further.
5. Conclusions
The results of this study should be interpreted with caution. Only individual CHMs were investigated, and the results cannot be extrapolated to predict the levels of contaminants that might be found in manufactured Chinese herbal products or over-the-counter supplements containing Chinese herbs. In addition, given the range between the more likely and more conservative assessments of contribution of these CHM samples to elevated background levels of exposure, further research is warranted to determine the form or valence of the heavy metals in raw CHMs, leaching behavior in traditional Chinese medicinal preparations, general exposure scenarios (i.e., acute vs. chronic), and assumed limits of relative source contribution of contaminants from CHMs (e.g., 1% or 10% of total daily intake). Data on these aspects will help establish and refine formal contaminant limits in herbal products.
With these caveats in mind, the following conclusions can be made:
Based on our assumptions of the mode of consumption of raw CHMs, the heavy metal and pesticide contamination levels in the vast majority of the 334 Chinese-grown herb samples in this study (i.e., 99% of samples tested for heavy metals; 95% of samples tested for pesticide residues) are likely to be of negligible concern;
More research, monitoring, and regulation of heavy metals and pesticides in raw CHMs and all manufactured products containing individual CHMs are advised, particularly for the contaminants cadmium, chromium, and chlorpyrifos;
Samples should be monitored for contaminants regardless of whether they are cultivated or collected from the wild;
More research should be done to corroborate the spatial patterns of contamination with sources of agricultural and industrial pollution; and,
Most importantly, given the contaminant levels observed in a large number of “herbs of commerce,” the international community, and not just the United States and China, should continue efforts (e.g., GP-TCM, 2011; WHO, 1998) to design and implement a governmentally-regulated quality assurance and surveillance program for contaminants in all products that contain Chinese (and other) herbal material. In this way, an internationally endorsed scheme can be implemented to identify herbs of commerce found to contain contaminants of potential clinical concern and the geographic regions, specific farms or factories, where the contaminated herbal material was collected or processed can be speedily identified. Such monitoring of plant-based materials will help protect countless consumers of herbal medicines worldwide and establish safeguards for both exporting and importing countries.
Supplementary Material
Highlights.
The majority of herbs in this study had contaminant levels of negligible concern.
Wild-collected samples had higher levels of contaminants than cultivated samples.
The presence of some contaminants was weakly correlated with collection location.
More research and monitoring of cadmium, chromium, and chlorpyrifos are advised.
Acknowledgments
This work was supported by grants from the National Institutes of Health National Cancer Institute (U19 CA128534), the National Center for Complementary and Alternative Medicine (AT03002 “PIRC”), and the Bernard Osher Foundation. The sponsors were not involved in the collection, analysis, or interpretation of the data; in writing the report; or the decision to submit the paper for publication. Dr. Kaptchuk was partially supported by a grant from the National Institutes of Health (K24 AT004095). Dr. Woolf was supported (in part) by the Region 1 Pediatric Environmental Health Specialty Unit (PEHSU) under a cooperative agreement award number 1U61TS000118-01 from the Agency for Toxic Substances and Disease Registry (ATSDR). The U.S. Environmental Protection Agency (EPA) supports the PEHSU by providing funds to ATSDR under Inter-Agency Agreement number DW-75-92301301-0. Neither EPA nor ATSDR endorse the purchase of any commercial products or services mentioned in PEHSU publications. We acknowledge guidance provided on dietary exposure assessment and risk assessment methodologies from David J. Miller, Bernard Schneider, Aaron Niman, and David Hrdy of U.S EPA’s Office of Pesticide Programs. We acknowledge NSF International Center for Applied Research, Ann Arbor, Michigan for performing the chemical analyses and for help with the toxicological assessment. Additionally, the authors would like to acknowledge comments from Drs Roger Davis (Harvard Medical School), Sumeeta Srinivasan (Harvard University Center for Geographic Analysis), and P. Michael Bolger (FDA, Chemical Hazards Assessment Team) on earlier drafts of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR (Agency for Toxic Substances and Disease Registry) [Internet] ATSDR Minimal Risk Levels for Hazardous Substances (MRLs) [cited 2010 Sept 16]. Available from: http://www.atsdr.cdc.gov/mrls/index.html.
- Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412–431. [PubMed] [Google Scholar]
- Ang HH, Lee EL, Matsumoto K. Analysis of lead content in herbal preparations in Malaysia. Hum Exp Toxicol. 2003;22:445–451. doi: 10.1191/0960327103ht382oa. [DOI] [PubMed] [Google Scholar]
- Anselin L. Local indicators of spatial association - LISA. Geogr Anal. 1995;27:93–115. [Google Scholar]
- Anselin L, Syabri I, Kho Y. GeoDa: An introduction to spatial data analysis. Geogr Anal. 2006;38:5–22. [Google Scholar]
- Au AM, Ko R, Boo FO, Hsu R, Perez G, Yang Z. Screening methods for drugs and heavy metals in Chinese patent medicines. B Environ Contam Tox. 2000;65:112–119. doi: 10.1007/s0012800102. [DOI] [PubMed] [Google Scholar]
- Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Cavaliere C, Rea P, Lynch ME, Blumenthal M. Herbal supplement sales rise in all channels in 2009. Herbalgram. 2010;86:62–65. [Google Scholar]
- CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) [Internet] Official Website. [cited 2010 Sept 16]. Available from: http://www.cites.org.
- Cooper K, Noller B, Connell D, Yu J, Sadler R, Olszowy H, et al. Public health risks from heavy metals and metalloids present in Traditional Chinese Medicines. J Toxicol Env Heal A. 2007;70:1694–1699. doi: 10.1080/15287390701434885. [DOI] [PubMed] [Google Scholar]
- CPC (Chinese Pharmacopoeia Commission) Pharmacopoeia of the Peoples Republic of China: 2005. Beijing, China: People’s Medical Publishing House; 2005. [Google Scholar]
- Dolan SP, Nortrup DA, Bolger PM, Capar SG. Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry. J Agr Food Chem. 2003;51:1307–1312. doi: 10.1021/jf026055x. [DOI] [PubMed] [Google Scholar]
- Egan SK, Bolger PM, Carrington CD. Update of US FDA’s Total Diet Study food list and diets. J Expo Sci Env Epid. 2007;17:573–582. doi: 10.1038/sj.jes.7500554. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, van Rompay M, et al. Trends in alternative medicine use in the United States, 1990–1997 - Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Harris ESJ, Littlefield BA, Cao SG, Craycroft JA, Scholten R, et al. Developing a library of authenticated Traditional Chinese Medicinal (TCM) Plants for systematic biological evaluation Rationale, methods and preliminary results from a Sino-American collaboration. Fitoterapia. 2011;82:17–33. doi: 10.1016/j.fitote.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002;23:136–139. doi: 10.1016/S0165-6147(00)01972-6. [DOI] [PubMed] [Google Scholar]
- Ernst E, Coon JT. Heavy metals in traditional Chinese medicines: A systematic review. Clin Pharmacol Ther. 2001;70:497–504. doi: 10.1067/mcp.2001.120249. [DOI] [PubMed] [Google Scholar]
- ESRI. ArcGIS 9.3.1. Redlands. United States: Environmental Systems Research Institute; 2009. [Google Scholar]
- EUP (European Union Pharmacopoeia) [Internet] European Union Pharmacopoeia Pesticides Database. [cited 2010 Oct 10]. Available from: http://ec.europa.eu/sanco_pesticides/public/index.cfm.
- Frankos VH, Street DA, O’Neill RK. FDA regulation of dietary supplements and requirements regarding adverse event reporting. Clin Pharmacol Ther. 2010;87:239–244. doi: 10.1038/clpt.2009.263. [DOI] [PubMed] [Google Scholar]
- GP-TCM (Good Practice in Traditional Chinese Medicine Research in the Post-genomic Era Consortium) [Internet] Official Website. [cited 2011 Feb 12]. Available from: http://www.gp-tcm.org.
-
Han XL, Zhang XB, Guo LP, Huang LQ, Li MJ, Sun YZ, et al. Statistical analysis of residues heavy metals in Chinese crude drugs. China Journal Of Chinese Materia Medica (
 ) 2008;33:2041–2048. [PubMed] [Google Scholar]
) 2008;33:2041–2048. [PubMed] [Google Scholar] - Hetherington LE, Brown TJ, Benham AJ, Bide T, Lusty PAJ, Hards VL, et al. World Mineral Production 2002–06. Nottingham, United Kingdom: British Geological Survey; 2008. [Google Scholar]
- Imahara H, Hatayama T, Kuroda S, Horie Y, Inoue E, Wakatsuki T, et al. Production of phytochelatins in Polygonum cuspidatum on exposure to copper but not to zinc. J Pharmacobio-Dynam. 1992;15:667–671. doi: 10.1248/bpb1978.15.667. [DOI] [PubMed] [Google Scholar]
- Jaggi S, Sood C, Kumar V, Ravindranath SD, Shanker A. Leaching of pesticides in tea brew. J Agr Food Chem. 2001;49:5479–5483. doi: 10.1021/jf010436d. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) WHO Technical Report Series No. 837. Geneva, Switzerland: WHO; 2000. Evaluation of Certain Food Additives and Contaminants. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) JECFA Seventy-second meeting; Rome. 16–25 February 2010; Geneva, Switzerland: WHO; 2010. [Google Scholar]
- Ko RJ. Adulterants in Asian patent medicines. New Engl J Med. 1998;339:847. doi: 10.1056/nejm199809173391214. [DOI] [PubMed] [Google Scholar]
- Koh HL, Woo SO. Chinese proprietary medicine in Singapore - Regulatory control of toxic heavy metals and undeclared drugs. Drug Safety. 2000;23:351–362. doi: 10.2165/00002018-200023050-00001. [DOI] [PubMed] [Google Scholar]
- Lai HY, Chen ZS. The EDTA effect on phytoextraction of single and combined metals-contaminated soils using rainbow pink (Dianthus chinensis) Chemosphere. 2005;60:1062–1071. doi: 10.1016/j.chemosphere.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lehotay SJ, de Kok A, Hiemstra M, van Bodegraven P. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int. 2005;88:595–614. [PubMed] [Google Scholar]
- Lendinez E, Lorenzo ML, Cabrera C, Lopez MC. Chromium in basic foods of the Spanish diet: seafood, cereals, vegetables, olive oils and dairy products. Sci Total Environ. 2001;278:183–189. doi: 10.1016/s0048-9697(01)00647-7. [DOI] [PubMed] [Google Scholar]
- Leung KSY, Chan K, Chan CL, Lu GH. Systematic evaluation of organochlorine pesticide residues in Chinese materia medica. Phytother Res. 2005;19:514–518. doi: 10.1002/ptr.1694. [DOI] [PubMed] [Google Scholar]
- Li B, Wu S, Lui C. China Pharmaceuticals: Investing in Traditional Chinese Medicine (TCM) Hong Kong, China: Morgan Stanley Research Asia/Pacific; 2009. [Google Scholar]
- Lin CG, Schaider LA, Brabander DJ, Woolf AD. Pediatric Lead Exposure From Imported Indian Spices and Cultural Powders. Pediatrics. 2010;125:E828–E835. doi: 10.1542/peds.2009-1396. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu YF, Wu Q, Goyer R, Waalkes MP. Mineral arsenicals in traditional medicines: Orpiment, realgar, and arsenolite. J Pharmacol Exp Ther. 2008a;326:363–368. doi: 10.1124/jpet.108.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shi JZ, Yu LM, Goyer RA, Waalkes MP. Mercury in traditional medicines: Is cinnabar toxicologically similar to common mercurials? Exp Biol M. 2008b;233:810–817. doi: 10.3181/0712-MR-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Lu FL, Chen YH, Tseng MC, Lo CF, Lin JH. Survey on heavy metals in raw material of Traditional Chinese Medicine (V) Annual Report BFDA Taiwan ROC (
 ) 2009;27:51–64. [Google Scholar]
) 2009;27:51–64. [Google Scholar] - Martena MJ, Van Der Wielen JCA, Rietjens IMCM, Klerx WNM, De Groot HN, Konings EJM. Monitoring of mercury, arsenic, and lead in traditional Asian herbal preparations on the Dutch market and estimation of associated risks. Food Addit Contam A. 2010;27:190–205. doi: 10.1080/02652030903207235. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Nat Health Stat Report. 2009;18:1–14. [PubMed] [Google Scholar]
- Nookabkaew S, Rangkadilok N, Satayavivad J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. J Agr Food Chem. 2006;54:6939–6944. doi: 10.1021/jf060571w. [DOI] [PubMed] [Google Scholar]
- NSF International. NSF International Standard/American National Standard #173 for Dietary Supplements. Ann Arbor, United States: NSF International; 2008. [Google Scholar]
- Pollard AJ, Powell KD, Harper FA, Smith JAC. The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci. 2002;21:539–566. [Google Scholar]
- Rai V, Kakkar P, Khatoon S, Rawat AKS, Mehrotra S. Heavy metal accumulation in some herbal drugs. Pharm Biol. 2001;39:384–387. [Google Scholar]
- Raman P, Patino LC, Nair MG. Evaluation of metal and microbial contamination in botanical supplements. J Agr Food Chem. 2004;52:7822–7827. doi: 10.1021/jf049150+. [DOI] [PubMed] [Google Scholar]
- Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy metal content of Ayurvedic herbal medicine products. JAMA. 2004;292:2868–2873. doi: 10.1001/jama.292.23.2868. [DOI] [PubMed] [Google Scholar]
- Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA. 2008;300:915–923. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid V, Bensky D, Ellis A, Barolet R. Chinese Herbal Medicine: Formulas and strategies. Seattle, United States: Eastland Press; 2009. [Google Scholar]
- Turan M, Bringu A. Phytoremediation based on canola (Brassica napus L.) and Indian mustard (Brassica juncea L) planted on spiked soil by aliquot amount of Cd, Cu, Pb, and Zn. Plant Soil Env. 2007;53:7–15. [Google Scholar]
- USDA (United States Department of Agriculture) Pesticide Data Program: Annual Summary, Calendar Year 2008. Washington, DC, United States: Agricultural Marketing Service, Science and Technology Programs; 2009. [Google Scholar]
- US EPA (United States Environmental Protection Agency) [Internet] Integrated Risk Information System (IRIS) [cited 2010 Nov 17]. Available from: http://www.epa.gov/iris.
- US FDA (United States Food and Drug Administration) Total Diet Study Results. Revision 4.1, Market Baskets 1991 through 2005. College Park, United States: US FDA, Center for Food Safety and Applied Nutrition; 2007. [Google Scholar]
- US FDA (United States Food and Drug Administration) [Internet] Pesticide Residues in Food and Feed - Enforcement Criteria (CPG Sec. 575.100) [updated 2009 Sept 12; cited 2010 Oct 10]. Available from: http://www.fda.gov/iceci/compliancemanuals/compliancepolicyguidancemanual/ucm123236.htm.
- US GAO (United States Government Accountability Office) Herbal Dietary Supplements: Examples of Deceptive or Questionable Marketing Practices and Potentially Dangerous Advice. Washington, DC, United States: US GAO; 2010. [Google Scholar]
- Wei S, Zhou Q, Mathews S. A newly found cadmium accumulator-Taraxacum mongolicum. J Hazard Mater. 2008;159:544–547. doi: 10.1016/j.jhazmat.2008.02.052. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Quality Control Methods for Medicinal Plant Materials. Geneva, Switzerland: WHO; 1998. [Google Scholar]
- Wong M, Tan P, Wee Y. Heavy metals in some Chinese herbal plants. Biol Trace Elem Res. 1993;36:135–142. doi: 10.1007/BF02783172. [DOI] [PubMed] [Google Scholar]
- Wong TC, Lee FS, Hu GL, Chang L, Wang X, Fu PP. A survey of heavy metal and organochlorine pesticide contaminations on commercial Lingzhi products. J Food Drug Anal. 2007;15:472–479. [Google Scholar]
- Wu J, Zou Y, Zhan X, Chen S, Lu G, Lai F. Survey of heavy metal pollution in four chinese crude drugs and their cultivated soils. B Environ Contam Tox. 2008;81:571–573. doi: 10.1007/s00128-007-9170-2. [DOI] [PubMed] [Google Scholar]
- Xue J, Hao L, Peng F. Residues of 18 organochlorine pesticides in 30 traditional Chinese medicines. Chemosphere. 2008;71:1051–1055. doi: 10.1016/j.chemosphere.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Yee SK, Chu SS, Xu YM, Choo PL. Regulatory control of Chinese proprietary medicines in Singapore. Health Policy. 2005;71:133–149. doi: 10.1016/j.healthpol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Zhao ZZ, Xiao PG, Xiao Y, Yuen JPS. Quality assurance of Chinese Herbal Medicines (CHMs) J Food Drug Anal. 2007;15:337–346. [Google Scholar]
- Zuin VG, Vilegas JHY. Pesticide residues in medicinal plants and phytomedicines. Phytother Res. 2000;14:73–88. doi: 10.1002/(sici)1099-1573(200003)14:2<73::aid-ptr577>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.