Abstract
Purpose
Intratumoral hypoxia is known to be associated with radioresistance and metastasis. The present study examined the effect of acute and chronic hypoxia on the metastatic potential of prostate cancer PC-3, DU145 and LNCaP cells.
Methods and Materials
Cell proliferation and clonogenicity were tested by MTT assay and colony formation assay, respectively. “Wound-healing” and Matrigel-based chamber assays were used to monitor cell motility and invasion. Hypoxia-inducible factor 1 alpha (HIF-1α) expression was tested by Western blot and HIF-1-target gene expression was detected by real time PCR. Secretion of matrix metalloproteinases (MMPs) was determined by gelatin zymography.
Results
When PC-3 cells were exposed to 1% oxygen (hypoxia) for various periods of time, chronic hypoxia (≥24 h) decreased cell proliferation and induced cell death. In contrast, prostate cancer cells exposed to acute hypoxia (≤6 h) displayed increased motility, clonogenic survival and invasive capacity. At the molecular level, both hypoxia and anoxia transiently stabilized HIF-1α. Exposure to hypoxia also induced the early expression of MMP-2, an invasiveness-related gene. Treatment with the HIF-1 inhibitor YC-1 attenuated the acute hypoxia-induced migration, invasion, and MMP-2 activity.
Conclusions
The length of oxygen deprivation strongly impacted the functional behavior of all 3 prostate cancer cell lines. Acute hypoxia in particular was found to promote a more aggressive metastatic phenotype.
Keywords: acute hypoxia, prostate cancer, metastasis, HIF-1α, MMP-2
INTRODUCTION
In cancer patients tumor hypoxia has been identified as a common environmental stress factor associated with resistance to radiation therapy (1) and poor prognosis (2). As cancers progress, tumor cells acquire the ability to adapt to hypoxic environments by co-opting blood vessel formation while also migrating and invading toward vessels (3). Clinical investigations have indicated hypoxia to be a common feature of most solid tumors, a characteristic that may relate to the incidence of cancer metastasis (4). Preclinical experimental evidence derived from animal models has demonstrated that exposure to hypoxia increases tumor metastatic ability in vivo (5). Tumor hypoxia has generally been classified into two types: chronic and acute hypoxia (6). Tumor cells residing at the limits of oxygen diffusion from functional blood vessels may experience chronic or “diffusion-limited” hypoxia. Such hypoxic conditions typically last for relatively long periods of hours or days (6). In contrast, some tumor cells may be exposed to short-term (minutes to hours), transient hypoxia as a result of intermittent blood flow due to tumor vasculature abnormalities (7). Such acute or “perfusion-limited” hypoxia is characterized by rapid reoxygenation and hypoxic-oxic cycles (8) shown to have periodicities of minutes, hours or days. Although both types of hypoxia occur in human tumors, their relative impact on the metastatic dissemination of cancer cells may vary (9).
HIF-1 is a key transcriptional factor that may serve as a surrogate marker of tumor oxygenation and response. In general, HIF-1 is believed to mediate a pleiotropic role under both aerobic and anaerobic conditions. In the presence of ambient oxygen tension, the HIF-1α subunit is degraded by the ubiquitin-proteasome system via binding to the von Hippel-Lindau (VHL) protein. Under hypoxic conditions, HIF-1α is rapidly stabilized and functionally activated (10). HIF-1 mediates key hypoxia-associated genes involved in angiogenesis, metabolism, survival and invasion (11). In some cancer types, HIF-1 can also be constitutively activated in an O2-independent manner under aerobic conditions as a consequence of dysregulated signaling pathways that involve hyperactivation of oncogenes or inactivation of tumor suppressor genes. Given the central role of HIF-1 in driving multiple cellular behaviors in response to environmental oxygen loss, HIF-1 serves as an attractive target for drug development (10).
As is the case in other solid tumors, intratumoral hypoxia is emerging as a common feature of prostate cancers that are associated with poor prognosis. HIF-1α has been shown to be activated in prostate cancer as compared to normal prostatic epithelium, suggesting a possible role for HIF-1α as a biomarker for premalignant lesions of the prostate (12). Upregulation of HIF-1α represents an early event in prostate carcinogenesis (13) that is highly correlated with the risk of metastases (14). Moreover, in prostate cancer patients treated with radiotherapy, increased HIF-1α expression is associated with reduced time to progression (15). Although the effects of hypoxia on prostate cancer cell function have been studied (16), there is limited information directly comparing the effects of acute and chronic hypoxia. The present study examined the impact of acute and chronic hypoxia on metastasis-associated cell functions and behaviors in human prostate cancer models.
METHODS AND MATERIALS
Cell culture and hypoxia
Human prostate cancer cells (PC-3, DU145, LNCaP) were purchased from American Type Culture Collection. Cells were maintained in appropriate media plus 10% fetal bovine serum (FBS) in humidified 5% CO2 at 37°C. For hypoxic culture conditions, cells were incubated in glass dishes in a modular incubator chamber (Oxygen Sensors, Gladwyne, PA) flushed with a gas mixture containing 1% O2 (hypoxia) or 0% O2 (anoxia) balanced with 5% CO2 and N2 at 37°C. For reoxygenation after hypoxic incubation, cells were transferred back to 5% CO2 and air. Cells were used for all experiments in 20 passages.
Cell proliferation
Cells were seeded into glass dishes (7×105/dish) and allowed to attach over night. Cells then were exposed to hypoxia (1% O2) for various durations. Adhered cells were trypsinized and counted using a hemocytometer. Cell proliferation was determined by comparing the cell number under hypoxic to normoxic conditions. Cell death was assessed by trypan blue exclusion in adhered and floating cell populations, and expressed as % of positive stained cells.
Cell motility
Cell motility was determined using an in vitro “wound-healing” assay. Cells were seeded in glass dishes (106/dish) and grown for 48 h to allow them to reach confluency. Scratches (~2 mm wide) were made in the confluent cell monolayer using a sterilized 1 ml pipette tip (8~10 scratches/sample). After scratching, cells were cultured under hypoxic (1% O2) or anoxic (0% O2) conditions for the desired period of time and then cultured under aerobic conditions until assessed. In some circumstances, cells were cultured under hypoxic/anoxic conditions before scratching the cell monolayers. Cell migration was then monitored and scored 24 h later.
Cell invasion
Cell invasion was detected as described previously (17). Briefly, cells (104) were seeded into Matrigel-coated invasion chambers (BD Biosciences, San Diego, CA) and incubated under hypoxia for desired periods of time. Cells that invaded to the underside of the filters were counted microscopically. Fold-changes in invasion were calculated as the ratio of invading cells under hypoxic to normoxic conditions.
Colony formation
Cells were exposed to 1% O2 for 0.5, 2, 6 or 24 h before seeded into 6-well plates (200 and 100/well in triplicate, respectively) and incubated under aerobic conditions for 14 days. Plates were stained with crystal violet and cell colonies (> 50 cells) were counted. Plating efficiency was calculated from the ratio of the number of colonies formed divided by the number of cells seeded.
Western blot
Preparation of whole cell lysates and Western blot analysis were described previously (17). Nuclear HIF-1α expression was tested from nuclear extracts prepared as described previously (18). Primary antibodies against HIF-1α (BD Bioscience) and β-actin (Sigma) were used.
Quantitative real time PCR (qPCR)
qPCR was performed as previously described (18) to determine the HIF-1α target gene expression level. Primers were designed as: vascular endothelial growth factor (VEGF), forward, 5′-GCCTTGCTGCTCTACCTCCAC-3′, reverse, 5′-ATGATTCTGCCCTCCTCCTTCT-3′; lysyl oxidase (LOX), forward, 5′-CAGCATACAGGGCAGATGTCAGA-3′, reverse, 5′-GTGTTGGCATCAAGCAGGTCA-3′; MMP-2, forward, 5′-TTGATGGCATCGCTCAGATC-3′, reverse, 5′-GCTTGTCACGTGGCGTCA-3′; MMP-9, forward, 5′-GAGGCGCTCATGTACCCTATGT-3′, reverse, 5′-CCGTGGCTCAGGTTCAGG-3′. Actin (an internal control): forward, 5′-CTCCTCCTGAGCGCAAGTACTC-3′, reverse, 5′-TCCTGCTTGCTGATCCACATC-3′.
Gelatin zymography
Subconfluent cells were incubated under normoxia or hypoxia in serum-free medium. Conditioned medium (CM) was concentrated by centrifuging at 2,000×g for 20 min using an Amicon Ultra Centrifugal filter device (cut-off molecular weight 10 kDa, Millipore). CM was normalized to equal cell numbers and separated on a zymogram gel (Bio-Rad) under non-denaturing and non-reducing conditions. The gel was washed with a renaturing buffer (2.5% Triton X-100) and incubated in a developing buffer (50 mM Tris-HCl, pH 7.5, 0.2 M NaCl and 5 mM CaCl2) at 37°C overnight. The gel was stained with Coomassie blue R-250 (0.1%) and destained for visualization. Bands were shown after image inversion by Photoshop CS2 and the density was quantified with the aid of the Image J software program (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented using a box plot with whiskers above and below the box indicating the 90th and 10th percentiles, respectively. The Kruskal-Wallis test was used to analyze the effect of hypoxic duration on cell functions. The Wilcoxon non-parametric rank sum test or Student’s t-test was applied to investigate difference between two individual groups. All statistical analysis was performed using GraphPad Prism 5.0 software (San Diego, CA). A threshold of p < 0.05 was defined as statistically significant.
RESULTS
Chronic but not acute hypoxia inhibits cell growth and induces cell death
Previous reports indicate that that long-term intratumoral hypoxia can lead to cell loss and necrosis (19, 20). In PC-3 cells, 24 h of hypoxia resulted in ~10% cell loss (p < 0.01), while hypoxia for 48 h significantly suppressed cell growth ~25% (p < 0.001) and enhanced cell death (p < 0.001) (Table 1). Short-term hypoxic exposure (2 – 6 h) had no effect on either cell growth or cell death (Table 1). Similar results were observed when PC-3 cells were exposed to anoxia (data not shown).
Table 1.
Impact of acute and chronic hypoxia on PC-3 cell proliferation and cell death
| Normoxia | Hypoxia
|
||||
|---|---|---|---|---|---|
| 2 h† | 6 h† | 24 h†† | 48 h§ | ||
| Cell proliferation (%)|| | 100.0 ± 0.0 | 99.8 ± 1.7 | 98.5 ± 3.1 | 89.1 ± 3.3** | 75.5 ± 3.7*** |
| Cell death (%)¶ | 2.5 ± 1.3 | 2.7 ± 1.5 | 2.7 ± 2.1 | 4.8 ± 2.1 | 17.3 ± 5.1*** |
Note:
Cells were cultured under hypoxic conditions (1% O2) for 2 or 6 h, and re-cultured under 20% O2 for 22 or 18 h, respectively. For cell proliferation, † and †† were compared with 24 h normoxia, and § was compared with 48 h normoxia.
Relative cell number under 24 h or 48 h normoxia was defined as 100%.
Cell death was expressed as the average number of trypan blue-positive cells after culturing under 20% O2 for 24 h and 48 h (there is no difference on trypan blue-positive cell number with these two durations). Data are presented as mean ± SD (n=3).
p < 0.01;
p < 0.001 vs. normoxia (t-test).
Acute hypoxia/anoxia promotes cell motility
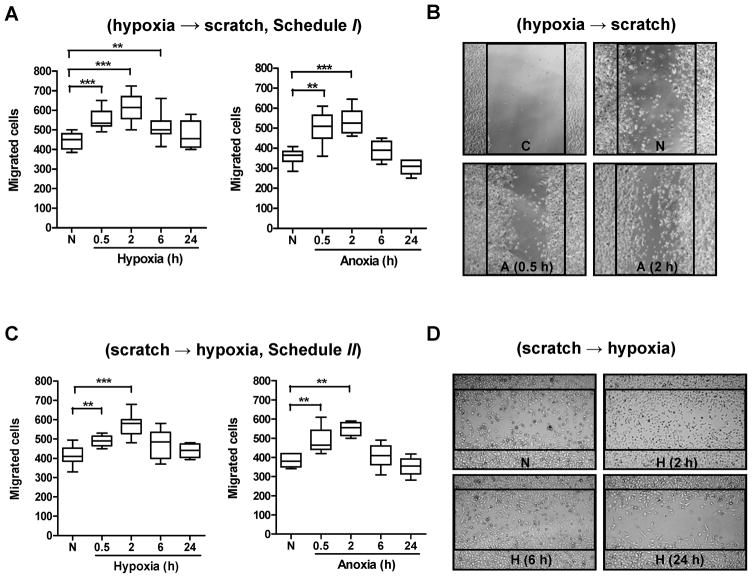
To test the effect of hypoxia or anoxia (the latter was investigated to mimic a scenario of complete vessel shut-down) on tumor cell motility, two treatment schedules were evaluated. In the first (schedule I), cells were exposed to hypoxia/anoxia before the “wound” was made (Fig. 1A and B), while in the second (schedule II), the cell layer was scratched before exposure to hypoxia/anoxia (Fig. 1C and D). The results showed that irrespective of the treatment schedule, hypoxia or anoxia significantly affected cell migration (p < 0.001, Kruskal-Wallis test). Furthermore, short-term exposure to hypoxia (0.5, 2 or 6 h) or anoxia (0.5 or 2 h) significantly increased cell movement. The greatest increase in cell migration occurred following a 2 h exposure to hypoxia (36.6±3.5%, p < 0.001 and 38.8±5.1%, p < 0.01 in schedule I and II, respectively) or anoxia (48.5±5.4%, p <0.01 and 40.6±3.0%, p <0.05 in schedule I and II, respectively) (Fig. 1). In contrast, chronic hypoxia or anoxia (24 h) failed to significantly enhance tumor cell migration (p > 0.05).
Fig. 1.
Motility of tumor cells exposed to acute and chronic hypoxia or anoxia. (A) PC-3 cells grown as monolayers were exposed to hypoxia or anoxia, then scratches were made and cells were incubated under normoxia for an additional 24 h. (B) Typical photographs of A are shown (original magnification, ×50). C=control (0 h after scratching); N=normoxia; A=anoxia. (C) PC-3 cell monolayers were scratched and then incubated under hypoxia/anoxia for 0.5, 2, 6 or 24 h, followed by re-culturing under normoxia for 23.5, 22, 18 or 0 h, respectively. (D) Typical photographs of C are shown (original magnification, ×50). N=normoxia; H=hypoxia. Cells that migrated into the denuded areas were scored from 4 random fields/sample with ≥3 independent samples. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n≥12, Wilcoxon test). The global effect of hypoxia on cell migration was analyzed by the Kruskal-Wallis test.
Hypoxia mediates prostate cancer cell invasion in a time-dependent manner
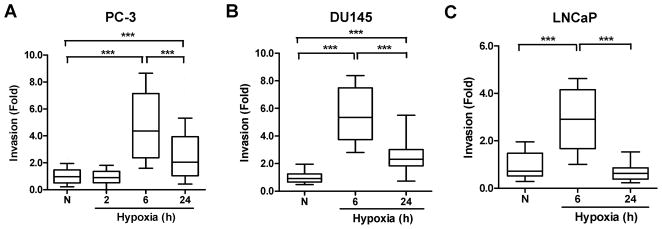
A 6 h exposure to hypoxic conditions significantly enhanced cell invasion in all 3 prostate cancer cell lines (p < 0.001, Kruskal-Wallis test, Fig. 2). Longer periods of hypoxia (24 h) also increased the ability of PC-3 cells to invade through a Matrigel layer (2.6±0.2-fold vs. normoxia, p < 0.001), but not as effectively as the 6 h exposure (4.7±0.6-fold vs. normoxia, p < 0.001; p < 0.001 vs. 24 h hypoxia, Fig. 2A); an observation also found in DU145 (Fig. 2B) and LNCaP cells (Fig. 2C). In contrast, very short periods of oxygen deprivation (2 h) did not alter PC-3 cell invasion (Fig. 2A) and extended hypoxia (48 h) resulted in a decreased invasive ability (data not shown).
Fig. 2.
Cell invasion under hypoxic environments. Prostate cancer cells were seeded into transwells containing Matrigel and the inserts were incubated under hypoxia for 2, 6, and 24 h, then re-culturing under aerobic conditions for 22, 18 and 0 h, respectively. Invasion was determined by counting the number of invading cells from 8 random fields/sample (original magnification, ×100), with 3 independent samples. N=normoxia. *, p < 0.05; ***, p < 0.001 (n=24, Wilcoxon test). The global effect of hypoxia on cell invasion was analyzed by the Kruskal-Wallis test.
Acute hypoxia enhances clonogenic survival
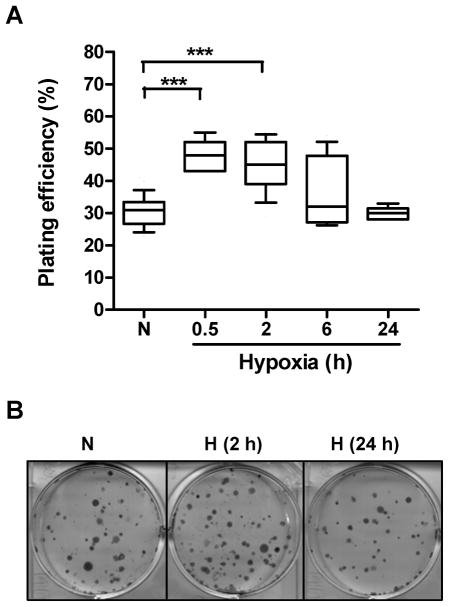
The impact of hypoxia on the ability of PC-3 cells to form macroscopic colonies was assessed using a clonogenic cell survival assay (Fig. 3). The results showed that compared to aerobic conditions (plating efficiency of 30.6±4.8 %), tumor cells pre-incubated under hypoxia for short periods of time (0.5 or 2 h) formed significant more colonies (plating efficiencies of 54.5±6.2% and 44.6±6.4% for 0.5 h and 2 h, respectively, p < 0.001, Fig. 3A). A 6 h hypoxia pretreatment resulted in a modest increase in plating efficiency (p = 0.075) while a 24 h hypoxic exposure had no effect (Fig. 3A and B).
Fig. 3.
Clonogenic cell survival under hypoxia. (A) PC-3 cells were exposed to 1% oxygen for 0.5, 2, 6 or 24 h, prior to culturing them in an aerobic environment (21% oxygen) for 14 days. Cell colonies (> 50 cells) were counted macroscopically (6 wells/sample with 3 independent samples). N=normoxia; H=hypoxia. ***, p < 0.001 (n=18, Wilcoxon test). (B) Typical wells in A are shown.
Hypoxia induces transient stabilization of HIF-1α, activates HIF-target gene expression and promotes MMP-2 secretion
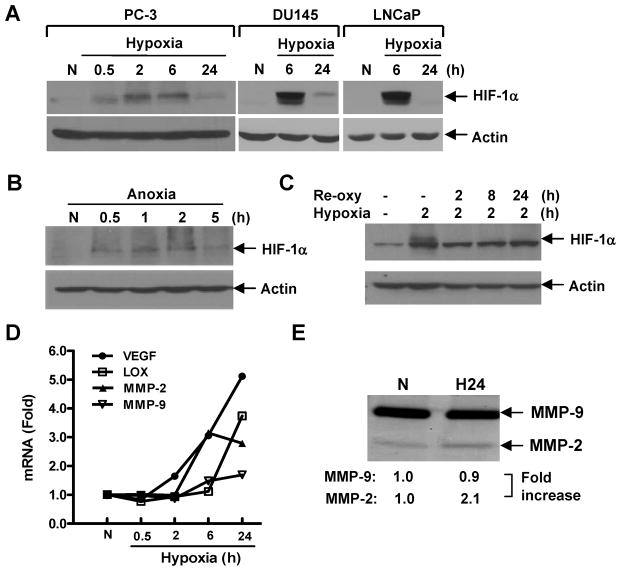
The HIF-1α expression in PC-3 cells was observed to accumulate at 30 min, reach a peak level at 2 h, and decrease with the longer exposure to hypoxia (Fig. 4A). In DU145 and LNCaP cells, the maximal expression of HIF-1α was found when cells were exposed to hypoxia for 6 h, with a significantly decayed expression at 24 h (Fig. 4A). A similar result was found under anoxic conditions, where nuclear HIF-1α increased from 0.5 to 2 h (highest expression at 1 h) and decreased at 5 h in PC-3 cells (Fig. 4B). The hypoxia-induced HIF-1α rapidly disappeared (within 2 h) when PC-3 cells were returned to normoxia (Fig. 4C). At the mRNA level, hypoxia activated the expression of multiple HIF-1-target genes involved in tumor angiogenesis and invasion including VEGF, MMP-2 and LOX (Fig. 4D). Notably, MMP-2 gene expression was significantly upregulated (3.2±0.2-fold) after PC-3 cells were exposed to hypoxia for 6~24 h whereas the activation of MMP-9 was only slightly altered. Consistent with the gene expression, the active form of MMP-2 (72 kDa), but not MMP-9 (92 kDa), showed elevated enzymatic activity after 24 h hypoxia, although the basal level of MMP-9 was much higher than that of MMP-2 (Fig. 4E).
Fig. 4.
Molecular response of prostate cancer cells under hypoxia. (A) PC-3, DU145 and LNCaP cells were exposed to 1% oxygen for various periods of time. (B) PC-3 cells were exposed to 0% oxygen for various periods of time. (C) PC-3 cells were exposed to 1% oxygen for 2 h followed by reoxygenation (Re-oxy). Whole cell lysates (A and C) or nuclear extracts (B) were analyzed by Western blot for HIF-1α expression using actin as a loading control. (D) PC-3 cells were exposed to 1% oxygen as in A and HIF-1-target gene expression was determined by qPCR. Bars, SD (n=3). (E) PC-3 cells were cultured under hypoxia or normoxia in serum-free medium for 24 h. Conditioned medium (from 2×105 cells) was harvested for gelatin zymography analysis. N=normoxia; H=hypoxia.
Hypoxia-induced cell migration and invasion is HIF-1-dependent
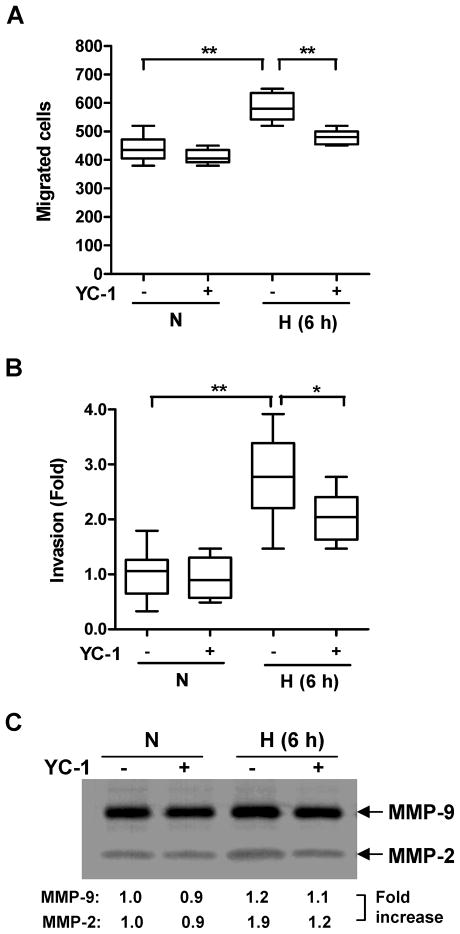
To examine the role of HIF-1α activation in hypoxia-induced cell invasion YC-1, a HIF-1 inhibitor reported to suppress HIF-1α activation (21), was applied to PC-3 cell exposed to short-term oxygen deprivation. YC-1 treatment (60 μM (22)) had little effect on cell migration when PC-3 cells were treated under normoxic conditions (p = 0.21) (Fig. 5A). However, the same dose of YC-1 significantly decreased 2 h hypoxia-induced cell migration (p < 0.01, Fig. 5A). Similarly, increased cell invasion after 6 h hypoxic exposure was significantly attenuated by YC-1 treatment (p < 0.05, Fig. 5B) as was the MMP-2 activity (Fig. 5C). The active MMP-9 level, however, remained unchanged by either hypoxia or YC-1 (Fig. 5C).
Fig. 5.
Effect of treatment with the HIF inhibitor YC-1 on PC-3 cell migration and invasion. PC-3 cells were untreated or pretreated with YC-1 (60 μM) for 1 h. (A) Cells then were exposed to 1% oxygen for 2 h prior to a “wound-healing” assay. After scratching the monolayer, the cells were incubated aerobically for 24 h. (B) After drug exposure, cells were seeded into the invasion chambers. Cells were then incubated under hypoxia for 6 h followed by normoxia for 18 h. In both A and B, YC-1 was in the medium throughout the experiments. Migrated (A) or invaded (B) cells were scored from 4 random fields/sample using 2 independent samples. *, p < 0.05; **, p < 0.01 (n=8, Wilcoxon test). (C) PC-3 cells were untreated or exposed to YC-1 as in B in serum-free medium. Conditioned medium (from 105 cells) was harvested for gelatin zymography. N=normoxia; H=hypoxia.
DISCUSSION
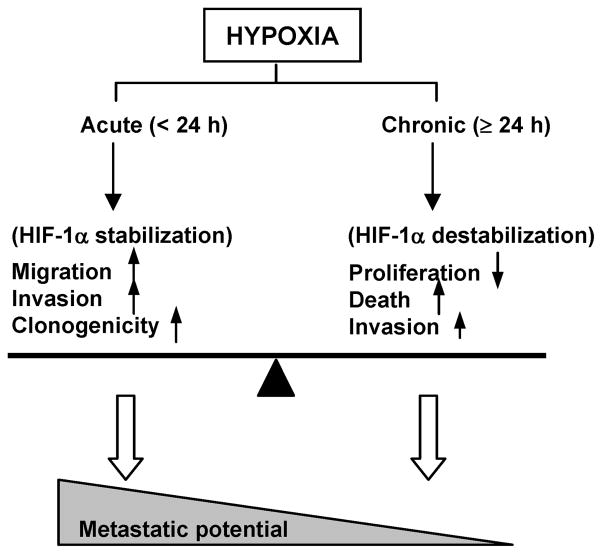
The relative contributions of acute versus chronic hypoxia on cancer metastasis remain controversial. While some studies appear to favor chronic hypoxia as the key factor (23, 24), others suggest that acute hypoxia may be equally or more critical in mediating a cancer cell’s metastatic behavior (9, 25, 26). The present study shows that in prostate cancer cells acute and chronic exposures to hypoxia trigger different cell responses. Whereas chronic (≥ 24 h) hypoxia appears to result primarily in decreased PC-3 cell proliferation and increased cell death (Table 1), acute hypoxia (≤ 6 h) notably enhances cell migration (Fig. 1), invasion (Fig. 2) and clonogenic survival (Fig. 3). These functional changes imply that the shorter (acute) oxygen deprivations are most effective at elevating the metastatic phenotype, suggesting that varying contributions of acute and chronic hypoxia to the microenvironment may significantly influence the metastatic potential of cancer cells (Fig. 6). The observed response of PC-3 cells under chronic hypoxia is consistent with other studies that have previously reported 48 h hypoxia to decreases invasion (27) and induce apoptosis (28) in this model. The present results together with data indicating that acutely hypoxic cells may be more resistant to radiation than cells exposed to chronic hypoxia (29), further serve to highlight the possible role of acute hypoxia in radiotherapy failure in prostate cancer.
Fig. 6.
Paradigm of hypoxia-mediated cell response in PC-3 cells. While chronic (≥24 h) hypoxia reduces cell proliferation, increases cell death and moderately enhances invasion, acute (< 24 h) hypoxia significantly promotes multiple metastasis-related functions including migration, invasion, and clonogenicity, leading to an increased metastatic potential. Such functional behaviors are partially associated with the expression of HIF-1α, which is transiently stabilized under acute hypoxia but destabilized under chronic hypoxia.
It is interesting that short-term oxygen deprivation enhanced the ability of PC-3 cells to migrate into a denuded area irrespective of whether the exposure to hypoxia occurred prior to or during the migratory phase (Fig. 1). These two conditions were investigated in an attempt to mimic the cyclic nature of acute hypoxia observed in tumors that results from transient occlusion of vessels and arteriolar vasomotion (25). When such fluctuations were exacerbated in tumor-bearing mice by exposing the animals to repeated short-term intervals of hypoxia, the frequency of lung metastases was markedly increased (25, 30). Consistent with other studies (31, 32), the present results support a role for the process of reoxygenation in acute hypoxia-mediated cell functions, given that cells exposed to hypoxia for short periods before being returned to normoxia displayed significantly elevated locomotive and invasive capacity, as well as increased clonogenicity; factors that would suggest the possibility of enhanced metastatic colonization. Although the current experiments do not allow us to distinguish which of the altered phenotypes will ultimately prove to be most relevant to the metastatic process, the findings do provide important evidence that short hypoxic exposures (< 6 h) readily facilitate key metastasis-related cellular functions.
At the molecular level, HIF-1α protein accumulation occurred over the same time frame (0.5~6 h for hypoxia; 0.5~2 h for anoxia) that also elevated functional cell behavior (migration and invasion). Hypoxic exposure did not induce the expression of motility-associated genes such as hepatocyte growth factor, transforming growth factor alpha or autocrine motility factor, nor triggered the activation of signaling molecules involved in migration (c-Met, data not shown); a finding inconsistent with other reports (33, 34). Hypoxia did however trigger the activation of VEGF and LOX, two HIF-1 target genes whose increased expression has been associated with elevated aggressiveness in prostate cancer (35, 36). MMP-2 expression, both transcriptional (Fig. 4D) and functional (Fig. 4E) also was increased by hypoxic exposure, indicating that MMP-2 may be involved in hypoxia-mediated cell invasiveness. This possibility was further supported by the observation that treatment with the HIF inhibitor YC-1 attenuated PC-3 cell migration (Fig. 5A) and invasion (Fig. 5B) in a manner that correlated with MMP-2 activation by acute hypoxia (Fig. 5C). Taken together, these results implicate HIF-1α as a crucial factor that facilitates both cellular and molecular events in transiently hypoxic PC-3 cells and suggest in particular that the metastatic phenotype associated with acute hypoxia exposure is at least in part HIF-1-dependent.
It should be noted that the elevated levels of HIF-1α observed following hypoxic exposure were rapidly destabilized once the tumor cells were returned to normoxic conditions (Fig. 4C). This suggests that the augmented cell functions resulting from acute hypoxia exposure may not require continuous HIF-1α activation. Speculation regarding the role of HIF-1α in the current experiments is further complicated by the fact that in PC-3 cells HIF-1α is constitutively expressed even under aerobic conditions (likely due to PTEN loss). Furthermore, it is conceivable that signaling pathways already activated by HIF-1 during hypoxia may be sustained during the reoxygenation phase even though HIF-1α has been inactivated, as has been suggested in other prostate cancer cell models (37).
Finally, it is worth noting that in the current study chronic hypoxia (24 h), despite having a negative effect on cell growth and only a minor impact on migration and clonogenicity, did increase tumor cell invasion and MMP-2 secretion. Hence these data may suggest an adaptation mechanism for cells that have experienced chronic hypoxia. Acquisition of invasive characteristics by tumor cells existing in chronically hypoxia regions of a tumor coupled with the notion that hypoxia selects for the survival of more aggressive tumor cells therefore could increase the risk of tumor progression (38). In keeping with such a possibility, a 24 h hypoxic exposure was recently shown in PC-3 cells to lead altered expression of multiple proteins that predict for the induction of the epithelial-mesenchymal transition (39).
CONCLUSIONS
The present studies have demonstrated that hypoxic exposure leads to highly similar outcomes in both molecular signaling and critical cell functional behavior in three human prostate cancer cell lines. Specifically, tumor cell behaviors associated with a metastatic phenotype were found to be correlated with HIF-1α expression. The results also support the notion that acute hypoxia plays a more crucial role than chronic hypoxia in mediating the metastatic potential of prostate cancer cells. Taken together our in vitro findings provide mechanistic insights that set the stage for in vivo investigations directed at determining the deleterious impact of tumor hypoxia on prostate cancer metastasis.
Acknowledgments
Grant support: National Cancer Institute Public Health Service Grant R01 (CA089655).
The authors acknowledge the assistance of Dr. Lori Rice, Sharon Lepler and Chris Pampo for technical support, and Dr. Cyndi Garvan for statistical analysis.
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 2.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26:333–339. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 3.Chaudary N, Hill RP. Hypoxia and metastasis. Clin Cancer Res. 2007;13:1947–1949. doi: 10.1158/1078-0432.CCR-06-2971. [DOI] [PubMed] [Google Scholar]
- 4.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Hill RP. Hypoxia enhances metastatic efficiency in HT1080 fibrosarcoma cells by increasing cell survival in lungs, not cell adhesion and invasion. Cancer Res. 2007;67:7789–7797. doi: 10.1158/0008-5472.CAN-06-4221. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 7.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 8.Kimura H, Braun RD, Ong ET, et al. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56:5522–5528. [PubMed] [Google Scholar]
- 9.Rofstad EK, Galappathi K, Mathiesen B, et al. Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clin Cancer Res. 2007;13:1971–1978. doi: 10.1158/1078-0432.CCR-06-1967. [DOI] [PubMed] [Google Scholar]
- 10.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 12.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 13.Pipinikas CP, Carter ND, Corbishley CM, et al. HIF-1alpha mRNA gene expression levels in improved diagnosis of early stages of prostate cancer. Biomarkers. 2008;13:680–691. doi: 10.1080/13547500802591992. [DOI] [PubMed] [Google Scholar]
- 14.Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- 15.Vergis R, Corbishley CM, Norman AR, et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–351. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 16.Marignol L, Coffey M, Lawler M, et al. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev. 2008;34:313–327. doi: 10.1016/j.ctrv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Dai Y, Siemann DW. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Mol Cancer Ther. 2010;9:1554–1561. doi: 10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, Liu M, Tang W, et al. A Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer. 2009;9:392–407. doi: 10.1186/1471-2407-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jaeger K, Merlo FM, Kavanagh MC, et al. Heterogeneity of tumor oxygenation: relationship to tumor necrosis, tumor size, and metastasis. Int J Radiat Oncol Biol Phys. 1998;42:717–721. doi: 10.1016/s0360-3016(98)00323-x. [DOI] [PubMed] [Google Scholar]
- 20.Rotin D, Robinson B, Tannock IF. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986;46:2821–2826. [PubMed] [Google Scholar]
- 21.Shin DH, Kim JH, Jung YJ, et al. Preclinical evaluation of YC-1, a HIF inhibitor, for the prevention of tumor spreading. Cancer Lett. 2007;255:107–116. doi: 10.1016/j.canlet.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Sun HL, Liu YN, Huang YT, et al. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-kappaB signaling to HIF-1alpha accumulation during hypoxia. Oncogene. 2007;26:3941–3951. doi: 10.1038/sj.onc.1210169. [DOI] [PubMed] [Google Scholar]
- 23.Thews O, Wolloscheck T, Dillenburg W, et al. Microenvironmental adaptation of experimental tumours to chronic vs acute hypoxia. Br J Cancer. 2004;91:1181–1189. doi: 10.1038/sj.bjc.6602066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alqawi O, Wang HP, Espiritu M, et al. Chronic hypoxia promotes an aggressive phenotype in rat prostate cancer cells. Free Radic Res. 2007;41:788–797. doi: 10.1080/10715760701361531. [DOI] [PubMed] [Google Scholar]
- 25.Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8908. [PubMed] [Google Scholar]
- 26.Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res. 2004;64:2054–2061. doi: 10.1158/0008-5472.can-03-3196. [DOI] [PubMed] [Google Scholar]
- 27.Ackerstaff E, Artemov D, Gillies RJ, et al. Hypoxia and the presence of human vascular endothelial cells affect prostate cancer cell invasion and metabolism. Neoplasia. 2007;9:1138–1151. doi: 10.1593/neo.07568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie S, Sakamoto S, Kyprianou N. Maspin modulates prostate cancer cell apoptotic and angiogenic response to hypoxia via targeting AKT. Oncogene. 2008;27:7171–7179. doi: 10.1038/onc.2008.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan N, Milosevic M, Bristow RG. Tumor hypoxia, DNA repair and prostate cancer progression: new targets and new therapies. Future Oncol. 2007;3:329–341. doi: 10.2217/14796694.3.3.329. [DOI] [PubMed] [Google Scholar]
- 30.Hill RP, De Jaeger K, Jang A, et al. pH, hypoxia and metastasis. Novartis Found Symp. 2001;240:154–165. doi: 10.1002/0470868716.ch11. discussion 165–158. [DOI] [PubMed] [Google Scholar]
- 31.Shi J, Wan Y, Di W. Effect of hypoxia and re-oxygenation on cell invasion and adhesion in human ovarian carcinoma cells. Oncol Rep. 2008;20:803–807. [PubMed] [Google Scholar]
- 32.Postovit LM, Abbott DE, Payne SL, et al. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103:1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- 33.Funasaka T, Raz A. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev. 2007;26:725–735. doi: 10.1007/s10555-007-9086-7. [DOI] [PubMed] [Google Scholar]
- 34.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 35.Balbay MD, Pettaway CA, Kuniyasu H, et al. Highly metastatic human prostate cancer growing within the prostate of athymic mice overexpresses vascular endothelial growth factor. Clin Cancer Res. 1999;5:783–789. [PubMed] [Google Scholar]
- 36.Stewart GD, Gray K, Pennington CJ, et al. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol Rep. 2008;20:1561–1567. [PubMed] [Google Scholar]
- 37.Khandrika L, Lieberman R, Koul S, et al. Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1alpha levels contribute to emergence of an aggressive phenotype in prostate cancer. Oncogene. 2009;28:1248–1260. doi: 10.1038/onc.2008.476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 39.Mak P, Leav I, Pursell B, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]