Abstract
Purpose
To estimate α/β, the parameter ratio from the linear-quadratic (LQ) model, for grade ≥2 late rectal toxicity among patients treated on Radiation Therapy Oncology Group (RTOG) protocol 94-06, and to determine whether correcting the rectal dose-volume histogram (DVH) for differences in dose per fraction, based on the LQ model, significantly improves the fit to these data of the Lyman-Kutcher-Burman (LKB) normal-tissue complication probability (NTCP) model.
Methods and Materials
The generalized LKB model was fitted to the grade ≥2 late rectal toxicity data in two ways: 1) using DVHs representing physical dose to rectum, and 2) using a modified approach in which dose bins in the rectal DVH were corrected for differences in dose per fraction using the LQ model, with α/β estimated as an additional unknown parameter. The analysis included only patients treated with the same treatment plan throughout radiotherapy, so that the dose per fraction to each voxel of rectum could be determined from the DVH. The likelihood ratio test was used to assess whether the fit of the LQ-corrected model was significantly better than the fit of the LKB model based on physical doses to rectum.
Results
The analysis included 509 of the 1084 patients enrolled on RTOG 94-06. The estimate of α/β from the LQ-corrected LKB model was 4.8 Gy, with 68% confidence interval 0.6 Gy to 46 Gy. The fit was not significantly different from the fit of the LKB model based on physical dose to rectum (P = 0.236).
Conclusions
The estimated fractionation sensitivity for grade ≥2 late rectal toxicity is consistent with values of α/β for rectum found previously in humans and rodents. However, the confidence interval is large, and there is no evidence that LQ-correction of the rectal DVH significantly changes the fit or predictions of the LKB model for this endpoint.
Keywords: prostate cancer, RTOG, rectal toxicity, linear-quadratic model, dose-volume histogram
INTRODUCTION
Since the earliest days of radiotherapy (RT), it has been recognized that the severity of normal-tissue toxicity depends, in part, on the number of dose fractions into which the total radiation dose is divided (1). The fractionation effect is most often quantified using the parameter ratio α/β from the linear-quadratic (LQ) model (2,3). Although alternative models may be needed in some settings, for example to describe the effects of low-dose hypersensitivity (4,5) or hypofractionation (6,7), the LQ model generally provides a reasonably good description of fractionation effects for doses per fraction up to at least 6 Gy.
Estimates of α/β have been derived for a wide range of tumor and normal-tissue endpoints (1, 3). Values of α/β for tumors and acute normal-tissue reactions are usually 10 Gy or higher, while α/β ratios for late reactions are typically close to 3 Gy. For the types of late rectal injury seen after external beam radiotherapy for prostate cancer, the only published estimate, to our knowledge, is that of Brenner (8), who reported a value of α/β = 5.4 Gy for grade ≥2 toxicity scored using RTOG criteria (9).
At the time most published estimates of α/β were derived, conformal RT techniques had not yet been developed, and standard RT delivered relatively uniform doses to the treatment field. Values of α/β computed from clinical data were based on changes in total isoeffective target dose (the dose corresponding to a specified level of tissue injury) after changes in prescribed dose per fraction. Any intra-patient variations in dose to normal tissue were disregarded, as were any differences among patients in the volumes of normal tissue irradiated. Both of these factors, however, may impact toxicity rates.
The goal of the present study was to derive an estimate of α/β for grade ≥2 late rectal toxicity using a novel strategy in which α/β is included as an additional parameter in the Lyman-Kutcher-Burman (LKB) normal-tissue complication probability (NTCP) model (10,11). This approach enabled us to take both fractionation effects and volume effects into account at the same time.
The rectal toxicity data analyzed in this study are from patients treated on RTOG 94-06, a large multi-institutional dose-escalation trial designed to determine the maximum tolerated dose for 3D-conformal radiotherapy (3D-CRT) of prostate cancer (12–16). In RTOG 94-06, the variations in prescribed fractional dose to prostate were modest (1.8 Gy versus 2 Gy). However, the use of 3D-CRT resulted in somewhat wider variations in delivered dose per fraction to different subvolumes across the rectum, ranging from 0 Gy up to 2.2 Gy, as reflected in the rectal DVH. The strategy of the current study was to utilize these variations in dose per fraction to estimate α/β for late rectal toxicity in the context of fitting the LKB model to data.
In view of the known impact of fractionation effects on normal-tissue toxicity, it has been suggested that the DVH should be adjusted for differences in dose per fraction when NTCP models are fitted to data (17). A further aim of our study, therefore, was to determine whether or not LQ-correction would lead to a significantly improved fit of the LKB model to grade ≥2 late rectal toxicity, compared to a fit based on physical doses to rectum. In addition, we investigated the difference in magnitude of the NTCP estimates obtained using the two approaches.
METHODS AND MATERIALS
Radiotherapy
Protocol RTOG 94-06 has been described in detail elsewhere (12–16). Briefly, the trial included 5 dose levels: 68.4 Gy, 73.8 Gy and 79.2 Gy consisting, respectively, of 38, 41, or 44 prescribed daily fractions of 1.8 Gy (levels I–III), and 74.0 Gy and 78.0 Gy consisting, respectively, of 37 or 39 prescribed daily fractions of 2 Gy (levels IV–V). Patients were stratified into 3 groups according to the estimated risk of seminal vesicle (SV) involvement (18). Patients with T1–T2 tumors and <15% risk of SV invasion (group 1) were treated to the prostate only. Patients with T1–T2 tumors and ≥ 15% risk of SV involvement (group 2) were treated to the prostate and bilateral SVs for the first 55.8 Gy (in dose levels I–III) or 54 Gy (in dose levels IV–V) and to the prostate only for the remainder of treatment. Patients with T3 tumors (group 3) were treated to the prostate plus bilateral SVs throughout therapy. The present study included only the patients treated on groups 1 and 3, for whom there was no reduction in field size during RT as there was in group 2. Patients in groups 1 and 3 who had revisions to the treatment plan during therapy, as happened in some cases for clinical reasons, were excluded. Therefore, all patients included in the analysis had the same treatment fields throughout therapy, and the dose per fraction to each voxel of rectum could be computed from the rectal DVH based on the total number of dose fractions received.
DVH data
Patients were simulated in supine position with individualized immobilization devices, and a treatment planning CT was acquired in the same position and under the same conditions (e.g. full versus empty bladder) as for treatment. The CT scan extended through the perineum from the level of the iliac crest, and included all tissues to be irradiated. CT scan thickness was ≤ 0.5 cm throughout the region containing the target volume and ≤ 1 cm outside. The rectum was contoured from the level of the ischial tuberosities to the rectosigmoid flexure. The DVH for rectum as a solid volume was computed from the rectal contour using the dose matrix provided by the participating institution. For cases in which the dose matrix did not encompass the entire rectum, the volume of rectum outside the dose matrix was added to the 0-Gy dose bin of the DVH for the purpose of including it in the calculation of total rectal volume.
Toxicity scoring and study endpoint
Patient follow-up on RTOG 94-06 was every 3 months for the first year, every 4 months during the second year, every 6 months during the next 3 years, and annually thereafter. Rectal toxicity was scored using the RTOG criteria (9), and late complications were defined to be those starting or persisting at least 120 days after RT. The endpoint for analysis in the present study was grade ≥2 late rectal toxicity. Time to grade ≥2 late rectal toxicity was computed from the start of RT, or censored at last follow-up for patients not experiencing the endpoint.
The toxicity data analyzed here were extracted from the RTOG 94-06 database in October 2007. This retrospective secondary analysis was approved by the RTOG Publications Committee and by the Institutional Review Boards of The University of Texas MD Anderson Cancer Center, the Washington University Medical Center, and the American College of Radiology.
Data analysis
The Lyman-Kutcher-Burman NTCP model (10,11), with unknown parameters TD50, m, and n, is given by the formula
| (1) |
where
| (2) |
and
| (3) |
In Eq. 3, Di is the physical dose to relative organ subvolume vi, and the sum extends over all dose bins in the DVH (19).
In the present study, we revised the LKB model to obtain an LQ-corrected version of the model in which the physical dose Di in equation (3) was replaced by LQ2Di, the dose biologically equivalent to Di if given in 2 Gy per fraction (17). According to the LQ model, LQ2Di is given by:
| (4) |
where di is the (physical) fractional dose to subvolume vi. The dose per fraction di was calculated by dividing Di by the number of dose fractions received. For this reason, it was important to limit the analysis to patients for whom each voxel of rectum received the same number of fractions throughout treatment. The value of the ratio α/β was estimated as an unknown parameter when the LQ-corrected LKB model was fitted to data. Note that in the LKB model fitted using the DVH for physical dose to rectum, the TD50 parameter also represents physical dose to rectum, whereas in the LQ-corrected model, the TD50 parameter represents dose given in 2 Gy per fraction. For clarity, therefore, we denote this parameter by LQ2TD50 in the LQ-corrected model with 2 Gy per fraction chosen as the reference fraction size. Any other reference fraction size could be used instead, of course, by substituting it in place of 2 Gy in Eq. 4.
The LKB model, using physical doses to rectum (Di), and the LQ-corrected LKB model, using doses corrected for differences in dose per fraction (LQ2Di; Eq. 4), were fitted to data using a mixture-model approach, described previously (20,21), in which the observed times to occurrence of toxicity are taken into account. The distribution in latent times to grade ≥2 late rectal toxicity among patients experiencing the endpoint was modeled using a lognormal density function f(t) with parameters μ and σ, which represent the mean and standard deviation, respectively, of the normal distribution with density function ln(f(t)):
| (5) |
Initially, μ and σ were fixed at the values estimated previously (21): μ = 0.442 and σ= 756 Subsequently, a fit was performed in which μ and σ were treated as unknown parameters (along with m, n, TD50 or LQ2TD50, and α/β) to be estimated from the data.
The generalized LKB (Eqs. 1–3, 5) and LQ-corrected LKB (using LQ2Di of Eq. 4 in place of Di in Eq. 3) models were fitted to the grade ≥2 late rectal toxicity data using maximum likelihood (ML) analysis (22). As described previously (20,21), the contribution to the likelihood for a patient experiencing toxicity at time τ was NTCP · f (τ), and for a patient followed to time τ without experiencing toxicity, the contribution to the likelihood was 1 − NTCP · F (τ), where F(t) is the cumulative distribution function corresponding to f(t). Confidence intervals for the ML parameter estimates were computed using the profile likelihood method (22). The likelihood ratio test was used to test whether the LQ-corrected LKB model provided a significantly better fit to the data than the LKB model based on physical dose. All statistical analyses were performed using Stata (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
RESULTS
Patients and follow-up
Of the 1084 patients enrolled on RTOG 94-06, data from 1010 patients were available for analysis, as described previously (21). In the present study, we excluded all patients treated on group 2, for whom there was a planned field reduction during the course of radiotherapy, as well as any other patient whose treatment plan was revised during therapy for any other reason. These exclusions left 509 patients available for this analysis. The numbers of patients in dose levels I–V were 42, 171, 93, 108, and 95, respectively.
At the time of data extraction from the RTOG database (October 2007), the median follow-up among patients in the cohort analyzed here was 7.6 years (range 3 months to 12.1 years). There were 77 patients who experienced grade ≥2 late rectal toxicity, of whom 13 had grade 3 toxicity and 1 had grade 4 toxicity.
Estimate of α/β
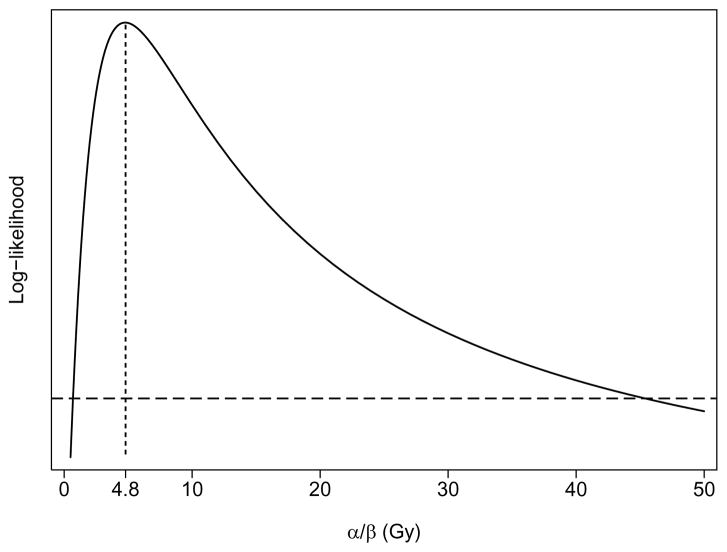
Table 1 lists the parameter estimates obtained by fitting the generalized LKB and LQ-corrected LKB models to the grade ≥2 late rectal toxicity data using, respectively, the DVHs representing physical dose to rectum (Di) or DVHs in which LQ corrections were made for differences in dose per fraction (using LQ2Di of Eq. 4 in place of Di in Eq. 3). Initially, 95% confidence intervals (CIs) were sought for each of the estimated parameters. However, the 95% CI for α/β was found to include all possible values >0. Accordingly, 68% CIs are reported instead. The estimate of α/β from the LQ-corrected version of the LKB model was α/β = 4.8 Gy, with 68% confidence interval 0.6 Gy to 46 Gy, as illustrated in Figure 1. Of note, however, is that use of LQ-corrected DVHs did not lead to a significant improvement in the fit of the LKB model to the data from this cohort (P = 0.236, likelihood ratio test).
Table 1.
Parameter estimates of the LKB model obtained using DVHs representing physical dose to rectum (Eq. 3) or using DVHs corrected for differences in dose per fraction based on the LQ model (Eq. 4).
| Parameter | Physical doses* | LQ-corrected doses* |
|---|---|---|
| TD50 (Gy) | 78.2 (75.6, 81.1) | --- |
| LQ2TD50 (Gy) | --- | 77.9 (74.5, 81.2) |
| m | 0.134 (0.105, 0.184) | 0.151 (0.113, 0.233) |
| n | 0.073 (0.048, 0.122) | 0.080 (0.049, 0.152) |
| α/β | --- | 4.8 (0.6, 46) |
68% profile-likelihood confidence intervals are shown.
Figure 1.
Solid curve: Profile log-likelihood (LL) from the fit of the generalized LQ-corrected Lyman-Kutcher-Burman model (Eqs. 1, 2, 4, 5), plotted as a function of the parameter ratio α/β from the LQ model. The maximum LL occurs at α/β =4.8 Gy, representing the best estimate of α/β, as indicated by the location of the vertical stippled line. The 68% profile-likelihood confidence interval for α/β = 4.8 Gy is represented by the range of α/β values between the points of intersection of the solid curve with the horizontal dashed line, and ranges from 0.6 Gy to 46 Gy.
For the analysis described above, the parameters μ and σ were fixed at the values obtained from an earlier analysis of the RTOG 94-06 data (21). However, a fit in which μ and σ were allowed to vary led to a nearly identical fit; rounded to two significant digits, all parameter estimates were the same as those listed in Table 1.
In fitting the LQ-corrected LKB model to data, we chose 2 Gy as the reference fraction size (Eq. 4), but, since 3 of the 5 dose levels in RTOG 94-06 had prescription doses of 1.8 Gy, this would also have been a natural choice. The corresponding estimate of the TD50 parameter using a reference fraction size of 1.8 Gy is LQ1.8TD50 = 80.3 Gy (68% CI 76.8 to 83.7 Gy). The remaining parameter estimates from the model fit (m, n, and α/β) are the same as those obtained using a reference fraction size of 2 Gy.
NTCP estimates
The parameter estimates listed in Table 1 were used to compute the estimated risk of grade ≥2 late rectal toxicity for each of the 509 patients in the study cohort, and the risk estimates for each patient from the two different model fits were compared. Using the parameters of the LKB model based on physical dose to rectum, NTCP values ranged from 1% to 42%, with a median risk of 14%. The absolute difference between these risk estimates and those obtained using the LQ-corrected LKB model was <2% on average, ranging from 5% smaller to 4% larger for the LQ-corrected model. Differences of ≥4% in the risk estimates from two models were seen only for patients with an estimated risk of grade ≥2 late rectal toxicity exceeding 25%. For patients with NTCP <10% by either model, the difference in the predictions of the two models was never larger than 2 percentage points.
DISCUSSION
An important finding of the present study is that the fit of the Lyman NTCP model to late rectal toxicity was not significantly improved (P=0.236) when DVHs representing physical dose to rectum were corrected for differences in dose per fraction, at least over the range of prescribed doses per fraction in RTOG 94-06 (1.8 versus 2 Gy per fraction). This is consistent with our previous analysis of the data from RTOG 94-06, in which volume effects, and not dose per fraction per se, appear to explain the increased toxicity in dose level V compared with levels I–IV (21). Rancati et al. also found little difference in the parameter estimates for several NTCP models for late rectal toxicity fitted using DVH data with and without fraction-size corrections, using an assumed value of α/β = 3 Gy (23). In their study, also, the range of prescribed doses per fraction was 1.8–2.0 Gy.
It is not surprising that the present study found no significant impact of correcting rectal DVHs for differences in dose per fraction when one considers the extraordinarily narrow range of doses per fraction contributing to the fit of the LKB model to late rectal toxicity. In both the present study and in our previous analysis of the data from RTOG 94-06 (21), we estimated that the volume parameter for the LKB model fitted to these data is n ≈ 0.08. For values of n that small, dose bins corresponding to <60 Gy in the rectal DVH have a negligible impact on the value of Deff computed for each patient and therefore have little impact on estimates of toxicity risk. Therefore, only the regions of rectum receiving the highest doses (>60 Gy) contribute to the risk of late rectal toxicity calculated using the LKB model. In the present cohort, the range of doses per fraction in voxels receiving total doses >60 Gy is quite narrow: 1.4 Gy to 2.2 Gy. In fact, if all dose bins with total dose >60 Gy are tabulated, the middle 50% (the interquartile range) received doses per fraction in a very narrow range from just 1.5 Gy to 1.6 Gy. The significance of correcting for differences in dose per fraction, and the associated impact on the resulting NTCP estimates, would likely be much greater in studies of rectal toxicity in which there was a wider range of prescribed doses per fraction than was the case here, or in studies of other normal tissue endpoints exhibiting a more pronounced volume effect, with LKB volume parameter closer to 1. For such tissues, tissue voxels across a wider range of the dose gradient will contribute to toxicity risk, and it may therefore be more important to correct for fractionation effects.
The estimate of α/β = 4.8 Gy for grade ≥2 late rectal toxicity derived in the current study is quite close to the value of α/β = 5.4 Gy reported previously for this endpoint by Brenner (8). Interestingly, the previous estimate was also based in part on data from RTOG 94-06. Specifically, Brenner used the overall toxicity rates reported after 1.8 Gy per fraction in dose levels I–II of RTOG 94-06 (10, 14), combined with the rates reported for three single-institution studies after 1.8 Gy (24), 2 Gy (25), or 3 Gy (26) to derive an estimate of α/β for late rectal toxicity.
The approach employed here was to estimate α/β by utilizing dose-volume information contained in the rectal DVH for each individual patient. Since we included α/β as an additional parameter in fitting the generalized LKB model to data, our estimate of α/β for late rectal toxicity depends in part on our choice of NTCP model, and could potentially be somewhat different if another NTCP model were used. At present, however, the LKB model appears to provide a good description for the dose-volume dependence of late rectal toxicity (23,27).
To our knowledge, our study and the study of Brenner (8) provide the only estimates of α/β for late rectal toxicity after modern external beam RT of prostate cancer, although a few older studies reported lower or upper bounds on α/β for very severe late bowel toxicity (stricture or perforation) based on treatment of other pelvic malignancies: >2.2 Gy (28), <6 Gy (29), and <8 Gy (30). Quite a few animal studies have reported α/β for late rectal toxicity in rodents, and the majority of those are in the 4–7 Gy range (31–37), consistent with our estimate. It has been suggested that α/β values in this range, intermediate between values generally expected for late endpoints (≈3 Gy) and acute endpoints (≈10 Gy), might indicate that late rectal injury is, at least in part, a consequential effect of acute toxicity (8,38,39 and references therein).
It should be emphasized that there is considerable uncertainty associated with the estimate of α/β for late rectal toxicity derived here. Although there is a clear peak in the likelihood function at α/β = 4.8 Gy (Figure 1), the 95% confidence interval encompasses all values >0, and the 68% confidence interval is also wide, ranging from 0.6 – 46 Gy. Although the present study adds to the evidence for α/β close to 5 Gy, it is not yet possible to conclude with certainty that α/β >3 Gy for grade ≥2 late rectal toxicity. In particular, a hypofractionated treatment regimen designed using α/β =4.8 Gy instead of α/β =3 Gy could lead to unexpectedly high rectal toxicity.
A more precise estimate of α/β for late rectal toxicity might be obtained from studies that avoid one or more of the many sources of uncertainty in the present data set. Examples include studies that take organ motion into account, to increase the accuracy with which the dose to each individual voxel of rectum is known; studies in which the endpoint is limited to rectal bleeding, since other symptoms included in the RTOG rectal endpoint (diarrhea, excess rectal mucus and bowel movements) may have different dose-volume dependencies than rectal bleeding; and analyses based on the dose to rectal wall instead of to whole rectum.
CONCLUSION
Although correcting for differences in fraction size did not have a significant impact on the fit of the Lyman-Kutcher-Burman NTCP model to the grade ≥2 late rectal toxicity data from protocol RTOG 94-06, where the prescribed doses per fraction were 1.8 Gy and 2 Gy, there are other scenarios in which knowledge of the α/β ratio could be of importance. The present study supports the conclusion that a value of α/β close to 4.8 Gy may be appropriate for this endpoint.
Acknowledgments
Supported in part by grants R01 CA104342, U24 CA81647, U10 CA21661, U10 CA37422 and U10 CA32115 from the National Cancer Institute, the National Institutes of Health.
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thames HD, Hendry JH. Fractionation in Radiotherapy. New York: Taylor & Francis; 1987. [Google Scholar]
- 2.Douglas BG, Fowler JF. The effect of multiple small doses of x rays on skin reactions in the mouse and a basic interpretation. Radiat Res. 1976;66:401–426. [PubMed] [Google Scholar]
- 3.Thames HD, Jr, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 4.Marples B, Joiner MC. The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat Res. 1993;133:41–51. [PubMed] [Google Scholar]
- 5.Smith LG, Miller RC, Richards M, et al. Investigation of hypersensitivity to fractionated low-dose radiation exposure. Int J Radiat Oncol Biol Phys. 1999;45:187–191. doi: 10.1016/s0360-3016(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49:4825–4835. doi: 10.1088/0031-9155/49/20/012. [DOI] [PubMed] [Google Scholar]
- 7.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1013–1015. doi: 10.1016/j.ijrobp.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 10.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–19. [PubMed] [Google Scholar]
- 11.Kutcher GJ, Burman C. Calculation of complication probability factors for nonuniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 12.Michalski JM, Purdy JA, Winter K, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys. 2000;46:391–402. doi: 10.1016/s0360-3016(99)00443-5. [DOI] [PubMed] [Google Scholar]
- 13.Michalski JM, Winter K, Purdy JA, et al. Preliminary evaluation of low-grade toxicity with conformal radiation therapy for prostate cancer on RTOG 9406 dose levels I and II. Int J Radiat Oncol Biol Phys. 2003;56:192–198. doi: 10.1016/s0360-3016(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 14.Michalski JM, Winter K, Purdy JA, et al. Toxicity after three-dimensional radiotherapy for prostate cancer with RTOG 9406 dose level IV. Int J Radiat Oncol Biol Phys. 2004;58:735–742. doi: 10.1016/S0360-3016(03)01578-5. [DOI] [PubMed] [Google Scholar]
- 15.Michalski JM, Winter K, Purdy JA, et al. Toxicity after three-dimensional radiotherapy for prostate cancer on RTOG 9406 dose Level V. Int J Radiat Oncol Biol Phys. 2005;62:706–713. doi: 10.1016/j.ijrobp.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Ryu JK, Winter K, Michalski JM, et al. Interim report of toxicity from 3D conformal radiation therapy (3D-CRT) for prostate cancer on 3DOG/RTOG 9406, level III (79.2 Gy) Int J Radiat Oncol Biol Phys. 2002;54:1036–1046. doi: 10.1016/s0360-3016(02)03006-7. [DOI] [PubMed] [Google Scholar]
- 17.Wheldon TE, Deehan C, Wheldon EG, et al. The linear-quadratic transformation of dose-volume histograms in fractionated radiotherapy. Radiother Oncol. 1998;46:285–295. doi: 10.1016/s0167-8140(97)00162-x. [DOI] [PubMed] [Google Scholar]
- 18.Roach M., 3rd Re: The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:1923–1924. doi: 10.1016/s0022-5347(17)35937-2. [DOI] [PubMed] [Google Scholar]
- 19.Mohan R, Mageras GS, Baldwin B, et al. Clinically relevant optimization of 3-D conformal treatments. Med Phys. 1992;19:933–944. doi: 10.1118/1.596781. [DOI] [PubMed] [Google Scholar]
- 20.Tucker SL, Liu HH, Liao Z, et al. Analysis of radiation pneumonitis risk using a generalized Lyman model. Int J Radiat Oncol Biol Phys. 2008;72:568–574. doi: 10.1016/j.ijrobp.2008.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker SLDL, Bosch WR, et al. Late rectal toxicity on RTOG 94-06: analysis using a mixture Lyman model. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2010.01.069. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan BJT. Analysis of Quantal Response Data. New York: Chapman & Hall; 1992. [Google Scholar]
- 23.Rancati T, Fiorino C, Gagliardi G, et al. Fitting late rectal bleeding data using different NTCP models: results from an Italian multi-centric study (AIROPROS0101) Radiother Oncol. 2004;73:21–32. doi: 10.1016/j.radonc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Skwarchuk MW, Jackson A, Zelefsky MJ, et al. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys. 2000;47:103–113. doi: 10.1016/s0360-3016(99)00560-x. [DOI] [PubMed] [Google Scholar]
- 25.Kuban D, Pollack A, Huang E, et al. Hazards of dose escalation in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1260–1268. doi: 10.1016/s0360-3016(03)00772-7. [DOI] [PubMed] [Google Scholar]
- 26.Akimoto T, Muramatsu H, Takahashi M, et al. Rectal bleeding after hypofractionated radiotherapy for prostate cancer: correlation between clinical and dosimetric parameters and the incidence of grade 2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys. 2004;60:1033–1039. doi: 10.1016/j.ijrobp.2004.07.695. [DOI] [PubMed] [Google Scholar]
- 27.Michalski JM, Gay H, Jackson A, et al. Radiation dose volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edsmyr F, Andersson L, Esposti PL, et al. Irradiation therapy with multiple small fractions per day in urinary bladder cancer. Radiother Oncol. 1985;4:197–203. doi: 10.1016/s0167-8140(85)80084-0. [DOI] [PubMed] [Google Scholar]
- 29.Bennett MR. The treatment of stage III squamous carcinoma of the cervix in air and hyperbaric oxygen. Br J Radiol. 1978;51:68. (abstract) [Google Scholar]
- 30.Singh K. Two regimes with the same TDF but differing morbidity used in the treatment of stage III carcinoma of the cervix. Br J Radiol. 1978;51:357–362. doi: 10.1259/0007-1285-51-605-357. [DOI] [PubMed] [Google Scholar]
- 31.Breiter N, Trott KR. Chronic radiation damage in the rectum of the rat after protracted fractionated irradiation. Radiother Oncol. 1986;7:155–163. doi: 10.1016/s0167-8140(86)80095-0. [DOI] [PubMed] [Google Scholar]
- 32.Dewit L, Oussoren Y, Bartelink H, et al. The effect of cisdiamminedichloroplatinum(II) on radiation damage in mouse rectum after fractionated irradiation. Radiother Oncol. 1989;16:121–128. doi: 10.1016/0167-8140(89)90029-7. [DOI] [PubMed] [Google Scholar]
- 33.Dubray BM, Thames HD. Chronic radiation damage in the rat rectum: An analysis of the influences of fractionation, time and volume. Radiother Oncol. 1994;33:41–47. doi: 10.1016/0167-8140(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 34.Gasinska A, Dubray B, Hill SA, et al. Early and late injuries in mouse rectum after fractionated x-ray and neutron irradiation. Radiother Oncol. 1993;26:244–253. doi: 10.1016/0167-8140(93)90266-b. [DOI] [PubMed] [Google Scholar]
- 35.Terry NH, Denekamp J. RBE values and repair characteristics for colo-rectal injury after caesium 137 gamma-ray and neutron irradiation. II. Fractionation up to ten doses. Br J Radiol. 1984;57:617–629. doi: 10.1259/0007-1285-57-679-617. [DOI] [PubMed] [Google Scholar]
- 36.van den Aardweg GJ, Olofsen-van Acht MJ, van Hooije CM, et al. Radiation-induced rectal complications are not influenced by age: A dose fractionation study in the rat. Radiat Res. 2003;159:642–650. doi: 10.1667/0033-7587(2003)159[0642:rrcani]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.van der Kogel AJ, Jarrett KA, Paciotti MA, et al. Radiation tolerance of the rat rectum to fractionated X-rays and pi-mesons. Radiother Oncol. 1988;12:225–232. doi: 10.1016/0167-8140(88)90265-4. [DOI] [PubMed] [Google Scholar]
- 38.Brenner DJ. Hypofractionation for prostate cancer radiotherapy – What are the issues? Int J Radiat Oncol Biol Phys. 2003;57:912–914. doi: 10.1016/s0360-3016(03)01456-1. [DOI] [PubMed] [Google Scholar]
- 39.Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]