Abstract
Cajanus cajan, a tropical shrub, serves as source of food and traditional medicines. The evaluation of aqueous and ethanol extracts for activity against measles virus and toxicity to embryonated chicken eggs was carried out in this study. In vivo and in vitro assay techniques using embryonated chicken eggs and tissue culture (Hep-2 cell lines) as media for both virus cultivation and anti-virus assay showed that a hot-water extract yielded higher activity against measles virus. The hot-water extract of the stem yielded a Log2 titre of 0.1 for the in vivo assay and an inhibition of cytopathic effect (CPE) in Hep-2 cells by 100% for the in vitro assay. At all concentrations of the extracts, there was a lowering of virus concentration (p = 0.05), indicated by hemagglutination (HA) titration, which is the advantage of HA titration over the tissue culture technique using CPE. This study validates embryonated chicken eggs as suitable media for anti-virus assay and the use of C. cajan in the treatment of some diseases of viral origin.
Introduction
Medicinal herbs have the potential for addressing multiple targets with minor side-effects, development of low resistance (due to selective pressure of infective agents) and cost effectiveness [3, 6, 15, 34]. Several plants have been studied for novel antibacterial [10, 21, 24, 31, 36], antifungal [18, 22, 27, 35], anti-parasitic [25, 28] and antiviral [6, 36] properties.
The antiviral properties of plants are rarely studied using laboratory-based assays to establish their efficacy in traditional medicine [6, 15]. Conventional techniques for evaluating antiviral agents include in vitro and in vivo methods. In vitro techniques include plaque inhibition/reduction assay, virus yield reduction assay, inhibition of virus-induced cytopathic effect (CPE), inhibition/reduction of the synthesis of virus-specific polypeptides, immunological assays for detecting viral antigens and viral enzyme inhibition-based assays [3, 6, 7, 32]. The in vivo methods include the use of ferrets, laboratory mice, cotton rats and chickens for measuring a number of parameters indicating the extent of inhibition of infection [29, 33]. The embryonated chicken egg system is a standard method for the propagation and isolation of egg-adapted viruses [20]. Antiviral agents have been successfully screened using embryonated chicken egg as media for both virus cultivation and inhibition assays [13, 33].
Cajanus cajan (L), commonly called pigeon pea, has many uses in traditional medicine. The leaves are prepared as infusion for anaemia, hepatitis, diabetes, urinary infections, yellow fever, and genital and other skin irritations, especially in females, while floral decoctions are used for bronchitis, coughs, pneumonia, dysentery, and menstrual disorders [5, 17]. In eastern Nigeria, leaf decoctions are used to treat measles, and this study was undertaken to empirically verify its potential efficacy. We evaluated aqueous and ethanol plant extracts for activity against measles virus and for toxicity to embryonated chicken eggs.
Materials and methods
Plant materials evaluated
Fresh plants were collected in July 2009 from a local farm at Orba, Udenu local government area of Enugu State, Nigeria. The plant was authenticated taxonomically by Mr. O. A. Ozioko of the Bioresources Development and Conservation Program (BDCP) Centre, Nsukka. Leaves, stems and roots were separated and thoroughly rinsed in running tap water. The roots and stems were cut into chunks, and all of the plant material was air dried at room temperature for a period of 14 days and pulverized.
Extraction of plant materials
A 50.0-g portion of the pulverized leaves, stems and roots was extracted by maceration in 200 ml of cold water and absolute ethanol (BDH) for 24 h. Hot-water extraction was carried out using a modification of the method of Okoli et al. [24]. Briefly, 50.0 g of the plant material was macerated in 200 ml of hot water (boiled to 100°C) and allowed to stand for 4 h with occasional shaking. Each extract was filtered through a Whatman No. 1 filter paper, and the pH was determined. The filtrate was concentrated by evaporation to dryness in a steady air current for 24 h in a previously weighed Petri dish, and the weight of the extract was determined. Each extract was subsequently exposed to UV rays for about 18 h, and sterility was ascertained by streaking on nutrient agar. Extracts were stored in sterile containers at room temperature.
Phytochemical screening
All of the extracts were screened for the presence of alkaloids, saponins, tannins, glycosides, flavonoids, reducing sugars, and carbohydrates using the methods of Trease and Evans [30] and Harbone [12].
Embryonated chicken eggs for virus cultivation and antivirus assays
Unvaccinated day-old chicks (pullets and cockerels) were obtained from Ota Farms, Ota Ogun State of Nigeria. The chicks were reared in laboratory cages and fed with commercial feed (Guinea feeds), applying strict hygienic husbandry practices. The birds were tested for hemagglutination inhibition (HI) antibodies to ensure that they were unexposed to infection by the test virus. Embryonated eggs (53 ± 0.6 g) were incubated within 5 days of laying. Egg incubation was in a humidified incubator at 37°C for 9-10 days with candling and manual turning of the egg thrice daily. Unfertilized eggs and eggs with dead embryos were discarded.
Hep-2 Cell lines for virus cultivation and antivirus assays
Human larynx epidermoid carcinoma (Hep-2) cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) to which L-glutamine (2 mmol l−1), penicillin (100 U ml−1) and streptomycin (100 mg ml−1) were added and supplemented with 10% heat-inactivated foetal bovine serum (FBS). The cell cultures were maintained in a humidified 5% CO2 atmosphere at 37°C in a T-75 flask (Bio-Rad).
Test virus strain
Cultivation in embryonated chicken eggs
Live attenuated measles virus (MV) was obtained in lyophilized ampoules from the Public Health Unit, University Collage Hospital, University of Ibadan, Nigeria. The freeze-dried MV was resuspended in 4.0 ml of sterile 0.01 M phosphate-buffered saline (pH 7.2), and 200 μl of the suspension was inoculated into the allantoic cavities of five 10-day-old embryonated chicken eggs. The eggs were incubated in a humidified incubator, turned thrice daily and candled once every 24 h. Eggs were discarded if embryo death occurred within 24 h [20]. For adaptation to the embryonated chicken egg, MV was passaged thrice in the allantoic cavity, and the allantoic fluid harvest was stored at −20°C to create a virus pool.
Cultivation in Hep-2 cell lines
A confluent monolayer of Hep-2 cell culture was washed twice with pre-warmed PBS, and a standard trypsinization procedure was carried out using 0.05% Trypsin 1:250 (Difco) to dislodge the cells [26]. DMEM was added to neutralize the action of the trypsin on the cells, and 0.2 ml of the reconstituted live attenuated MV was inoculated into Hep-2 tissue culture in a T-75 flask (Bio-Rad). The culture was incubated in a humidified 5% CO2 incubator at 37°C and monitored daily for CPE. Fluid was pooled from cultures with marked CPE to obtain stock viral suspensions.
Chicken embryo lethality assay
The various extracts of C. cajan were each diluted to the following concentrations: 400, 200, 100, 50, 25, 12.5 and 6.25 mg ml−1 in sterile PBS. A single dose of 100 μl of each dilution was inoculated into the albumin at the sharp pole of 10-day-old embryonated chicken eggs of seven serial groups according to their concentrations; a control group was inoculated with sterile PBS. The eggs were incubated in a humidified incubator at 37°C for 4 days, the viability of the chick embryos was checked by candling the eggs daily, and embryo death was recorded.
Spot hemagglutination
Using a 25-mm circle card (ANTEC, UK), 100 μl of the allantoic fluid harvest was mixed with 100 μl of standard washed Macaca mulatta red blood cells (mRBCs), and the card was rocked for about 8 minutes. A reactive allantoic fluid showed definite clumping of the mRBCs, and hemagglutination (HA) titration was then performed to measure the virus titre. Non-reactive allantoic fluid showed an even suspension of particles.
Hemagglutination assay
Twofold serial dilutions of MV were made in 50 μl PBS using U-shaped 96-well microtiter plates. Fifty μl of 0.6% suspension of Macaca mulatta red blood cells (mRBCs) was added to each well, and the contents were mixed by gently agitating the plate. The plate was incubated at room temperature (~27°C) until the control wells showed complete setting of mRBCs. The HA titer was read as the reciprocal of the dilution of virus in the last well showing complete HA.
Screening of extracts for antiviral activity
The allantoic cavity of ten-day-old ECE was inoculated with 200 μl of MV suspension corresponding to EID50 ml−1. Subsequently, 100 μl of the 250 mg ml−1 C. cajan extracts were inoculated into the albumen, adopting the improved ECE model of [33]. The same procedure was repeated with C. cajan extracts being inoculated into the allantoic cavity. Five replicates of each assay were done [11]. Eggs were incubated at 37°C in a humidified incubator and candled daily to observe embryo status. If embryo death occurred later than 24 h after inoculation, eggs were chilled for 4 h at 4°C, and the allantoic fluid was harvested and spot-checked for HA. Embryos that survived for 96 h were chilled for 4 h at 4°C, and the allantoic fluid was harvested and spot-checked for HA.
Similarly, 100 μl of MV suspension was added to a 48-well microtiter tissue culture plate (Corning) containing 400 μl Hep-2 cells in the growth medium, and the culture was incubated in a humidified 5% CO2 incubator at 37°C for 1 h. Afterwards, 100 μl of 250 mg ml−1 C. cajan was added as described previously, and the culture was re-incubated and monitored daily for CPE using an inverted microscope.
Concentration-dependent assay of extracts for antivirus activity
The same procedure used for screening C. cajan extracts for activity against MV was repeated, but the extracts were subjected to doubling dilution, yielding seven concentrations from 250 to 3.91 mg ml−1, and inoculated through the allantoic cavity. This procedure was used with all extracts (roots, stems and leaves) obtained with ethanol and hot- and cold-water extraction, and the allantoic fluid harvests were subjected to HA titration. Similarly, 100 μl of each concentration of the extract was introduced into microtiter tissue culture plates containing Hep-2 tissue culture and MV. In this case, only stem and root extracts were used. The culture was observed daily for CPE using an inverted microscope.
Statistical analysis
The egg infectivity dose (EID50 ml−1) was calculated following egg infectivity with twofold serial dilutions of five concentrations, while the lethality assay was calculated following inoculation of extracts into the albumen with twofold serial dilutions of eight concentrations. For both the EID50 and LD50, probit analysis [1, 8, 9] was employed, while ANOVA was used for comparing the mean values of treatment groups.
Results
The pH of extracts (Table 1) ranged from 4.65 to 5.51; cold-water extracts had the lowest value (4.45-4.81) and hot-water extracts the highest value (5.12-5.51). Likewise, the percentage yields of the extracts ranged from 6.45 to 12.19, with the cold-water extracts giving yields of 8.51-12.19% and the ethanol extracts giving yields of 7.28-11.52%. The toxicity test with chicken embryos showed an LD50 range of 602.0-742.0 mg ml−1, with ethanol extracts showing the lowest toxicity (706.0-741.0 mg ml−1) (see Table 1). The in vivo hemagglutination screening tests of C. cajan for anti-MV activity (Table 2) at a concentration of 250 mg ml−1 were all negative irrespective of the extraction method used, which included hot-water extract of the leaves (LHW), ethanol extract of the leaves (LET), hot-water extract of stems (SHW), ethanol extract of stems (SET), hot-water extract of roots (RHW) and ethanol extract of leaves (RET). Allantoic fluid harvests for groups treated with cold water extract of leaves (LWC), cold-water extract of stems (SWC) and cold-water extract of roots (RWC) all showed hemagglutination when tested by the spot HA technique. When tested by in vitro assays, LHW, LET, SHW, SET, RHW and RET inhibited CPE in Hep-2 tissue culture inoculated with MV at 250 mg ml−1 (Table 3), while some degree of CPE was observed in the microtitre wells containing cells treated with cold-water extracts (LWC, SWC and RWC).
Table 1.
The pH, percentage yield and LD50 of Cajanus cajan extracts
| Plant material | Extract | pH | % yield | LD50 (mg/ml) |
|---|---|---|---|---|
| Leaves | LWC | 4.81 | 12.19 | 639.0 |
| LHW | 5.14 | 9.15 | 616.0 | |
| LET | 5.19 | 11.52 | 742.0 | |
| Stems | SWC | 4.45 | 10.19 | 651.0 |
| SHW | 5.12 | 7.45 | 634.0 | |
| SET | 5.31 | 9.76 | 719.0 | |
| Roots | RWC | 4.65 | 8.51 | 602.0 |
| RHW | 5.51 | 6.45 | 626.0 | |
| RET | 5.22 | 7.28 | 707.0 |
LWC = cold-water extracts of the leaves; LHW = hot-water extracts of the leaves; LET = ethanol extracts of the leaves; SWC = cold-water extracts of the stem; SHW = hot-water extract of the stem; SET = ethanol extracts of the stem; RWC = cold-water extracts of the root; RHW = hot-water extracts of the root; RET = ethanol extracts of the root
Table 2.
In vivo screening of C. cajan at 250 mg ml−1 for anti-MV in ECE employing spot HA on allantoic fluid
| Inoculation route | Plant extract | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LWC | LHW | LET | SWC | SHW | SET | RWC | RHW | RET | PBS control | Virus control | |
| Albumen | + | − | − | + | − | − | + | − | − | − | + |
| Allantoic cavity | + | − | − | + | - | − | + | − | − | − | + |
+ = Presence of agglutination
− = Absence of agglutination
Table 3.
In vitro screening of C. cajan at 250 mg ml−1 for anti-MV activity in Hep-2 tissue culture
| Activity and morphology | Plant extract | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LWC | LHW | LET | SWC | SHW | SET | RWC | RHW | RET | Virus control | |
| Anti-MV Activity | − | + | + | − | + | + | − | + | + | − |
| Hep-2 cell morphology | R | N | N | R | N | N | R | N | N | R |
+ = Absence of cytopathic effect; − = Presence of cytopathic effect; R = Cells where round in shape (cytopathic effect was observed); N = Cells where normal in shape (cytopathic effect was not observed)
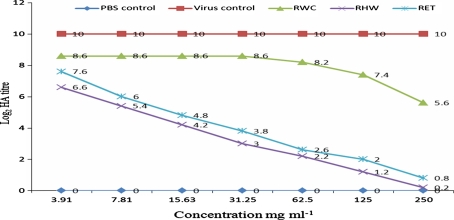
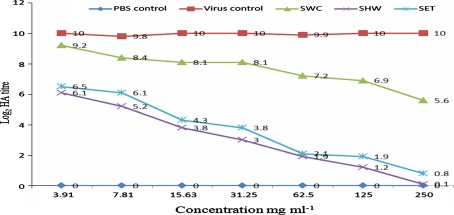
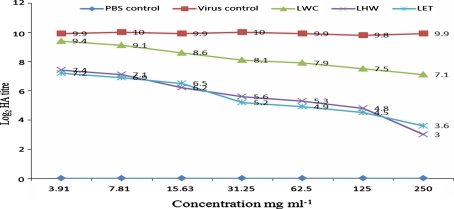
When C. cajan extracts were tested in vivo for anti-MV activity, the following log2 HA titres (p = 0.05) of allantoic fluid harvests were obtained: 0.2-6.6 (RHW), 0.8-7.6 (RET), and 5.6-8.6 (RWC) for root extracts; 0.1-6.1 (SHW), 0.8-6.5 (SET), and 5.6-9.2 (SWC) for stem extracts; and 3-7.4 (LHW), 3.6-7.2 (LET), and 7.1-9.4 (LWC) for leaf extracts (see Figs. 1, 2 and 3). The negative control (PBS) yielded no virus growth, as expected, while the positive virus control showed virus multiplication as the HA titre increased from an inoculum concentration of 3 to a value of 10 ± 0.2 (Log2).
Fig. 1.
HA titre of the in vivo evaluation of root extracts of C. cajan for anti-MV activity. PBS represents phosphate buffer control; RWC represents cold-water extract of the root; RHW represents hot-water extract of the root; RET represents ethanol extract of the root
Fig. 2.
HA titre of the in vivo evaluation of stem extracts of C. cajan for anti-MV activity. PBS represents phosphate buffer control; SWC represents cold-water extract of the stem; SHW represents hot-water extract of the stem; SET represents ethanol extract of the stem
Fig. 3.
HA titre of the in vivo evaluation of leaf extracts of C. cajan for anti-MV activity. PBS represents phosphate buffer control; LWC represents cold-water extract of the leaves; LHW represents hot-water extract of the leaves; LET represents ethanol extract of the leaves
The anti-MV activity of C. cajan on Hep-2 tissue culture in vitro showed a complete inhibition of CPE with the root extracts (ethanol and hot water) at concentrations of 250, 125 and 62.50 mg ml−1 (Table 4). Similarly, the stem extracts (ethanol and hot water) at 250 and 125 mg ml−1 showed complete inhibition of CPE on Hep-2 cells. However, at 31.25-3.91 mg ml−1, the root and stem extracts showed a varying percentage of CPE inhibition. On the other hand, the cold-water extracts (root and stem) showed 59-30% inhibition of CPE at 250 mg ml−1 (Table 4). The phytochemical analysis of C. cajan extracts revealed the presence of flavonoids in all fractions, with higher concentrations being present in hot-water extracts. Carbohydrates and reducing sugars were also present in all fractions in various amounts, while tannins, alkaloids and glycosides were present only in some fractions (Table 5).
Table 4.
In vitro anti-MV activity of C. cajan extracts
| Concentration (mg/ml) | % inhibition of cytopathic effect by C. cajan extracts | |||||
|---|---|---|---|---|---|---|
| SWC | SHW | SET | RWC | RHW | RET | |
| 3.91 | − | + | + | − | + | + |
| 7.81 | − | + | + | − | + | + |
| 15.63 | − | + + | + + | − | + + | + |
| 31.25 | − | ++ | + + | − | + + | ++ |
| 62.50 | − | ++ | + + | − | +++ | +++ |
| 125 | + | +++ | +++ | − | +++ | +++ |
| 250 | + | +++ | +++ | + | +++ | +++ |
| Extract control | N | N | N | N | N | N |
| PBS control | N | N | N | N | N | N |
+++ = 100–80% inhibition of CPE; ++ = 79–60% inhibition of CPE; + = 59–30% inhibition of CPE; − = 29–0% inhibition of CPE; N = cells were normal in shape
Table 5.
Phytochemical composition of Cajanus cajan extracted with ethanol and water (hot and cold)
| Phytochemicals | Leaves | Stems | Roots | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LWC | LHW | LET | SWC | SHW | SET | RWC | RHW | RET | |
| Alkaloids | − | − | − | − | + | + | − | − | − |
| Tannins | − | + | + | + | ++ | ++ | − | + | ++ |
| Saponins | + | − | ++ | + | − | ++ | − | − | + |
| Flavonoids | + | +++ | ++ | ++ | +++ | ++ | ++ | +++ | ++ |
| Carbohydrates | + | ++ | + | + | ++ | + | + | ++ | ++ |
| Glycosides | ++ | + | ++ | + | + | ++ | − | − | − |
| Reducing sugars | ++ | + | ++ | + | + | ++ | + | + | ++ |
+ = Present in trace amounts; ++ = Present in moderate amounts; +++ = Present in high amounts; − = Not detected
Discussion
The pH of C. cajan extracts (4.45-5.51) agrees with pH values reported for most plant extracts [2, 4, 19]. Differences in percentage yields obtained by the different extraction methods did not correlate with activity, since extracts with the highest yield were the least active. Caution must be used in interpreting the lethality assay, since different parts of the plant yielded different values, indicating an uneven distribution of secondary metabolites. The partitioning of secondary metabolites may have resulted in variable activity and not toxicity, although the variations observed with different plant parts and different extraction solvents were not statistically significant at a level of 0.05. The LD50 values were three times higher than the test value, implying that embryo death was not the result of extract toxicity but was due to the MV used to experimentally infect the ECE. This agrees with the fact that the control groups in the tissue culture assay that were treated with the extracts alone did not show any deleterious effects, since Hep-2 cells grew normally. One limitation of phytotherapy in traditional medicine is the absence of dose standardization. The efficacy of concoctions varies with different herbal doctors, and so does the dose they recommend. Furthermore, the belief that herbal remedies are not toxic is another complicating factor for establishing dose standardization. The activity of C. cajan extracts against MV may be due to constituents such as flavonoids, tannins and alkaloids found to be present in the extracts and known to possess antiviral activity [14, 16]. Attributing the activity to flavonoids may be unjustified, since they were detected in fairly high amounts in all of the extracts, although anti-MV activity varied significantly. At present, it is not clear which plant constituents, singly or in synergy, are responsible for the observed anti-MV activity. Separation of the different constituents present in the extracts will be necessary to identify the active substances. Different routes of administration (allantoic cavity or albumen) of the herbal extracts in the in vivo method did not appear to influence the outcome of extract activity since they produced similar results. The in vivo anti-MV assay showed that the hot-water and ethanol extracts of C. cajan roots and stems possessed a significant activity against MV, since it reduced the hemagglutination titre to almost undetectable levels (0.2 and 0.1). The cold water extracts showed little inhibitory activity against MV although they had a very similar composition. The in vitro assay similarly showed that hot-water and ethanol extracts inhibited MV replication, as indicated by the absence of CPE at higher extract concentrations. In vivo inhibition was demonstrated both by CPE and by agglutination of Macaca mulatta red cells.
Conclusion
The antiviral activity observed at all concentrations of hot-water and ethanol extracts indicates that certain constituents of C. cajan possess anti-MV properties, and the identity of the active substances should be further analyzed. Since MV infection can be overcome by the development of an adequate immune response, the exact potential efficacy of C. Cajan extracts should be further analyzed.
Acknowledgments
The authors are grateful to the Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare, Alice, South Africa, for the facilities used in the course of manuscript preparation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Alfred MW, Gilbert MS. Probit analysis of data for infection with echovirus-12 should generate a straight line. J Infect Dis. 1985;152(3):649–650. doi: 10.1093/infdis/152.3.649. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R, Meda ME, Cassiolato MA, Pavan M, Mário M (2001) Alleviating Soil Acidity through Plant Organic Compounds. Braz Arch Biol Technol 44(1):185–189
- 3.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dnyaneshwar WP, Chavan KJ Bhushan P (2003) DNA isolation from fresh and dry plant samples with highly acidic tissue extracts. Plant Mol Biol Rep 21:467–467
- 5.Duke JA. Handbook of legumes of world economic importance. New York: Plenum Press; 1981. [Google Scholar]
- 6.Esimone CO, Grunwald T, Wildner O, Nchinda G, Tippler B, Proksch P, Uberla K. Invitro pharmacodynamic evaluation of antiviral medicinal plants using a vector-based assay technique. J Appl Microbiol. 2005;99:1346–1355. doi: 10.1111/j.1365-2672.2005.02732.x. [DOI] [PubMed] [Google Scholar]
- 7.Fernando LM, Fabricio JB, Walter ARJ, João CP, Carlos N, Rosa ECL. The in vitro antiviral activity of an aliphatic nitro compound from Heteropteris aphrodisiaca. Microbiol Res. 2008;163(2):136–139. doi: 10.1016/j.micres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Finney DJ. Probit analysis. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- 9.Finney DJ. Statistical method in biological assay. London: Charles Griffin & Co; 1978. [Google Scholar]
- 10.Firas A, Al-Bayati C. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J Ethnopharmacol. 2008;116:403–406. doi: 10.1016/j.jep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Gomez KA, Gomez AA (1984). Statistical procedures for agricultural research. In: An international rice research institute book, 2nd edn. Wiley, New York
- 12.Harbone JB. Phytochemical methods: a guide in modern techniques of plant analysis. London: Chapman & Hall; 1984. pp. 221–232. [Google Scholar]
- 13.Heartl A, Suerbrei A, Stelzner A, Wutzler P. Influenza infection of the embryonated hen’s egg-an alternative model for in vivo evaluation of antiviral compounds. Arzneimittel Forschung-Drug Research. 2004;54(2):130–134. doi: 10.1055/s-0031-1296948. [DOI] [PubMed] [Google Scholar]
- 14.Hu K, Kobayashi H, Dong A, Iwasaki S, Yoa X. Antifungal, antimycotic and anti-HIV-1 agents from the roots of Wilkstroemia indica. Planta Medica. 2000;66:564–567. doi: 10.1055/s-2000-8601. [DOI] [PubMed] [Google Scholar]
- 15.Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma SC, But PH, Ooi VE, He YH, Lee SH, Lee SF, Lin RC. Antiviral amentoflavone from Selaginella sinensis. Biol Pharm Bull. 2001;24:311–312. doi: 10.1248/bpb.24.311. [DOI] [PubMed] [Google Scholar]
- 17.Morton JF. The pigeon pea (Cajanus cajan Millsp.), a high protein tropical bush legume. HortScience. 1976;11(1):11–19. [Google Scholar]
- 18.Mudassir AZ, Sidney AC. Biologically active traditional medicinal herbs from Balochistan, Pakistan. J Ethnopharmacol. 2005;96(1–2):331–334. doi: 10.1016/j.jep.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Nwodo UU, Ngene AA, Iroegbu CU. Effects of fractionation on antibacterial activity of crude extracts of Tamarindus indica. Afr J Biotechnol. 2010;9(42):7108–7113. [Google Scholar]
- 20.OIE (2004). Newcastle disease. Manual of standard for diagnostic tests and vaccines. Office International des Epizootics, Paris, pp 221–232
- 21.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okigbo RN, Mmeka EC. Antimicrobial effects of three tropical plant extracts on Staphylococcus aureus, Escherichia coli and Candida albicans. Afr J Tradit Complim Altern Med. 2008;5(3):226–229. doi: 10.4314/ajtcam.v5i3.31277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoli AS, Iroegbu CU. Evaluation of extracts of Anthocleista djalonensis, Nauclea latifolia and Uvaria afzalii for activity against bacterial isolates from cases of non-gonococcal urethritis. J Ethnopharmacol. 2004;92(1):135–144. doi: 10.1016/j.jep.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Okoli AS, Okeke MI, Iroegbu CU, Ebo PU. Antibacterial activity of Harungana madagascariensis leaf extract. Phytother Res. 2002;16(2):174–179. doi: 10.1002/ptr.991. [DOI] [PubMed] [Google Scholar]
- 25.Paolo MG. Traditional antihelmintic, antiparasitic and repellent uses of plants in Central Italy. J Ethnopharmacol. 1999;68(1–3):183–192. doi: 10.1016/s0378-8741(99)00089-6. [DOI] [PubMed] [Google Scholar]
- 26.Parker RC. Methods of tissue culture. 3. New York: P. B. Hoeber, Inc.; 1961. [Google Scholar]
- 27.Paster N, Menasherov M, Ravid U, Juven B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J Food Prot. 1995;58(1):81–85. doi: 10.4315/0362-028X-58.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Quinlan MB, Quinlan RJ, Nolan JM. Ethnophysiology and herbal treatment of intestinal worm in Dominica West India. J Ethnopharmacol. 2002;80(1):75–83. doi: 10.1016/S0378-8741(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 29.Sidwell RW, Smee DF. In vitro and in vivo systems for the study of influenza virus inhibitors. Antivir Res. 2000;48:1–6. doi: 10.1016/S0166-3542(00)00125-X. [DOI] [PubMed] [Google Scholar]
- 30.Trease GE, Evans WC (1978). Phytochemistry: introduction and general methods. In: Pharmacognosy, 11th edn, pp 227–247
- 31.Ugonabo JAC, Nwodo UU, Ngene AA, Nwuche CO, Wopara RK. Studies on the antibacterial properties of the leaf extracts of Chromolaena odorata (L.) King and Robinson (Asteraceae) Bioresearch. 2007;5:228–230. [Google Scholar]
- 32.Vlietinck AJ, Vanden Berghe DA. Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacol. 1991;32:141–153. doi: 10.1016/0378-8741(91)90112-Q. [DOI] [PubMed] [Google Scholar]
- 33.Wang JX, Zhou JY, Yang QW, Li X, Piao YA, Li HY. An improved embryonated chicken egg model for the evaluation of antiviral drugs against influenza A virus. J Virol Methods. 2008;153(2):218–222. doi: 10.1016/j.jviromet.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Williams JE. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on Una de Gato and Sangre de Grado. Altern Med Rev. 2001;6:567–579. [PubMed] [Google Scholar]
- 35.Yin MC, Cheng WS. Inhibition of Aspergillus niger and Aspergillus flavus by some herbs and spices. J Food Prot. 1998;61(1):123–125. doi: 10.4315/0362-028x-61.1.123. [DOI] [PubMed] [Google Scholar]
- 36.Yu L, Guo-Song C, Yong C, Jun L. Inclusion complexes of Azadirachtin with native and methylated cyclodextrins: solubilization and binding ability. Bioorganic Med Chem. 2005;13:4037–4042. doi: 10.1016/j.bmc.2005.03.051. [DOI] [PubMed] [Google Scholar]