Abstract
Salt-losing tubulopathies with secondary hyperaldosteronism (SLT) comprise a set of well-defined inherited tubular disorders. Two segments along the distal nephron are primarily involved in the pathogenesis of SLTs: the thick ascending limb of Henle’s loop, and the distal convoluted tubule (DCT). The functions of these pre- and postmacula densa segments are quite distinct, and this has a major impact on the clinical presentation of loop and DCT disorders – the Bartter- and Gitelman-like syndromes. Defects in the water-impermeable thick ascending limb, with its greater salt reabsorption capacity, lead to major salt and water losses similar to the effect of loop diuretics. In contrast, defects in the DCT, with its minor capacity of salt reabsorption and its crucial role in fine-tuning of urinary calcium and magnesium excretion, provoke more chronic solute imbalances similar to the effects of chronic treatment with thiazides. The most severe disorder is a combination of a loop and DCT disorder similar to the enhanced diuretic effect of a co-medication of loop diuretics with thiazides. Besides salt and water supplementation, prostaglandin E2-synthase inhibition is the most effective therapeutic option in polyuric loop disorders (e.g., pure furosemide and mixed furosemide–amiloride type), especially in preterm infants with severe volume depletion. In DCT disorders (e.g., pure thiazide and mixed thiazide–furosemide type), renin–angiotensin–aldosterone system (RAAS) blockers might be indicated after salt, potassium, and magnesium supplementation are deemed insufficient. It appears that in most patients with SLT, a combination of solute supplementation with some drug treatment (e.g., indomethacin) is needed for a lifetime.
Keywords: Bartter syndrome, Gitelman syndrome, Salt-losing tubulopathies, Classification, Prostaglandins, Secondary hyperaldosteronism, Differential diagnosis, Treatment
Introduction
Basic renal physiology and mechanisms of solute reabsorption in the distal nephron
The preservation of electrolyte homeostasis and thus water balance is vital to the entire organism. It is the primary responsibility of the kidney to maintain this vital milieu interior. The primary urine is formed by glomerular filtration. Because of their small size, salts fall through the glomerular filter and thus need to be reabsorbed in the renal tubule. Around one third of filtered salt load is reabsorbed in the distal nephron: about 25% in the thick ascending limb (TAL) of Henle`s loop (loop) and around 10% in the distal convoluted tubule (DCT) and the cortical collecting duct (CCD). The primary role of the TAL is the concentration of salt in the interstitium as a prerequisite for countercurrent exchange and the urinary concentration mechanism. This segment is practically impermeable to water and actively pumps large portions of sodium chloride out of the filtrate, generating the hypertonicity of the interstitium that drives countercurrent exchange. The DCT plays an important role in fine-tuning renal excretion not only of sodium chloride but especially of divalent cations such as calcium and magnesium. The DCT can be further subdivided into an early segment (DCT1), a late portion (DCT2), and the connecting tubule (CNT) that leads over to the CCD. These subsegments are characterized by the expression of different ion transport proteins responsible for salt and divalent cation reabsorption.
The reabsorption capacity of the total distal nephron needs to be regulated and even fine-tuned depending on nutritional intake and/or extrarenal losses of salt and water. One of the best-studied checkpoints of this fine-tuning process along the distal nephron is the macula densa (MD). It is a key player in coupling renal hemodynamics with tubular reabsorption in a way that enables chloride concentration monitoring in the tubular fluid and thereby provides a feedback mechanism that matches glomerular filtration with tubular salt load [tubuloglomerular feedback (TGF)]. Regulation of glomerular arterial resistance is achieved partly by modulation of the renin-angiotensin II system and intrarenal cyclo-oxygenase (COX)-2 activity [1–3]. Thus, genetic or acquired defects of distal tubular functions, which are quite specific and unique for individual nephron segments, will have a major impact on the clinical presentation of pre- and post-MD salt-loosing tubulopathies (SLTs) or of loop and DCT disorders, respectively. In the first case, the water-impermeable TAL – with its greater salt reabsorption capacity and herewith its crucial role in TGF – is impaired, leading acutely to major salt and water losses similar to the effect of high-ceiling loop diuretics. In the second case, DCT, with a minor capacity for salt reabsorption, is impaired, leading to extracellular volume depletion. For some time, this can be compensated for by hyperaldosteronism but at the expense of potassium imbalance.
Salt reabsorption in the thick ascending limb (TAL) of Henle’s loop (loop)
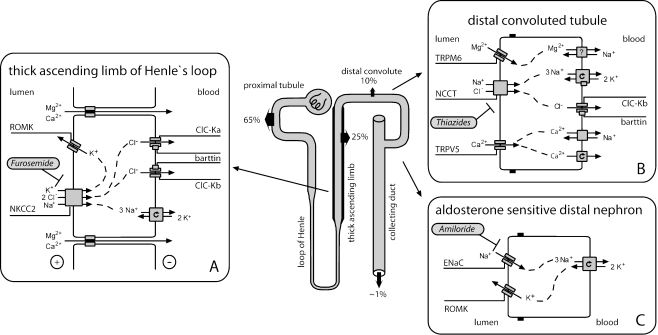
In the TAL, sodium and chloride are actively taken up into tubular cells via the electroneutral sodium–potassium-2-chloride co-transporter NKCC2 (encoded by the SLC12A1 gene) that is the target of loop diuretics such as furosemide (Fig. 1a). Sodium is then actively pumped out of the TAL cell by basolateral sodium–potassium–adenosine triphosphatase (Na-K-ATPase), whereas chloride leaves the cell basolaterally through specific chloride channels termed ClC-Ka and ClC-Kb (encoded by the CLCNKA and CLCNKB genes). The operation of both chloride channels is dependent on an accessory protein, the β-subunit barttin. In contrast, potassium is recycled across the apical membrane back into the tubular fluid through the potassium-permeable ion channel, the renal outer medullary potassium channel, Kir 1.1 (ROMK), encoded by the KCNJ1 gene). Active salt reabsorption thereby produces a lumen-positive transepithelial potential, which serves as the driving force for the passive paracellular reabsorption of calcium and magnesium in this tubular segment.
Fig. 1.
Salute reabsorption in the thick ascending limb (TAL) of Henle`s loop, the distal convoluted tubule (DCT), and the aldosterone-sensitive distal nephron (ASDN). In the TAL (a), sodium chloride is reabsorbed by furosemide-sensitive sodium–potassium-2-chloride co-transporter (NKCC2) together with potassium, which has to be recycled via the renal outer medullary potassium channel, Kir 1.1 (ROMK) into the tubular lumen. Calcium and magnesium are reabsorbed passively via the paracellular pathway driven by lumen-positive transepithelial potential. In the DCT (b), salt reabsorption occurs via thiazide-sensitive sodium cotransporter (NCCT). As in the TAL, sodium is extruded basolaterally by sodium–potassium–adenosine triphosphatase (Na-K-ATPase), and chloride leaves the cell through chloride channels (ClC). Reabsorption of magnesium and calcium in the DCT is active and transcellular in nature, consisting of uptake through selective ion channels (TRPM6 and TRPV5, respectively). In the ASDN (c), ROMK potassium channels – in addition to their role in the TAL – are essential for potassium ion secretion in exchange for the electrogenic reabsorption of sodium via amiloride-sensitive epithelial sodium channels (ENaC) under the influence of aldosterone
Solute reabsorption in the distal convoluted tubule (DCT)
As in the TAL, transepithelial salt transport requires the activity of the basolateral Na-K-ATPase (Fig. 1b). In the early DCT, the energy provided by the electrochemical gradient for sodium is utilized by apically expressed sodium chloride cotransporter NCCT (encoded by the SLC12A3 gene) for the uptake of chloride against its electrochemical gradient together with sodium. Chloride then passively exits the tubular cell, as in the TAL, through basolaterally expressed chloride channels (mainly ClC-Kb). Thiazide diuretics therapeutically inhibit the activity of NCCT. The NCCT is expressed mainly in DCT1 with gradually decreasing expression in DCT2 where its expression slightly overlaps with more distally located epithelial sodium channels (ENaC). Magnesium reabsorption in DCT is active and transcellular in nature and involves an apical entry into the DCT cell probably through a specific ion channel, which is formed by TRPM6, a member of the transient receptor-potential ion-channel family [4]. The process of basolateral extrusion is still unknown at the molecular level. As the hypocalciuria observed in patients with NCCT defects is also present in thiazide-treated TRPV5−/− mice, which lack the apical calcium channel in the DCT required for active calcium reabsorption, the hypocalciuria was attributed to an increase in calcium reabsorption in the proximal tubule during phases of volume depletion [5]. This mechanism was confirmed directly by micropuncture studies in this murine knockout model. At the same time, the authors of that study observed a critical down-regulation of TRPM6 expression as a possible mechanism for renal magnesium wasting in DCT disorders.
Salt reabsorption in the aldosterone-sensitive distal nephron (ASDN)
Unlike in the TAL and early DCT in which transcellular sodium reabsorption is directly coupled to chloride transport, in the more distally located nephron segments, sodium can also be reabsorbed separately from chloride through apically located amiloride-sensitive ENaC (Fig. 1c). Expression of these channels is under direct influence of aldosterone. Again, sodium uptake is driven by the action of basolateral Na-K-ATPase. For reasons of electroneutrality, each sodium ion that enters the tubular cell requires a secreted cation. Therefore, sodium reabsorption by ENaC is coupled to potassium secretion via ROMK potassium channels in the apical membrane of CCD cells.
Short historical overview and introduction of pharmacology-based classification
As Bartter’s group was the first to identify marked hyperaldosteronism in patients with SLTs and to recognize the contribution of this factor to the disordered potassium and acid-base homeostasis, the term Bartter syndrome (BS) was introduced for this condition [6]. The DCT variant of BS, later referred to as Gitelman syndrome (GS), began to be characterized when measurements of magnesium levels in blood and calcium levels in urine of young adult patients were introduced in the diagnostic workup of SLTs [7]. Hypomagnesemia can now be interpreted retrospectively as the cause of tetany, carpopedal spasms, and a positive Chvostek’s sign, already mentioned in Bartter’s index patients [6].
In contrast, the full-blown loop variant of BS was fatal until the mid-1980s with progress of neonatology. Before then, only incomplete phenotypes may have survived and/or could have been studied [8]. The detailed description of the complete clinical phenotype of a loop disorder stems from the pediatricians and neonatologists Ohlssen and Seyberth [9, 10], who highlighted the antenatal onset of the disease. Seyberth instituted the life-saving treatment with indomethacin as soon as the fatal role of dramatically elevated prostaglandin E2 (PGE2) formation was discovered in the sequelae of loop dysfunction. Thereafter, the term hyperprostaglandin E syndrome (HPS) was introduced [10, 11].
In the past 15 years, mutations in seven or more different genes have been identified as being responsible for SLTs. Besides careful clinical observations and innovative physiological concepts, molecular genetics and pharmacology have made this progress possible. Syndromic and genetic terminology, genes, affected gene products, and key features of clinical presentation are displayed in Table 1. This traditional terminology is based on the two major clinical syndromes: BS and GS, as well as on the chronology of their first genetic characterization. In addition, a pharmacology-based classification and pharmacotype terminology for SLTs were developed and introduced in 2008 [12]. This newer terminology is presented together with the affected tubular segments and the pharmacological classification in relation to the key features of clinical presentation in Table 2. This classification is based on three major subgroups of inherited SLTs. One is the thiazide-like DCT disorders, traditionally referred to as GS and classic BS or BS type III. A second is the more severe polyuric and furosemide-like loop disorders, traditionally referred to as antenatal BS/HPS or BS types I and II. The third is the combination of both tubular disorders, traditionally referred to as antenatal BS/HPS with sensorineural deafness (BSND), or BS type IV.
Table 1.
Syndromic and genetic terminology
| Syndromic terminology | Genetic terminology | Gene | Gene product affected | Key features of clinical presentationa |
|---|---|---|---|---|
| Bartter syndrome | ||||
| aBS/HPS | BS type I | SLC12A1 | NKCC2 | Polyhydram., polyuria, hypercalciuria, NC |
| aBS/HPS | BS type II | KCNJ1 | ROMK | Polyhydram., polyuria, hypercalciuria, NC, transient hyperkalemia |
| cBS | BS type II | CLCNKB | CIC-Kb | Hypochlor., mild hypomagnesemia, FTT in infancy |
| BSND | BS type IV | BSND | Barttin | Polyhydram., polyuria, hypochlor., hypomagnesemia, SND, CRF |
| ADH | BS type V | CASR | CaSR | Hypocalcemia, hypomagnesemia, (polyuria) |
| BSND | CLCNKA + B | CIC-Ka + b | Polyhydram., polyuria, hypochlor., hypomagnesemia, SND, CRF | |
| Gitelman syndrome | GS | SLC12A3 | NCCT | Hypomagnesemia, hypocalcuria, growth retardation |
| EAST syndrome | EAST syndrome | KCNJ10 | Kir 4.1 | Hypomagnesemia, hypocalcuria, EAST syndrome |
BS Bartter syndrome, aBS antenatal Bartter syndrome, HPS hyperprostaglandin E syndrome, cBS classic Bartter syndrome, BSND Bartter syndrome with sensorineural deafness, ADH autosomal dominant hypocalcemia, GS Gitelman syndrome, EAST syndrome, epilepsy, ataxie, sensorineural deafness, and tubulopathy, polyhydram polyhydramnios, NC medullary nephrocalcinosis, hypochlor. hypochloremia, FTT failure to thrive, NKCC sodium–potassium-2-chloride co-transporter SND sensorineural deafness, CRF chronic renal failure. ROMK renal outer medullary potassium channel, Kir 1.1, CaSR calcium-sensing receptor, CIC chloride channels, NCCT sodium chloride cotransporter
aHypochloremic alkalosis and hypokalemia is an ubiquitary finding and is therefore not mentioned separately
Table 2.
New terminology and pharmacological classification
| Type of disorder (gene product affected) | Affected tubular segment | Pharmacotype | Polyhydramnios | Key features of clinical presentationa |
|---|---|---|---|---|
| Loop disorders | ||||
| L1 type (NKCC2) | TAL | Furosemide type | +++ | Polyuria, hypercalciuria, NC |
| L2 type (ROMK) | TAL/CCDb | Furosemide-amiloride type | +++ | Polyuria, hypercalciuria, NC, transient hyperkalemia, |
| DCT disorders | ||||
| DC1 type (NCCT) | DCT | Thiazide type | – | Hypomagnesemia, hypocalciuria, growth retardation |
| DC2 type (ClC-Kb) | DCT/TALb | Thiazide-furosemide type | + | Hypochloremia, mild hypomagnesemia, FTT in infancy |
| DC3 type (Kir 4.1) | DCT | Thiazide type | – | Hypomagnesemia, hypocalciuria, EAST sydrome |
| Combined disorders | ||||
| L-DC1 type (ClC-Ka + b) | TAL + DCT | Furosemide-thiazide type | +++ | Polyuria, hypochloremia, mild hypomagnesemia, SND, CRF |
| L-DC2 type (barttin) | TAL + DCT | Furosemide-thiazide type | +++ | Polyuria, hypochloremia, mild hypomagnesemia, SND, CRF |
TAL thick ascending limb of Henle`s loop, DCT distal convoluted tubule, CCD cortical collecting duct, NC medullary nephrocalcinosis, FTT failure to thrive, EAST syndrome, epilepsy, ataxia, sensorineural deafness, and tubulopathy, SND sensorineural deafness, CRF chronic renal failure
aHypochloremic alkalosis and hypokalemia is an ubiquitary finding and is therefore not mentioned separately
bThis affected tubular segment is not of equal importance.
One of the advantages of the pharmacology-based classification system is that young medical students appear to be familiar with the mode of action and adverse reactions of the classical diuretics, such as loop diuretics, thiazides, and potassium-sparing diuretics [13–16]. Moreover, the pharmacology-based classification might not only be helpful in finding the most leading diagnostic criteria but might provide some useful therapeutic concepts. It is also hoped that this classification will cope with and be adapted accordingly to newer discoveries, such as entirely new functional proteins, functional consequences of loss-of-function mutation of critical gene products, or additional function of an affected gene product somewhere else in the nephron or in the body. Thus, this classification system and corresponding terminology based on pharmacology as well as anatomy and physiology is applied in addition to the traditional terminology throughout this review.
Distal nephron disorders
Loop disorders: a major subgroup of inherited SLTs
Clinical presentation is governed by global dysfunction of the TAL, the major part of the nephron’s urine concentration machinery, due its water impermeability and unique sodium chloride reabsorption abilities. Failures here lead inevitably to marked polyuria with all its consequences, especially in infancy and even in utero. Thus, the very first pathognomonic finding of a loop disorder is the development of a severe maternal polyhydramnios within the second trimenon due to fetal saluretic polyuria. The excessive amniotic fluid volume almost always leads to premature delivery in the first part of the third trimester of pregnancy.
Bartter syndrome type I, or pure furosemide type (L1 type), also referred to as antenatal Bartter syndrome, hyperprostaglandin E syndrome
The key symptoms besides life-threatening polyuria shortly after birth include isosthenuria or even hyposthenuria, hyperprostaglandinuria, and hypokalemic alkalosis [17]. Within the first weeks of life, nearly all patients develop medullary nephrocalcinosis in parallel with persistently high urinary calcium excretion. This phenotype mimics well the pharmacological profile of furosemide treatment in preterm infants [18]. As such, it was fitting that Lifton’s group identified mutations in the SLC12A1 gene coding for the furosemide target molecule, the furosemide-sensitive NKCC2 [19]. Thus, in pharmacology-based terminology, this tubulopathy is called the pure furosemide type of loop disorder [12, 15].
Bartter syndrome type II, or the mixed furosemide-amiloride type (L2 type), also referred to as antenatal Bartter syndrome, hyperprostaglandin E syndrome
In addition to NKCC2 mutations, ROMK defects are responsible for a typical loop disorder [20, 21]. The difference between these two disorders is a transient hyperkalemia within the first days of life of patients with this mixed type of loop disorder. This curious phenomenon reflects the contribution of ROMK to distal net potassium excretion [17]. Later, other CCD potassium channels apparently compensate, and patients become hypokalemic although significantly less severe compared with other patients with SLTs. This phenotypical characteristic matches well results observed with the popular diuretic regimen combining the strong saluretic and kaliuretic furosemide with the potassium-sparing amiloride.
DCT disorders, a major subgroup of inherited SLTs
Disorders of the DCT markedly differ from the above-described loop disorders in terms of age of onset, severity of clinical manifestations, absence of a major urinary concentrating defect, and associated electrolyte abnormalities. Tubular disorders affecting the DCT share with loop disorders the features of renin–angiotensin–aldosterone system (RAAS) activation and hypokalemia but exhibit hypomagnesemia and reduced urinary calcium excretions, as observed during long-term treatment with thiazides [22]. In contrast to the transepithelial salt transport, the etiology of the coexistence of hypomagnesemia and hypocalciuria is still not completely understood (see below). Whereas sodium enters the DCT cell together with chloride by the action of the thiazide-sensitive NCCT cotransporter, both ions leave the cell separately via Na-K-ATPase and ion channels (ClC-Kb), respectively (Fig. 1b). Disorders of salt handling in the DCT involve disturbances in both apical sodium and chloride uptake as well as basolateral extrusion.
DCT disorder with apical uptake defect
Gitelman syndrome, or the pure thiazide type (DC1 type), also referred to as familial hypokalemia–hypomagnesemia)
In its first report on tubular salt-loosing disorders in 1996, Lifton’s group elucidated the underlying genetic defect in a hypokalemic, hypomagnesemic variant of SLTs [23]. Inactivating mutations in the SLC12A3 gene coding for apically expressed thiazide-sensitive NCCT caused this DCT disorder. Initially, it was considered a relatively benign variant of salt-wasting disorders during infancy and early childhood, which usually becomes symptomatic at school age and finally during adolescence or adulthood with mild symptoms, such as muscular weakness, fatigue, salt craving, or signs of increased neuromuscular excitability such as cramps or tetany. Young patients are often diagnosed accidentally during a diagnostic workup because of growth retardation, constipation, or enuresis, but also by family history [24]. However, over time and in a subgroup of young male patients, the thiazide type of tubular disorder is not so benign [25–27]. Patients may suffer from significant reduction in quality of life, more or less related to more severe sequelae of the primary disorder. These include hypokalemic rhabdomyolysis, seizures, cardiac arrhythmias, or chondrocalcinosis. For example, chondrocalcinosis, which affects mainly the knees, elbows, and shoulders and might lead to consultation with a rheumatologist [28], is thought to be the result of chronic hypomagnesemia [27].
Based on the large number of patients with >140 mutations of the NCCT gene and the already available long-term experience, the natural history of GS or the pure thiazide-type disorder is highly heterogeneous in terms of age of clinical diagnosis and the nature and severity of biochemical abnormalities and severity of clinical manifestation, even when a common underlying mutation is present [26, 27, 29]. Moreover, hypocalciuria and hypomagnesemia might change during the life cycle of a given patient, reflecting environmental changes or compensatory mechanisms. Hypomagnesemia and hypocalciuria were considered pathognomonic for the NCCT defect. However, this laboratory constellation is also observed in other disorders primarily affecting active transcellular magnesium reabsorption or active transcellular salt reabsorption in the DCT (see below). Therefore, the combination of hypomagnesemia with hypocalciuria might be considered a DCT signature rather than being NCCT or GS specific.
DCT disorders with basolateral extrusion defect
Besides the NCCT defect, two additional defects have been identified as causes for a DCT disorder: the mixed thiazide–furosemide and the mixed kidney–brain type; Both are based on a basolateral extrusion defect.
Bartter syndrome type III or the mixed thiazide–furosemide type (DC2 type), also referred to as classic Bartter syndrome
Initially, when Lifton’s group identified mutations in the chloride channel gene CLCNKB as another cause of SLT, it was considered that chloride reabsorption in the loop is primarily and exclusively impaired [30]. However, later, the entire spectrum of the clinical presentation with a strong DCT signature became apparent, leading to the term classic BS [31]. This term was chosen to keep this disorder clearly apart from the more severe and life-threatening loop disorders, the antenatal BS/HPS. The mixed thiazide-furosemide-like clinical presentation of a tubular disorder with ClC-Kb-defect is most likely explained by differences in the expression of the chloride channels ClC-Ka and ClC-Kb in the distal nephron. Expression of both chloride channels occurs in the TAL with an exclusive expression of ClC-Ka in the thin ascending limb and predominant expression of ClC-Kb in more distal nephron segments, i.e., the DCT. Thus, there is a potential of compensation for an isolated ClC-Kb defect in the TAL by ClC-Ka, whereas no such option exists in the DCT [32, 33]. That is why in contrast to the traditional terminology, BS type III is primarily regarded as a DCT disorder in the new terminology.
The phenotype of this mixed thiazide type of DCT disorder can be described as follows [17, 24, 31, 34]: After an uneventful neonatal period, patients usually present with failure to thrive. At first presentation, electrolyte derangements are usually pronounced, because renal salt wasting progresses slowly and is virtually not accompanied by evident polyuria, which delays medical consultation. Laboratory examination can reveal extremely low plasma chloride concentrations associated with hyponatremia and severe hypokalemic alkalosis. Up to one third of patients might be affected with prenatal polyhydramnios that, however, is less pronounced and rarely requires amniocentesis or leads to extreme prematurity [17, 31]. Accordingly, symptoms consistent with dysfunction of the TAL, such as hyposthenuric or isosthenuric polyuria or hypercalciuria, are rare findings. Few patients develop medullary nephrocalcinosis. Clearly, the majority of patients share symptoms with patients with a pure thiazide type of disorder, such as postnatal manifestation, a largely preserved renal concentrating capacity, low plasma levels of magnesium, and diuretic insensitivity to thiazide administration [17, 24, 31, 35]. This makes it quite difficult to differentiate between these two disorders: BS type III and GS. Unfortunately, genetic findings are not able to explain the entire spectrum of phenotypic variability of this mixed type of tubular disorder [29, 31, 36, 37]. The phenotypic heterogeneity might also reflect an individual variance in distribution of ClC-Kb in the distal nephron or a potential for activating alternative routes of basolateral chloride secretion.
EAST/SeSAME syndrome, or mixed kidney–brain type (DC3 type)
Very recently, two independent groups described a complex syndrome combining epilepsy, ataxia, mental retardation, sensorineural deafness, and renal salt wasting for which they introduced the acronyms EAST, or SeSAME [38, 39]. Besides RAAS stimulation and hypokalemic alkalosis, the renal phenotype includes a largely preserved urinary concentrating ability as well as hypomagnesemia and hypocalciuria resembling the above-mentioned DCT signature. Autosomal-recessive EAST/SeSAME syndrome was found to be caused by loss-of-function mutations in KCNJ10, coding for Kir4.1, a member of the inwardly rectifying potassium channel family. Kir4.1 expression was demonstrated in glial cells in several parts of the brain, including the spinal cord, and in the stria vascularis of the inner ear, explaining the observed central nervous phenotype and deafness in affected patients. In kidney, Kir4.1 is expressed in DCT, connecting tubule (CT), and CCD [40]. In these segments, Kir4.1 localizes to the basolateral membrane and is supposed to function together with Na-K-ATPase and allows for a recycling of potassium ions entering the tubular cells and counters movement of the extruded sodium [39]. This is another example of a DCT disorder that demonstrates a similar clinical presentation concerning the renal phenotype despite a different gene defect. This phenomenon can be explained by ion transport mechanisms that are tightly coupled to each other, meaning that loss-of-function mutations affecting one element of active transepithelial transport potentially lead to the complete breakdown of salt reabsorption in the affected epithelial cells in the DCT.
Combined disorders, a combination of both major subgroups of inherited SLTs
Bartter syndrome type IV, or furosemide–thiazide types with ear involvement (L-DC1 and L-DC2 type), also referred to as antenatal Bartter syndrome or hyperprostaglandin E with sensorineural deafness
A combined defect of salt reabsorption in the TAL and DCT leads to the clinically most severe variant of tubular salt-wasting disorders, which fortunately is much less common than any other SLT. It is caused by a defect in chloride transport both in the TAL and DCT by disruption of the function of basolateral ClC-Ka and ClC-Kb. After the initial description of a defect in barttin, an essential subunit of both chloride channels [41], genetic heterogeneity was demonstrated by the occurrence of a digenic defect of both ClC-Ka and ClC-Kb [32]. The combined impairment of basolateral chloride transport in the TAL and DCT mimics the concerted action of furosemide and thiazides. In addition, defective chloride transport via ClC-Ka and ClC-Kb also leads to sensorineural deafness.
Typically, these disorders manifest prenatally, with the development of a maternal polyhydramnios due to fetal polyuria, beginning close to the end of the second trimester of pregnancy. As in all loop disorders, polyhydramnios accounts for preterm labor and extreme prematurity. Postnatally, patients exhibit excessive salt and water losses and are at high risk of hypovolemic hypotension or even shock. Plasma chloride decreases to rather low levels, similar to the SLT with ClC-Kb defect. Polyuria and isosthenuria or hyposthenuria are present, as in other loop disorders. However, response to indomethacin treatment, which has been shown to be highly effective in NKCC2 and ROMK defects, is – for unknown reasons – unsatisfactory, necessitating extensive fluid and electrolyte therapy. In contrast to patients with NKCC2 and ROMK defects, patients with these combined disorders (BS type IV) exhibit only transitory hypercalciuria but commonly proceed to progressive renal failure, although medullary nephrocalcinosis is usually absent [24, 42].
Diagnostic considerations
Possible misdiagnoses
As the first step when considering the diagnosis of an SLT-like clinical presentation, nonrenal diseases such as cystic fibrosis, chloride diarrhea, chronic vomiting, and laxative abuse need to be excluded. When a renal tubular disorder is more closely considered, the not so uncommon misdiagnoses – such as nephrogenic diabetes insipidus for any loop disorder or pseudohypoaldosteronism for the mixed furosemide–amiloride subtype (BS type II) – have to be excluded. In nephrogenic diabetes insipidus, pure renal water wastage is not associated with the development of polyhydramnios and hyponatremia [43], and in pseudohypoaldosteronism, hyperreninemic hyperaldosteronism is associated with persistent hyperkalemic acidosis, as opposed to the development of hypokalemic alkalosis in ROMK defects [17, 44, 45].
Differential diagnosis
As shown in Table 2, there are robust signs and symptoms to differentiate between loop and DCT disorders [17, 24]. Excessive maternal polyhydramnios (3–15 l) and often with the need for amniocentesis, massive polyuria in early childhood (>15 ml/kg/day) with a urine osmolality <300 mOsmol/kg and persistent hypercalciuria (>8 mg/kg/day) with nephrocalcinosis are all indicative for loop disorders. In contrast, hypocalciuria (<2 mg/kg/day) and a maximal urine osmolality clearly >400 mOsmol/kg associated with neuromuscular irritability and tetany in patients not much younger than school-age children strongly suggest a DCT disorder. However, the combined furosemide–thiazide type of tubular disorder (BS type IV) with ear involvement is an exception. Despite reinforced diuresis, the net effect of a hypercalciuric loop defect combined with a hypocalciuric DCT defect will ultimately result in normocalciuria with no parenchymal calcification of the renal medulla. There are also some deviations from this diagnostic rule as a result of residual function of the mutated channels or transporters, such as a partial defect of NKCC2, which has been associated with a late-onset manifestation beyond childhood [46]. Moreover, as mentioned before, a minority of patients with ClC-Kb defect have some features of a rudimentary loop disorder [17]. In particular, African Americans might have a higher tendency to this mixed kind of clinical presentation [47].
For the accurate diagnosis of SLTs under routine clinical conditions, fractional clearance studies during hypotonic saline diuresis to assess distal chloride reabsorption [48] or diuretic response tests [49] in patients with suspected SLT-like disease are not very helpful or may even be sometimes dangerous. In the first case, induced hypotonic diuresis may cause a further drop in serum potassium levels, and in the second case, the additional pharmacological blockade of compensatory mechanisms will cause almost total failure of salt reabsorption in the distal tubule [50]. Most valuable for differential diagnosis is the patient’s medical history. Severe prenatal manifestation is the hallmark for all loop disorders. Transient hyperkalemia is a special feature of the mixed furosemide–amiloride type (BS type II or L2 type), and sensorineural deafness characterizes combined tubular disorders (BS type IV or L-DC1 and L-DC2 types). Also, the mixed kidney–brain type of the DCT disorders (EAST syndrome or DC3 type) with the complex neurological phenotype should allow a straightforward diagnosis. However, because of the great variability of the clinical presentation and the significant overlap between the pure thiazide type (GS or DC1 type) and the mixed thiazide–furosemide type (BS type III or DC2 type), the correct diagnosis of these DCT disorders can often be made only by genetic analysis.
Secondary SLTs
There is also a variety of unrelated disorders associated with secondary SLTs. Some are inherited; others are acquired dysfunctions. Often, the exact pathological mechanism of salt wasting is not well understood and is occasionally overlooked as a concomitant feature of the primary disease. Autosomal dominant hypocalcemia is caused by gain-of-function mutations in the calcium-sensing receptor (CaSR), which is highly expressed at the basolateral membrane of the TAL [51, 52]. This receptor negatively regulates the passive reabsorption of divalent cations. Activation of the CaSR by interstitial concentrations of calcium and magnesium provokes inhibition of active, transcellular salt reabsorption and thereby decreases transepithelial lumen-positive potential and paracellular divalent cation reabsorption in the TAL [53, 54]. Thus, CaSR activation might have the potential to cause a certain degree of loop dysfunction. That is why sometimes this disorder is also referred to as BS type V (see Table 1). However, similar interactions and mechanisms might also exist in other disorders that can be associated with dysfunction of salt transport in the distal tubule, such as Sjögren’s syndrome [55], Dent’s disease [56], sarcoidosis [57], Kearns–Sayre syndrome [58], and cystinosis [59]. Finally, several drugs may cause SLT-like or Bartter-like adverse reactions, such as aminoglycosides, prostaglandins, and cytotoxic drugs (e.g., cisplatin) [60–62]. However, the most frequent drugs involved are diuretics, especially when adolescents chronologically abuse them.
Therapeutic options
Supplementation
Usually, lifelong supplementation of salt and water is essential for all patients with SLTs. Potassium-rich diet or direct potassium supplementation needs to be considered, particularly in patients with muscular weakness, cardiac arrhythmias, and/or constipation. This might not be the case in patients with a loop disorder of the mixed furosemide–amiloride type (BS type II) if adequately treated with indomethacin (see below). For patients with hypomagnesemia associated with tetany, cramps, paresthesias, and joint and muscle pain, magnesium supplementation is always warranted [27]. However, this is a major therapeutic challenge because of the limited intestinal tolerance for oral magnesium administration [17].
Pharmacotherapy
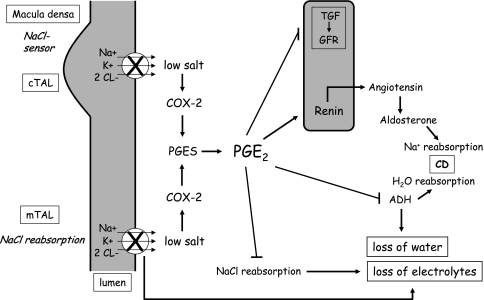
For quite some time, therapeutic interventions might have been too exclusively focused on potassium levels following the hypothesis that hypokalemia is the preceding event for increased prostaglandin production in patients with SLTs [63]. Thus, potassium supplementation in combination with the aldosterone antagonist spironolactone and/or potassium-sparing diuretics has been recommended as the first therapeutic option for patients with SLTs [64]. Probably the most convincing clinical evidence that the sequence of events is just the other way round was demonstrated by the hyperprostaglandinuric, hyperkalemic, preterm neonates with a ROMK defect in the first weeks of life [17]. Today, we know more about the role of prostaglandins in the underlying pathological mechanism, particularly of loop disorders. The pivotal role of renal PGE2 in the pathogenesis of loop disorders is presented briefly (Fig. 2). To sense tubular chloride concentration, MD cells take advantage of essentially the same repertoire of transport proteins as found in the salt-reabsorbing TAL cells. MD binding through genetically disrupted apical salt (chloride) entry – for example, through defective NKCC2 – incorrectly signals low tubular salt concentration with resultant counterregulation by interfering with tubuloglomerular feedback and the attendant disinhibition of glomerular filtration [1–3, 65]. The overwhelming salt load caused by this prostaglandin-mediated glomerular overfiltration might constitute one of the most important mechanisms underlying the severe salt and water wasting in loop disorders. Moreover, the high tubular salt load is a major stimulus for even more COX-2-mediated PGE2 production, which causes an additional direct inhibition of tubular salt and water reabsorption [24, 65, 66]. The actual trigger for this PGE2 overproduction at the tubular site might also be the transcellular chloride concentration gradient and some additional stimuli, such as tubular shear stress along the entire distal nephron [67]. This concept might explain why salt and water supplementation alone without concomitant inhibition of renal prostaglandin synthesis does not improve but even aggravates salt and water wasting of a loop disorder. In this situation, a vicious cycle is started.
Fig. 2.
Simplified scheme to explain how prostaglandin E2 (PGE2) plays a pivotal role in the pathogenesis of salt and water wasting in loop disorders. The genetic knockout of active transcellular transport impairs salt (chloride) detection by low intracellular salt content and cell shrinkage in the macula densa (MD), with the consequence of cyclooxygenase-2 (COX-2) and prostaglandin E2-synthase (PGES) activation. Overproduced PGE2 interferes with tubuloglomerular feedback (TGF) through disinhibition of glomerular filtration, which increases glomerular filtration rate (GFR). In parallel, PGE2 inhibits antidiuretic hormone (ADH) action on water reabsorption at the level of the collecting duct (CD) and activates the renin–angiotensin–aldosterone system (RAAS) in an attempt to increase salt reabsorption. However, PGE2 antagonizes this by inhibiting tubular salt reabsorption in addition to the genetic defect directly at the tubular site and thereby actually aggravates renal salt wasting. cTAL cortical thick ascending limb of Henle’s loop, mTAL medullary thick ascending limb of Henle's loop
Consequently, in loop disorders, the supplementation of wasted salt and water ought to be accompanied by pharmacological suppression of elevated PGE2 synthesis in the kidney [67]. As a chronic treatment, indomethacin appears to be one of the most appropriate therapeutic options. The effect of indomethacin is particularly pronounced in patients with a mixed furosemide–amiloride type of loop disorder (BS type II). After titration of the least toxic but still efficacious dose (sometimes <1 mg/kg body weight/day), this therapy appears to be convenient, as no additional potassium supplementation or RAAS blockers are needed in the majority of patients [17]. Besides the beneficial effects on renal salt and water wasting, effective indomethacin treatment significantly improves failure to thrive and growth, particularly in the first years of treatment. This effect is observed in patients with loop disorders as well as in those with DCT disorders [68, 69]. DCT disorders are not always associated with markedly elevated renal PGE2 synthesis [17, 24]. This is especially the case in adult patients [70]. This might be one reason that PGE synthesis inhibitors have not been tested as frequently in DCT disorders compared with those with loop disorders. There is, in fact, a great need for randomized, well-controlled clinical trials with the different pharmacotherapeutic options and combinations in patients with SLTs, especially with DCT disorders [71].
Only in the case of persistent hypokalemia (plasma potassium <3.0 mmol/l) that occurs despite adequate and tolerated inhibition of prostaglandin synthesis and salt and potassium supplementation, for symptomatic antihypokalemic therapy, one might consider the use of drugs that interfere with the RAAS, such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or direct renin inhibitors [72–74]. However, close monitoring of renal function and blood pressure is absolutely warranted, particularly as a therapeutic agent is phased in. This add-on therapy might have an additional beneficial effect on proteinuria, which becomes a growing problem in the long run in patients with SLTs [75, 76]. However, it should be mentioned again that no such well-defined clinical trials have been conducted. Moreover, the off-label use of all of these antihypokalemic and antiproteinuric orphan medicines needs to be considered carefully.
At the end of this discussion of various aspects of the pharmacotherapeutic interventions in patients with SLT, the attempt to treat either hypokalemia or hypercalciuria with a potassium- or calcium-sparing diuretic ought to be mentioned. This symptomatic and in part paradoxical diuretic treatment is harmful to patients with diuretic-like salt and water wasting tubulopathies [77–79]. In both cases, this pharmacological approach attenuates or even abolishes essential compensatory mechanisms in segments of the distal nephron that are not genetically affected. In this situation, volume contraction appears to be worsened and, in the case of a potassium-sparing diuretic, a sudden shift from hypokalemia to hyperkalemia might occur, particularly when renal function is critically reduced in a hypovolemic state or during additional extrarenal fluid losses (e.g., diarrhea and vomiting).
Prognosis
In contrast to loop disorders, which are most severe during the perinatal period and in early infancy with improving stability later in life, DCT disorders or the Gitelman-like syndromes have the tendency of aggravation during adulthood [25]. That means in patients with DCT disorders that the therapeutic efforts might have to be intensified over time, whereas in patients with loop disorders (BS I and II), treatment can be tapered down. This was observed at our institution in patients with loop disorders when medications were withdrawn for a few days under controlled conditions in intervals of 3–4 years [10, 80, 81]. These withdrawal tests have the additional advantage that one may identify patients with a rather mild loop disorder [46] or realize that one might have been dealing with a transient loop dysfunction during the perinatal period [82]. Some reports about long-term follow-ups from various single groups and centers with experiences in managing patients with SLT are available [25, 75, 76, 81]. Unfortunately, until now, larger cohorts of patients from various centers and institutions have not been enrolled in international registries. Such registries are essential for making clearer prognostic statements. However, the following risks and possible adverse drug reactions during the patient’s entire life span are listed:
Prolonged use of prostaglandin synthesis inhibitors can be associated with increased gastrointestinal intolerance or even toxicity [75, 83, 84]. Use of more selective COX-2 inhibitors has been evaluated as a better option [85–87]. However, administration of these compounds seems to increase cardiovascular risk [88]. For the present time, indomethacin, which appears to be most efficacious and reasonably well tolerated by children, remains the drug of choice. Sometimes, the combined use of medicines that control gastric acidity and the integrity of gastric mucosa, such as prostaglandin analogues or proton-pump inhibitors, might be indicated.
There is always a certain risk of secondary renal failure during chronic volume contraction, especially in patients with polyuric SLTs and noncompliance concerning the medication [75]. Fortunately, renal function is usually protected from irreversible renal damage if close patient monitoring during long-term indomethacin treatment is provided [81]. However, the special natural history and prognosis of combined loop and DCT disorders (BS type IV), which are prone to renal failure, needs to be considered [42].
Cardiac arrhythmias and QT prolongation induced by hypokalemia and hypomagnesemia might put patients with DCT disorders (GS and BS type III) at risk of sudden cardiac death [89–91]. For this reason, several commonly used medicines that prolong the QT interval, such as macrolides, antihistamines, some antitussives, antimycotics, psychotropics, and β2-agonists, should be avoided. Compilations of commonly used drugs with QT-prolonging effect are available [92].
Growth retardation is not uncommon in patients with DCT disorders [75, 93]. Delayed growth appears to be a quite common observation in a subgroup of severely affected male patients with the pure thiazide type of DCT disorder (GS) [26].
Questions
(Answers appear following the reference list.)
-
The pregnancy of a 28-year-old woman with one previous miscarriage is complicated by idiopathic polyhydramnios, which was first recognized by routine ultrasound at the end of the second trimester. After therapeutic amniocentesis (estimated amniotic fluid volume of 10 l) and rupture of membranes at 30 weeks of gestation, an acute Caesarean section was performed. The delivered male infant was appropriate for gestational age and showed an uneventful postnatal adaptation, except for a mild respiratory distress syndrome requiring a positive end-expiratory pressure device. However, a few days later, he developed hyponatremia, hyperkalemia, hyposthenuria, hypercalciuria, and a weight loss from birthweight by >15%.
The diagnosis most likely is:- Nephrogenic diabetes insipidus
- Loop disorder with NKCC2 defect (BS type 1)
- Loop disorder with ROMK defect (BS type II)
- Combined loop and DCT disorder with barttin defect (BS type IV)
- Combined loop and DCT disorder with a defect in ClC-Ka and ClC-Kb
- What is the most appropriate pharmacotherapeutic intervention in a patient with polyuric and hypercalciuric salt-losing tubulopathy associated with hyperaldosteronism?
- Potassium-sparing diuretics
- Calcium-sparing diuretics
- COX-2 inhibitors
- Prostaglandin synthesis inhibitors
- ACE inhibitors and/or AR blockers
- Which patient with a salt-losing tubulopathy is most likely at risk to develop end-stage renal failure later in life?
- Combined loop and DCT disorder with barttin defect (BS type IV)
- Loop disorder with ROMK defect (BS type II)
- DCT disorder with ClC-Kb defect (BS type III)
- DCT disorder with NCCT defect (GS)
- Loop disorder with NKCC2 defect (BS type I)
- Which special subtype of salt-losing tubulopathy is least likely to be associated with chronic hypercalciuria and nephrocalcinosis?
- NKCC2 defect (BS type I)
- ClC-Kb defect (BS type III)
- ROMK defect (BS type II)
- Barttin defect (BS type IV)
- What is the most convenient way to differentiate between renal and extrarenal salt losses?
- Plasma electrolyte measurement
- Urine osmolality
- Urinary sodium and/or chloride levels
- Sweat chloride test
- What is the most unlikely complication or sequelae of a DCT disorder with an apical uptake defect (GS)?
- Hypokalemic rhabdomyolysis
- Nephrolithiasis
- Growth retardation
- Cardiac arrhythmias
- Chondrocalcinosis
Footnotes
Answers
1. (c)
2. (d)
3. (a)
4. (d)
5. (c)
6. (b)
Contributor Information
Hannsjörg W. Seyberth, Phone: +49-6341-944655, Email: seyberth@staff.uni-marburg.de
Karl P. Schlingmann, Email: schlingm@med.uni-marburg.de
References
- 1.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274:R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 2.Deng A, Wead LM, Blantz RC. Temporal adaptation of tubuloglomerular feedback: effects of COX-2. Kidney Int. 2004;66:2348–2353. doi: 10.1111/j.1523-1755.2004.66033.x. [DOI] [PubMed] [Google Scholar]
- 3.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol. 2010;21:1093–1096. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 5.Nijenhuis T, Vallon V, Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartter F, Pronove P, Gill J, Jr, MacCardle R. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 7.Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–235. [PubMed] [Google Scholar]
- 8.Fanconi A, Schachenmann G, Nussli R, Prader A. Chronic hypokalaemia with growth retardation, normotensive hyperrenin-hyperaldosteronism ("Bartter's syndrome"), and hypercalciuria. Report of two cases with emphasis on natural history and on catch-up growth during treatment. Helv Paediatr Acta. 1971;26:144–163. [PubMed] [Google Scholar]
- 9.Ohlsson A, Sieck U, Cumming W, Akhtar M, Serenius F. A variant of Bartter's syndrome. Bartter's syndrome associated with hydramnios, prematurity, hypercalciuria and nephrocalcinosis. Acta Paediatr Scand. 1984;73:868–874. doi: 10.1111/j.1651-2227.1984.tb17793.x. [DOI] [PubMed] [Google Scholar]
- 10.Seyberth HW, Rascher W, Schweer H, Kuhl PG, Mehls O, Scharer K. Congenital hypokalemia with hypercalciuria in preterm infants: a hyperprostaglandinuric tubular syndrome different from Bartter syndrome. J Pediatr. 1985;107:694–701. doi: 10.1016/S0022-3476(85)80395-4. [DOI] [PubMed] [Google Scholar]
- 11.Seyberth HW, Koniger SJ, Rascher W, Kuhl PG, Schweer H. Role of prostaglandins in hyperprostaglandin E syndrome and in selected renal tubular disorders. Pediatr Nephrol. 1987;1:491–497. doi: 10.1007/BF00849259. [DOI] [PubMed] [Google Scholar]
- 12.Seyberth HW. An improved terminology and classification of Bartter-like syndromes. Nat Clin Pract Nephrol. 2008;4:560–567. doi: 10.1038/ncpneph0912. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz I. Molecular pathogenesis of Bartter's and Gitelman's syndromes. Kidney Int. 1998;54:1396–1410. doi: 10.1046/j.1523-1755.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Seyberth H, Soergel M, Koeckerling A. Hypokalaemic tubular disorders: the hyperprostaglandin E syndrome and Gitelman-Bartter syndrome. Oxford: Oxford University Press; 1998. [Google Scholar]
- 15.Reinalter SC, Jeck N, Peters M, Seyberth HW. Pharmacotyping of hypokalaemic salt-losing tubular disorders. Acta Physiol Scand. 2004;181:513–521. doi: 10.1111/j.1365-201X.2004.01325.x. [DOI] [PubMed] [Google Scholar]
- 16.Unwin RJ, Capasso G. Bartter's and Gitelman's syndromes: their relationship to the actions of loop and thiazide diuretics. Curr Opin Pharmacol. 2006;6:208–213. doi: 10.1016/j.coph.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Peters M, Jeck N, Reinalter S, Leonhardt A, Tönshoff B, Klaus GG, Konrad M, Seyberth HW. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112:183–190. doi: 10.1016/S0002-9343(01)01086-5. [DOI] [PubMed] [Google Scholar]
- 18.Hufnagle KG, Khan SN, Penn D, Cacciarelli A, Williams P. Renal calcifications: a complication of long-term furosemide therapy in preterm infants. Pediatrics. 1982;70:360–363. [PubMed] [Google Scholar]
- 19.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 20.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 21.Karolyi L, International Study Group for Bartter-like Syndromes Mutations in the gene encoding the inwardly-rectifying renal potassium channel, ROMK, cause the antenatal variant of Bartter syndrome: evidence for genetic heterogeneity. [published erratum appears in Hum Mol Genet 1997 Apr;6(4):650] Hum Mol Genet. 1997;6:17–26. doi: 10.1093/hmg/6.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Hollifield JW. Thiazide treatment of systemic hypertension: effects on serum magnesium and ventricular ectopic activity. Am J Cardiol. 1989;63:22G–25G. doi: 10.1016/0002-9149(89)90214-2. [DOI] [PubMed] [Google Scholar]
- 23.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 24.Jeck N, Schlingmann KP, Reinalter SC, Kömhoff M, Peters M, Waldegger S, Seyberth HW. Salt handling in the distal nephron: lessons learned from inherited human disorders. Am J Physiol Regul Integr Comp Physiol. 2005;288:R782–R795. doi: 10.1152/ajpregu.00600.2004. [DOI] [PubMed] [Google Scholar]
- 25.Cruz DN, Shaer AJ, Bia MJ, Lifton RP, Simon DB. Gitelman's syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int. 2001;59:710–717. doi: 10.1046/j.1523-1755.2001.059002710.x. [DOI] [PubMed] [Google Scholar]
- 26.Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ, Dahan K, Devuyst O. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol. 2007;18:1271–1283. doi: 10.1681/ASN.2006101095. [DOI] [PubMed] [Google Scholar]
- 27.Knoers NV, Levtchenko EN. Gitelman syndrome. Orphanet J Rare Dis. 2008;3:22. doi: 10.1186/1750-1172-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smilde TJ, Haverman JF, Schipper P, Hermus AR, Liebergen FJ, Jansen JL, Kloppenborg PW, Koolen MI. Familial hypokalemia/hypomagnesemia and chondrocalcinosis. J Rheumatol. 1994;21:1515–1519. [PubMed] [Google Scholar]
- 29.Lin SH, Cheng NL, Hsu YJ, Halperin ML. Intrafamilial phenotype variability in patients with Gitelman syndrome having the same mutations in their thiazide-sensitive sodium/chloride cotransporter. Am J Kidney Dis. 2004;43:304–312. doi: 10.1053/j.ajkd.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 31.Konrad M, Vollmer M, Lemmink HH, Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschenes G, Antignac C, Guay-Woodford L, Knoers NV, Seyberth HW, Feldmann D, Hildebrandt F. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol. 2000;11:1449–1459. doi: 10.1681/ASN.V1181449. [DOI] [PubMed] [Google Scholar]
- 32.Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S. Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med. 2004;350:1314–1319. doi: 10.1056/NEJMoa032843. [DOI] [PubMed] [Google Scholar]
- 33.Kramer BK, Bergler T, Stoelcker B, Waldegger S. Mechanisms of Disease: the kidney-specific chloride channels ClCKA and ClCKB, the Barttin subunit, and their clinical relevance. Nat Clin Pract Nephrol. 2008;4:38–46. doi: 10.1038/ncpneph0689. [DOI] [PubMed] [Google Scholar]
- 34.Brochard K, Boyer O, Blanchard A, Loirat C, Niaudet P, Macher MA, Deschenes G, Bensman A, Decramer S, Cochat P, Morin D, Broux F, Caillez M, Guyot C, Novo R, Jeunemaitre X, Vargas-Poussou R. Phenotype-genotype correlation in antenatal and neonatal variants of Bartter syndrome. Nephrol Dial Transplant. 2009;24:1455–1464. doi: 10.1093/ndt/gfn689. [DOI] [PubMed] [Google Scholar]
- 35.Nozu K, Iijima K, Kanda K, Nakanishi K, Yoshikawa N, Satomura K, Kaito H, Hashimura Y, Ninchoji T, Komatsu H, Kamei K, Miyashita R, Kugo M, Ohashi H, Yamazaki H, Mabe H, Otsubo A, Igarashi T, Matsuo M. The pharmacological characteristics of molecular-based inherited salt-losing tubulopathies. J Clin Endocrinol Metab. 2010;95:E511–E518. doi: 10.1210/jc.2010-0392. [DOI] [PubMed] [Google Scholar]
- 36.Jeck N, Konrad M, Peters M, Weber S, Bonzel KE, Seyberth HW. Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res. 2000;48:754–758. doi: 10.1203/00006450-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Zelikovic I, Szargel R, Hawash A, Labay V, Hatib I, Cohen N, Nakhoul F. A novel mutation in the chloride channel gene, CLCNKB, as a cause of Gitelman and Bartter syndromes. Kidney Int. 2003;63:24–32. doi: 10.1046/j.1523-1755.2003.00730.x. [DOI] [PubMed] [Google Scholar]
- 38.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, Hoff W, Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- 41.Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 42.Jeck N, Reinalter SC, Henne T, Marg W, Mallmann R, Pasel K, Vollmer M, Klaus G, Leonhardt A, Seyberth HW, Konrad M. Hypokalemic salt-losing tubulopathy with chronic renal failure and sensorineural deafness. Pediatrics. 2001;108:E5. doi: 10.1542/peds.108.1.e5. [DOI] [PubMed] [Google Scholar]
- 43.Bichet DG. Hereditary polyuric disorders: new concepts and differential diagnosis. Semin Nephrol. 2006;26:224–233. doi: 10.1016/j.semnephrol.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Finer G, Shalev H, Birk OS, Galron D, Jeck N, Sinai-Treiman L, Landau D. Transient neonatal hyperkalemia in the antenatal (ROMK defective) Bartter syndrome. J Pediatr. 2003;142:318–323. doi: 10.1067/mpd.2003.100. [DOI] [PubMed] [Google Scholar]
- 45.Nozu K, Fu XJ, Kaito H, Kanda K, Yokoyama N, Przybyslaw Krol R, Nakajima T, Kajiyama M, Iijima K, Matsuo M. A novel mutation in KCNJ1 in a Bartter syndrome case diagnosed as pseudohypoaldosteronism. Pediatr Nephrol. 2007;22:1219–1223. doi: 10.1007/s00467-007-0468-4. [DOI] [PubMed] [Google Scholar]
- 46.Pressler CA, Heinzinger J, Jeck N, Waldegger P, Pechmann U, Reinalter S, Konrad M, Beetz R, Seyberth HW, Waldegger S. Late-onset manifestation of antenatal Bartter syndrome as a result of residual function of the mutated renal Na+−K+−2Cl- co-transporter. J Am Soc Nephrol. 2006;17:2136–2142. doi: 10.1681/ASN.2005101071. [DOI] [PubMed] [Google Scholar]
- 47.Schurman SJ, Shoemaker LR. Bartter and Gitelman syndromes. Adv Pediatr. 2000;47:223–248. [PubMed] [Google Scholar]
- 48.Rodriguez-Soriano J, Vallo A, Castillo G, Oliveros R. Renal handling of water and sodium in infancy and childhood: a study using clearance methods during hypotonic saline diuresis. Kidney Int. 1981;20:700–704. doi: 10.1038/ki.1981.199. [DOI] [PubMed] [Google Scholar]
- 49.Colussi G, Bettinelli A, Tedeschi S, Ferrari ME, Syren ML, Borsa N, Mattiello C, Casari G, Bianchetti MG. A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol. 2007;2:454–460. doi: 10.2215/CJN.02950906. [DOI] [PubMed] [Google Scholar]
- 50.Sassen MC, Jeck N, Klaus G. Can renal tubular hypokalemic disorders be accurately diagnosed on the basis of the diuretic response to thiazide? Nat Clin Pract Nephrol. 2007;3:528–529. doi: 10.1038/ncpneph0576. [DOI] [PubMed] [Google Scholar]
- 51.Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG. Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet. 1994;8:303–307. doi: 10.1038/ng1194-303. [DOI] [PubMed] [Google Scholar]
- 52.Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens. 2003;12:527–532. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaitre X, Paillard M, Planelles G, Dechaux M, Miller RT, Antignac C. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–2266. doi: 10.1097/01.ASN.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 55.Casatta L, Ferraccioli GF, Bartoli E. Hypokalaemic alkalosis, acquired Gitelman's and Bartter's syndrome in chronic sialoadenitis. Br J Rheumatol. 1997;36:1125–1128. doi: 10.1093/rheumatology/36.10.1125. [DOI] [PubMed] [Google Scholar]
- 56.Besbas N, Ozaltin F, Jeck N, Seyberth H, Ludwig M. CLCN5 mutation (R347X) associated with hypokalaemic metabolic alkalosis in a Turkish child: an unusual presentation of Dent's disease. Nephrol Dial Transplant. 2005;20:1476–1479. doi: 10.1093/ndt/gfh799. [DOI] [PubMed] [Google Scholar]
- 57.Yu TM, Lin SH, Ya-Wen C, Wen MC, Chen YH, Cheng CH, Chen CH, Chin CS, Shu KH. A syndrome resembling Bartter's syndrome in sarcoidosis. Nephrol Dial Transplant. 2009;24:667–669. doi: 10.1093/ndt/gfn600. [DOI] [PubMed] [Google Scholar]
- 58.Emma F, Pizzini C, Tessa A, Giandomenico S, Onetti-Muda A, Santorelli FM, Bertini E, Rizzoni G. "Bartter-like" phenotype in Kearns-Sayre syndrome. Pediatr Nephrol. 2006;21:355–360. doi: 10.1007/s00467-005-2092-5. [DOI] [PubMed] [Google Scholar]
- 59.Caltik A, Akyuz SG, Erdogan O, Bulbul M, Demircin G. Rare presentation of cystinosis mimicking Bartter's syndrome: reports of two patients and review of the literature. Ren Fail. 2010;32:277–280. doi: 10.3109/08860221003592804. [DOI] [PubMed] [Google Scholar]
- 60.Chrispal A, Boorugu H, Prabhakar AT, Moses V. Amikacin-induced type 5 Bartter-like syndrome with severe hypocalcemia. J Postgrad Med. 2009;55:208–210. doi: 10.4103/0022-3859.57407. [DOI] [PubMed] [Google Scholar]
- 61.Talosi G, Katona M, Turi S. Side-effects of long-term prostaglandin E(1) treatment in neonates. Pediatr Int. 2007;49:335–340. doi: 10.1111/j.1442-200X.2007.02380.x. [DOI] [PubMed] [Google Scholar]
- 62.Panichpisal K, Angulo-Pernett F, Selhi S, Nugent KM. Gitelman-like syndrome after cisplatin therapy: a case report and literature review. BMC Nephrol. 2006;7:10. doi: 10.1186/1471-2369-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartter F. On the pathogenesis of Bartter’s syndrome. Miner Electrolyte Metab. 1980;3:61–65. [Google Scholar]
- 64.Rodriguez-Soriano J. Tubular disorders of electrolyte regulation. In: Barratt TM, Avner ED, Harmon WE, editors. Pediatric nephrology. Baltimore: Lippincott Williams & Wilkins; 1999. pp. 545–563. [Google Scholar]
- 65.Nüsing RM, Seyberth HW. The role of cyclooxygenases and prostanoid receptorsin furosemide-like salt losing tubulopathy: the hyperprostaglandin E syndrome. Acta Physiol Scand. 2004;181:523–528. doi: 10.1111/j.1365-201X.2004.01326.x. [DOI] [PubMed] [Google Scholar]
- 66.Nüsing RM, Treude A, Weissenberger C, Jensen B, Bek M, Wagner C, Narumiya S, Seyberth HW. Dominant role of prostaglandin E2 EP4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin E syndrome/antenatal Bartter syndrome. J Am Soc Nephrol. 2005;16:2354–2362. doi: 10.1681/ASN.2004070556. [DOI] [PubMed] [Google Scholar]
- 67.Kömhoff M, Tekesin I, Peters M, Leonhard A, Seyberth HW. Perinatal management of a preterm neonate affected by hyperprostaglandin E2 syndrome (HPS) Acta Paediatr. 2005;94:1690–1693. doi: 10.1080/08035250510043897. [DOI] [PubMed] [Google Scholar]
- 68.Seidel C, Reinalter S, Seyberth HW, Scharer K. Pre-pubertal growth in the hyperprostaglandin E syndrome. Pediatr Nephrol. 1995;9:723–728. doi: 10.1007/BF00868723. [DOI] [PubMed] [Google Scholar]
- 69.Liaw LC, Banerjee K, Coulthard MG. Dose related growth response to indometacin in Gitelman syndrome. Arch Dis Child. 1999;81:508–510. doi: 10.1136/adc.81.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gladziwa U, Schwarz R, Gitter AH, Bijman J, Seyberth H, Beck F, Ritz E, Gross P. Chronic hypokalaemia of adults: Gitelman's syndrome is frequent but classical Bartter's syndrome is rare. Nephrol Dial Transplant. 1995;10:1607–1613. [PubMed] [Google Scholar]
- 71.European Medicine Agency (2006) List of paediatric needs in nephrology as established by the Paediatric Working Party http://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp
- 72.Hene RJ, Koomans HA, Dorhout Mees EJ, Stolpe A, Verhoef GE, Boer P. Correction of hypokalemia in Bartter's syndrome by enalapril. Am J Kidney Dis. 1987;9:200–205. doi: 10.1016/s0272-6386(87)80055-0. [DOI] [PubMed] [Google Scholar]
- 73.Stolpe A, Verhoef GE, Hene RJ, Koomans HA, Vijver JC. Total body potassium in Bartter's syndrome before and during treatment with enalapril. Nephron. 1987;45:122–125. doi: 10.1159/000184092. [DOI] [PubMed] [Google Scholar]
- 74.Bell DS. Successful utilization of aliskiren, a direct renin inhibitor in Bartter syndrome. South Med J. 2009;102:413–415. doi: 10.1097/SMJ.0b013e31819b8673. [DOI] [PubMed] [Google Scholar]
- 75.Bettinelli A, Borsa N, Bellantuono R, Syren ML, Calabrese R, Edefonti A, Komninos J, Santostefano M, Beccaria L, Pela I, Bianchetti MG, Tedeschi S. Patients with biallelic mutations in the chloride channel gene CLCNKB: long-term management and outcome. Am J Kidney Dis. 2007;49:91–98. doi: 10.1053/j.ajkd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Puricelli E, Bettinelli A, Borsa N, Sironi F, Mattiello C, Tammaro F, Tedeschi S, Bianchetti MG. Long-term follow-up of patients with Bartter syndrome type I and II. Nephrol Dial Transplant. 2010;25:2976–2981. doi: 10.1093/ndt/gfq119. [DOI] [PubMed] [Google Scholar]
- 77.Phillips DR, Ahmad KI, Waller SJ, Meisner P, Karet FE. A serum potassium level above 10 mmol/l in a patient predisposed to hypokalemia. Nat Clin Pract Nephrol. 2006;2:340–346. doi: 10.1038/ncpneph0201. [DOI] [PubMed] [Google Scholar]
- 78.Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol. 2006;104:p73–p80. doi: 10.1159/000094001. [DOI] [PubMed] [Google Scholar]
- 79.Chadha V, Alon US. Hereditary renal tubular disorders. Semin Nephrol. 2009;29:399–411. doi: 10.1016/j.semnephrol.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 80.Leonhardt A, Timmermanns G, Roth B, Seyberth HW. Calcium homeostasis and hypercalciuria in hyperprostaglandin E syndrome [see comments] J Pediatr. 1992;120:546–554. doi: 10.1016/S0022-3476(05)82480-1. [DOI] [PubMed] [Google Scholar]
- 81.Reinalter SC, Gröne HJ, Konrad M, Seyberth HW, Klaus G. Evaluation of long-term treatment with indomethacin in hereditary hypokalemic salt-losing tubulopathies. J Pediatr. 2001;139:398–406. doi: 10.1067/mpd.2001.117007. [DOI] [PubMed] [Google Scholar]
- 82.Reinalter S, Devlieger H, Proesmans W. Neonatal Bartter syndrome: spontaneous resolution of all signs and symptoms. Pediatr Nephrol. 1998;12:186–188. doi: 10.1007/s004670050433. [DOI] [PubMed] [Google Scholar]
- 83.Schachter AD, Arbus GS, Alexander RJ, Balfe JW. Non-steroidal anti-inflammatory drug-associated nephrotoxicity in Bartter syndrome. Pediatr Nephrol. 1998;12:775–777. doi: 10.1007/s004670050545. [DOI] [PubMed] [Google Scholar]
- 84.Vaisbich MH, Fujimura MD, Koch VH. Bartter syndrome: benefits and side effects of long-term treatment. Pediatr Nephrol. 2004;19:858–863. doi: 10.1007/s00467-004-1527-8. [DOI] [PubMed] [Google Scholar]
- 85.Kleta R, Basoglu C, Kuwertz-Broking E. New treatment options for Bartter's syndrome. N Engl J Med. 2000;343:661–662. doi: 10.1056/NEJM200008313430915. [DOI] [PubMed] [Google Scholar]
- 86.Nüsing RM, Reinalter SC, Peters M, Kömhoff M, Seyberth HW. Pathogenetic role of cyclooxygenase-2 in hyperprostaglandin E syndrome/antenatal Bartter syndrome: therapeutic use of the cyclooxygenase-2 inhibitor nimesulide. Clin Pharmacol Ther. 2001;70:384–390. [PubMed] [Google Scholar]
- 87.Reinalter SC, Jeck N, Brochhausen C, Watzer B, Nüsing RM, Seyberth HW, Komhoff M. Role of cyclooxygenase-2 in hyperprostaglandin E syndrome/antenatal Bartter syndrome. Kidney Int. 2002;62:253–260. doi: 10.1046/j.1523-1755.2002.00435.x. [DOI] [PubMed] [Google Scholar]
- 88.Kömhoff M, Klaus G, Nazarowa S, Reinalter SC, Seyberth HW. Increased systolic blood pressure with rofecoxib in congenital furosemide-like salt loss. Nephrol Dial Transplant. 2006;21:1833–1837. doi: 10.1093/ndt/gfl096. [DOI] [PubMed] [Google Scholar]
- 89.Scognamiglio R, Negut C, Calo LA. Aborted sudden cardiac death in two patients with Bartter's/Gitelman's syndromes. Clin Nephrol. 2007;67:193–197. doi: 10.5414/cnp67193. [DOI] [PubMed] [Google Scholar]
- 90.Malafronte C, Borsa N, Tedeschi S, Syren ML, Stucchi S, Bianchetti MG, Achilli F, Bettinelli A. Cardiac arrhythmias due to severe hypokalemia in a patient with classic Bartter disease. Pediatr Nephrol. 2004;19:1413–1415. doi: 10.1007/s00467-004-1611-0. [DOI] [PubMed] [Google Scholar]
- 91.Pachulski RT, Lopez F, Sharaf R. Gitelman's not-so-benign syndrome. N Engl J Med. 2005;353:850–851. doi: 10.1056/NEJMc051040. [DOI] [PubMed] [Google Scholar]
- 92.Arizona Center for Education and Research on Therapeutics (2010) QT drug list by risk groups: Drugs that prolong the QT interval and/or induce torsades de points ventricular arrhythmia http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm
- 93.Rudin A. Bartter's syndrome. A review of 28 patients followed for 10 years. Acta Med Scand. 1988;224:165–171. doi: 10.1111/j.0954-6820.1988.tb16755.x. [DOI] [PubMed] [Google Scholar]