HSP90 regulates cyclical glucocorticoid receptor activity, cofactor recruitment, histone acetylation and transcriptional pulsing at the Period 1 promoter in response to ultradian glucocorticoid exposure.
Abstract
Glucocorticoid (GC) hormones are secreted from the adrenal gland in a characteristic pulsatile pattern. This ultradian secretory activity exhibits remarkable plasticity, with distinct changes in response to both physiological and stressful stimuli in humans and experimental animals. It is therefore important to understand how the pattern of GC exposure regulates intracellular signaling through the GC receptor (GR). We have previously shown that each pulse of ligand initiates rapid, transient GR activation in several physiologically relevant and functionally diverse target cell types. Using chromatin immunoprecipitation assays, we detect cyclical shifts in the net equilibrium position of GR association with regulatory elements of GC-target genes and have investigated in detail the mechanism of pulsatile transcriptional regulation of the GC-induced Period 1 gene. Transient recruitment of the histone acetyl transferase complex cAMP response element-binding protein (CREB) binding protein (CBP)/p300 is found to precisely track the ultradian hormone rhythm, resulting in transient localized net changes in lysine acetylation at GC-regulatory regions after each pulse. Pulsatile changes in histone H4 acetylation and concomitant recruitment of RNA polymerase 2 precede ultradian bursts of Period 1 gene transcription. Finally, we report the crucial underlying role of the intranuclear heat shock protein 90 molecular chaperone complex in pulsatile GR regulation. Pharmacological interference of heat shock protein 90 (HSP90) with geldanamycin during the intranuclear chaperone cycle completely ablated GR's cyclical activity, cyclical cAMP response element-binding protein (CREB) binding protein (CBP)/p300 recruitment, and the associated cyclical acetylation at the promoter region. These data imply a key role for an intact nuclear chaperone cycle in cyclical transcriptional responses, regulated in time by the pattern of pulsatile hormone.
In the intact animal, the endogenous secretion of glucocorticoids (GC) from the adrenal gland occurs in a distinctive circhoral pattern with pulses at approximately hourly intervals (1, 2). This hormone profile interacts directly with individual stress responses (3, 4) and is modulated by physiological parameters, such as age, sex, and lactation (5), as well as pathophysiological processes associated with immunological, metabolic, cardiovascular, and affective dysfunction (6, 7). Because virtually every organ system in the body has GC receptor (GR) expression, it is important to understand how individual cells and tissues read the digital signal from pulses of GC hormones and indeed how they terminate their response when hormone levels rapidly diminish.
The classic static model of gene regulation involving prolonged binding of GR to DNA at specific GC regulatory elements (GRE) in target gene promoters has been superseded by a more dynamic model of nuclear receptor action (8, 9). Single cell imaging and fluorescent recovery after photobleaching technology have revealed that rapid chromatin exchange occurs in a timescale of seconds with GR binding causing chromatin remodeling and allowing a cycle of transcription to proceed. The chromatin transition results in ejection of GR from the DNA template, before GR can bind again (10–12). These studies have provided fascinating new insights into the real-time kinetics of GR interactions with the chromatin template, yet provide less information about the overlying slow cycling of the receptor at equilibrium position at individual DNA regulatory sites within the promoter regions of physiologically relevant “natural” target genes.
We have recently proposed that physiological GR function requires the ligand to be presented to target cells in discrete pulses, which are necessary for the establishment and maintenance of optimally regulated gene activation (13). We have shown that exposure of cells to pulses of their physiologically relevant ligand (cortisol for human HeLa cells and corticosterone for rat HTC and mouse AtT-20 cells) results in cyclical GR activation (14). In this manuscript, we now elucidate pulse-directed slow cycling of GR at GC regulatory regions in the promoters of the Period 1 (PER1), metallothionein I (MT1), tyrosine aminotransferase (TAT), and proopiomelanocortin (POMC) genes. Furthermore, we explore the mechanisms underlying the transcriptional pulsing phenomenon associated with the ultradian GC rhythm. GC pulse-directed cyclical regulation of the PER1 gene has been pursued in specific detail, and we have found that the robust cyclical transcriptional activity of GR at the PER1 gene involves cyclical actions of cAMP response element-binding protein (CREB) binding protein (CBP)/p300, rapid and reversible acetylation of histone H4, and cycles of RNA polymerase 2 (RNA Pol2) recruitment to the PER1 promoter region. Finally, we report that the intranuclear chaperone cycle is a necessary and integral feature of the cyclical transcriptional activity at the PER1 promoter. When the chaperone cycle is disrupted by heat shock protein 90 (HSP90) inhibition with geldanamycin (GA), pulsatile GR transcriptional activity is ablated at the primary step of ligand-induced GR activation. Subsequent steps of GR association with regulatory regions in the PER1 promoter, recruitment of CBP/p300, increased acetylation status, and production of nascent RNA ceases in the absence of an intact molecular chaperone cycle.
Ours is the first report of ultradian GC rhythm-regulated cyclical activity of the histone acetyl transferases (HAT) CBP/p300, with remarkably rapid and reversible pulsatile changes in histone H4 acetylation status, consequent rhythmic changes in RNA Pol2 recruitment, and the associated pulsatile production of nascent RNA from a GC-target gene.
Results
Ultradian GR slow cycling at the chromatin template
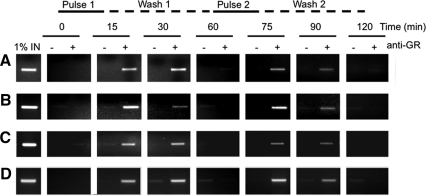
Three physiologically relevant and functionally diverse cell types, the pituitary-derived corticotroph AtT-20, the hepatocyte-derived HTC, and the cervical epithelial cancer cell HeLa, have previously been chosen to assess GR activation kinetics to pulses of natural GC. In all three cell lines, each pulse of GC hormone initiated a “wave” of GR activation and DNA association, which decreased rapidly after hormone washout, returning to baseline levels before addition of the next pulse 45 min later (14). Because these results measured GR binding to synthetic oligonucleotides containing the canonical GRE sequence, it was important to confirm the results using chromatin immunoprecipitation (ChIP) assay to measure GR association with GRE sites in “real” target genes. Therefore, we tested whether the endogenous ligands corticosterone and cortisol could also direct cycles of enhanced GR association with chromatin at specific GRE in natural GC-regulated gene promoters. Primers were designed to DNA regions flanking known GRE in the human PER1 gene promoter, the mouse MT1 gene promoter, the mouse POMC gene promoter, and the rat TAT gene promoter. Pulses of cortisol or corticosterone were applied to each relevant cell type (human HeLa cells for PER1, mouse pituitary corticotroph AtT-20 cells for POMC and MT1, and liver hepatoma HTC cells for TAT). Cells were fixed with 1% formaldehyde at defined time points throughout the time course, and ChIP assays were used to determine the net GR association detectable at each time point. A distinct pattern emerged from the ChIP studies, with GR oscillating between a predominantly chromatin-associated form and a predominately dissociated form (Fig. 1). This pattern was evident at each of the regulatory DNA regions analyzed. Furthermore, the highly consistent pattern observed followed the temporal kinetics of GR activation determined by GRE oligonucleotide binding of prepared nuclear extracts in the TransAM assay (14). Interestingly, the dynamics of activated GR at the chromatin template were similar for all GRE tested, including the negative GRE of the GC repressed gene POMC. The rapid exchange of GR at the chromatin template (15) allows receptor to constantly sense intracellular hormone concentrations and respond quickly to the ultradian pulses of ligand. These highly responsive networks impose a distinctive temporal periodicity in regulation on GR action. The temporal regulation was conserved across four different genes, irrespective of whether they were induced or repressed by GC, as well as across three different species and cells derived from three different tissue types.
Fig. 1.
Activated GR associates in a pulsatile fashion with the regulatory elements of GC target genes involved in HPA axis regulation, protein metabolism, basic cellular function, and peripheral clock resetting. In vivo ChIP assays showed that the same regime of pulses applied to each cell line caused cyclical changes in GR chromatin association activity at GRE of natural target gene promoters, including the GRE in the mouse MT1 gene promoter in the AtT-20 line (A), human PER1 gene promoter in the HeLa line (B), and the rat TAT in the HTC line (C), as well as the negative GRE of the mouse POMC gene promoter in the corticotroph-derived AtT-20 line (D).
Cyclical recruitment of CBP/p300 and pulsatile histone H4 acetylation during ultradian transcriptional regulation
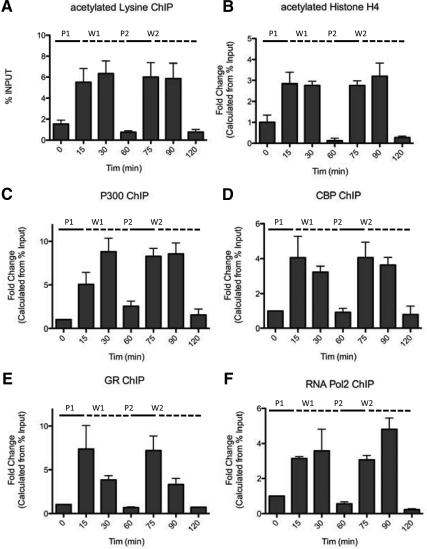
The Clock gene PER1 has been particularly well characterized as a responsive target for pulsatile hormone treatment (13, 16). Therefore, PER1 was chosen for a detailed study of the underlying mechanisms of its pulsatile transcriptional regulation. Generalized local acetylation status at the PER1 promoter proximal GRE site was first assessed by ChIP, using antiacetylated lysine antibody. Local acetylation fluctuated significantly with each pulse of hormone addition, correlating well with the described transcriptional pulse profile. General acetylation increased at 15 min and was maximal at 30 min, relative to the start of each 15-min pulse (Fig. 2A). This increased acetylation was rapidly reversible, decreasing to baseline levels by 60 min. Histone H4 is a known target for acetylation (17). Increased histone H4 acetylation is associated with recruitment of chromatin remodeling factors of the SWItch/Source NonFermentable family such as Brahma-related gene 1, which allows access of RNA Pol2 and auxiliary transcription machinery to the transcription start site (18, 19) and is often correlated with transcriptional activity (20). ChIP assay using a specific acetylated histone H4 antibody confirmed pulsatile acetylation of this structural nucleosome core protein (Fig. 2B). The HAT complex members CBP and p300 were also assessed using ChIP and were found to undergo cyclical recruitment to the PER1 GRE region (Fig. 2, C and D). The pattern of both CBP and p300 recruitment closely tracked the GR slow cycling profile (Fig. 2E) and in turn was closely followed by cyclical RNA Pol2 recruitment (Fig. 2F). The pattern of cyclical Pol2 recruitment was consistent with our previously described pulses of PER1 transcription in cell lines and rat liver (13) and rat hippocampus (16).
Fig. 2.
Rapid and reversible acetylation of histone H4 at the PER1 promoter during cyclical recruitment of GR, CBP, p300, and RNA Pol2. A, An increase in generalized protein acetylation at the proximal GRE-containing region of the PER1 promoter was detected at 15 min and was maximal at 30 min, relative to the start of each 15-min pulse. B, ChIP assay using a specific acetylated histone H4 antibody confirmed H4 as an acetylation target. The HAT complex members CBP (C) and p300 (D) were both found to undergo cyclical recruitment to the PER1 GRE region. The pattern of both CBP and p300 recruitment closely tracked (E) the GR slow cycling profile and in turn was closely followed by (F) cyclical RNA Pol2 recruitment. P, Pulse; W, wash.
An exclusively intranuclear activity of GR is dependent on an intact HSP90 chaperone complex
Nuclear HSP90 sustains GR residence time and confers protection from proteasome-dependent nuclear clearance
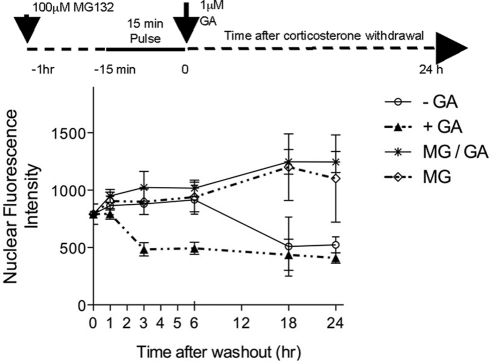
Potential mechanisms for the dynamic responsiveness to pulsatile corticosterone administration were tested in the AtT-20 cell line. To test whether pulsatile GR responses were due to regulated nuclear cytoplasmic shuttling, high-powered analysis using the IN Cell 1000 Analyzer (GE Healthcare, Princeton, NJ) (21) was performed to quantitate GR cytoplasmic to nuclear translocation and nuclear retention kinetics. Initial studies with the IN Cell 1000 Analyzer revealed that constant corticosterone exposure resulted in rapid translocation of GR from the cytoplasm to the nucleus, with redistribution completed at 10–15 min of treatment onset (data not shown). Further incubation times of up to 60 min did not increase the nuclear/cytoplasmic ratio. Next, a single pulse of hormone was added to cells. Interestingly, nuclear GR levels remained maximal for several hours after hormone withdrawal (Fig. 3) and then slowly cleared from the nucleus by 24 h consistent with previous reports in cultured cell experiments (22). Treatment with the HSP90 inhibitor GA, applied to the cells after the first pulse when GR nuclear translocation was complete, resulted in an increased clearance of GR from the nucleus during the acute phase of the time course (first 3 h of hormone withdrawal). The GA-induced accelerated nuclear GR clearance was reversed by preincubation with the specific 19S proteasome inhibitor MG132.
Fig. 3.
HSP90 and the proteasome have opposing effects on GR nuclear retention in AtT-20 cells after a single corticosterone pulse. IN Cell 1000 analysis data presented graphically here show time (hours) after ligand withdrawal. The solid trace (open circles) represents corticosterone-treated cells, treated with vehicle after ligand washout. Here, GR was retained in the nucleus for up to 6 h after ligand washout. Clearance from the nucleus then occurred slowly and was complete by 24 h after ligand washout. The dashed trace (filled triangles) represents treatment with 1 μm GA after the 15-min corticosterone pulse was washed from the cells (termed posttreatment). Inhibition of HSP90, after the GC pulse, resulted in an increased rate of clearance of GR from the nucleus. The effect of HSP90 posttreatment was reversed by preincubation of the cells with the proteasome inhibitor MG132; the solid trace (stars) represents preincubation with 10 μm MG132 before the 15-min corticosterone pulse and GA posttreatment. The dashed trace (open diamonds) represents preincubation with 10 μm MG132 before the 15-min corticosterone pulse, in the absence of GA treatment. MG132 appeared to affect both the GA-related, accelerated GR nuclear clearance as well as the slower naturally occurring GR nuclear clearance after hormone withdrawal.
Therefore, it appears that in the AtT-20 cell line, HSP90 and the related molecular chaperones act to protect GR from some form of proteasome-dependent clearance mechanism. Due to reports that the proteasome acts as a mobility factor (23, 24), we cannot rule out the possibility that the observed effect of MG132 is due to a lack of nuclear export resulting from immobilization of GR within the nucleus. However, the data strongly indicate that the HSP90 chaperone acts to effectively retain GR within the nucleus, suggesting that pulse responsiveness may occur in the intranuclear recycling loop first proposed by DeFranco and co-workers (25).
Ultradian activity of GR occurs in an exclusively intranuclear circuit
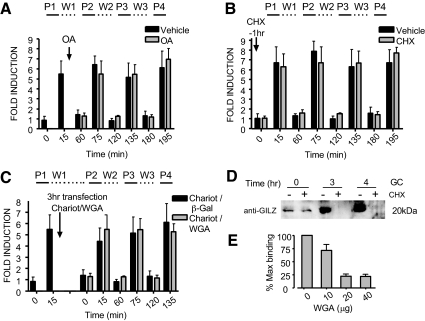
To conclusively establish whether GR responsiveness to pulsatile GC treatment occurred independently of cytoplasmic transit, we examined whether the cyclic changes in GR activity required reimport of exported GR with each pulse of corticosterone. Treatment with okadaic acid, a nuclear translocation inhibitor for recycled cytoplasmic GR (26), did not decrease GR cyclical activity (Fig. 4A). Similarly, treating AtT-20 cells with cyclohexamide (CHX), an inhibitor of de novo protein synthesis (27), did not reduce cycles of GR reactivation. Therefore, new synthesis of cytoplasmic GR was not necessary for pulsatile GR activity (Fig. 4B). Finally, to ensure that all active transport through the nuclear pore was inhibited, cells were transfected with wheat germ agglutinin (WGA) using the protein transfection reagent Chariot (Active Motif, Carlsbad, CA) (28). WGA was delivered into the cells after nuclear translocation was complete but before the next pulse of corticosterone. Even when all active transit via the nuclear pore was inhibited, the GR activation-deactivation cycle continued during pulsatile GC treatment (Fig. 4C). Experimental controls for the efficacy of CHX and WGA are shown in Fig. 4, D and E, respectively.
Fig. 4.
Each cycle of GR reactivation is exclusively intranuclear and does not require transit through the cytoplasm. A, Treatment with okadaic acid (OA) after nuclear translocation was complete had no effect on the subsequent cyclical waves of GR activation measured by TransAM GRE binding of nuclear extract prepared from cells at each indicated time point. B, CHX inhibition of de novo protein synthesis had no effect on the subsequent intranuclear GR cyclical DNA association activity. C, All active transport through the nuclear pore was inhibited by transfection of the cells with the lectin WGA using the protein transfection reagent Chariot (Active Motif) after the initial pulse-directed GR translocation is complete. Subsequent intranuclear cyclical DNA association activity was unaffected by WGA transfection. The bacterial protein β-galactosidase (β-GAL) was used as a transfection control. D, Protein extracts from cells treated with CHX in part (B) were Western blotted to PVDF membrane and probed for the GC-inducible leucine zipper protein (GILZ) to verify de novo protein synthesis had been ablated. Clearly, this dose of CHX inhibited expression of GILZ protein during the time course of the experiment. E, Transfection of WGA before the first pulse of corticosterone inhibited nuclear translocation of GR and GRE binding in the TransAM assay. P, Pulse; W, wash.
Pulsatile GR responsiveness depends on an intact nuclear chaperone complex
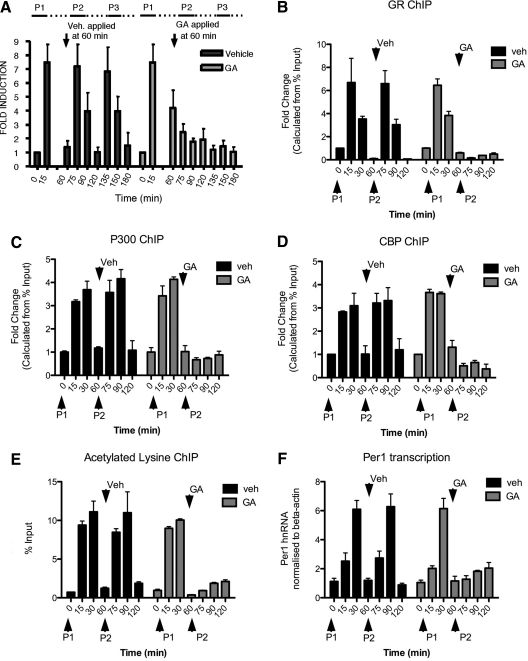
The molecular mechanism underlying the pulsatile hormone-directed slow cycling activity of GR and the subsequent effects on cyclical transcriptional activity at the PER1 gene were next addressed. Based on work that describes the HSP90 p23 molecular chaperone complex recruitment to GRE in rat liver HTC cells (29), we reasoned that the chaperone complex might play an integral role in GR transcriptional responsiveness to pulses of ligand. The chaperone complex centers around a direct HSP90-GR interaction, and the specific inhibitor of this interaction GA works rapidly within the timeframe of the physiological interpulse interval. Therefore, to test whether the chaperone complex is involved in pulsatile GR activity, GA was applied to cells during pulsatile GC exposure. It was surmised that if GR is reactivated by each pulse of corticosterone in the nuclear compartment, then it must reassociate with the chaperone complex to retain the ligand binding cleft in the correct conformation for ligand rebinding, receptor reactivation, and DNA binding. Therefore, the final set of experiments was to inhibit HSP90 with GA after completion of the first cycle of GR activation and inactivation but while all the GR were still within the nuclear compartment. This was an important aspect of the experimental design, because GA treatment is known to inhibit ligand binding and GR activation in the cytoplasmic compartment and thus cytoplasmic to nuclear translocation. Figure 5A shows that GA treatment (postcorticosterone withdrawal) completely abrogated further responsiveness of GR to pulses of corticosterone. It should be noted that the absolute levels of nuclear GR are not yet depleted in the first 2 h of GA treatment (see Fig. 3). These results strongly support a role for chaperone activity in GR reactivation by ligand within the nucleus, a function previously well described for HSP90 in the cytoplasm for the initial round of GR activation (30). Because GR action was completely inhibited at the first stage of ligand-induced activation, it was hypothesized that all subsequent transcriptional events would also be inhibited. The consequence of GA treatment on downstream transcriptional events was confirmed to be a complete ablation of ultradian cyclical transcriptional activity at the PER1 gene. Figure 5 shows that the first pulse (before GA treatment) causes recruitment of GR to the chromatin template at the GRE site (Fig. 5B), recruitment of CBP (Fig. 5C) and p300 (Fig. 5D), increased acetylation (Fig. 5E), and a pulse of nascent PER1 RNA production (Fig. 5F). After GA treatment, neither GR, CBP, or p300 were detected at the GRE site, and acetylation and the transcription rate remained at low basal levels. All cyclical transcriptional activity was inhibited, consistent with a lack of GR responsiveness to pulsatile hormone addition.
Fig. 5.
Inhibition of HSP90 with GA renders GR unable to be reactivated by subsequent pulses of CORT and ablates cyclical downstream transcriptional events. A, Treatment of AtT-20 cells with GA after nuclear translocation was complete and after GR dissociation from the DNA template (i.e. at 45 min after corticosterone withdrawal) resulted in failure of the GR to be subsequently reactivated by further GC pulses. Activated GR was measured in nuclear extracts in the TransAM assay. B, ChIP assay revealed that GR association with the PER1 GRE followed the expected kinetics observed in Fig. 1 for the first pulse. After treatment with GA, GR could not be detected at the PER1 GRE during the second corticosterone pulse. The HAT p300 (C) and CBP (D) were both recruited to the GRE, closely after GR. This association was maximal at 15 and 30 min but reduced to baseline levels by 60 min. The transient recruitment of p300 and CBP to the GRE was ablated after treatment with GA. E, The pulsatile changes in net lysine acetylation at the PER1 GRE was negated by GA treatment. F, Pulsatile transcription (measured here by quantitative PCR of PER1 hnRNA) was ablated by GA treatment. P, Pulse; Veh, vehicle.
Discussion
Repeated pulses of natural GC ligand initiate and maintain rapid and transient pulsatile activation of GR, and cyclical changes in GR chromatin association profiles at specific DNA regulatory elements in endogenous GC regulated gene promoters. In the case of PER1, pulsatile GR action was associated with the consequent cyclical recruitment of CBP, p300, and RNA Pol2, cyclical increases in histone H4 acetylation, and pulses of nascent PER1 RNA. Interestingly, the related steroid receptor, the estrogen receptor (ER), has been reported to act in a cyclical transcriptional mechanism. ER cycling on target gene promoters was shown to occur in an ordered, cyclical manner modulated by estradiol and other agonist compounds (31, 32). The cyclic assembly and disassembly during the hormonal response shows a periodicity of 40 min for estradiol-bound ER, with some of the cofactors reloading again within 2 h of the original hormone treatment. In stark contrast to the findings with ER, GR and its associated transcriptional coactivators have been shown to remain constantly loaded on the mouse mammary tumor virus promoter over 2 h during constant hormone treatment (33, 34). Taken together, these reports strongly suggest that ER exhibits an intrinsic cyclical activity, whereas GR does not. We therefore propose the alternative mechanism for GR function. Here, the ligand acts as the determining factor for cyclical activity at the target gene promoter. The stochastic GR action at the chromatin template, which has been so well elucidated by the Hager lab (8, 9, 15, 34), appears to work optimally with the deterministic pattern of pulsatile ligand exposure to establish and maintain this striking cyclical transcriptional activity.
Notably, the cyclical activation profile of GR to pulsatile GC administration was found to be independent of nuclear cytoplasmic shuttling. In fact, after the first pulse, GR remained confined to the nuclei of AtT-20 cells for several hours, consistent with the previously reported nuclear export rate of t1/2 = 8–9 h (22, 35). Our finding agrees with many reports derived from different methodologies (25, 35, 36), notably including a study describing continuous ligand responsiveness in a GR mutant selectively retained in the nucleus via addition of the heterologous simian virus 40 nuclear localization sequence (37). Our IN Cell 1000, high content imaging has enabled robust quantification of this phenomenon, because over 10,000 cells were analyzed simultaneously and without experimental bias (21, 38).
It has been appreciated for many years that the nuclear chaperone complex is critically important for nuclear retention of GR (39), because inhibition of HSP90 with GA significantly reduced GR nuclear localization. This study indicates that a significant proportion of “unchaperoned” nuclear GR appears to undergo some form of proteasome-dependent clearance, because pretreatment with the proteasome inhibitor MG132 greatly reduced the effect of GA on GR clearance from the nucleus and restored GR nuclear retention. These findings strongly implicate the HSP90 chaperone assembly involvement in retention of GR within the nucleus, enabling an intranuclear circuit capable of responding to pulsatile ligand exposure.
Although models describing an intranuclear recycling mechanism for GR have been in existence for over 10 yr (25, 36, 39), our study places the mechanism in a physiologically relevant context related to the naturally occurring ultradian GC secretory rhythm. The original studies by DeFranco and co-workers, based on a series of elegant experiments in digitonin-treated, cytosol-free cells, showed a deoxyribonuclease sensitive, high affinity binding of GR to the nucleus in the presence of GC. This changed to a more loosely associated binding after ligand washout. Upon hormone readdition, GR reacquired its deoxyribonuclease sensitive, high affinity state, thus providing compelling although indirect evidence of cycles of DNA association. Further evidence to support this model derives from live cell imaging studies from Hager and co-workers (40) showing green fluorescent protein-GR localized to an artificial array of GRE in a 200-copy tandem repeat of the mouse mammary tumor virus promoter. Stavreva et al. (13) have recently used this model system to investigate the effect of ultradian patterns of ligand exposure on GR activity and established a “gene pulsing” effect dependent on pattern of ligand exposure to the target cell and independent of cytoplasmic export. We have now extended this study to include pulse-directed GR activity on real GC target genes in physiologically relevant and functionally diverse GC target cell types. Direct evidence for defined cyclical changes in the set-point of GR's chromatin association activity, regulated by pulses of the natural GC corticosterone and cortisol, is presented. We further show that an exclusively intranuclear chaperone cycle is critically involved in generating nuclear GR responsiveness to pulsatile GC treatment.
Our data strongly support a model that places the HSP90/p23 molecular chaperone complex in the nuclear compartment and fundamental for GR intranuclear function. HSP90 and p23 have both been implicated in many diverse and significant chromatin-associated functions (25, 29, 30, 35, 39–41). We also present the first evidence for a significant physiological role of these molecular chaperones as key regulators of GR cyclical transcriptional activity during ultradian hormone rhythmicity. Our results strongly indicate that the chaperone activity of HSP90 is required for GR reactivation by ligand in the nucleus, because inhibition of HSP90 significantly attenuated GR responsiveness to subsequent pulses of corticosterone (Fig. 5). We suggest that the function of the nuclear chaperone complex in this context is most likely to retain the GR ligand binding cleft in the correct conformation for ligand rebinding and receptor reactivation.
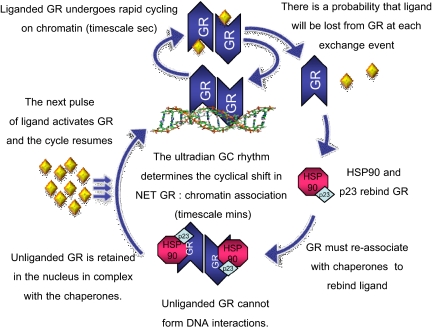
Our model for GR activation during basal pulsatility at the circadian peak is described in Fig. 6. Here, the activity of GC target cells is regulated by discrete pulses of ligand in an intranuclear circuit mediated by the chaperone complex. The receptor exchanges rapidly with binding sites in accessible chromatin domains during the process of chromatin remodeling (9, 12); at some point during this exchange process, ligand is lost, and the receptor must reenter the chaperone cycle to rebind hormone. Each pulse-directed GR activation cycle initiates a new cycle of cofactor recruitment, histone acetylation, and transcriptional activity (at the PER1 gene studied). Inhibition of intranuclear HSP90 inhibits the cyclical transcriptional process at the most fundamental stage, GR activation by ligand. These data highlight the key role of the molecular chaperone cycle in this cyclical transcriptional process. The optimal functioning of this finely regulated system is strictly dependent upon three critical and interrelated mechanistic factors: the stochastic action of GR, the deterministic action of ultradian ligand pattern, and the regulatory action of the HSP90 molecular chaperone circuit. The physiological outcome of this form of stringent temporal control of the transcriptional profile of target cells is to maintain a highly responsive system, optimal for meeting the rapidly changing demands of the organism.
Fig. 6.
Model of intranuclear GR responsiveness to pulses of GC. The first pulse of endogenous GC, corticosterone or cortisol, resulted in nuclear translocation and enhanced association at specific GRE sequences at the chromatin template. p300 and CBP are recruited to this regulatory site, increasing acetylation at histone H4 and the association of activated RNA Pol2. At this phase (pulse peak), there is an increased rate of transcription of the PER1 gene. As hormone is cleared (the half-life of corticosterone is <10 min in vivo), the cells can sense the declining levels of ligand, because the endogenous ligand off-rate from GR is relatively rapid. The mechanism underlying this acute response to declining ligand is the rapid GR cycling on chromatin (timescale sec). At each exchange event, there is a probability that ligand will be lost from GR. Unliganded GR will then reenter the chaperone cycle, with the reassociation of the molecular chaperones HSP90 and p23. Unliganded GR is then retained within the nuclear compartment in complex with these molecular chaperones. At this phase (pulse trough), GR and cofactors are not chromatin associated, and the acetylation state returns rapidly to basal levels. RNA Pol2 association at the promoter is also decreased to basal levels, and the transcription rate is low. The next pulse of ligand can activate the intranuclear pool of GR, enabling another cycle of transcriptional activity, because the chaperones hold the ligand binding cleft in the correct conformation. Inhibition of the HSP90 chaperone assembly affects the intranuclear chaperone cycle at multiple points. Inhibition of a productive HSP90-GR interaction prevents pulsatile GR reactivation by directly acting at the ligand binding step. Therefore, all cyclical transcriptional activities initiated by GR as a pioneer factor at the chromatin template ceases. NET, The amount of GR (measurable by ChIP) at the chromatin template at equilibrium position.
Materials and Methods
Cell culture
Frozen stocks of the mouse corticotroph AtT-20 (D16v-F2), human HeLa, rat liver HTC, and human lung-derived A549 cell lines were obtained from the European Collection of Cell Cultures (Salisbury, UK). All cells were cultured in 75-cm2 tissue culture flasks incubated at 37 C in humidified air containing 5% (vol/vol) CO2 until the cells reached approximately 75% confluency. Culture media (DMEM with l-glutamine and 4.5 g/liter glucose; GIBCO, Paisley, UK), were supplemented with 10% (vol/vol) heat inactivated fetal calf serum (Sigma, Welwyn Garden City, UK), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (GIBCO). Before corticosterone treatment, the cultures were washed (3×) in PBS, and for 16–24 h before hormone treatments, cells were maintained in serum and phenol red-free DMEM/F12 medium (GIBCO) supplemented with 100 μg/ml BSA (fraction V) (GIBCO) and 10 μg/ml transferrin (Sigma). Hormone treatments (corticosterone, 100 nm; cortisol, 200 nm) and washes were also performed in this hormone-free culture medium formulation.
Nuclear extraction
Nuclear fractions were prepared as previously described (42). All procedures were performed at 4 C and on ice. Cells were lysed in 300 μl S1 buffer [10 mm HEPES (pH 7.9), 10 mm KCl, 1.5 mm MgCl2, and 0.1 mm EDTA (pH 8)], supplemented with 0.5 mm dithiothreitol, 0.2 mm Na orthovanadate, 2 mm NaF, 0.2% Nonidet P-40, and Complete Protease Inhibitor (Roche Diagnostics Ltd., UK). Nuclear proteins were extracted in 1.2 pellet volumes of S2 buffer [10 mm HEPES (pH 7.9), 400 mm NaCl, 1.5 mm MgCl2, 0.1 mm EDTA (pH 8), and 5% glycerol], supplemented with 0.5 mm dithiothreitol, 0.2 mm Na orthovanadate, 2 mm NaF, and Complete Protease Inhibitor and stored at −80 C. Protein concentrations were determined by bicinchoninic acid assay (Pierce, Rockford, IL).
GR-DNA binding assay
A commercially available ELISA-based transcription factor binding assay kit (TransAM GR; Active Motif) was used to measure GRE binding activity for each nuclear sample. The amount of total protein loaded for each sample was determined by bicinchoninic acid assay, and as an additional normalization control to more accurately measure the integrity of each sample, an aliquot of nuclear extract from each sample was also processed for nuclear factor Y (NF-Y) DNA binding activity (TransAM NF-Y; Active Motif). NF-Y is a ubiquitous transcription factor that showed no significant difference between the treatment groups. DNA binding assay kits were used in accordance with the manufacturer's instructions. Briefly, nuclear extracts (20 μg for GR, 5 μg for NF-Y) were incubated in wells of the 96-well plates coated with GR or NF-YA binding consensus oligonucleotide sequence for 1 h, then incubated with the supplied primary anti-GR or NF-Y antibody (1:1000) for 1 h, then with a peroxidase-conjugated secondary antibody (1:1000) for 1 h. After the substrate was added, color development was read at 450 nm, and the OD of both GR and NF-Y were recorded. The OD results obtained from the GR assay were normalized relative to OD results from the NF-Y assay. Data were then expressed as fold induction relative to time zero.
Chromatin immunoprecipitation
ChIP was performed using buffers prepared according to the EZ ChIP kit (Upstate, Charlottesville, VA) with some modification to the protocol. A wash in PBS (pH 7.4) terminated treatment before cells were fixed in 1% (vol/vol) formaldehyde in PBS (10 min, room temperature). Cross-linking was quenched by addition of glycine to 0.125 m for 5 min, before fixing solution was removed fully by three washes in ice-cold PBS supplemented with 2 mm NaF and 0. mm Na orthovanadate. Cells were scraped into sodium dodecyl sulfate lysis buffer supplemented with NaF, Na orthovanadate, and Complete Protease Inhibitor, then sonicated with a Branson Sonifier 450 to an average chromatin fragment size of 2 kb with 10-sec pulses at 10% output; 70 μg chromatin per ChIP reaction was diluted 1:10 in ChIP dilution buffer and rotated 2 h at 4 C with 60 μl protein A agarose beads (Upstate). Precleared lysate was rotated at 4 C overnight with 2 μg H-300 rabbit anti-GR (AtT-20 and HeLa) or 2 μg M-20 rabbit anti-GR (HTC). Negative controls were incubated with 2 μg nonimmune rabbit IgG (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) under identical conditions. Antibody-GR-DNA complexes were collected on 60 μl protein A agarose beads (2 h, 4 C), then washed once with the low salt buffer, twice with a 300 mm NaCl variant on the kit high salt buffer, once with LiCl buffer, and twice with TE buffer (pH 8.0) to remove nonspecific binding. Complexes were eluted off beads by shaking in elution buffer (0.1 mm NaHCO3 and 1% sodium dodecyl sulfate) before reversal of cross-links overnight at 65 C under high salt (0.2 m NaCl) conditions. Ribonuclease (Roche Diagnostics Ltd.) and proteinase K (Sigma) digestion removed specific contaminants before eluted DNA was extracted once in 25:24:1 phenol-chloroform-isoamyl alcohol and once in 24:1 chloroform-isoamyl alcohol (Sigma). DNA was ethanol precipitated overnight, washed in 70% ethanol, and finally eluted in nuclease-free water (Ambion, Huntingdon, UK) ready for PCR amplification. Primers (Invitrogen, Paisley, UK) were designed in regions flanking described GRE in the PER1 and Mt1 and POMC promoters, and the GRE at −5.4 kb in the TAT promoter. Amplification was inside the linear range.
Primer sequences 〈m, mus; h, human; r, rat):
mPOMC, forward (F) CGCTAAGCCTCTGTCCAGTT
mPOMC, reverse (R) GTCCCTGTCGCTCTTCTCTC
mMTI, F GGCTCTGCAGATGACACAGA
mMTI, R GGGTGTTTGCCAAGTAGGAA
hPER1, F GCAGATGGGAGTCCTGAAAA
hPER1, R GAGGCTGGAGAGACTGGAGA
rTAT, F GACTCCTCTGGCTTTCATCG
rTAT, R TGCATAACTGTGGCAACCAT.
Cofactor and acetylation ChIP assays
Replicate chromatin samples were prepared as above and used for ChIP assays with antiacetylated lysine (9441L; Cell Signaling, Beverly, MA), anti-CBP (sc-369X; Santa Cruz Biotechnology, Inc.), p300 (sc-584X; Santa Cruz Biotechnology, Inc.), anti-RNA Pol2 (Active Motif AM39233), and antiacetylated histone H4 (Active Motif AM39243).
The amount of immunoprecipitated DNA resulting from each ChIP was analyzed by quantitative PCR using Sybr Green assay (Applied Biosystems, Foster City, CA) with the following primers:
mPER1, F CAGGAATTTTTGGCTTTTGTACAG
mPER1, R GCCCAGGACATGCACACA.
Preparation of cell cultures for IN Cell image and data acquisition
For IN Cell imaging experiments, AtT-20 Cells were grown in 96-well Corning Costar plates (Appleton Woods, Birmingham, UK) that had been precoated with Poly-d-lysine (Sigma) and seeded to 5000 cells per well. For hormone withdrawal experiments (unless specified), cells were pretreated with 100 nm corticosterone for 1 h, washed with PBS (3×), and then maintained in hormone-free culture medium.
Immunocytochemistry
Treatments were terminated by washing the cultures in PBS (pH 7.4) and were then fixed using 4% (wt/vol) paraformaldehyde (Sigma) in 0.1 m PBS for 20 min. After two more washes with PBS, cells were permeablized with 0.1% (vol/vol) Triton X-100/PBS (Sigma) for 3 min and washed (2×) in PBS. Nonspecific protein binding was blocked with 1% (wt/vol) BSA/PBS for 20 min at room temperature before incubating with the primary antibody. GR immunoreactivity was detected using M-20 rabbit anti-GR (Santa Cruz Biotechnology, Inc.), at a dilution of 1:500 in 1% (wt/vol) BSA/PBS. After overnight incubation at 4 C, the cells were washed (3×) with PBS and stained with donkey antirabbit Alexa Fluor 488-conjugated IgG (Invitrogen, Molecular Probes, Paisley, UK) at 1:500 dilution in 1% (wt/vol) BSA/PBS and incubated in the dark for a further 2 h at room temperature. Before nuclear staining, the cells were washed in PBS and then counterstained by incubating in Hoechst 33342 (Invitrogen, Molecular Probes) at 3:10,000 in 0.1 m PBS for 10 min followed by a further wash in PBS before IN Cell analysis.
Demarcation and quantification of nuclear and cytoplasmic compartments
Digital images were acquired from four designated fields per well giving a total collection area of not less than 2.4 mm2. Typically, images of 1000–4000 cells per well were captured using a ×10 (numerical aperture, 0.45) objective (plan apochromat) and D360-nm (Hoechst) and S475-nm (Alexa Fluor 488) excitation filters. Images of nuclear and cytoplasmic regions were monitored through 460- and 535-nm emission filters, respectively, and quantified using the IN Cell 1000 Analyzer software (Dual Area Analysis Algorithm version 1.0; GE Healthcare). Background fluorescence from surrounding cell-free regions was subtracted to provide the fluorescence intensity for the cytosol or nucleus. These values were then used to calculate the GR nuclear and cytoplasmic fluorescence intensities for each individual cell.
Statistical procedure and data presentation
Unless otherwise stated, figures show data from four to eight replicate wells per treatment condition (4000 cells per well) (TransAM, IN Cell), data pooled from three separate experiments (IN Cell), or a single representative experiment of at least three with similar outcomes (ChIP). All data are expressed as mean ± sem. Curve fitting for gene expression experiments was performed with GraphPad Prism 4 (GraphPad Software, San Diego, CA).
Acknowledgments
We thank the invaluable technical assistance of Professor C. A. McArdle and Dr. C. J. Caunt in the use of the IN Cell 1000 Analyzer and Benjamin Flynn for excellent technical assistance in the ChIP assays.
This work was supported by the Wellcome Trust Program Grant Reference 089647/Z/09/Z (to S.L.L. and B.L.C.-C.) and the Needham Cooper Charitable Trust (C.L.G. and D.M.K.). The IN Cell 1000 Analyzer was purchased with funds from the Wellcome Trust Equipment Grant 078407 (to S.L.L. and C.A.M.) and the Applied Biosystems 7500 System for running quantitative PCR experiments with the Wellcome Trust Equipment Grant 075548/Z/04/Z (to S.L.L.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: GR;
Coregulators: CBP | p300;
Ligands: Corticosterone.
Footnotes
- CBP
- CREB binding protein
- ChIP
- chromatin immunoprecipitation
- CHX
- cyclohexamide
- CREB
- cAMP response element-binding protein
- ER
- estrogen receptor
- F
- forward
- GA
- geldanamycin
- GC
- glucocorticoid
- GR
- GC receptor
- GRE
- GC regulatory element
- h
- human
- HAT
- histone acetyl transferase
- HSP90
- heat shock protein 90
- m
- mus
- MT1
- metallothionein I
- NF-Y
- nuclear factor Y
- PER1
- Period 1
- POMC
- proopiomelanocortin
- r
- rat
- R
- reverse
- RNA Pol2
- RNA polymerase 2
- TAT
- tyrosine aminotransferase
- WGA
- wheat germ agglutinin.
References
- 1. Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. 2008. The significance of glucocorticoid pulsatility. Eur J Pharmacol 583:255–262 [DOI] [PubMed] [Google Scholar]
- 2. Young EA, Abelson J, Lightman SL. 2004. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrin 25:69–76 [DOI] [PubMed] [Google Scholar]
- 3. Windle RJ, Wood SA, Lightman SL, Ingram CD. 1998. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology 139:4044–4052 [DOI] [PubMed] [Google Scholar]
- 4. Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. 1998. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139:443–450 [DOI] [PubMed] [Google Scholar]
- 5. Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa AP, Ingram CD, Lightman SL. 1997. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female ratsduring non-disrupted maternal activity. J Neuroendocrinol 9:407–414 [DOI] [PubMed] [Google Scholar]
- 6. Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. 2001. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol 13:905–911 [DOI] [PubMed] [Google Scholar]
- 7. Young EA, Carlson NE, Brown MB. 2001. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology 25:267–276 [DOI] [PubMed] [Google Scholar]
- 8. McNally JG, Müller WG, Walker D, Wolford R, Hager GL. 2000. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287:1262–1265 [DOI] [PubMed] [Google Scholar]
- 9. Hager GL, Nagaich AK, Johnson TA, Walker DA, John S. 2004. Dynamics of nuclear receptor movement and transcription. Biochim Biophys Acta 1677:46–51 [DOI] [PubMed] [Google Scholar]
- 10. Keeton EK, Fletcher TM, Baumann CT, Hager GL, Smith CL. 2002. Glucocorticoid receptor domain requirements for chromatin remodeling and transcriptional activation of the mouse mammary tumor virus promoter in different nucleoprotein contexts. J Biol Chem 277:28247–28255 [DOI] [PubMed] [Google Scholar]
- 11. Fletcher TM, Ryu BW, Baumann CT, Warren BS, Fragoso G, John S, Hager GL. 2000. Structure and dynamic properties of a glucocorticoid receptor-induced chromatin transition. Mol Cell Biol 20:6466–6475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fletcher TM, Xiao N, Mautino G, Baumann CT, Wolford R, Warren BS, Hager GL. 2002. ATP-dependent mobilization of the glucocorticoid receptor during chromatin remodeling. Mol Cell Biol 22:3255–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. 2009. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lightman SL, Conway-Campbell BL. 2010. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev 11:710–718 [DOI] [PubMed] [Google Scholar]
- 15. Hager GL, McNally JG, Misteli T. 2009. Transcription dynamics. Mol Cell 35:741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. 2010. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol 22:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito T, Ikehara T, Nakagawa T, Kraus WL, Muramatsu M. 2000. p300-mediated acetylation facilitates the transfer of histone H2A-H2B dimers from nucleosomes to a histone chaperone. Gene Dev 14:1899–1907 [PMC free article] [PubMed] [Google Scholar]
- 18. Bouazoune K, Miranda TB, Jones PA, Kingston RE. 2009. Analysis of individual remodeled nucleosomes reveals decreased histone-DNA contacts created by hSWI/SNF. Nucleic Acids Res 37:5279–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinke H, Hörz W. 2003. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol Cell 11:1599–1607 [DOI] [PubMed] [Google Scholar]
- 20. Lee DY, Hayes JJ, Pruss D, Wolffe AP. 1993. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 72:73–84 [DOI] [PubMed] [Google Scholar]
- 21. Caunt CJ, Rivers CA, Conway-Campbell BL, Norman MR, McArdle CA. 2008. Epidermal growth factor receptor and protein kinase C signaling to ERK2: spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem 283:6241–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haché RJ, Tse R, Reich T, Savory JG, Lefebvre YA. 1999. Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. J Biol Chem 274:1432–1439 [DOI] [PubMed] [Google Scholar]
- 23. Schaaf MJ, Cidlowski JA. 2003. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol Cell Biol 23:1922–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deroo BJ, Rentsch C, Sampath S, Young J, DeFranco DB, Archer TK. 2002. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol Cell Biol 22:4113–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang J, Liu J, DeFranco DB. 1997. Subnuclear trafficking of glucocorticoid receptors in vitro: chromatin recycling and nuclear export. J Cell Biol 137:523–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeFranco DB, Qi M, Borror KC, Garabedian MJ, Brautigan DL. 1991. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol 5:1215–1228 [DOI] [PubMed] [Google Scholar]
- 27. Satav JG, Katyare SS, Fatterparker P, Sreenivasan A. 1977. Study of protein synthesis in rat liver mitochondria use of cycloheximide. Eur J Biochem/FEBS 73:287–296 [DOI] [PubMed] [Google Scholar]
- 28. Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. 2004. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010–1014 [DOI] [PubMed] [Google Scholar]
- 29. Freeman BC, Yamamoto KR. 2002. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296:2232–2235 [DOI] [PubMed] [Google Scholar]
- 30. Yang J, DeFranco DB. 1996. Assessment of glucocorticoid receptor-heat shock protein 90 interactions in vivo during nucleocytoplasmic trafficking. Mol Endocrinol 10:3–13 [DOI] [PubMed] [Google Scholar]
- 31. Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. 2003. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- 32. Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. 2003. Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11:695–707 [DOI] [PubMed] [Google Scholar]
- 33. Li X, Wong J, Tsai SY, Tsai MJ, O'Malley BW. 2003. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol 23:3763–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Becker M, Baumann C, John S, Walker DA, Vigneron M, McNally JG, Hager GL. 2002. Dynamic behavior of transcription factors on a natural promoter in living cells. EMBO Rep 3:1188–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tago K, Tsukahara F, Naruse M, Yoshioka T, Takano K. 2004. Regulation of nuclear retention of glucocorticoid receptor by nuclear Hsp90. Mol Cell Endocrinol 213:131–138 [DOI] [PubMed] [Google Scholar]
- 36. Liu J, Xiao N, DeFranco DB. 1999. Use of digitonin-permeabilized cells in studies of steroid receptor subnuclear trafficking. Methods 19:403–409 [DOI] [PubMed] [Google Scholar]
- 37. Picard D, Salser SJ, Yamamoto KR. 1988. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell 54:1073–1080 [DOI] [PubMed] [Google Scholar]
- 38. Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. 2008. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem 283:26612–26623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J, DeFranco DB. 1999. Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol Endocrinol 13:355–365 [DOI] [PubMed] [Google Scholar]
- 40. Stavreva DA, Müller WG, Hager GL, Smith CL, McNally JG. 2004. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol Cell Biol 24:2682–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toogun OA, Dezwaan DC, Freeman BC. 2008. The hsp90 molecular chaperone modulates multiple telomerase activities. Mol Cell Biol 28:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Revest JM, Di Blasi F, Kitchener P, Rougé-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. 2005. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci 8:664–672 [DOI] [PubMed] [Google Scholar]