Abstract
Background & Aims
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) reduce weight and improve glucose metabolism in obese patients, although it is not clear if metabolic changes are independent of weight loss. We investigated alterations in glucose metabolism in rats following RYGB or VSG.
Methods
Rats underwent RYGB or VSG and were compared to sham-operated rats that were fed ad lib or pair-fed with animals that received RYGB. Intraperitoneal glucose tolerance and insulin sensitivity tests were performed to assess glycemic function, independent of the incretin response. A hyperinsulinemic euglycemic clamp was used to compare tissue-specific changes in insulin sensitivity following each procedure. A mixed-meal tolerance tests was used to assess the effect of each surgery on post-prandial release of GLP-1(7–36) and glucose tolerance, and were also performed in rats given the GLP-1 receptor antagonist exendin9–39.
Results
Following RYGB or VSG, glucose tolerance and insulin sensitivity improved, in proportion to weight loss. Hepatic insulin sensitivity was significantly better in rats that received RYGB or VSG, compared with rats fed ad lib or pair-fed, whereas glucose clearance was similar in all groups. During the mixed-meal tolerance test, plasma levels of GLP-1(7–36) and insulin were greatly and comparably increased in rats that received RYGB and VSG, compared with those that were pair-fed or fed ad lib. Administration of a GLP-1 receptor antagonist prevented improvements in glucose and insulin responses after a meal among rats that received RYGB or VSG.
Conclusion
In obese rats, VSG is as effective as RYGB at increasing secretion of GLP-1 and insulin secretion and at improving hepatic sensitivity to insulin; these effects are independent of weight loss.
Keywords: obesity, surgery, weight reduction, diabetes
Introduction
Bariatric surgery is currently the most effective therapy for sustained weight loss in obese patients. The Roux-en-Y gastric bypass (RYGB) procedure, with a gastric pouch draining into the mid-jejunum and pancreatico-biliary flow diverted 8 to the distal jejunum, is the most commonly performed surgery in the US 1. However, another type of surgery, the vertical sleeve gastrectomy (VSG), a surgery in which 80% of the stomach is removed along the greater curvature but intestinal anatomy is unaltered, is gaining in popularity 1. On average, RYGB and VSG patients can expect to lose ≥60% of their excess body weight and of those with preoperative type 2 diabetes ≥80% go into remission after surgery 2–4. In fact, normal fasting glucose concentrations and favorable changes in insulin sensitivity often occur before any substantial weight loss 5–7. This profile has led to the hypothesis that these surgeries improve blood glucose parameters beyond what would be expected from hypophagia and weight loss alone. It is noteworthy that although RYGB and VSG involve very distinct manipulations of the GI tract many of the short- and long-term outcomes of these procedures are similar3.
In addition to heightened insulin sensitivity, post-surgical increases in the release of intestinal hormones and especially of glucagon-like peptide-1 (GLP-1)7–36 are also thought to confer major improvements in glucose homeostasis after surgery 3, 8–10. GLP-1(7–36) is released from L cells in the distal gut in response to nutrients 11. GLP-1(7–36) acts at a G-protein-coupled receptor to limit postprandial glucose excursions by augmenting insulin release, inhibiting endogenous glucose production, and decreasing gastric emptying 12. The reason that RYGB surgery changes postprandial hormone profiles is not clear, but may result from changing intestinal functional anatomy such that undigested chyme is delivered closer to the distal small intestine where L-cells are in greater abundance. In this study, we asked whether VSG enhances postprandial GLP-1(7–36) levels and improves glucose homeostasis to the same degree as RYGB surgery, or whether, based on the surgical alteration of the small intestine, RYGB confers additional metabolic benefits. We therefore directly compared the effects of RYGB and VSG on glucose homeostasis and assessed weight-independent effects using a group of sham-operated rats that were pair-fed to the RYGB surgical group.
Methods
Animals
Male Long-Evans rats (n=54) (Harlan Laboratories, Indianapolis, IN; 250–300 g) were individually housed and maintained on a 12/12-h light/dark cycle (lights off at 1400 h) at 25 °C and 50–60% humidity. All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Prior to surgery rats were given ad libitum access to water and a high-fat butter diet (Research Diets, New Brunswick, NJ, 4.54 kcal/g; 41% fat) previously documented to produce metabolic impairments 13. After 8-weeks on the HFD the assigned surgical groups (RYGB, VSG, ad lib-fed Sham or sham-operated pair-fed) were counterbalanced based on fat mass.
Surgical Procedures
Bariatric Surgeries
VSG was performed in anesthetized rats (isoflurane) to remove ~80% of the stomach as described previously 14. To perform the RYGB, anesthetized rats had a laparotomy and the jejunum was transected 30 cm from the ligament of Treitz. A longitudinal antimesenteric incision was made 10 cm distal to the transected bowel and was connected to the afferent limb with a running 7-0 Vicryl® Absorbable Suture (Ethicon Endo-Surgery, Somerville, NJ). The stomach was isolated and the fundus was excised by making a vertical cut along the edge of the corpus with an ETS-FLEX 35 mm staple gun (Ethicon Endo-Surgery, Somerville, NJ). A second staple line was placed across the waist of the stomach creating a gastric pouch that was ~10% the size of the original stomach. The distal remnant was returned to the peritoneal cavity and an incision was made on either side of the gastric pouch that spared the vascular architecture. The efferent limb of the transected jejunum was connected to the gastric pouch with a running 8-0 Prolene non-absorbable suture. After reintegrating the gastric pouch into the peritoneal cavity the abdominal wall was closed in layers using a running stitch and a running subcuticular stitch, respectively. For the sham surgery, after the laparotomy a section of jejunum was isolated and cut 30 cm beyond the ligament of Treitz. The two halves were then anastomosed end-to-end and the laparotomy was closed in layers.
Post Operative Care
Subcutaneous injections of Metacam (0.25mg/100g BW once daily for 4-days), gentamicin (0.8 mg/100g BW on the day of surgery), Buprenex (0.3 ml 2X per day for 5-days), and warm saline (10 ml and 5 ml 2X per day for days 0–3 and 4–5, respectively) were given to all post-operative rats. A wire grate was used for 5-days post-operatively to prevent rats from eating their bedding.
Food Intake and Body Weight
3-days pre-operatively the high fat diet was replaced with Ensure Plus™ liquid Diet (1.41 kcal/g; 29% Fat) (Abbott Nutrition, Columbus, OH) for the remainder of the study. Pair-fed rats were given access to the amount of food eaten by the RYGB animals on the previous day. All rats were given access to food for 18-h each day (Food on at 1400-h; food off at 0800-h). Body composition was assessed using an EchoMRI analyzer (Houston, TX) ~1 week pre- and at 28-days and 105-days post-operatively.
Insulin Tolerance Test
After a full recovery (4-weeks after surgery), 6-h fasted rats were given an IP injection of insulin (0.5 U/kg) delivered in 1 ml/kg saline. Blood glucose was measured at baseline (0), 15, 30, 45 and 60-min after injections with a hand-held glucose analyzer (Accu chek, Roche Diagnostics, Indianapolis, Indiana).
Glucose Tolerance Test
5-weeks after surgery, 6-h fasted rats were given an IP injection of 50% dextrose (1.5 g/kg). Blood glucose was measured at baseline (0), 15, 30, 45, 60 and 120-min. Plasma insulin was measured at 0, 15 and 60-min.
Hyperinsulinemic Euglycemic Clamp Studies
Another cohort of rats was generated as described above except that catheters were placed in the carotid artery and jugular vein immediately after the respective gastrointestinal surgery. Glucose turnover was assessed 2-weeks after surgery in 6-h fasted rats, with a primed, continuous infusion of HPLC purified [3-3H]glucose (Perkin Elmer Life Sciences, Boston, MA) as described previously 15. Briefly, a primed (8 mU/kg/min), continuous (4 mU/kg/min) infusion of insulin was given and a variable rate of dextrose was infused to maintain euglycemia. Insulin was analyzed at 100 and 240-min. Glucose turnover (glucose rates of appearance, glucose production, and glucose utilization) were calculated according to previous methods 15.
Mixed Meal Tolerance Test
Approximately 5-months after surgery, 6-h fasted rats were gavaged with 2.5 ml (3.7 kcal) of Ensure Plus Liquid diet. The caloric load was based on the animals’ previously determined average 15-min intake of Ensure Plus liquid diet (Data not shown). Blood glucose was measured and 180 μl of blood was collected into tubes containing 20 μl of anti-proteolytic cocktail (4.65g EDTA + 92mg aprotinin + 40000U of Heparin in 50ml saline) every 15-min for 1-h. GLP-1(7–36) was measured by an electrochemiluminescence assay (Meso Scale Discovery, Gaithersburg, Md). Six weeks later the experiment was repeated in rats pretreated with an IP injection of the selective GLP-1 receptor antagonist, Exendin(9–39) (American Peptide Company, CA; 50 μg/kg), 30-min prior to baseline.
Statistical Analysis
The data were analyzed using 2-way and 1-way mixed-model ANOVAs where appropriate. Significant differences between treatments were followed up with Bonferroni Multiple comparisons tests. In all cases statistical significance was set at P<0.05. The data are displayed as mean ± SEM.
Results
RYGB and VSG Reduce Food Intake and Body Weight Comparably
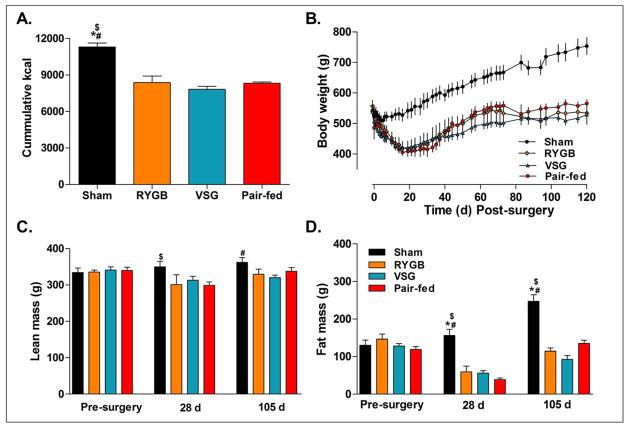
RYGB and VSG both reduced food intake and body weight relative to ad lib sham-operated controls (Figure 1A-B), P<0.05. This was primarily due to body fat which was significantly decreased in RYGB, VSG, and pair-fed groups at 28-days and 105-days post-op. Lean mass was slightly reduced in pair-fed at 28-days and in VSG at 105-days post-op compared to sham animals (Figure 1C-D). There were no significant differences in body weight between pair-fed, RYGB and VSG rats at any time point (P>0.05).
Figure 1.
A. Cummulative food intake in Sham (n=10), RYGB (n=4), VSG (n=7), and Pair-fed (n=10) rats after 120-days. Average cumulative food intake was significantly higher in Sham operated rats vs. RYGB, VSG, and Pair-fed animals, P<0.05. B. Body weight was also significantly higher in Sham rats compared to the other treatment groups, P<0.05. C. Lean tissue mass was slightly higher in Sham operated rats vs. RYGB rats after 28-days, P<0.05, and slightly higher than in VSG rats after 105-days, P<0.05. D. Fat tissue mass was substantially higher in Sham vs. all other treatment groups at 28-days, P<0.05, and 105-days, P<0.05, after surgery. Bonferroni’s Post-test; *P<0.05 vs. Pair-fed, $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
RYGB and VSG improve IP Insulin Sensitivity and Glucose Tolerance In Proportion to Weight Loss
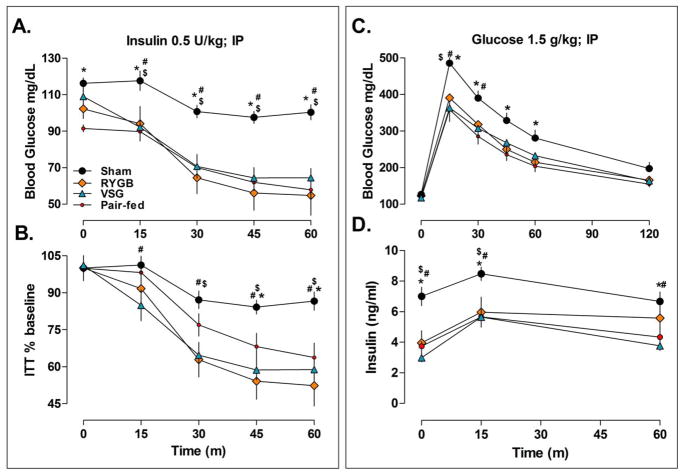
In order to assess peripheral insulin sensitivity and glucose tolerance, independent of the gut, IP insulin and glucose tolerance tests were performed. In response to insulin, blood glucose fell to significantly lower levels in RYGB, VSG, and pair-fed rats compared to Sham when expressed as an absolute value (Figure 2A) or as a percentage of baseline (Figure 2B), P<0.05. During an IP glucose tolerance test (Figure 2C), blood glucose levels were similar among RYGB, VSG and pair-fed rats, all of which were significantly lower than sham levels, P<0.05. Plasma insulin during the IP glucose tolerance test was also significantly lower in RYGB, VSG and pair-fed rats compared with shams at baseline (0), 15 and 60-min, P<0.05 (Figure 2D). These data indicate that IP insulin sensitivity and glucose tolerance after RYGB and VSG surgery occurred in proportion to weight loss.
Figure 2.
IP insulin sensitivity (A-B) and glucose tolerance (C-D) are improved in proportion to weight loss after RYGB and VSG surgeries. A. Blood glucose levels before (time 0) and after an IP injection of insulin in Sham (n=10), RYGB (n=4), VSG (n=7), and Pair-fed rats (n=10). RYGB, VSG, and Pair-fed rats were more sensitive to effect of insulin on circulating glucose levels than ad lib fed Sham rats, P<0.05. B. The same data expressed as a percentage of baseline shows that changes in insulin sensitivity were not due to differences in basal circulating glucose. C. Blood glucose levels before (time 0) and after an IP injection of glucose show that RYGB, VSG, and Pair-fed rats also had lower glucose excursions and D. plasma insulin levels relative to sham operated rats in response to peripherally administered glucose. Bonferroni’s Post-test; *P<0.05 vs. Pair-fed, $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
Hepatic Insulin Sensitivity is Improved in RYGB and VSG Independent of Changes in Body Weight
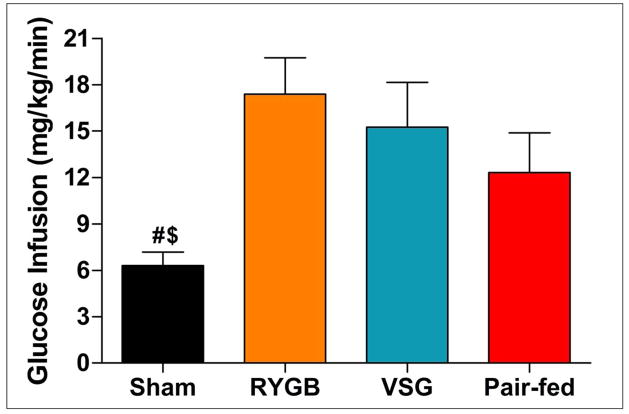
To examine tissue-specific effects of RYGB and VSG on insulin sensitivity, an additional cohort of animals was generated and studied during a hyperinsulinemic euglycemic clamp. On the day of the clamp, basal glucose levels were similar among groups (sham 162±5 mg/dL, RYGB, 157±9 mg/dL, VSG 150±9 mg/dL, pair-fed 164±12 mg/dL), P =0.68, whereas plasma insulin levels were significantly higher in sham rats (3.5±0.3 ng/ml) relative to RYGB (1.7±0.2 ng/ml), VSG (1.9±0.3 ng/ml) and pair-fed animals (1.4±0.4 ng/ml), P<0.0001. During the clamp (final 40-min) glucose (sham 141±2 mg/dL, RYGB 133±3 mg/dL, VSG 139±7 mg/dL, pair-fed 137±6 mg/dL), P=0.75, and insulin levels (sham 9.5±0.5 ng/ml, RYGB 8.3±1.3 ng/ml, VSG 8.3±0.9 ng/ml, pair-fed 6.6±0.9 ng/ml), were similar among treatments, P>0.05. The average glucose infusion rate over the final 40 min was significantly greater in RYGB and VSG rats relative to shams, P<0.05 (Figure 3). The glucose infusion rate in the pair-fed rats was not significantly different compared to either surgical group or to the sham rats, P>0.05. Endogenous glucose production was similar at baseline across treatments. During the clamp endogenous glucose production was significantly inhibited in RYGB and VSG, but not sham or pair-fed rats, P<0.05 (Figure 4A). Glucose clearance was similar among treatments at baseline and increased similarly during the clamps (Figure 4B). Significant reductions in endogenous glucose production indicate that RYGB and VSG surgeries increase hepatic insulin sensitivity independent of changes in body weight.
Figure 3.
Whole body insulin sensitivity as measured by hyperinsulinemic euglycemic clamp. Average glucose infusion rate in Sham (n=8), RYGB (n=7), VSG (n=6), and Pair-fed (n=7) rats during steady state (final 40-min) clamp conditions. Glucose infusion rates were significantly increased in RYGB and VSG vs. ad lib fed Sham rats, P<0.05. Differences between RYGB, VSG, and Pair-fed rats were non-significant, P>0.05. Bonferroni’s Post-test; $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
Figure 4.
A. Endogenous glucose production during hyperinsulinemic euglycemic was similar at baseline between Sham (n=8), RYGB (n=7), VSG (n=6), and Pair-fed (n=7) rats, P>0.05. However, during steady state conditions glucose production was significantly reduced in RYGB and VSG rats vs. Sham and Pair-fed rats, P<0.05. B. Glucose clearance was similar between treatments at baseline and during the final 40-min of the clamp, P>0.05. Bonferroni’s Post-test; *P<0.05 vs. Pair-fed, $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
RYGB and VSG increase postprandial plasma GLP-1(7–36) and Insulin
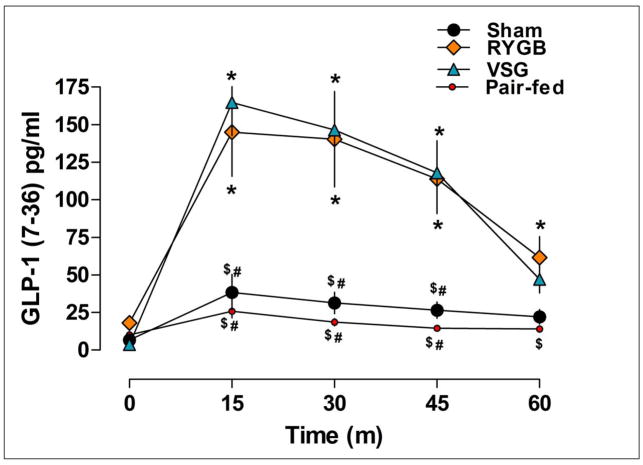
Postprandial GLP-1(7–36) and insulin release were assessed in response to the mixed-meal (Ensure) tolerance test. Basal GLP-1(7–36) levels were similar among treatments, P>0.05 (Figure 5). 15-min after the gavage of Ensure, RYGB and VSG rats had significantly higher circulating GLP-1(7–36) than pair-fed and Sham animals, P<0.05, and these increases were sustained through 60-min. Thus, GLP-1(7–36) secretion was increased to a similar extent by RYGB and VSG surgery and this was independent of changes in body weight.
Figure 5.
Active levels of the incretin hormone GLP-1(7–36) before (time 0) and after an oral gavage of Ensure Plus liquid diet in Sham (n=8), RYGB (n=6), VSG (n=6), and Pair-fed (n=9) rats. Post-prandial plasma levels of GLP-1(7–36) were significantly higher in RYGB and VSG rats relative to Sham and Pair-fed animals (15, 30, 45-min) P<0.05. RYGB rats also had significantly higher GLP-1(7–36) relative to Pair-fed rats after 60-min. Differences between Sham and Pair-fed rats were not significant, P>0.05. Bonferroni’s Post-test; *P<0.05 vs. Pair-fed, $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
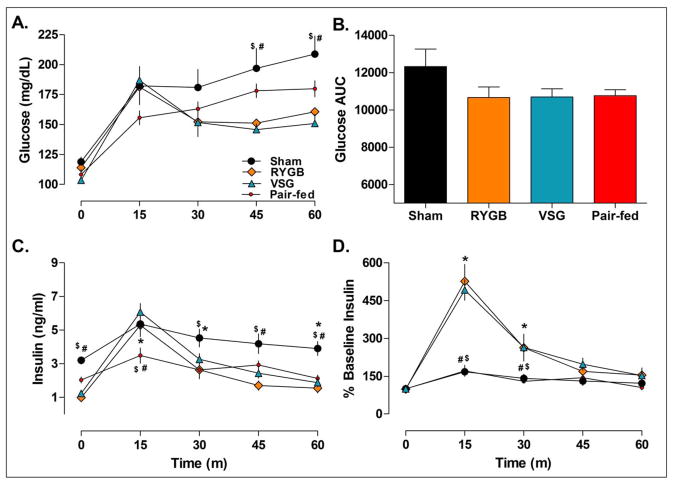
In response to Ensure, glucose levels were similar among all groups at 15-min, however, unlike sham and pair-fed rats, the RYGB and VSG rats sharply declined such that at 30-min, glucose levels were significantly lower in RYGB and VSG rats relative to their 15-min time point, P<0.05 (Figure 6A). In contrast, blood glucose levels continued to rise in Sham and pair-fed rats such that after 60 min blood glucose levels had risen significantly relative to their 15 min time point, P<0.05. When analyzed as AUC, RYGB, VSG and pair-fed animals had improved meal tolerance rative to sham operated rats (Figure 6B).
Figure 6.
Blood glucose (A-B) and plasma insulin (C-D) levels before (time 0) and after an oral gavage of Ensure Plus liquid diet in Sham (n=8), RYGB (n=6), VSG (n=6), and Pair-fed (n=9) rats. A. Blood glucose levels were significantly lower in RYGB and VSG rats relative to ad lib fed Sham animals at 45 and 60-min, P<0.05. B. Differences in oral glucose tolerance were not significant between treatments when the data were expressed as AUC, P>0.05. C. Plasma insulin levels were significantly lower at baseline in RYGB and VSG rats relative to ad lib fed Sham animals, P<0.05. Pair-feeding reduced plasma insulin levels relative to ad lib fed Sham rats at 15, 30 and 60-min, P<0.05 D. Insulin excursions expressed as a percentage of baseline showed that RYGB and VSG produced similar increases in postprandial insulin release that were not seen in Pair-fed animals. Bonferroni’s Post-test; *P<0.05 vs. Pair-fed, $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
Next, we examined whether the enhanced GLP-1(7–36) release in VSG and RYGB rats secondarily increased insulin levels (Figure 6C). At baseline, plasma insulin levels were significantly lower in RYGB and VSG rats relative to sham animals, P<0.05, and were not significantly different from pair-fed rats. Consistent with the hypothesis that the incretin effect was greater in RYGB and VSG rats, plasma insulin levels were significantly higher compared with pair-fed animals, after 15-min, P<0.05. When expressed as a percentage of baseline (Figure 6D), insulin excursions were higher in RYGB and VSG rats at 15 and 30-min, P<0.01, vs. both sham and pair-fed animals. These results indicate that the GLP-1(7–36) response was increased in VSG to the same extent as with RYGB surgery. Further, this effect occurred independently of food intake or body weight.
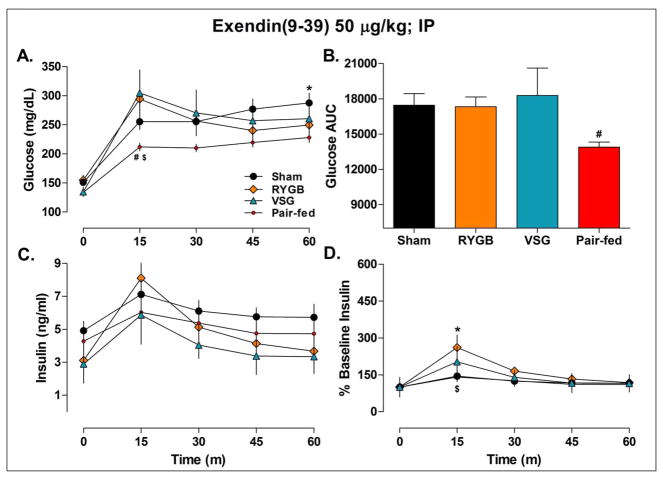
To assess the degree to which GLP-1(7–36) signaling affected the glucose and insulin levels in RYGB and VSG rats, we repeated the mixed-meal tolerance test in the presence of the GLP-1(7–36) receptor antagonist, Exendin(9–39). The improvements in glucose after Ensure were blunted in RYGB and VSG rats relative to ad lib-fed Sham animals at each time point (Figure 7A). The VSG rats also had significantly greater glucose AUC responses compared to the pair-fed rats, P<0.05 (Figure 7B). In RYGB and VSG rats, blood glucose levels did not decline, relative to the 15-min time point, until 45-min, P<0.05, and they were significantly higher than levels in pair-fed rats after 15-min, P<0.05. These data imply that, despite the presence of a GLP-1(7–36) receptor antagonist, the improvements in oral glucose tolerance were maintained in pair-fed, but not in RYGB and VSG rats, relative to ad lib-fed Sham animals.
Figure 7.
Blood glucose (A-B) and plasma insulin (C-D) levels before (time 0) and after an oral gavage of Ensure Plus liquid diet in rats pretreated with the GLP-1(7–36) receptor antagonist Exendin(9–39). Sham (n=8), RYGB (n=6), VSG (n=6), and Pair-fed (n=9) rats. A. AUC glucose was significantly lower in Pair-fed vs. VSG rats, P<0.05. B. Glucose excursions were significantly lower in Pair-fed animals vs. RYGB and VSG rats after 15-min, P<0.05, and Pair-fed vs. ad lib fed Sham operated rats after 60-min, P<0.05. C. Plasma insulin levels were not significantly different between treatments, P>0.05. D. Insulin levels expressed as a percentage of baseline were significantly higher in RYGB vs. Pair-fed and Sham rats at 15-min, P<0.05, but at no other time. *P<0.05 vs. Pair-fed, $P<0.05 vs. RYGB, #P<0.05 vs. VSG.
Pretreatment with Exendin(9–39) also blunted differences in insulin levels between RYGB, VSG, and sham-operated ad lib and pair-fed animals, P>0.05 (Figure 7C). When the data were expressed as a percentage of baseline, VSG rats were not significantly different from any of the other treatment groups, P>0.05. Although insulin excursions in RYGB rats were higher than pair-fed and ad lib-fed Sham animals at 15-min, P<0.05 (Figure 7D), this increase was substantially reduced relative to what was seen in the previous experiment (261±61% vs. 526±67%). Taken together, these data support the view that increased GLP-1(7–36) signaling is responsible for a majority of the improved meal tolerance and insulin secretion produced by RYGB and VSG.
Discussion
Initially developed to preserve the pylorus and reduce the likelihood of dumping syndrome in patients undergoing biliopancreatic diversion, a bypass procedure that is similar to RYGB 16, VSG is gaining in popularity as a stand-alone procedure. This is likely due to the fact that that VSG is less complicated and invasive, requires less follow-up than RYGB 2 and is comparably effective in terms of weight loss and the resolution of diabetes 2–4. Our data add to these findings as this is the first report to directly compare the weight-independent effects of RYGB with VSG on glucose handling and insulin sensitivity. Surprisingly, VSG increased post-prandial GLP-1(7–36) and insulin release to the same degree as occurred with RYGB during a mixed-meal tolerance test. The increased GLP-1(7–36) was not the result of reduced body weight or caloric intake as pair-feeding had no significant effect on postprandial GLP-1(7–36) or insulin release. IP glucose tolerance and insulin sensitivity improved in proportion to weight loss. However, when insulin sensitivity was assessed using the more sensitive hyperinsulinemic euglycemic clamp technique, it was revealed that hepatic insulin sensitivity was increased beyond the benefit produced by pair-feeding in both procedures. The fact that both procedures were similarly effective was surprising as the improvements in glucose homeostasis produced by RYGB surgery are thought to result primarily from the surgical alteration of the gastrointestinal anatomy, rather than from gastric restriction and weight loss per se.
Increased GLP-1(7–36) is often implicated as a mechanism for the improvements in glucose homeostasis after bariatric surgery. Several studies have reported increased GLP-1(7–36) after RYGB 3, 7–10 and one study has reported increased GLP-1(7–36) after VSG 3. While it is true that RYGB 3, 7, 9, 10 and VSG 3 both increase postprandial insulin release, the data linking the increase in GLP-1(7–36) to insulin secretion have only been correlative. In our study, along with the increase in GLP-1(7–36), we saw higher insulin excursions during a mixed-meal tolerance test in RYGB and VSG rats, but not during an IP glucose tolerance test, which is consistent with the likelihood that the enhanced insulin release is driven by an enhanced incretin effect rather than from the direct actions of circulating glucose on β-cells per se. To assess the degree to which increased GLP-1(7–36) release in RYGB and VSG rats improved oral glucose tolerance, we performed a mixed-meal tolerance test in the presence of Exendin(9–39). We found that the majority of the incretin effect after both RYGB and VSG was prevented by blocking GLP-1(7–36) receptors. If antagonizing GLP-1(7–36) receptors had produced a general impairment in oral glucose tolerance, then bariatric and pair-fed animals would have been equally affected during the mixed meal tolerance test. However, pair-fed animals had significantly lower blood glucose levels relative to each bariatric group, consistent with the conclusion that RYGB and VSG animals were disproportionately sensitive to the effects of Exendin(9–39). Together, these data are the first to establish that the large increase in GLP-1(7–36) after RYGB and VSG is responsible for the increased insulin secretion during a meal, and also establish that the incretin effect is an important weight-loss independent effect of both surgeries.
The most common example of a weight-loss independent effect of RYGB is the rapid improvement in glucose homeostasis following surgery. In RYGB, diabetic patients have been found to have normalized glucose and significant improvements in insulin resistance less than 1-week after surgery 5, 6. Although not as well characterized, VSG appears to produce similar effects, with improved fasting glucose and insulin levels in as little as 3-days 6. In that study, additional weight loss produced no further changes in glucose or insulin, and similar results were not observed in obese humans that were fed a similar diet after undergoing cholecystectomy. The rapidity of the effects has been taken as evidence that insulin sensitivity increases independent of the weight loss produced by these surgeries 6. One caveat is that prior studies poorly controlled for the effect of caloric restriction on insulin sensitivity or did not control for it at all. Campos et al. used standardized caloric restriction to control for weight loss produced by RYGB surgery, and with a hyperinsulinemic euglycemic clamp they found increased glucose infusion rate, an index of whole body insulin sensitivity, that correlated with the magnitude of weight loss 7. Thus, the clinical data suggest that there are both weight loss-dependent and -independent effects of both RYGB and VSG. However, the difficulty in controlling for caloric restriction after bariatric surgery may explain contradictory findings.
In our study, the weight-independent effects of bariatric surgery were not detected during IP glucose and insulin tolerance tests. However, the most sensitive technique for assessing insulin sensitivity, the hyperinsulinemic euglcyemic clamp, showed that RYGB and VSG surgeries enhanced hepatic insulin sensitivity beyond the improvement produced by pair-feeding. Laferrere et al.9 found that oral glucose tolerance was significantly better in Type 2 diabetic patients that underwent RYGB, compared to control subjects that lost a similar amount of weight through dieting. To some extent, we have similar findings in our rodent model. In our mixed-meal tolerance test, although differences in blood glucose levels between bariatric rats and pair-fed animals never reached significance, in the surgical rats glucose significantly decreased while in the pair-fed rats glucose actually increased from 15–60 min after the gavage, indicating that the pair-fed rats had an impaired inability to clear the glucose load. Thus, improved hepatic insulin sensitivity may explain part, or all, of the weight loss-independent effect observed by Laferrere et al 9.
While a role of GLP-1(7–36) on insulin secretion is expected, its role in improving insulin sensitivity is less clear. Interestingly, over-expressing GLP-1(7–36) in mice has been reported to increase hepatic insulin sensitivity and the expression of insulin receptor protein substrates 17. Additionally, a peripheral infusion of GLP-1(7–36) has been shown to suppress hepatic glucose production in humans 18, and we have previously observed that GLP-1(7–36) increases hepatic insulin sensitivity through actions in the hypothalamus in rat 15. Thus, it is possible that the pharmacological increases in GLP-1(7–36) seen with RYGB and VSG have important insulin-sensitizing effects specifically at the liver. Additional studies that examine the molecular underpinnings of RYGB and VSG-induced increases in hepatic insulin sensitivity are needed.
Taken together, the present data suggest that that GLP-1(7–36) is critical to the effect of both surgeries on glucose homeostasis. However, it is worth pointing out that GLP-1(7–36) is only one of a number of factors that are altered after bariatric surgery that could potentially improve glucose homeostasis. RYGB surgery slightly increases postprandial levels of gastric inhibitory polypeptide (GIP), in humans 9, 19 but not in rats 10, whereas the effect of VSG on GIP is unknown. In humans 3, 7, 19 and in rodents 10, prandial peptide YY(3–36) and amylin release are also increased after both surgeries, and all of these hormones have favorable actions on glucose homeostasis, although only GLP- 1(7–36) and GIP increase insulin release.
The fact that these different surgeries produced comparable improvements in metabolism is surprising. While the cause(s) of these metabolic improvements cannot be ascertained from these experiments, and we cannot entirely eliminate the possibility of subtle differences between VSG and RYGB that these experiments were unable to detect, it seems unlikely that specific alterations of the size of the stomach, or rerouting of the chyme through the intestines, are key. Rather, common endocrine or other changes resulting from the surgical procedures must be invoked.
In RYGB, nutrients bypass the duodenum and upper intestine and enter directly into the lower jejunum. The decreased time that it takes for nutrients to reach the GLP-1(7–36) producing L-cells of the lower bowel presumably results in enhanced GLP-1(7–36) release. In contrast, VSG may increase GLP-1(7–36) through actions in the proximal bowel. The possibility that enhanced nutrient delivery into the foregut increases GLP-1(7–36) release seems inconsistent with the fact that the number of L-cells in the gut increases as you move distally towards the colon. However, since under normal feeding conditions GLP-1(7–36) is rapidly released before most of the glucose reaches the lower gut during a meal, it seems likely that glucose activates the release of GLP-1(7–36) through means other than direct contact with L-cells specifically within the ileum 20. Consistent with this hypothesis Rocca and Brubaker demonstrated that the secretion of GLP-1(7–36) from the ileum is regulated by a complex neuroendocrine loop, in which nutrients in the duodenum induce GLP-1(7–36) release from ileal L-cells via vagal innervation21.
One possibility is that faster gastric emptying rates, as occurs in both RYGB22 and VSG23, result in increased rate of nutrient entry into the intestine and thus increased GLP-1(7–36) release. Indeed, faster gastric emptying rates have been associated with higher weight loss after RYGB surgery24, and the altered glucose pattern in our study, in which peak glucose levels occurred early in both RYGB and VSG rats, suggests faster gastric emptying rates in these animals. Other, gastric surgeries, such as partial gastrectomy and gastroenterostomy, also increase gastric emptying and produce exaggerated GLP-1(7–36) and insulin release25 that are similar in shape and magnitude to the profile produced by RYGB and VSG in the present study. At a first pass, the fact that increased gastric emptying stimulates the release of a hormone that has been suggested to inhibit gastric emptying and motility may seem paradoxical. However, it is unclear if gastric emptying and intestinal motility would be even higher if not for the increased release of GLP-1(7–36) in RYGB and VSG patients, and studies designed to assess these parameters are needed.
In conclusion, RYGB and VSG produced weight loss-dependent increases in whole body insulin sensitivity, and weight loss-independent increases in hepatic insulin sensitivity, postprandial GLP-1(7–36) and insulin release. Results from the mixed-meal tolerance test suggest that GLP-1(7–36) is critical to the metabolic benefit of both procedures on postprandial glucose levels. We hypothesize that increased GLP-1(7–36) after both VSG and RYGB is important in mediating improved insulin secretion and hepatic insulin sensitivity. Our results also show that RYGB and VSG produce similar outcomes in terms of effects on food intake and body weight, and support clinical data showing that these procedures are equally effective with respect to the resolution of type 2 diabetes. Because VSG is a technically simpler operation and requires less recovery time than RYGB, and because VSG is not associated with the malabsorption of micronutrients, VSG may represent the new operation of choice for many bariatric patients.
Acknowledgments
Grant Support: This work was supported by a grant from Ethicon Endo-Surgery, Inc. and NIEHS ESO10957 (SS). APC is supported by a CIHR fellowship.
Abbreviations
- GLP-1
Glucagon Like Peptide 1
- RYGB
Roux-en-Y gastric bypass
- VSG
Vertical Sleeve Gastrectomy
Footnotes
Adam P. Chambers: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript, revision of the manuscript
Lene Jessen: Acquisition of Data
Karen K Ryan: Acquisition of data
Stephanie Sisley: Acquisition of Data
Hilary E. Wilson-Pérez: Acquisition of Data
Margaret A Stefater: Technical support
Shrawan Gaitonde: Technical support
Joyce E. Sorrell: Technical support
Mouhamadoul Toure: Technical support
Jose Berger: Technical support
David A. D’Alessio: Important Intellectual Content and interpretation of data
Stephen C. Woods: Important Intellectual Content, interpretation of data, and drafting of the manuscript, critical revision of the manuscript
Randy J. Seeley: Important Intellectual Content, interpretation of data, study concept and design, critical revision of the manuscript
Darleen A. Sandoval: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript
Disclosure: Drs. Sandoval, Seeley, Woods, and D’Alessio receive funding from Johnson & Johnson. The remaining authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. Jama. 2005;294:1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 2.Lakdawala MA, Bhasker A, Mulchandani D, Goel S, Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg. 20:1–6. doi: 10.1007/s11695-009-9981-9. [DOI] [PubMed] [Google Scholar]
- 3.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–41. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 4.Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 24:1005–10. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 5.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–81. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 6.Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, Basso N. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 20:50–5. doi: 10.1007/s11695-009-0017-2. [DOI] [PubMed] [Google Scholar]
- 7.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 151:1588–97. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goke R, Fehmann H, Goke B. Glucagon-like peptide-1 (7–36) amide is a new incretin/enterogastrone candidate. Journal of Clinical Investigation. 1991;135:135–144. doi: 10.1111/j.1365-2362.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: Effects of exogenous glucagon-like peptide 1 (GLP-1)-(7–36) amide in type 2 diabetic patients. Journal of Clinical Endocrinology and Metabolism. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 13.Woods SC, Seeley RJ, Rushing PA, D’Alessio DA, Tso P. A controlled high-fat diet induces an obese syndrome in rats. Journal of Nutrition. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 14.Stefater M, Perez-Tilve D, Chambers A, Wilson-Perez H, Sandoval D, Berger J, Toure M, Tscoep M, Woods S, Seeley R. Sleeve Gastrectomy Induces Loss of Weight and Fat Mass in Obese Rats, But Does Not Affect Leptin Sensitivity. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate GLP-1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008 doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagace M, Marceau P, Marceau S, Hould FS, Potvin M, Bourque RA, Biron S. Biliopancreatic Diversion with a New Type of Gastrectomy: Some Previous Conclusions Revisited. Obes Surg. 1995;5:411–418. doi: 10.1381/096089295765557511. [DOI] [PubMed] [Google Scholar]
- 17.Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, Yoon JW, Jun HS. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671–9. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 18.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–7. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 19.Bose M, Teixeira J, Olivan B, Bawa B, Arias S, Machineni S, Pi-Sunyer FX, Scherer PE, Laferrere B. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2:47–55. doi: 10.1111/j.1753-0407.2009.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann C, Goke R, Richter G, Fehman HC, Arnbold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 21.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–94. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz M, Collins PJ, Harding PE, Shearman DJ. Gastric emptying after gastric bypass. Int J Obes. 1986;10:117–21. [PubMed] [Google Scholar]
- 23.Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19:1515–21. doi: 10.1007/s11695-009-9954-z. [DOI] [PubMed] [Google Scholar]
- 24.Akkary E, Sidani S, Boonsiri J, Yu S, Dziura J, Duffy AJ, Bell RL. The paradox of the pouch: prompt emptying predicts improved weight loss after laparoscopic Roux-Y gastric bypass. Surg Endosc. 2009;23:790–4. doi: 10.1007/s00464-008-0069-8. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen JJ, Orskov C, Holst JJ. Secretion of glucagon-like peptide-1 and reactive hypoglycemia after partial gastrectomy. Digestion. 1994;55:221–8. doi: 10.1159/000201151. [DOI] [PubMed] [Google Scholar]