Abstract
Background
Hemoglobinopathies are among the most common inherited diseases around the world. They have become much more common recently in northern and central Europe, including Germany, due to immigration.
Method
Selective review of the literature with consideration of national guidelines.
Results
The hemoglobinopathies encompass all genetic diseases of hemoglobin. They fall into two main groups: thalassemia syndromes and structural hemoglobin variants (abnormal hemoglobins). α- and β-thalassemia are the main types of thalassemia; the main structural hemoglobin variants are HbS, HbE and HbC. There are many subtypes and combined types in each group. The highly variable clinical manifestations of the hemoglobinopathies range from mild hypochromic anemia to moderate hematological disease to severe, lifelong, transfusion-dependent anemia with multiorgan involvement. Stem-cell transplantation is the preferred treatment for the severe forms of thalassemia. Supportive, rather than curative, treatment consists of periodic blood transfusions for life, combined with iron chelation. Drugs to treat the symptoms of sickle-cell disease include analgesics, antibiotics, ACE inhibitors and hydroxyurea. Blood transfusions should be given only when strictly indicated. More than 90% of patients currently survive into adulthood. Optimally treated patients have a projected life span of 50 to 60 years.
Conclusion
Hemoglobinopathies are a public health issue in today’s multiethnic German population. Adequate care of the affected patients requires a wide variety of diagnostic and therapeutic measures.
With approximately 7% of the worldwide population being carriers, hemoglobinopathies are the most common monogenic diseases and one of the world’s major health problems (1, 2, e1, e2). They were originally found mainly in the Mediterranean area and large parts of Asia and Africa (3). International migration has spread them from those areas all over the world. In many parts of Europe today, hemoglobin (Hb) defects are classified as endemic diseases (3) (table 1).
Table 1. Prevalence of hemoglobinopathy gene carriers in the world’s population (1– 3, 6, e1, e2).
| Region | Gene carriers |
| Africa | 5 to 30% |
| Arab nations | 5 to 40% |
| Up to 60% regionally | |
| Central Asia and India | 10 to 20% |
| South-East Asia | 5 to 40% |
| Up to 70% regionally | |
| USA and Central America | 5 to 20% |
| Italy | 7 to 9% |
| Greece | 6 to 7% |
| Turkey | 7 to 10% |
| Germany, | Among total population: 0.5 to 1% |
| Great Britain, | Among immigrants: 5% |
| Portugal, | |
| Spain, | |
| France, | |
| the Netherlands, | |
| Belgium, | |
| Scandinavian countries | |
| Albania, | 2 to 5% |
| the former Yugoslavia, | |
| Croatia, | |
| Bosnia-Herzegovina, | |
| Bulgaria | |
| Russia | Rare |
| Transcaucasia | Up to 5% |
Germany is one of the countries in which hemoglobinopathies have increased in recent years (4– 7). There are no epidemiological studies on their frequency. The following statements can be made regarding gene carriers: prevalence among the 9 million immigrants from countries with a high risk of hemoglobinopathies as a whole is 4.5%, giving a figure of 400 000 for the number of hemoglobinopathy gene carriers (6). The total number of patients diagnosed with these diseases in the author’s laboratory from 1970 to 2010 is 5831.
This review article should be considered an extension of the original article “Hemoglobinopathies in Germany—a longitudinal study over four decades,” also published in Deutsches Ärzteblatt International (6). It aims to provide a brief summary of the most important clinical pictures and indicate the features that can be used to identify those with these diseases with low-level symptoms, but not gene carriers in good health, in general practice (Tables 2, 3). Topical grounds for the publication of this article are the increase in the number of people affected, which has implications for care provision, and the fact that optimum treatment can give patients a steadily-increasing projected life span. As a result, medical treatment is becoming more and more part of adult medicine, rather than pediatrics alone.
Table 2. Diagnoses, gene types, hematological findings, and cardinal symptoms of thalassemia syndromes (2, 4, 9).
| Inheritance status/diagnosis/phenotype | Arrangement of α-globin genes | Red blood cell count | Hemoglobin pattern | Cardinal symptoms |
| α-thalassemias | ||||
| Normal findings | αα/αα | Hb normal, MCH normal | Normal | No symptoms |
| Heterozygous α+-thalassemia | – α/αα | Hb normal, | Normal | No symptoms |
| = α-thalassemia minima | MCH <27 pg | Slight changes to blood count | ||
| Homozygous α+-thalassemia | – α/– α | Hb normal or low, | Normal | Mild anemia |
| = α-thalassemia minor | MCH <26 pg | Significant changes to blood count | ||
| Heterozygous α0-thalassemia | – –/αα | Hb normal or low, | Normal | Mild anemia |
| = α-thalassemia minor | MCH <24 pg | Significant changes to blood count | ||
| Mixed heterozygosity, | – –/– α | Hb 8 to 10 g/dL, | HbH ≈ 10 to 20% | Variable chronic hemolytic anemia |
| α + /α 0 -thalassemia | MCH <22 pg | |||
| = HbH disease | ||||
| Homozygous α0-thalassemia | – –/– – | Hb <6 g/dl, | Hb Bart’s 80 to 90%, | Life-threatening fetal anemia |
| = Hb Bart’s hydrops fetalis | MCH <20 pg | Hb Portland ≈ 10 to 20%, | Generalized hydrops | |
| HbH <1% | ||||
| β-thalassemias | ||||
| Heterozygous β-thalassemia | β++ | Hb ♂ 9 to 15 g/dL | HbA2 >3.2% | Mild anemia |
| = β-thalassemia minor | β + | Hb ♀ 9 to 13 g/dL | HbF 0.5 to 6% | |
| β 0 | MCV 55 to 75 fl | |||
| MCH 19 to 25 pg | ||||
| Homozygous β-thalassemia | β+/β+ | Hb <7 g/dl | HbA2 variable | Severe illness with long-term |
| = β-thalassemia major | β 0 /β 0 | MCV 50 to 60 fl | HbF 70 to 90% | transfusion-dependent anemia |
| Compound heterozygous | β + /β 0 | MCH 14 to 20 pg | ||
| β-thalassemia | ||||
| = β-thalassemia major | ||||
| Mild homozygous or compound | β+/β+ | Hb 6 to 10 g/dL | HbA2 variable | Moderate disease |
| heterozygous β-thalassemia | β + /β ++ | MCV 55 to 70 fl | HbF up to 100% | Variable transfusion dependency |
| = β-thalassemia intermedia | β + /β 0 | MCH 15 to 23 pg | ||
| β 0 /β 0 + influential factors | ||||
TABLE 3. Diagnoses, gene types, hematological findings, and cardinal symptoms of the main hemoglobin disorders (2, 3, 10, 13).
| Diagnosis | Gene type | Red blood cell (RBC) count | Hemoglobin pattern | Cardinal symptoms |
| Sickle-cell disease | HbSS | Hb 6 to 9 g/dL | HbS = 55 to 90% | Sickle-cell crises/pain crises |
| Normochromic sickle cells | HbA2 >3.5% | Acute organ syndromes | ||
| Positive hemolysis parameters | HbF = <10 to >20% | Chronic hemolytic anemia | ||
| HbS heterozygosity | HbAS | Normal | HbS = 35 to 40% | No apparent illness |
| HbA2 ≥ 3.5% | ||||
| Sickle-cell β+-thalassemia | HbS β+-thalassemia | Hb 9 to 12 g/dL | HbS >55% | Variable, mild sickle-cell disease |
| Hypochromia, microcytosis | HbF >20% | |||
| HbA2 >3.5% | ||||
| Sickle-cell β0-thalassemia | HbS β0-thalassemia | Hb 6 to 10 g/dL | HbS >80% | Severe sickle-cell disease |
| Hypochromia, microcytosis | HbF <20% | |||
| HbA2 >3.5% | ||||
| HbSC disease | HbSC | Hb 10 to 13 g/dL | HbS ≈ 50% | Weak symptoms of sickle-cell disease |
| Target cells | HbC ≈ 50% | Chronic hemolytic anemia | ||
| MCV <75 fl | HbF <5% | |||
| HbC disease | HbCC | Hb 10 to 12 g/dL | HbC >95% | Pain crises |
| Target cells | HbA2 ≈ 2.5% | Organ events | ||
| MCV <75 fl | HbF ≈ 0.5% | Chronic hemolytic anemia | ||
| MCHC >35 g/dL | ||||
| HbC heterozygosity | HbAC | Normal | HbC ≈ 50% | No apparent disease |
| HbA ≈ 47% | ||||
| HbA2 = 3% | ||||
| HbE heterozygosity | HbAE | Hb normal or slightly low | HbE = 25 to 35% | Mild, hypochromic anemia |
| Hypochromia | ||||
| HbE disease | HbEE | Hb 10 to 14 g/dL | HbE >95% | Mild anemia |
| High RBC count | HbA2 ≈ 2.5% | Hemolysis caused by infections/ medical drugs | ||
| MCH 20 pg | HbF <3% | |||
| MCV 65 fl | ||||
| Target cells | ||||
| HbE β+-thalassemia | HbE β+-thalassemia | Hb low to varying degree | HbE + HbA2 = 25 to 80% | Variable, intermediate, |
| Hypochromia | HbF = 6 to 50% | hypochromic anemia | ||
| Microcytosis | HbA = 5 to 60% | |||
| HbE β0-thalassemia | HbE β0-thalassemia | Hb <8 g/dl | HbE up to 85% | As for β-thalassemia major |
| MCV <60 fl | HbA2 <5% | |||
| MCH <22 pg | HbF = 15 to 25% | |||
| Hemoglobinopathies with unstable Hb | HbX = approximately 150 different variants HbX/HbA | Hb variable to significantly‧anemic; | HbX ≈ 20% | Variable, sometimes transfusion-dependent chronic hemolytic anemia |
| Heinz bodies; | HbA2 ≈ 3 to 4% | |||
| Hemolysis caused by viral infections/medical drugs | HbF <5% | |||
| Abnormal hemoglobins with disruptions to O2 transportation function | Multiple variants | Polycythemia | Varies according to type of abnormality | Congenital cyanosis with HbM abnormalities |
| Increased MetHb | Congenital polycythemia with Hb abnormalities with high O2 affinity |
Indications for hemoglobin testing
Hemoglobin testing is particularly indicated in the following situations (8, 9):
Microcytic hypochromic anemia after iron deficiency has been ruled out
Chronic hemolytic anemia
Vascular obliteration crises of unclear etiology in patients from areas in which HbS and/or HbC is widespread
Drug-induced anemia
Erythrocytosis and/or cyanosis caused by hematological factors
Hydrops fetalis of unclear etiology
Prevention (testing of family members, diagnosis of partners for genetic counseling)
Prenatal diagnosis.
Generalized hemoglobin electrophoresis for all cases of anemia cannot be justified on the basis of expediency or financial considerations, particularly in those with no background of migration.
Diagnosis
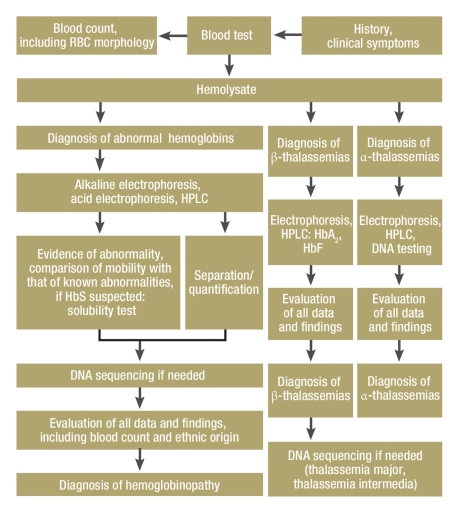
Hemoglobinopathy diagnosis in routine practice involves a red blood cell (RBC) count with erythrocyte indices, and a hemoglobin test (hemoglobin electrophoresis and/or chromatography) (Tables 2, 3). Specialized tests in facilities qualified for the purpose are often required (6, 9, 11, e3, e4). The eFigure shows a testing plan for step-by-step diagnosis and also includes indications for DNA testing.
eFigure.
Indications for DNA testing
Hb abnormalities:
To identify rare abnormalities; for clarification in the absence of electrophoretic or chromatographic separation; as part of genetic enquiries (families, partners, prenatal diagnosis); mixed forms of more than one hemoglobinopathy.
Thalassemias:
To determine the genetic type of β-thalassemia major; molecular diagnosis of β-thalassemia intermedia; mixed forms of hemoglobinopathy; suspected silent β-thalassemia gene carriers; diagnosis of α-thalassemias; as part of genetic enquiries (families, partners, prenatal diagnosis).
RBC, red blood cell
Basic types of hemoglobinopathy
The umbrella term “hemoglobinopathy” includes all genetic hemoglobin disorders. These are divided into two main groups as follows:
Thalassemia syndromes
Structural hemoglobin variants (abnormal hemoglobins).
Both are caused by mutations and/or deletions in the α- or β-globin genes. When gene defects cause Hb synthesis disorders, this gives rise to thalassemia. Hemoglobin structure in these cases is normal. When they cause changes in Hb structure, this gives rise to abnormal hemoglobin (5, 6, 11). There are also many mixed forms that combine features of both groups, e.g. β0/β+-thalassemias, HbSC disease and HbE α-thalassemias. The common features of the pathophysiology and various disease patterns are limited, and as a result so are the possibilities for summarizing them.
Thalassemia syndromes
This term includes all thalassemic Hb synthesis disorders (table 2). These are autosomal recessive conditions. α- and β-thalassemias have the greatest clinical significance (5, 11, 12).
Heterozygous thalassemia carriers are not completely healthy: they always have symptoms that require clarification with mild, iron-refractory, microcytic hypochromic anemia. Homozygous major forms are accompanied by serious, hypochromic hemolytic anemias and complex diseases.
α-thalassemias
α-thalassemias are caused by an α-globin chain synthesis defect. At the molecular level, they result from partial (α+) or total (α0) deletions, or more rarely mutations, of one or more of the four α-globin genes (αα/αα). They occur mainly in Africa, Arab nations, and, more frequently, South-East Asia (5).
Diagnostic criteria and cardinal symptoms: there are four clinical pictures of α-thalassemia, according to the number of genes affected by loss of function (5, 12). All of them become manifest perinatally (table 2):
Clinically inapparent α-thalassemia minima (heterozygous α+-thalassemia, -α/αα). This can be identified on the basis of mild hypochromia revealed in a blood count, with a barely measurable reduction in Hb values.
α-thalassemia minor (heterozygous α0-thalassemia, –/αα, or homozygous α+-thalassemia, -α/-α) with mild anemia, hypochromia, microcytosis.
HbH disease (compound heterozygous α+/α0-thalassemia with three inactive α-genes, –/-α), moderate hypochromic hemolytic anemia with splenomegaly. Anemic crises are caused by viral infections and oxidants (drugs). Complications include cardiac problems, gallstones, lower leg ulcers, and folic acid deficiency.
Hb Bart’s hydrops fetalis (homozygous α0-thalassemia) with very serious hemolytic anemia already present in utero and marked by a lack of any α-globin chain synthesis (–/–), with hydrops and ascites. This is fatal if not treated.
β-thalassemias
β-thalassemia syndromes (table 2) are the result of insufficient (β+) or absent (β0) production of β-globin chains. Their molecular causes are β-globin gene mutations. Most patients come from Mediterranean countries, South-East Europe, Arab nations, and Asia. Hematological changes become manifest from between the ages of three months and six months onwards (5, 6, 13).
Diagnostic criteria and cardinal symptoms:
Thalassemia minor (heterozygous β-thalassemia) with mild, microcytic hypochromic anemia (2)
Thalassemia intermedia (mild homozygous or mixed heterozygous β-thalassemia) of moderate severity and with a varying need for transfusions; typical complications are skeletal deformities and tumorous masses as a result of massive hyperplastic erythropoiesis (2)
Thalassemia major (severe homozygous or mixed heterozygous β-thalassemia) (13) with long-term, transfusion-dependent anemia (table 4); untreated children die before the age of 10. Thalassemia major entails a risk of iron overload and multiorgan involvement. As a result of treatment, the full clinical picture is no longer seen in Germany (2, 13). Optimally treated patients have a projected life span of 50 to 60 years.
Table 4. Initial diagnosis and schedule for monitoring as part of transfusion and iron removal therapy for β-thalassemia major (2, 20).
| When | From age | |
| Initial diagnosis and counseling | ||
| – Complete blood count | ||
| – Ferritin, transferrin saturation | ||
| – Hemolysis parameters | ||
| – Hb testing, DNA testing if needed | On diagnosis | |
| – Blood groups | ||
| – Family appraisal | ||
| – Information on the disease | ||
| – Genetic counseling | ||
| – Complete blood count | Before and after each transfusion | |
| – Antibody detection test | Every two months | 1 year onwards |
| – Serology: hepatitis B/C, HIV, CMV | Annual status assessment | 1 year onwards |
| Iron metabolism | ||
| – Ferritin, transferrin saturation | At every transfusion | 1 year onwards |
| Liver function, liver iron | ||
| – GOT, GPT, γGT, bilirubin (total/direct) | Annual status assessment | 1 year onwards |
| – Albumin, cholinesterase, Quick | Annual status assessment | 1 year onwards |
| – Liver iron (biopsy, MRI) | Biannual status assessment | 10 years onwards |
| Cardiology | ||
| – Echocardiography | Annual status assessment | 1 year onwards |
| – ECG, long-term ECG | Annual status assessment | 1 year onwards |
| – Cardiac MRI (if possible), chest X-ray | Annual status assessment | Adulthood |
| Endocrinology | ||
| – Percentile growth curve | Every transfusion | 1 year onwards |
| – Puberty stages, bone age/mineralization | Annual status assessment | 10 years onwards |
| – Testosterone/estradiol, LH, FSH, prolactin, cortisol | Annual status assessment | 13 to 15 years onwards |
| – Oral glucose tolerance test | Annual status assessment | 10 years onwards |
| – Combined pituitary function test | Annual status assessment | 10 years onwards |
| – Thyroid parameters | Annual status assessment | 10 years onwards |
| – Serum calcium, phosphate | Monthly | 10 years onwards |
Abnormal hemoglobins
This group of autosomal dominant inherited hemoglobin disorders is caused by structural defects resulting from an altered amino acid sequence in the α or β chains (3, 10, 14). Clinicians must distinguish between clinically harmless Hb abnormalities and those that cause illness (table 3). These latter are divided into the following four well-defined groups:
Variants with a tendency to aggregate and with sickle cell formation, e.g. the sickle syndromes (14)
Variants with abnormal hemoglobin synthesis, e.g. HbE (2, 10)
Variants with a tendency to precipitate and with hemolysis (unstable hemoglobins), e.g. Hb Köln (15)
Variants with abnormal oxygen transportation and congenital polycythemia, e.g. Hb Johnstown (16, 17), or with congenital cyanosis (abnormal methemoglobins, HbM abnormalities, e.g. M Iwate) (2).
The forms in the third and fourth groups cause serious illness when heterozygous. When homozygous, they are fatal.
The main Hb abnormalities, both worldwide and among immigrants living in Germany, are HbS, HbC, and HbE. The large groups of rare Hb abnormalities that occur only in isolated cases all over the world should also be monitored. These are often accompanied by hemolysis, polycythemia, and/or cyanosis. Identifying these is an important part of differential diagnosis of hematological diseases where other efforts towards diagnosis have proved inconclusive (3, 6, 10, 11).
HbS and sickle-cell disease
The term “sickle-cell disease” includes all manifestations of abnormal HbS levels (proportion of HbS >50%). These include homozygous sickle-cell disease (HbSS) and a range of mixed heterozygous hemoglobinopathies (HbS/β-thalassemia, HbSC disease, and other combinations) (14).
According to the International Nomenclature, the previously commonly-used term “sickle-cell anemia” should not be used, as the dominant aspects of the disease are vascular obliterations and the organ damage they cause, not anemia.
HbS is the most dangerous of all hemoglobinopathies. The sickle cells caused by a lack of oxygen lead to vascular obliterations, so infarctions with tissue death can occur in almost all organs (skin, liver, spleen, bone, kidneys, retina, CNS). Chronic hemolytic anemia can usually be well tolerated (14). Aplastic crises are seen with severe anemia following viral infections (2).
Diagnostic criteria and cardinal symptoms: Symptoms begin before the age of one, with chronic hemolytic anemia and developmental disorders (table 3). The main problems are pain crises (sickle-cell crises) that can affect the back, extremities, thorax, abdomen, and CNS in particular. Patients are also dangerously susceptible to infection, particularly by pneumococci, hemophilus, salmonellae, klebsiellae, and mycoplasmae. Sepsis, osteomyelitis, and meningeal events, sometimes with cardiac involvement, frequently result in death. Spleen crises, acute thoracic syndrome (ATS), and strokes are also fatal in more than a few cases. These issues cause serious organ damage.
Optimally treated patients can have a projected life span of 50 to 60 years.
Heterozygous HbS gene carriers are not affected clinically or hematologically (18).
HbC abnormality and HbC disease
HbC homozygosity, or HbC disease, progresses in a similar way to sickle-cell disease, but is less serious (2, 3). Variable hemolytic anemia is the most dominant form. Heterozygous HbC gene carriers enjoy complete clinical health (table 3).
HbE abnormality and HbE disease
HbE is an extremely common Hb variant native to South-East Asia. Its disease pattern is similar to that of β-thalassemias. Hb is also unstable, which means that hemolysis can be caused by viral infections and medications (table 3). HbE is often combined with thalassemias, which may result in serious major-form hemoglobinopathies (2, 3, 10).
HbE homozygosity (HbE disease): Typically, this is moderate, microcytic hypochromic anemia with possible hemolysis due to exogenous causes.
HbE heterozygosity: Patients have variable hypochromic anemia similar to that found in β-thalassemia minor.
Treatment for β-thalassemias
β-thalassemia major
After being diagnosed, patients should be referred to a hematology center for counseling and to decide on treatment, and, if appropriate, for regular diagnosis appraisal (table 4).
The current international standard treatment (18– 20) is based on the results of studies conducted at large sites in England (5) and the USA (3) and is stated in available child and adolescent medicine guidelines (20) (AWMF/II/025–017.htm).
Curative treatment: Hematopoietic stem-cell transplantation is the first-line treatment if a donor can be found (21).
Supportive treatment: Supportive treatment for thalassemia major includes lifelong regular transfusions combined with effective iron removal (20). Hemosiderosis-related organ damage requires specific treatment (22, 23).
Transfusion therapy: Repeat hemoglobin concentration levels of less than 8 g/dL are an indication for beginning transfusion therapy. The target baseline hemoglobin level is 9 to 10.5 g/dL. The recommended frequency of transfusions is usually one every three weeks. Transfusion volume is usually 12 to 14 mg/kg body weight (BW) with an RBC concentrate hematocrit of 60%. Target Hb levels are 13 to 13.5 g/dL.
-
Drug treatment to remove iron (chelation therapy): Iron removal is indicated when serum ferritin concentration repeatedly exceeds 1000 ng/mL (20).
Iron removal using deferoxamine: Standard deferoxamine treatment is a daily subcutaneous infusion (over several hours) at a dose of (20) – 40 – (50) mg/kg BW, 5 to 7 days per week. Dose adjustment is based on monthly testing of serum ferritin concentration. Patients must be closely monitored for potential side effects of deferoxamine (reduced growth, bone damage, high-frequency hearing loss, retinal damage) (20).
Iron removal with deferasirox: Deferasirox is a well-tolerated iron chelator in tablet form and has taken on a central role in iron removal therapy (22, 23). However, this is a relatively new drug of which no long-term studies have been conducted. A standard dose of 20 mg/kg BW/day is recommended for patients with β-thalassemia major who are receiving long-term transfusion therapy. This dose must be adjusted on the basis of monthly measuring of ferritin levels. The main side effects (close monitoring required!) are kidney failure, agranulocytosis, and liver failure (22, 23). Liver iron levels must be measured every two years (table 4).
Splenectomy is indicated for tumorous enlargement of the spleen with increased need for transfusions and hypersplenism.
β-thalassemia intermedia
Transfusion therapy is indicated for patients with complications as a result of major increases in erythropoiesis, and patients with anemia and an inability to maintain stable hemoglobin levels of more than 8 g/dL. In such cases, lifelong continuous transfusion therapy should be considered, rather than transfusions at intervals, combined with appropriate chelation therapy (24).
β-thalassemia minor
For severe anemia, folic acid supplements (0.5 mg/day orally) may be considered (2). Iron supplements are contraindicated unless there is simultaneous iron deficiency.
Treatment for α-thalassemias
α-thalassemia minima and minor do not require treatment. Iron supplements are contraindicated (except in cases of iron deficiency) (20).
Treatment for HbH disease depends on the severity of the clinical picture, which can vary widely. Transfusions are rarely indicated. Anemia requires regular substitution with folic acid (e.g. 5 mg/week) (2, 20). Iron supplements are contraindicated (unless there is simultaneous iron deficiency).
For Hb Bart’s syndrome, transfusions are required in utero and continuously after birth. Where possible, stem-cell transplantation is performed (12, 20).
Treatment for sickle-cell disease
Following diagnosis, patients should be referred to a hematology center for counseling and to decide on treatment, and, if appropriate, for regular diagnosis appraisal (table 5). The current standard treatment (18) is based on the results of studies conducted at large sites in England (5, e6, e7) and the USA (14) and is stated in available guidelines (18) (AWMF/II/025–016.htm).
Table 5. Initial diagnosis and schedule for long-term monitoring of patients with sickle-cell disease (2, 18).
| When | |
| Initial diagnosis | |
| – Complete blood count | |
| – Hb testing | |
| – DNA testing if needed | |
| – Ferritin, transferrin saturation | On diagnosis |
| – Blood groups | |
| – Hemolysis parameters | |
| – Family appraisal | |
| – Information on the disease | |
| – Genetic counseling | |
| Hematological tests | |
| – Complete blood count | At every doctor’s appointment |
| – Ferritin, transferrin saturation | Annually |
| – Red blood cell antibodies | Before each transfusion |
| General clinical examination | |
| – Before the age of 6 months | Monthly |
| – 6 months to 1 year | Every two months |
| – 1 to 5 years | Every three months |
| – 5 years onwards, adults | Every four months |
| Examinations of specific organs | |
| Liver/gall bladder | |
| – Liver function | Annually |
| – Hepatitis: antibodies, antigen | Annually |
| – Ultrasound | Biannually |
| Kidneys | |
| – Urine testing | Annually |
| – Urea, serum creatinine | Annually |
| – Ultrasound (from 10 years onwards) | Annually |
| Heart | |
| – ECG | Biannually |
| – Echocardiogram | Biannually |
| Lungs (from 5 years onwards) | |
| – Chest X-ray | Biannually |
| – Lung function test | Biannually |
| – Blood gas test | Biannually |
| Eyes (from 10 years onwards) | |
| – Background | Annually |
| – Vision | Annually |
| Endocrinology | |
| – Thyroid | Annually |
| – Gonads | Annually |
Curative treatment
Allogeneic stem-cell transplantation may be used for children under 16 (21, e9). Indications are CNS infarction, particularly severe, frequent pain crises, or frequent occurence of acute thoracic syndromes. For older patients, transplantation is usually not an option due to a lack of donors and the high risks of transplantation.
Symptomatic treatment
Analgesics: first-line treatment for pain crises consists of sufficient administration of fluids and analgesics appropriate to the level of pain (paracetamol, metamizol, possibly also codeine or tramal, or even morphine).
With proteinuria above 0.5 g/24 hours, ACE inhibitors can inhibit progression of glomerulonephritis or glomerulosclerosis.
Antibiotics are also administered, particularly for pneumococcal infection with suspected sepsis and for salmonella infection with suspected osteomyelitis.
Hydroxyurea (18, e7, e8) is the only substance to date that can reduce the number and severity of pain crises (in 70% to 75% of patients) and decrease the number of episodes of acute thoracic syndrome and mortality. The initial dose of 15 mg/kg/day can be increased to 35 mg/kg/day. Due to severe side effects (18), sickle-cell patients may only be treated with hydroxyurea when it is strictly indicated, following patient education, if birth control is used (for women of reproductive age), with regular blood counts (initially every two weeks and then monthly), and with careful documentation of side effects. Side effects include cytopenia revealed in a blood count, hyperpigmentation, weight gain, opportunistic infections, azoospermia in approximately 80% of men (even years after the end of treatment), and marked hypomagnesemia. It is also believed that there is a teratogenic effect (18).
Transfusion therapy is subject to strict indications (18). Unfortunately, these rules are often broken. Single transfusions are indicated for major splenic sequestration, aplastic crises, and acute thoracic syndrome, as well as before major surgery (Hb must be increased to 10 g/dL!). Partial exchange transfusions to reduce the proportion of HbS are indicated for acute organ failure or vascular occlusions, but rarely for refractory pain crises.
The main indication for long-term transfusion programs (to maintain low proportions of HbS in the blood long-term) is a CNS infarction. Sickle-cell patients receiving frequent transfusions must receive chelation therapy.
Splenectomy: homozygous sickle-cell diseases lead to spleen sclerosis and functional asplenia even in childhood. Patients with HbS β-thalassemia undergo splenectomy following splenic sequestrations or in the event of hypersplenism.
Prevention
Affected children must receive all vaccinations recommended by STIKO, Germany’s Standing Vaccination Commission, and 7-valent pneumococcal vaccinations from the age of two months onwards (also see guidelines).
Prophylactic penicillin must also be administered from the age of three months onwards, for at least five years.
The most important guidelines for psychosocial treatment are stated in the eTable.
eTable. Psychosocial support for patients with hemoglobinopathies.
| Main elements | |
| On diagnosis |
|
| On beginning iron chelation therapy |
|
| During adolescence |
|
| On career selection and incorporation into the workplace |
|
| Continuously |
|
Evidence available on treatment for hemoglobinopathies
The evidence available in the literature is consistent and ranges from meta-analyses of controlled studies to field reports (5, 7, 13, 14). The evidence supports stem-cell transplantation (21), transfusion therapy (5, 7), and treatment for secondary hemosiderosis (22, 23) to treat thalassemias; and symptomatic treatment as a whole, including hydroxyurea treatment, to treat sickle-cell disease (8, 14, 25).
Conclusion
The projected life span and quality of life of patients with severe hemoglobin disorders can be significantly improved using advanced treatment methods. In contrast to other European countries, in Germany there is unfortunately still no comprehensive overall approach to optimum diagnosis and treatment strategies. One problem that has barely been addressed is continued treatment after patients have moved from pediatric or adolescent care to adult care. It would be particularly desirable to establish hemoglobinopathy care centers at which interdisciplinary teams care for patients, as happens in neighboring countries (25).
Key Messages.
The umbrella term “hemoglobinopathy” includes all genetic hemoglobin disorders.
The two main groups are thalassemia syndromes and structural Hb variants (abnormal hemoglobins). The main types of thalassemia are α- and β-thalassemia. The main types of abnormal hemoglobin are HbS, HbE, and HbC. Within these main types there are several subtypes, with differing disease patterns.
Nowadays more than 90% of patients survive into adulthood. Optimally treated patients can have a projected life span of 50 to 60 years.
Stem-cell transplantation is the first-line treatment for β-thalassemia major. Conventional treatment consists of lifelong transfusions, consistent iron chelation therapy, and long-term follow-up care.
Treatment for sickle-cell disease focuses on treatment for pain crises/sickle-cell crises using effective analgesics and administration of fluids, and on the consequent antibiotic treatment of infections. Hydroxyurea treatment successfully reduces crises in many patients. Red blood cell transfusions are subject to strict indications. Organ damage must be treated as patients increase in age.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
The author declares that no conflict of interest exists.
References
- 1.Weatherall DJ. Hemoglobinopathies worldwide: Present and future. Curr Mol Med. 2008;8:592–599. doi: 10.2174/156652408786241375. [DOI] [PubMed] [Google Scholar]
- 2.Kleihauer E, Kohne E, Kulozik AE. Anomale Hämoglobine und Thalassämie-Syndrome Grundlagen und Klinik. Landsberg: Ecomed Verlagsgesellschaft; 1996. [Google Scholar]
- 3.Steinberg MH, Forget BG, et al., editors. Disorders of hemoglobin: genetics, pathophysiology and clinical management. Cambridge University Press; 2001. [Google Scholar]
- 4.Kulozik AE. Hemoglobinopathies are on the increase. Dtsch Arztebl Int. 2010;107:63–64. doi: 10.3238/arztebl.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weatherall DJ, Clegg JB. The thalassaemia syndromes. 4th Edition. Oxford: Blackwell Science Ltd; 2001. [Google Scholar]
- 6.Kohne E, Kleihauer E. Hemoglobinopathies in Germany—a longitudinal study over four decades. Dtsch Arztebl Int. 2010;107:65–72. doi: 10.3238/arztebl.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cario H, Stahnke K, Sander S, Kohne E. Epidemiological situation and treatment of patients with thalassemia major in Germany: results of the German multicenter-thalassemia study. Ann Hematol. 2000;79:7–12. doi: 10.1007/s002770050002. [DOI] [PubMed] [Google Scholar]
- 8.Dickerhoff R, von Rücker A, Maschmeyer G, Heimpel H. Probleme erwachsener Sichelzellpatienten in Deutschland. Dtsch med Wschr. 2009;134:1179–1184. doi: 10.1055/s-0029-1222586. [DOI] [PubMed] [Google Scholar]
- 9.Thomas L, editor. Labor und Diagnose. 8th edition. Frankfurt: TH-Books Verlagsgesellschaftt; 2011. Hämoglobinopathien. [Google Scholar]
- 10.Weatherall DJ, Clegg JB, Higgs DR, Wood WG. The hemoglobinopathies. In: Scriver CR, Beauder AL, Sly WS, Valle D, Graw-Hill Mc, editors. The metabolic basis of inherited disease. 8th edition. New York: 2000. [Google Scholar]
- 11.Herklotz R, Risch L, Huber AR. Hämoglobinopathien - Klinik und Diagnostik von Thalassämien und anomalen Hämoglobinen. Therapeutische Umschau. 2006;1:35–46. doi: 10.1024/0040-5930.63.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Higgs DR, Weatherall DJ. The Alpha Thalassaemias. Cell Mol Life Sci. 2009;66:1154–1162. doi: 10.1007/s00018-008-8529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivieri NF. The ß-Thalassemias. N Engl J Med. 1999;341:99–109. doi: 10.1056/NEJM199907083410207. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg MH. Sickle cell anemia, the first molecular disease: Overview of molecular etiology, pathophysiology, and therapeutic approaches. Sci world J. 2008;8:1295–1324. doi: 10.1100/tsw.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohba Y. Unstable hemoglobins. Hemoglobin. 1990;14 doi: 10.3109/03630269009031998. [DOI] [PubMed] [Google Scholar]
- 16.Petrides PE, Beykirch MK, Kohne E. The high oxygen-affinity Hemoglobin Johnstown 109 (G11) ValLeu in a German kindred with an elevated erythrocyte hemoglobin conten: Potential interaction with HFE mutation. Blood Cells Mol Dis. 2008;40(2):180–182. doi: 10.1016/j.bcmd.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Wajcman H, Galactéros F. Hemoglobins with high oxygen affinity leading to erythrocytosis, new variants and new concepts. Hemoglobin. 2005;29:91–106. [PubMed] [Google Scholar]
- 18.Dickerhoff R. Sichelzellkrankheit. Leitlinien Kinderheilkunde und Jugendmedizin. 2006;I 1:1–7. [Google Scholar]
- 19.Kulozik AE. ß-Thalassämie: Bewährtes und Neues in der Diagnostik und Therapie. Mschr Kinderheil. 1996;144:850–862. [Google Scholar]
- 20.Cario H, Kohne E. ß-Thalassämie, a-Thalassämie. Leitlinien Kinderheilkunde und Jugendmedizin. 2006;I 2a, I 2b:1–11. 1–3. [Google Scholar]
- 21.Storb RF, Lucarelli G, McSweeney PA, Childs RW. Hematopoietic cell transplantation for benign hematological disorders and solid tumors. Hematol (Am Soc Hematol Educ Program) 2003:372–397. doi: 10.1182/asheducation-2003.1.372. [DOI] [PubMed] [Google Scholar]
- 22.Gattermann N. The treatment of secondary hemochromatosis. Dtsch Arztbl Int. 2009;106:499–504. doi: 10.3238/arztebl.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cario H, Janka-Schaub G, et al. Recent developments in iron chelation therapy. Klin Päd. 2007;219:158–165. doi: 10.1055/s-2007-973845. [DOI] [PubMed] [Google Scholar]
- 24.Taher A, Isma´eel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006;37:12–20. doi: 10.1016/j.bcmd.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Sickle Cell Society, www.sicklecellsociety.org. London: Standards for the clinical care of adults with sickle cell disease in the UK 2008. [Google Scholar]
- E1.Loukopoulos D, Kollia P. Worldwide distribution of beta-thalassemia. In: Steinberg MH, Forget BG, et al., editors. Disorders of hemoglobin: genetics, pathophysiology and clinical management. Cambridge University Press; 2001. [Google Scholar]
- E2.Giordano PC, Harteveld CL, Heister AJGM, Batelaan D, van Delft P, Plug R, Losekoot M, Bernini LF. The molecular spectrum of beta-thalassemia and abnormal hemoglobins in the allochthonous and autochthonous dutch population. Community Genet. 1998;1:243–251. doi: 10.1159/000016170. [DOI] [PubMed] [Google Scholar]
- E3.Kohne E. Diagnostik von Hämoglobinopathien. J Lab Med. 2004;28:400–409. [Google Scholar]
- E4.Huber AR, Ottiger C, Risch L, Regenass St, Hergersberg M, Herklotz R. Thalassämie-Syndrome: Klinik und Diagnose. Schweiz Med Forum. 2004;4:947–952. [Google Scholar]
- E5.Old JM. Screening and genetic diagnosis of haemoglobin disorders. Blood Reviews. 2003;17:43–53. doi: 10.1016/s0268-960x(02)00061-9. [DOI] [PubMed] [Google Scholar]
- E6.Amrolia PJ, Almeida A, Halsey C, et al. Therapeutic challenges in childhood sickle cell disease Part 1: current and future treatment options. Br J Haematol. 2003;120:725–736. doi: 10.1046/j.1365-2141.2003.04143.x. [DOI] [PubMed] [Google Scholar]
- E7.Amrolia PJ, Almeida A, Davies SC, et al. Therapeutic challenges in childhood sickle cell disease Part 2: a problem oriented approach. Br J Haematol. 2003;120:737–743. doi: 10.1046/j.1365-2141.2003.04144.x. [DOI] [PubMed] [Google Scholar]
- E8.Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358:1362–1369. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- E9.Iannone R, Ohene-Frempong K, Fuchs EJ, et al. Bone marrow transplantation for sickle cell anemia: Progress and prospects. Petiatr Blood Caner. 2005;44:436–440. doi: 10.1002/pbc.20169. [DOI] [PubMed] [Google Scholar]