Abstract
Background & Aims
Rb1 encodes a cell cycle regulator that is functionally disrupted in most human cancers. Pancreatic ductal adenocarcinomas (PDACs) have a high frequency of mutations in KRAS and INK4A/CDKN2A that might allow cells to bypass the regulatory actions of RB. To determine the role of loss of RB function in PDAC progression, we investigated the effects of Rb disruption during pancreatic malignant transformation initiated by oncogenic Kras.
Methods
We generated mice with pancreas-specific disruption of Rb, in the absence or presence of oncogenic Kras, to examine the role of RB in pancreatic carcinogenesis.
Results
In the presence of oncogenic Kras, loss of Rb from the pancreatic epithelium accelerated formation of pancreatic intraepithelial neoplasia (PanIN), increased the frequency of cystic neoplasms, and promoted rapid progression toward PDAC. Early-stage cancers were characterized by acute pancreatic inflammation, associated with up-regulation of pro-inflammatory cytokines within the pancreas. Despite of the presence of markers associated with oncogene-induced senescence, low-grade PanIN were highly proliferative and expressed high levels of p53. Pancreatic cancer cell lines derived from these mice expressed high levels of cytokines and transcriptional activity of p53 was impaired.
Conclusions
Rb encodes a tumor suppressor that attenuates progression of oncogenic Kras-induced carcinogenesis in the pancreas by mediating the senescence response and promoting activity of the tumor suppressor p53.
Keywords: proliferation, transformation, signaling, chemokine
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the US.1 PDAC arises from precursor lesions, predominantly pancreatic intraepithelial neoplasia (PanIN) that progress from low grade, PanIN-1A and -1B, to intermediate and high grade, PanIN-2 and -3, respectively.2
The biological aggressiveness of PDAC derives from cancer cells that harbor driver mutations in several key genes, including the KRAS oncogene (~95%), and the TP53 (~80%), SMAD4 (~50%) and INK4A/CDKN2A (~85%) tumor suppressor genes (TSGs).3 INK4A/CDKN2A encodes p16Ink4a, which activates the tumor suppressive functions of RB, a nuclear phosphoprotein encoded by the RB1 TSG.4,5 In PDAC cases where INK4A/CDKNA is not mutated, it is silenced epigenetically.3 Thus, there is a near universal loss of p16Ink4a in PDAC. PDAC is also associated with overexpression of multiple tyrosine kinase receptors and their ligands,6 which, when super-imposed on the presence of oncogenic Kras, loss of p16Ink4a, and high levels of cyclin D1,7 could impede RB function. These observations suggest that RB inactivation may be of crucial importance in PDAC.
PDAC mouse models that recapitulated human disease were originally generated by targeting a conditionally mutated Kras allele (LSL-KrasG12D) to pancreatic progenitors, using Pdx1 and Ptf1a promoters.8 In these mice, PanIN progress slowly and at a low frequency towards PDAC by ~ 36 weeks of age. To delineate the role of RB in PDAC, we used RbLoxP/LoxP (RbL/L) mice9 to generate compound mutant Pdx1-Cre;LSL-KrasG12D;RbL/L (Rb/K) mice that carry activated, oncogenic Kras and deleted RB in the pancreas. While RB deletion alone does not alter pancreatic histology, it accelerates the induction of oncogenic Kras-associated neoplasia, and progression to PDAC. In early cancer stages, mice exhibit acute pancreatic inflammation and increased expression of pro-inflammatory cytokines, produced, in part, by the cancer cells. Low-grade lesions co-express senescence and proliferation markers suggesting that OIS is bypassed. All lesions are highly proliferative and display high levels of wild-type p53, which we show in vitro is transcriptionally inactive. Thus, loss of RB function in the context of oncogenic Kras leads to a pro-inflammatory pancreatic microenvironment, impaired OIS and enhanced cell proliferation, and p53 dysfunction, resulting in marked acceleration of pancreatic carcinogenesis.
Materials and Methods
Detailed materials and methods are described in the Supplementary Material.
Genetically modified mice and animal care
Pdx1-Cre and conditional RbL/L and LSL-KrasG12D mice were previously described.8–10 Animal experiments were approved by the Institutional Animal Care and Use Committee at Dartmouth College.
Immunohistochemistry
Mice were perfused with PBS then 10% formalin, pancreata dissected, fixed overnight and paraffin-embedded. Immunohistochemistry was performed using standard protocols.
Senescence-associated β–Galactosidase staining
Cryosections from K and Rb/K pancreata were prepared and stained in parallel under exactly the same conditions,11 yielding highly reproducible results.
Immnoblotting
Immunoblotting was performed as described.12
Primary cell line preparation and p53 mutation analysis
Primary cell lines were prepared as described.13 RNA was isolated with the RNeasy Mini kit (Qiagen, Valencia, CA) and cDNA was prepared with the Superscript III First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Sequencing was performed by the Molecular Biology Core at Dartmouth College (Hanover, NH).
Rb recombination and KrasG12D expression
Rb recombination9 and KrasG12D expression were analyzed as described.14
Quantitative reverse-transcription polymerase chain reaction
Pancreatic RNA was isolated using the low-temperature guanidine isothiocyanate method.15,16 Taqman expression assays (Applied Biosystems, Foster City, CA) were performed on cell line and pancreatic cDNA.
Proliferation assays
Cell growth was monitored as described.17
Luciferase assays
Reporter constructs were transfected into murine primary cell lines using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Luciferase activity was evaluated using the Dual Luciferase Assay kit (Promega, Madison, WI) and a LMaxII microplate reader (Molecular Devices, Sunnyvale, CA).
Results
RB deletion synergizes with oncogenic Kras to accelerate pancreatic carcinogenesis
By crossing Pdx1-Cre mice10 with RbL/L mice9, animals carrying a deletion of Rb in the pancreatic epithelium, and subsequently in all pancreatic cell types were generated. These mice were born at the expected frequency, exhibiting ~80% efficiency of Rb recombination in the pancreas (Fig.S1A), and had an average lifespan. No abnormalities in the pancreatic cytoarchitecture were observed (Fig.S1B). Thus, as in mouse models null for other pancreatic TSGs,14,18–21 RB inactivation per se does not affect pancreatic development or induce neoplasia.
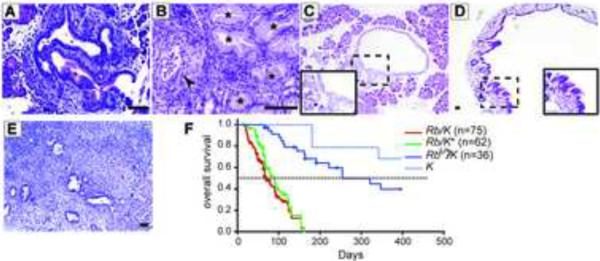
Although no phenotype was detected at embryonic day 15 and postnatal day 1, as early as postnatal week 1, pancreatic Rb deletion concomitant with KrasG12D (Rb/K mice) activation led to the development of high-grade PanIN (Fig. 1A). By 2 weeks, low and high-grade PanIN (Fig. 1B) were frequent, often occurring in conjunction with low- and high-grade cystic neoplasms (Fig. 1C–D), and one of 5 mice developed PDAC (Fig. 1E). Nearly 20% of Rb/K mice died during the first month of life, displaying severe cachexia and atrophic pancreata. Histological analysis of 10 such pancreata, collected from moribund mice, showed both low- and high-grade lesions, and large cysts, suggesting that these mice were developing PDAC and cystic changes. Overall, Rb/K mice had a median survival of ~10 weeks (Fig. 1F). Excluding animals that died during the first month of life and had no necropsy (12 mice) did not significantly alter the Kaplan-Meier survival plot (Fig. 1F).
Figure 1.
Rb/K animals develop early pancreatic lesions.
(A) One week-old mouse: PanIN-3. (B–E) Two week-old mice: (B) low- (*) and high-grade PanIN with microinvasion (arrowhead); (C) MCN-like and (D) IPMN-like cystic neoplasms; (E) PDAC. (F) Kaplan-Meier survival curves: Rb/K mice have a shorter lifespan (red line) compared to RbL/+/K (blue line) and K mice (dashed blue line). Rb/K survival was similar when mice that died within postnatal month 1 without necropsy were excluded (green line). Survival for K mice was derived from a previous study.8 Scale bars, 50 μm.
In mice expressing oncogenic Kras with Rb haploinsufficiency (Pdx1-Cre;LSL-KrasG12D;RbL/+, RbL/+/K mice) PanIN and cystic neoplasms progressed slowly, and median survival increased to 36 weeks (Fig. 1F and Table 1). Nonetheless, compared to Pdx1-Cre;LSL-KrasG12D mice (termed K), PanIN progression in RbL/+/K mice was faster, cyst formation was more common (Fig. S2), and survival was greatly reduced (Table 1 and Fig. 1F).8,14 Although Pdx1-Cre is also expressed in the duodenum, only low to moderate dysplasia, manifested by the presence of pseudostratified epithelium and rare luminal mitosis, was observed in the duodenal mucosa of Rb/K animals (not shown).
Table 1.
PDAC progression in the absence of one or 2 alleles of Rb
| Genotype | ADM | Cysts | PanIN1 | PanIN2 | PanIN3 | PDAC |
|---|---|---|---|---|---|---|
| Pdx1-Cre;LSL-KrasG12D;RbL/L | ||||||
| 2–3 weeks (n=5) | 5/5 | 2/5 (1*) | 5/5 | 1/5 | 1/5 | 1/5 |
| 1 month (n=11) | 10/11 | 6/11 (2*, 1**) | 7/11 | 4/11 | 3/11 | 1/11 |
| 2 months (n=10) | 9/10 | 8/10 (7*) | 10/10 | 5/10 | 3/10 | 3/10 |
| 3 months (n=13) | 11/13 | 8/13 (6*, 1**) | 12/13 | 8/13 | 7/13 | 6/13 |
| 4–5 months (n=6) | 6/6 | 3/6 (2*, 1**) | 6/6 | 4/6 | 4/6 | 4/6 |
|
| ||||||
| Pdx1-Cre;LSL-KrasG12D;RbL/+ | ||||||
| 2–3 months (n=7) | 6/7 | 3/7 | 7/7 | 2/7 | 0/7 | 0/7 |
| 4–5 months (n=10) | 10/10 | 1**/10 | 10/10 | 4/10 | 0/10 | 0/10 |
| 6–8 months (n=5) | 5/5 | 2/5 | 5/5 | 4/5 | 2/5 | 1/5 |
|
| ||||||
| Pdx1-Cre;LSL-KrasG12D | ||||||
| 2 months (n=8) | 4/8 | 0/8 | 4/8 | 0/8 | 0/8 | 0/8 |
| 2–4 months (n=16) | 12/16 | 2/16 | 15/16 | 2/16 | 1/16 | 1/16 |
| 4–6 months (n=19) | 13/19 | 0/19 | 19/19 | 2/19 | 0/19 | 0/19 |
| 6–10 months (n=16) | 15/16 | 3/16 | 16/16 | 1/16 | 1/16 | 1/16 |
Cyst diameter between 1–5 mm
cyst diameter > 5 mm
note that none of the cysts observed in Pdx1-Cre;LSL-KrasG12D mice are MCNs.
Histologic characteristics of pancreatic lesions in Rb/K mice
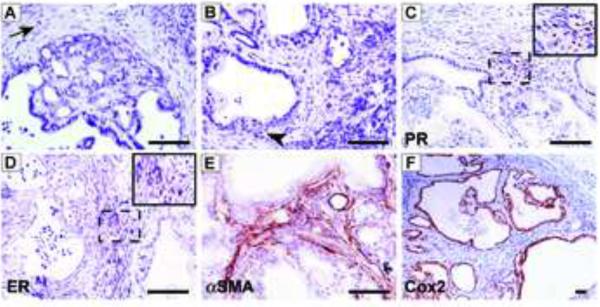
Akin to humans,22 PanIN in Rb/K mice developed in a peripheral location that did not initially involve the main duct or its large branches. These PanIN were CK19-positive and accumulated mucins as evidenced by Alcian blue staining (Fig. S3A–B). Wnt and Notch signaling pathways were upregulated, as shown by strong β-catenin and Hes1 immunoreactivity, respectively (Fig. S3C–D), and increased Sonic Hedgehog (Shh) expression was also observed (not shown). Rb/K mice frequently developed pancreatic cystic neoplasms (Table 1). Most of these cysts displayed mucin-rich and CK19-positive columnar epithelium (Fig. S3A–B) with low to high-grade dysplasia (Fig. 1C, 2A–B), and were surrounded by an “ovarian-like” stroma with spindle-shaped cells expressing progesterone (PR) and estrogen (ER) receptors (Fig. 2C–D). Thus, these cysts were similar to mucinous cystic neoplasia (MCN) in humans.23,24 The stroma also harbored activated stellate cells that were rich in α-smooth muscle actin (Fig. 2E). Wnt and Notch pathways were also activated in these MCN-like lesions, (Fig. S3C–D) and Cox2 was increased (Fig. 2F).
Figure 2.
Characteristics of epithelial and stromal compartments of MCN-like lesions.
(A) Low- to moderate-grade lesions in Rb/K mice display disorganized epithelia, without clear nuclear atypia, and are surrounded by hypercellular stroma (arrow). (B) High-grade lesions display nuclear atypia and loss of basement membrane (arrowhead). (C–E) Surrounding stroma expresses (C) PR, (D) ER and (E) αSMA. (F) Lesions overexpress Cox2 (F). Scale bar, 50 μm.
Three of 40 mice had a single, large cyst (~1.0 cm in diameter) in the head of the pancreas, in close proximity to the biliary duct from which it appeared to derive (not shown). These displayed papillary epithelium with nuclear stratification, minimal to moderate dysplasia (Fig. 1D), and stroma devoid of ER and PR (not shown), reminiscent of intraductal papillary mucinous neoplasia (IPMN) in humans.23 In one case, the IPMN-like lesion occurred in conjunction with PanIN-2 and -3, and MCN-like lesions.
PanIN and MCN-like lesions in Rb/K mice rapidly progress to PDAC
While normal acinar and ductal cells were not proliferative, there was a marked increase in proliferation in all PanIN in Rb/K pancreata: 62.9%+/−2% and 76.7%+/−4% of cells were Ki67-positive in PanIN-1 (Fig. S3E) and PanIN-2/-3, respectively. By contrast, proliferation of low-grade PanIN in K mice is only 16%+/−1.8%.8 In another mouse model, MCN-like lesions exhibited increased proliferation indices (2 to 10%).20 In Rb/K pancreata, however, this figure was 57%+/−2% (Fig. S3E), and foci of acinar-to-ductal metaplasia (ADM) were also proliferative (22%+/−2%). Inasmuch as RB controls cell proliferation by inhibiting the G1-S transition,25 these observations suggest that RB attenuates oncogenic Kras-activated mitogenic pathways in the pancreas.
In Rb/K mice, invasive PDAC developed with high frequency ranging from 20% (n=5) at 2 weeks to ~70% (n=6) at 4–5 months. Microinvasion was observed in association with both high-grade PanIN and MCN, as evidenced by basement membrane disruption (Fig. 1B, 2B) and CK19-expressing dysmorphic cells within the adjacent stroma (Fig. S4A–B), indicating that both lesions could progress to cancer. Altogether, 14/40 Rb/K mice developed PDAC (Table 1), which were highly proliferative with a collagen-rich stroma (Fig. S4C–D). Thus, aside from the absence of metastases, PDAC in Rb/K mice recapitulated many histologic features of human PDAC. RbL/+/K mice also developed PDAC but at very low frequency (1/37 after 12 months).
Rb/K mice develop marked inflammatory infiltrates
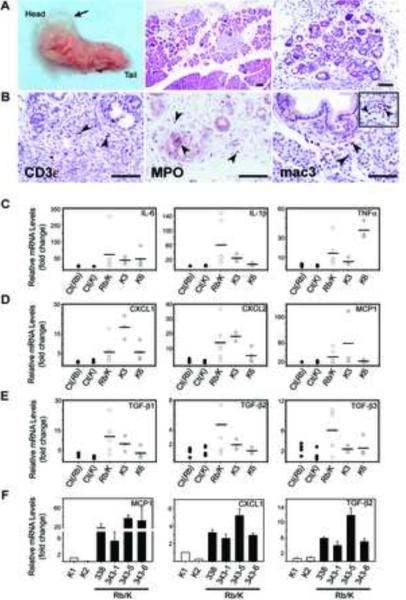
Marked inflammatory infiltrates were observed in 1/5 pancreata of 1 week-old Rb/K mice (not shown), and 3/5 pancreata of 2 week-old mice, with occasional foci of ADM (Fig. 3A). These pancreata exhibited marked edema with separation of lobules, and harbored many macrophages (Mac3-positive) both near and away from neoplastic lesions (Fig. 3B). T cells (CD3ε-positive) and neutrophils (MPO-positive) were also abundant (Fig. 3B), but B cells were absent (Pax5-negative, not shown). Although inflammatory changes were less frequent by postnatal week 8, some mice still exhibited inflammatory infiltrates and, occasionally, gross edema (Fig. 3A). Edematous changes and marked inflammation were never observed at early stages in K or RbL/+/K mice (Table S1), suggesting that full inactivation of Rb was required to induce the inflammatory reaction.
Figure 3.
Rb/K pancreata display acute pancreatic inflammation.
(A) Two month-old mouse (left panel): gross edema. (A–B) Two week-old mice: (A) diffuse (middle panel) and focal inflammation around neoplasms and ADM foci (middle and right panels); (B) CD3ε-positive T cells, MPO-positive neutrophils, and Mac3-positive macrophages adjacent to PanIN, and in “normal-appearing” pancreas (inset). Scale bar, 50 μm. (C–E) Pro-inflammatory cytokines are up-regulated in 3 week-old Rb/K pancreata (open circles), and 3- (K3) and 6 (K6) month-old K mice (shaded circles) compared to their littermate controls (black circles, Ct(Rb) and Ct(K)). Horizontal bars denote mean expression levels. (F) MCP1, CXCL1 and TGF-β2 mRNA levels were markedly elevated in all Rb/K (solid bars) compared to K cells (open bars).
Cytokine expression was examined next in 3 week-old Rb/K pancreata. When compared with littermate controls (including RbL/L and Pdx1Cre;RbL/L), the mRNA levels for the pro-inflammatory cytokines IL-6, IL-1β, TNF-α, CXCL1, CXCL2 and MCP1, were highly elevated (Fig. 3C–D). Additionally, the immune modulating growth factors, TGF-β1, -β2 and -β3 were up-regulated in 50 to 60% of Rb/K pancreata (Fig. 3E). These cytokines were also elevated in K pancreata compared to their littermate controls (Fig. 3C–E), but only at stages where low-grade lesions (3 months) and pronounced ADM-associated inflammation (6 months) are common (Table S1 and Fig. S2). On average, cytokine levels were similar in K and Rb/K pancreata. However, 1/3 to 1/6 of the Rb/K mice exhibited dramatic increases in IL6, IL-1β and CXCL2, which correlated with the presence of extensive inflammation histologically (not shown). TGF-β1, -β2 and -β3 levels were also higher in Rb/K pancreata.
To study the possibility that the inflammation was directly related to RB deletion in the cancer cells, two primary cell lines were established from 2 month-old Rb/K pancreata (Rb/K338 and 343). Several clones (Rb/K343-1 to -6) were isolated from Rb/K343 cells. As controls, we generated cell lines from K pancreata (K1 and K2). RB was absent in all Rb/K cell lines (Fig. S5A–C) and KrasG12D expression was confirmed in all cell lines. Although all cell lines formed tumors in nude mice, except for K2 cells, none were derived from grossly visible tumors suggesting that they originated from early stage malignancies. All experiments were performed on early passage cells (<12),8,14,20,21 to minimize alterations in culture. In Rb/K cells, CXCL1, MCP1, and TGF-β2 mRNA levels were increased 4-fold, 5- to 35-fold, and 7-fold respectively, compared to K cells (Fig. 3F). The levels of TGF-β1, -β3 and other assayed cytokines were similar between Rb/K and K cells, whereas IL-6 and IL-1β levels were very low in all cell lines.
Rb deletion leads to a bypass of KrasG12D-induced senescence
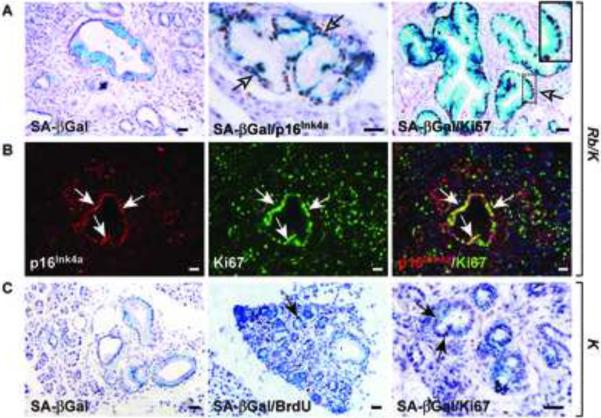
Ras-asscociated oncogenic stress can lead to OIS,26 a process that causes irreversible cell cycle arrest. Given the involvement of RB in senescence,27,28 we next examined senescence status in Rb/K and K pancreata. Senescence-associated β-Galactosidase (SA-βGal), an early senescence marker, and p16Ink4a, an essential component of senescence, were detected in PanIN-1 in both Rb/K and K mice. (Fig. 4A–C). Not all PanIN displayed SA-βGal activity, and adjoining cells were always negative, underscoring SA-βGal staining specificity. Dramatically, in Rb/K pancreata, SA-βGal-and p16Ink4a-positive PanIN-1 cells were highly proliferative, as evidenced by frequent overlap with Ki67 immunoreactivity (Fig. 4A–B). Indeed, the vast majority of SA-βGal-expressing cells co-expressed Ki67. By contrast, in K pancreata only rare proliferating cells were detected in PanIN-1, and none of them expressed SA-βGal (Fig. 4C) or p16Ink4a (not shown). Interestingly, p16Ink4a was elevated in all grade PanIN and MCN-like lesions in Rb/K mice, but was markedly decreased in proliferating PDAC cells (Fig. S6A–C), indicating that p16Ink4a is lost during progression from PanIN-3 to PDAC.
Figure 4.
Senescence is bypassed in Rb/K pancreata.
(A) Senescence-associated β–Galactosidase (SA-βGal) activity (left panel) co-localizes (arrows) with p16Ink4a (center panel) and Ki67 (right panel) in low-grade Rb/K PanIN. (B) Most p16Ink4a-expressing cells (red) co-express (arrows) Ki67 (green) as shown by the overlay (right panel). (C) In K pancreata, low-grade PanIN display SA-βGal activity (left panel), but rare BrdU incorporation (center panel), and Ki67 expression (right panel, arrows). Scale bar, 50 μm.
No consistent increases in the expression of the putative senescence markers Dec1 and DcR2 were observed in PanIN-1 in either mouse model, nor were there differences in trimethylated histone K9 immunoreactivity, a marker of heterochromatization (not shown). Given these inconclusive results, microarray studies were carried out to compare the expression of senescence-associated genes in Rb/K and K cells. There were highly significant, 10-, 7-, 25- and 5.3-fold increases in the mRNA levels encoding p19ARF, insulin-like growth factor binding protein 7 (IGFBP7), caveolin-1 (CAV1) and p15, respectively, in proliferating Rb/K cells, all of which have been associated with cellular senescence (Fig S7).29–32 These observations suggest that in Rb/K mice, senescence is initiated, but cells do not arrest and, instead, escape senescence and exhibit increased proliferation.
Rb deletion in the presence of oncogenic Kras leads to dysregulation of p53 function
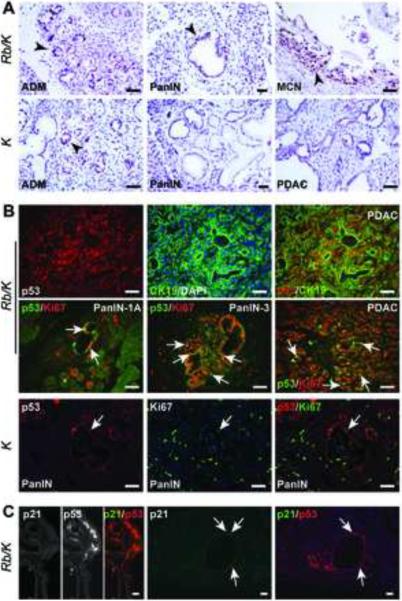
p53 is a crucial mediator of senescence and cell cycle arrest that is activated in response to diverse cellular stresses.33–35 Therefore, we evaluated p53 expression in Rb/K and K pancreata, and in derived cell lines. Strong, nuclear p53 immunoreactivity was present in all lesions (Fig. 5A) and PDAC (Fig. 5B) in Rb/K pancreata, frequently overlapping with Ki67 (Fig. 5B). By contrast, K pancreata had significantly less p53-positive cells (Fig. S8A), where it was primarily confined to non-proliferative ADM foci and stromal cells (Fig. 5A–B). p21CIP1/WAF1 (p21), a key p53 target that elicits growth inhibition and a senescence response,36 was present at very low levels in Rb/K pancreata, infrequently in neoplastic cells, and rarely co-localizing with p53 (Fig. 5C), suggesting that the low levels of p21 in Rb/K pancreata derived through p53-independent mechanisms.37,38
Figure 5.
RB loss together with KrasG12D activation causes p53 accumulation.
(A) In Rb/K pancreata, p53 (arrowheads) is abundant in ADM, PanIN and MCN-like lesions, but only in ADM foci in K pancreata. (B) Double immunofluorescence reveals that p53-expressing cells (red) are mainly epithelial (CK19-positive; green), and are highly proliferative (Ki67-positive; green) in low- and high-grade PanIN, and PDAC (arrows exemplify co-expression). In K pancreata (lower panels), p53 expression (red) is rare, and does not overlap (arrows) with Ki67 (green). (C) p21 expression is very low in lesions and rarely overlaps with p53 (arrows). The exposure time for p21 is 3-fold longer than for p53. Scale bar, 25 μm.
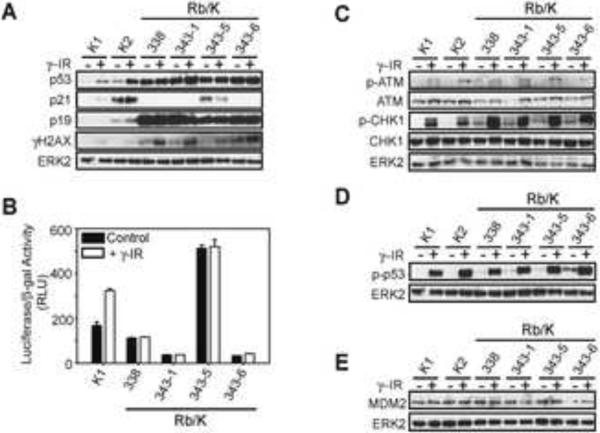
To further delineate p53 dysregulation in Rb/K pancreata, p53 and p21 expression was examined in Rb/K cell lines. In spite of high p53 levels, p21 mRNA levels were markedly decreased in Rb/K cells (Fig. S8B), and p21 protein was evident in only one cell line (Rb/K343-5; Fig. 6A). Following DNA damage induction by γ-irradiation, the expected increase in p53 and p21 was seen in K, but not Rb/K cells (Fig. 6A). Low p21 levels in the face of high p53 suggested that p53 might be transcriptionally inactive. Indeed, with the exception of Rb/K343-5, all Rb/K cell lines had low basal p53 transcriptional activity, which failed to increase following γ-irradiation, whereas K1 cells exhibited increased activity (Fig. 6B). None of the 7 Rb/K cell lines harbored a mutated p53 gene, as determined by cDNA sequencing. It was important, therefore, to confirm the loss of p53 transcriptional activity. Accordingly, we assessed the expression levels of several p53 target genes in Rb/K cells, including p21, Mdm2, and pro-apoptotic and pro-survival genes, many of which were significantly down-regulated (Fig. S8B–D).
Figure 6.
p53 is dysfunctional in Rb/K cells.
(A) Western blots on Rb/K and K lysates show that p53 and p19ARF are highly elevated in Rb/K cells, and p21 is not induced by γ-irradiation in Rb/K cell lines (except Rb/K343-5). Following γ-irradiation, γ-H2AX, a DNA damage marker, increases in all cell lines; (B) p53 transcriptional activity is very low in Rb/K cell lines except Rb/K343-5; (C) DNA damage response pathways are induced by γ-irradiation in K and Rb/K cells; (D) γ-irradiation induced p53 phosphorylation (serine 18); (E) MDM2 levels are similar in all cell lines.
To determine whether the DNA damage response pathways that activate p53 were functional, we next examined γH2AX, phospho-ATM (S1981) and phospho-CHK1 (S345) expression following γ-irradiation. Post-irradiation all three markers were increased in Rb/K and K cells (Fig. 6A;C), in conjunction with increased phosphorylation of p53 on Ser18 (Fig. 6D). Thus, Rb/K and K cells maintained an intact DNA damage response upstream of p53.34
We next compared p53 turnover in K and Rb/K cells. In K cells, activated p53 protein was below the level of detection within 45 min of cycloheximide (2 μg/ml) addition, whereas in Rb/K cells p53 levels remained markedly elevated even after a 6 hour incubation with 10 μg/ml cycloheximide (not shown), underscoring the marked p53 stability in Rb/K cells. Inasmuch as p19ARF is a major regulator of p53 protein stability, preventing Mdm2 from targeting p53 for degradation, we next compared the expression of p19ARF and Mdm2 in these cells. Although Mdm2 levels were similar in Rb/K and K cells at the protein and RNA level (Fig. 6E and S8B), in congruence with the array results (Fig. S7), p19ARF was elevated in Rb/K cells (Fig. 6A). Thus, given the normal p53 mRNA levels (Fig. S8E), p53 accumulation in Rb/K cells could be explained by p19ARF-mediated sequestration of Mdm2 and subsequent p53 protein stabilization.
The loss of p53 transcriptional activity suggested that p53-dependent DNA repair mechanisms could also be altered in Rb/K cells, potentially leading to chromosomal instability (CIN). Moreover, impaired p53 function has been reported to lead to CIN in another PDAC model.21 All Rb/K cell lines displayed an increased number of centrosomes and aberrant spindle formation in mitotic cells whereas none of the K cells showed >2 centrosomes in any mitotic cell (Fig. S9A). Abnormal mitotic events were also observed in advanced PDAC in Rb/K pancreata.(Fig. S9A). Moreover, SKY analysis showed that Rb/K cells had major chromosomal disruption, including amplification and translocation, and the presence of centric fragments (Fig. S9B). Thus, in the presence of oncogenic Kras, RB loss leads to CIN.
Discussion
A crucial feature of neoplastic transformation is loss of cell-cycle control. Active, hypo-phosphorylated RB is a key tumor suppressor that prevents cell-cycle progression,39,40 and alterations in the RB pathway have been identified in nearly every human malignancy.41 Multiple pathways regulate RB phosphorylation and modulate its activity.42 While the presence of oncogenic Kras could inactivate RB and enhance transformation, in some cell types, ras-induced oncogenic transformation requires RB.43 Given these divergent possibilities, and the need to assess the role of RB in PDAC, we examined the consequence of Rb deletion on Kras-induced pancreatic carcinogenesis.
In this study, RB inactivation alone did not affect pancreas development, nor induce pancreatic neoplastic transformation. However, concomitant Kras activation resulted in rapid development of PanIN and MCN-like cystic papillary neoplasms, which occurred in conjunction with marked pancreatic inflammation, pancreatic atrophy, rapid progression to PDAC at a high frequency, and a median survival of 10 weeks. Thus, Rb/K mice exhibit one of the most lethal phenotypes of all PDAC mouse models,8,14,18–21,44 underscoring RB's tumor suppressive role in the pancreas. RbL/+/K animals also displayed accelerated lesion progression and decreased survival. However, there was rare progression to PDAC, indicating that loss of both Rb alleles, and therefore full inactivation, is required to maximally promote Kras-driven oncogenesis. Signaling pathways that have been reported as being activated in human PanIN, such as Wnt, Notch and Shh, were upregulated in PanIN and MCN-like neoplasms in Rb/K mice, underscoring the relevance of the Rb/K model to human PDAC. Moreover, MCN was previously characterized in only one mouse model.19 Thus, Rb/K mice would also allow for improved studies of the pathobiology of MCN.
The acute inflammation in Rb/K mice was associated with increased expression of pro-inflammatory cytokines, including IL-6, which helps recruit neutrophils and facilitates Kras-mediated neoplastic transformation in various cell types by promoting cell survival,45 and IL-1β and TNF-α, which contribute to neutrophil activation.46 These cytokines were also expressed at high levels in K pancreata, but only in older mice, and they were never associated with edematous changes, as observed in 1 month-old Rb/K pancreata. Moreover, only Rb/K cells expressed high levels of CXCL1, TGF-β2 and MCP-1. CXCL1 and TGF-βs are neutrophil chemoattractants,46 and MCP1, which is also produced by injured acinar cells during the early stages of acute pancreatitis, is a potent monocyte chemoattractant.47 Thus, Rb/K cells produce and potentially release pro-inflammatory cytokines, a hallmark of a senescence-associated secretory phenotype (SASP).48,49 These findings suggest that in Rb/K mice, transformed cells produce high levels of chemokines that recruit inflammatory cells, and induce a highly pro-inflammatory microenvironment, leading to the observed pancreatitis-like changes and abundant inflammation.
We and others have shown that acute and chronic pancreatitis promote cancer progression in oncogenic Kras-driven PDAC mouse models.50–53 Moreover, inflammation and neoplastic transformation often appeared concomitantly in Rb/K mice, suggesting that these processes were interdependent. Given the importance of inflammation in cancer progression, Rb/K mice are an excellent model for investigating the relationship between oncogenic Kras, pancreatic inflammation, and PDAC initiation and progression.
Just as low-grade PanIN in Rb/K pancreata exhibited senescence markers (p16Ink4a and SA-βGal) and enhanced proliferation, proliferating Rb/K cells displayed activation of a senescence program, evidenced by increased p19ARF, IGFBP7, caveolin-1 and p15 expression. Increased expression of p19Arf was most likely due to deregulated E2F activity in the absence of RB, which could sequester Mdm2 and impede p53 degradation.54 Moreover, in Rb/K cells, p53 was phosphorylated on Ser18 in the absence of exogenous stress and caveolin-1 was overexpressed, and both alterations disrupt p53:Mdm2 interactions.29,55 These observations may explain why Rb/K cells exhibited increased p53 protein levels but normal p53 RNA levels.
In spite of high p53 levels in Rb/K cells, and the absence of mutations in its coding region, p53 was dysfunctional and could neither enforce senescence nor suppress proliferation. Several lines of evidence support this conclusion, and indicate that these perturbations were also present in Rb/K pancreata. First, p53 did not activate transcription in a reporter assay, and analysis of a panel of p53 target genes demonstrated that several classes of these genes were down-regulated in Rb/K cells. Second, Rb/K cells exhibited chromosomal instability (CIN), consistent with defective DNA repair observed in the absence of functional p53. Third, p21 expression was markedly attenuated in Rb/K lesions and rarely co-localized with p53, which correlated with low, un-inducible p21 expression in vitro. By contrast, in fibroblasts, RB loss up-regulates p21, which cooperates with p16Ink4a to preserve oncogenic ras-induced senescence.28 Silencing of p21 in these cells induces senescence bypass that is not compensated for by the RB family members, p107/p130.28 Thus, it is likely that senescence escape in early PanIN lesions in Rb/K mice is aborted due to the failure of p53 to up-regulate p21.
It is not clear whether p53 is dysfunctional due to failure to bind DNA and recruit transcriptional partners, or due to repressive post-translational modifications. Nonetheless, despite the absence of RB and dysfunctional p53, p16Ink4a inactivation was still required for progression to PDAC. Although it is generally assumed that the main role of p16Ink4a is to regulate RB, these data argue that p16Ink4a and RB have unique and independent tumor suppressor functions.
In summary, our study reveals several previously unappreciated functions of RB in PDAC progression. In the presence of an activated KrasG12D allele, RB prevents neoplastic cells from bypassing senescence and impedes inflammatory alterations that occur as a result of SASP. Moreover, RB promotes p53 functions to attenuate tumor progression. These findings underscore the importance of devising strategies that target RB pathway disruptions56 in PDAC, and indicate that the Rb/K mouse model could be useful for screening drugs designed to activate p53, and/or prevent pancreatic inflammation and cancer progression.
Supplementary Material
Acknowledgement
This research was supported by U.S. Public Health Service Grant CA-R37-075059, awarded by the National Cancer Institute to M. K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict of interest to disclose for all authors.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hansel DE, Kern SE, Hruban RH. Molecular Pathogeneis of Pancreatic Cancer. Annu Rev Genomics Hum Genet. 2003;4:237–256. doi: 10.1146/annurev.genom.4.070802.110341. [DOI] [PubMed] [Google Scholar]
- 4.Friend SH, Bernards R, Rogelij S, Weinberg RA, Rappaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 5.Lee WH, Shew JY, Hong FD, Sery TW, Donoso LA, Young LJ, Brookstein R, Lee EY. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987;329:642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- 6.Preis M, Korc M. Kinase signaling pathways as targets for intervention in pancreatic cancer. Cancer Biol Ther. 2010;9 doi: 10.4161/cbt.9.10.11534. [DOI] [PubMed] [Google Scholar]
- 7.Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG. Overexpression of Cyclin D1 in Human Pancreatic Carcinoma Is Associated with Poor Prognosis. Cancer Res. 1997;57:1634–1637. [PubMed] [Google Scholar]
- 8.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 9.Marino S, Vooijs M, van der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 10.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 11.Carrière C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci. 2007;104:4437–4442. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleeff J, Korc M. Up-regulation of Transforming Growth Factor (TGF)-β Receptors by TGF-β1 in COLO-357 Cells. J Biol Chem. 1998;273:7495–7500. doi: 10.1074/jbc.273.13.7495. [DOI] [PubMed] [Google Scholar]
- 13.Seeley ES, Carrière C, Goetze T, Longnecker DS, Korc M. Pancreatic Cancer and Precursor Pancreatic Intraepithelial Neoplasia Lesions Are Devoid of Primary Cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han JH, Stratowa C, Rutter WJ. Isolation of Full-Length Putative Rat Lysophospholipase cDNA Using Improved Methods for mRNA Isolation and cDNA cloning. Biochemistry. 1986;26:1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- 16.Chang S-C, Brannon PM, Korc M. Effects of Dietary Manganese Deficiency on Rat Pancreatic Amylase mRNA Levels. J Nutr. 1990;120:1228–1234. doi: 10.1093/jn/120.10.1228. [DOI] [PubMed] [Google Scholar]
- 17.Neupane D, Korc M. 14-3-3σ Modulates Pancreatic Cancer Cell Survival and Invasiveness. Clin Can Res. 2008;14:7614–7623. doi: 10.1158/1078-0432.CCR-08-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, Hanahan D, Redston MS, Chin L, Depinho RA. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–46. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA, Hingorani SR. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–43. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Hruban RH, Rustgi AK, Brentnall TA, Tempero MA, Wright CV, Tuveson DA. Pancreatic cancer in mice and man: the Penn Workshop 2004. Cancer Res. 2006;66:14–7. doi: 10.1158/0008-5472.CAN-05-3914. [DOI] [PubMed] [Google Scholar]
- 23.Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: Demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatology. 2004;2:1026–1031. doi: 10.1016/s1542-3565(04)00450-1. [DOI] [PubMed] [Google Scholar]
- 24.Yoshiaki M, Kenichiro U, Hiroki O, Yasuo H, Takeshi S, Taijiro S. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery. 2006;140:448–453. doi: 10.1016/j.surg.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67:293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- 26.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras Provokes Premature Cell Senescence Associated with Accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 27.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, Dickins RA, Narita M, Zhang M, Lowe SW. Dissecting the Unique Role of the Retinoblastoma Tumor Suppressor during Cellular Senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 Regulates the Antagonistic Pleiotropic Properties of Cellular Senescence through a Novel Mdm2/p53-Mediated Pathway. Cancer Research. 2009;69:2878–2886. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 31.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF Induces Senescence and Apoptosis through Pathways Mediated by the Secreted Protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in Senescence Induction by BRAF. Cell. 2010;141:746–747. doi: 10.1016/j.cell.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farnebo M, Bykov VJN, Wiman KG. The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun. 2010;396:85–89. doi: 10.1016/j.bbrc.2010.02.152. [DOI] [PubMed] [Google Scholar]
- 34.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 35.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 36.El-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, Wiman KG, Mercer WE, Kastan MB, Kohn KW, Elledge SJ, Kinzler KW, Vogelstein B. WAF1/CIP1 Is Induced in p53-mediated G1 Arrest and Apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 37.Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D. Induction of WAF1/CIP1 by a p53-independent Pathway. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 38.Johnson M, Dimitrov D, Vojta P,J, Barrett JC, Noda A, Pereira-Smith O,M, Smith J,R. Evidence for a p53-independent pathway for upregulation of SDI1/CIP1/WAF1/p21 RNA in human cells. Mol Carcinog. 1994;11:59–64. doi: 10.1002/mc.2940110202. [DOI] [PubMed] [Google Scholar]
- 39.Hatakeyama M, Weinberg RA. The role of RB in cell cycle control. Prog Cell Cycle Res. 1995;1:9–19. doi: 10.1007/978-1-4615-1809-9_2. [DOI] [PubMed] [Google Scholar]
- 40.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi A, Ohtani N, Hara E. Irreversibility of cellular senescence: dual roles of p16INK4a/Rb-pathway in cell cycle control. Cell Division. 2007;2:10. doi: 10.1186/1747-1028-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams JP, Stewart T, Li B, Mulloy R, Dimova D, Classon M. The retinoblastoma protein is required for ras-induced oncogenic transformation. Mol Cell Biol. 2006;26:1170–1182. doi: 10.1128/MCB.26.4.1170-1182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton JP, Jamieson NB, Karim SA, Athineos D, Ridgway RA, Nixon C, McKay CJ, Carter R, Brunton VG, Frame MC, Ashworth A, Oien KA, Evans TRJ, Sansom OJ. LKB1 Haploinsufficiency Cooperates With Kras to Promote Pancreatic Cancer Through Suppression of p21-Dependent Growth Arrest. Gastroenterology. 2010;139:586–597. e6. doi: 10.1053/j.gastro.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ancrile B, Lim K-H, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parekh T, Saxena B, Reibman J, Cronstein BN, Gold LI. Neutrophil chemotaxis in response to TGF-beta isoforms (TGF-beta 1, TGF-beta 2, TGF-beta 3) is mediated by fibronectin. J Immunol. 1994;152:2456–66. [PubMed] [Google Scholar]
- 47.Brady M, Bhatia M, Christmas S, Boyd MT, Neoptolemos JP, Slavin J. Expression of the chemokines MCP-1/JE and cytokine-induced neutrophil chemoattractant in early acute pancreatitits. Pancreas. 2002;25:260–269. doi: 10.1097/00006676-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Coppe J-P, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez P-Y, Campisi J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 50.Carrière C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res. Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, Bi Y, Grote T, Longnecker DS, Logsdon CD. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–82. 1082 e1–6. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–89. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–748. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- 55.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 56.Knudsen ES, Wang JYJ. Targeting the RB-pathway in Cancer Therapy. Clin Cancer Res. 2010;16:1094–1099. doi: 10.1158/1078-0432.CCR-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.