Abstract
Many studies from our laboratories and others have shown that STAT5 expression and activity are increased during adipogenesis of murine and human adipocytes. Ectopic expression of STAT5A in fibroblasts or preadipocytes can confer or enhance adipogenesis. To determine, if STAT5A also plays a role in adipogenesis in vivo, we injected athymic mice with Swiss 3T3 cells expressing an empty pBABE retrovirus, Swiss cells expressing a pBABE retrovirus containing STAT5A, or 3T3-F442A preadipocytes. Athymic mice injected with either 3T3-F442A cells or Swiss 3T3 cells expressing STAT5A resulted in fat pad formation at the site of injection. However, mice injected with Swiss cells containing the parent retroviral vector did not have any observable fat pads. An analysis of the ectopic fat pads obtained from the Swiss 3T3 STAT5A mice revealed abundant expression of PPARγ and adiponectin. The protein levels of both of these fat cell markers were comparable to expression levels in epididymal fat pads. These results demonstrate that STAT5A can promote adipogenesis in vivo in this model system which supports a role of this transcription factor in adipocyte development in the whole animal.
Introduction
The first studies on STAT expression in 3T3-L1 cells revealed that STATs 1, 5A and 5B were substantially induced during murine adipogenesis (1). Similar results were observed during the in vitro differentiation of human preadipocytes (2). In addition, the ectopic expression of C/EBPs β and δ in non-precursor cells results in an induction of adipogenesis (3) that is accompanied by an induction in STAT5A and STAT5B protein levels (4). These two STAT5 proteins are also coordinately regulated with both PPARγ and C/EBPα in differentiating 3T3-L1 cells under a variety of different conditions (5). In 3T3-F442A preadipocytes, the ability of growth hormone to modulate adipogenesis is attenuated by STAT5 anti-sense oligonucleotides (6). Also, constitutively active STAT5 is capable of replacing the requirement for growth hormone in adipogenesis of these cells (7). Ectopic expression of STAT5A enhances adipogenesis in 3T3-L1 preadipocytes (8) and confers adipocyte development in non-precursor fibroblast cell lines (9). The increased expression of STAT5 proteins is not typically observed until after the induction of C/EBPα and PPARγ, yet the activation of STAT5 proteins in preadipocytes occurs prior to the induction in expression of PPARγ in 3T3-L1 cells (9). Both STAT5 proteins are tyrosine phosphorylated and translocate to the nucleus within 15 minutes after the induction of adipogenesis (9, 10). Transgenic deletion of STAT5A, STAT5B, or both STAT5 genes in mice resulted in significantly reduced fat pad sizes compared to wild-type mice (11). Coupled with the variety of in vitro studies summarized above and observations that STAT5 null mice have fat pads one-fifth normal size (11), the data suggest that STAT5 proteins are important effectors of adipogenesis both in vitro and in vivo. To date, there are no studies on tissue specific knockout of STAT5 genes in adipocytes and the phenotype of the STAT5 null mice could be attributed to developmental effects of STAT5 that are independent of direct effects on preadipocyte differentiation.
Over twenty-five years ago, Green and Kehinde demonstrated that subcutaneous injection of 3T3-F442A preadipocytes into athymic mice gave rise to fat pads at the site of injection (12). It was shown that the implanted 3T3-F442A preadipocytes, rather than endogenous preadipocytes, gave rise to these ectopic fat pads in athymic mice (13). The utility of the athymic mice model system was used to study the role of STAT5A in adipogenic development. Our previous studies have shown that fibroblasts which do not normally differentiate into adipocytes can undergo adipogenesis in vitro if they ectopically express STAT5A (9). Hence, we used these fibroblast cell lines in athymic mice as a model system to evaluate if STAT5A could play a role in adipogenesis in the whole animal. Our studies revealed both PPARγ and adiponectin are expressed in ectopic fat pads generated from 3T3-F442A preadipocytes or from Swiss 3T3 cells expressing STAT5A. Although PPARγ expression has been observed in ectopic fat pads in nude mice (14), to our knowledge this is the first observation that adiponectin is expressed in ectopic adipose tissue generated from preadipocytes in athymic mice. In summary, the data obtained in this specific model system indicate that STAT5A plays a role in adipogenesis in vivo.
Materials and Methods
Materials
Dulbecco's Modified Eagle's Media (DMEM) was purchased from Life Technologies. Bovine sera and other cell culture reagents were purchased from Sigma. Both the PPARγ monoclonal (catalogue # sc-7273) antibody and the STAT 5A polyclonal (catalogue #sc-1081) antibody were purchased from Santa Cruz. The ERK p44 antibody (catalogue #sc-93) was a rabbit polyclonal from Santa Cruz. The adiponectin antibody (catalogue # PA1-054) was a rabbit polyclonal obtained Thermo Scientific. HRP-conjugated secondary antibodies were purchased from Jackson Immunoresearch. Enhanced chemiluminescence (ECL) kit was purchased from Pierce. Nitrocellulose was purchased from BioRad.
Retroviral-mediated transfection in nonprecursor cells
Retroviral mediated stable expression of pBabe parental vector and pBabeSTAT5A was generated in Swiss 3T3 cells. Recombinant retroviruses were produced as described (15, 16). Briefly, BOSC-23 packaging cells were transiently transfected with 20μg of each retroviral construct. At 8 and 24 h after transfection, the media were changed to DMEM and 10% calf serum, and at 48 h, the virus-containing media were collected, filtered (0.45 μm), and supplemented with 4 μg/ml polybrene. Target cells were incubated for 10 h with the retrovirus-containing solution. Puromycin (2.5 μg/ml) selection was initiated at 48 h and continued for 2 weeks and freezedown stocks were prepared and later used to generate the cells used for these studies.
Injection of Athymic mice
Sub-confluent 3T3-F442A preadipocytes or Swiss 3T3 cells containing either a pBabe parental vector or pBabeSTAT5A were trypsinized and resuspended in DMEM containing 10% fetal calf serum. Cells were centrifuged to remove trypsin and then resuspended in DMEM. Approximately 55 day old Crl:NU/NU-nuBR athymic mice (Charles River Laboratories) were injected over the sternum with 3 × 107 cells using a 25 gauge needle. Animals were housed in micro-isolator cages for 6 weeks before being sacrificed by CO2 inhalation followed by cervical dislocation.
Rodent Adipose Tissue Isolation
Animals were euthanized by cervical dislocation, and tissues were immediately removed and frozen in liquid nitrogen. Frozen tissues were homogenized in a buffer containing 150 mM NaCl, 10 mM Tris, pH 7.4, 1mM EGTA, 1mM EDTA, 1% Triton-X 100, 0.5% Igepal CA-630 (Nonidet P-40), 1 μM PMSF, 1 μM pepstatin, and 50 trypsin inhibitory milliunits of aprotinin, 10 μM leupeptin, and 2 mM sodium vanadate. Homogenates were centrifuged for 10 minutes at 2,000 × g to remove any debris and insoluble material and then analyzed for protein content. All animal studies were carried out with protocols which were reviewed and approved by institutional animal care and use committees.
Gel Electrophoresis and Western Blot Analysis
Proteins were separated in 7.5% polyacrylamide (acrylamide from National Diagnostics) gels containing sodium dodecyl sulfate (SDS) according to Laemmli (17) and transferred to nitrocellulose membrane in 25 mM Tris, 192 mM glycine, and 20% methanol. Following transfer, the membrane was blocked overnight in 4% milk at 4°C. Results were visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence.
Results
The nude athymic mice are a suitable model system for studying the development of preadipocytes in vivo because they lack T cells and do not reject foreign cells from mice or other species. To determine the adipogenic potential of STAT5A in vivo, we generated non precursor Swiss 3T3 cells that contained an empty retroviral vector or STAT5A. Our previous studies had shown that ectopic expression of STAT5A in several fibroblast cell lines confers their ability to differentiate into adipocytes (9). We prepared subconfluent non-adipogeneic Swiss 3T3 cells that contained the parent retroviral vector and proadipogenic Swiss 3T3 cells that contained STAT 5A to inject into athymic mice. As a positive control for the formation of ectopic fat pads, we used 3T3-F442A preadipocytes. Six weeks after the injection of cells, the mice were sacrificed. In two separate experiments, we observed that athymic mice injected with either the 3T3-F442A cells or the Swiss 3T3 cells expressing STAT5A resulted in the formation of a fat pad at the site of injection (see Table 1). Athymic mice with and without ectopic fat pads are shown in Figure 1A. Of note, mice injected with Swiss 3T3 cells with the empty retrovirus (our negative control) did not result in any ectopic adipose tissue formation.
Table I.
| Cell Type | Swiss 3T3 Empty Vector | Swiss 3T3 STAT5A | 3T3-F442A | |
|---|---|---|---|---|
| Experiment #1 | # of animals injected | 5 | 5 | 5 |
| # ectopic fat pads | 0 | 3 | 3 | |
| Experiment #2 | # of animals injected | 5 | 5 | 5 |
| # ectopic fat pads | 0 | 3 | 5 |
Figure 1. Swiss 3T3 fibroblasts expressing STAT5A can result in the formation of ectopic fat pads in athymic mice.
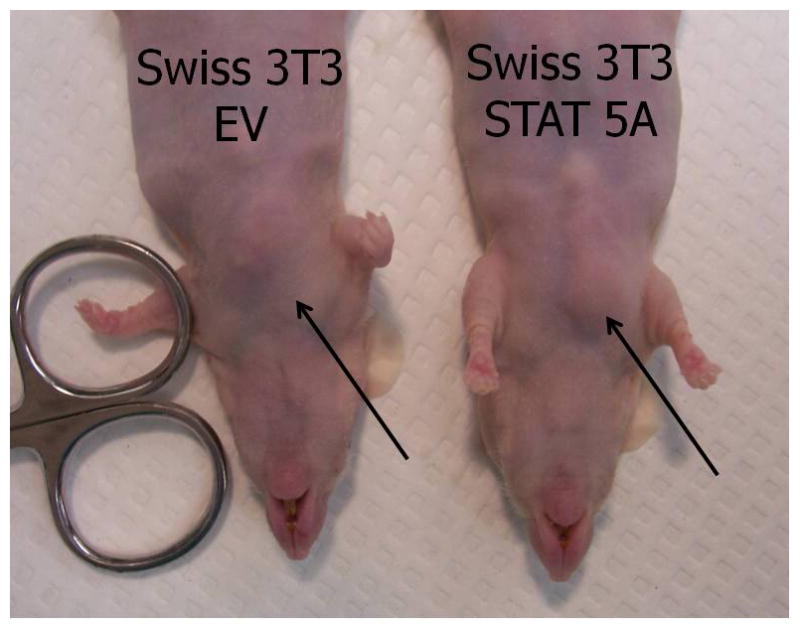
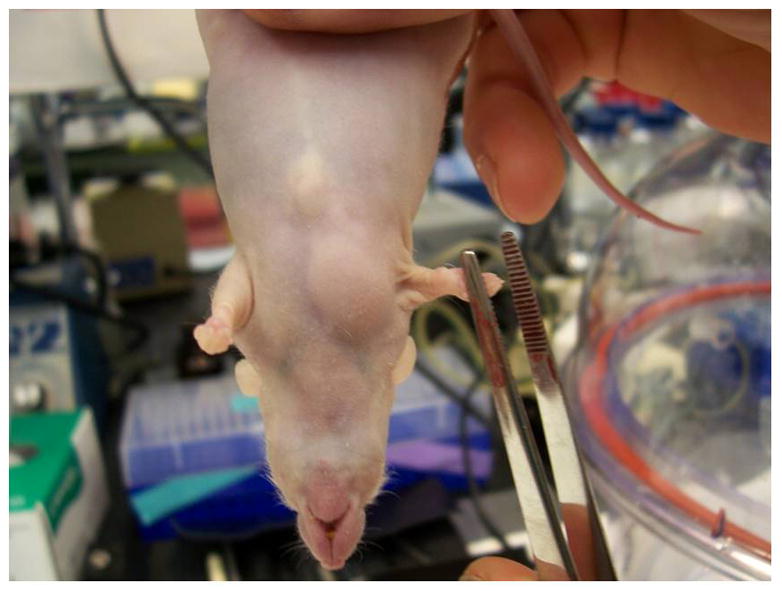
Sub-confluent Swiss 3T3 cells containing either an empty vector (EV) or STAT5A were trypsinized and resuspended in DMEM containing 10% fetal calf serum. Cells were centrifuged to remove trypsin and then resuspended in DMEM. Approximately 55 day old Crl:NU/NU-nuBR athymic mice were injected over the sternum with 3 × 107 cells. Animals were housed in micro-isolator cages for 6 weeks before being sacrificed by CO2 inhalation followed by cervical dislocation. The left hand panel shows mice 6 weeks after injection. The site of injection is indicated by the arrow. No ectopic fat pads were observed in mice injected with Swiss 3T3 cells containing empty vector (EV). Whenever an ectopic fat pad was present, it was found at the site of injection in mice injected with Swiss 3T3 cells containing STAT5A or 3T3-F442A cells. The panel on the right shows a visible ectopic fat pad in a mouse injected with STAT5A containing Swiss 3T3 cells.
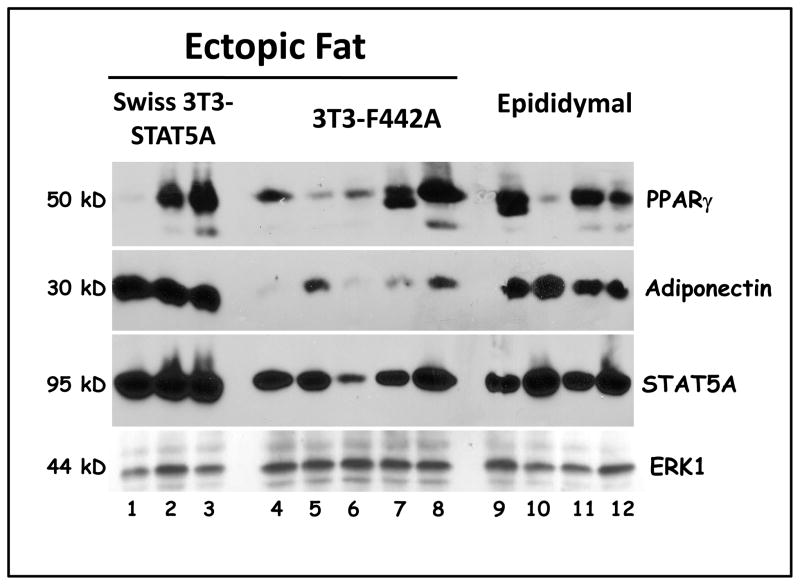
The ectopic fat pads were used for basic histological analysis and to prepare protein extracts. The data in Figure 2 indicates that the ectopic fat pads from 3T3-F442A cells (panels A–C) or Swiss 3T3 containing STAT 5A (panels D–F) are primarily composed of lipid containing cells. Western blot analysis of the ectopic fat pads obtained from the mice injected with either 3T3-F442A preadipocytes or Swiss 3T3-STAT5A cells revealed that these tissues express specific fat cell markers, including PPARγ and adiponectin. As shown in Figure 3, we analyzed three ectopic pads from mice injected with Swiss 3T3-STAT5A cells (lanes 1–3) and five ectopic fat pads from athymic mice injected with 3T3-F442A preadipocytes (lanes 4–8). Each of these lanes represents an ectopic fat pad from a different nude mouse from our second experiment listed in Table I. The expression of adipocyte markers was compared to epididymal fat pads obtained from these mice (lanes 9–12). As shown in Figure 3, PPARγ was expressed in two of the three fat pads generated from Swiss-3T3 STAT5A cells. In addition, these ectopic fat pads expressed adiponectin and showed increased levels of STAT5A (lanes 1–3). As noted above, we did not observe the formation of any ectopic fat pads in mice injected with Swiss-3T3 empty vector. Hence, there was no tissue to analyze. The fat pads generated from 3T3-F442A cells had varying amount of PPARγ and substantially lower levels of adiponectin (lanes 4–8) as compared to epididymal fat pads (lanes 9–12) or ectopic fat pads generated from Swiss STAT5A cells. The protein levels of the adipocyte markers that we examined in the epididymal fat pads of athymic mice were equivalent to what we observed in epididymal fat pads isolated from wildtype C57 Bl/6 mice (data not shown). ERK p44 expression is shown to indicate equivalent loading of protein samples.
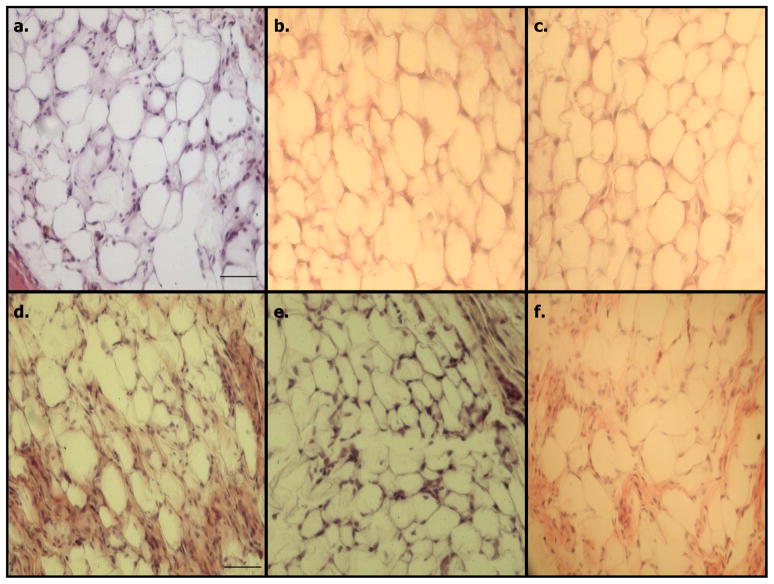
Figure 2. Morphology of ectopic fats produced in athymic mice.
Ectopic fat pads were removed from athymic mice for histological analysis. Ectopic fat pads were generated as described in the legend for Figure 1. A small portion of each pad was cut off with a razor blade and the fat pad section was fixed in formalin, embedded in paraffin, and sections were stained with hematoxylin and eosin. Panels A–C are fat pads generated from mice injected with 3T3-F442A cells and panels D–F represent ectopic fat pads obtained from mice injected with Swiss 3T3 cells containing STAT5A. The size bar in panels A and D is 50 um. No ectopic fat pads were produced in mice injected with Swiss 3T3 cells containing empty vector.
Figure 3. Ectopic fat pads express adipose tissue markers.
Epididymal and ectopic fat pads found at the site of injection were rapidly excised from athymic mice. The tissues were immediately frozen in liquid nitrogen and the tissues were stored at −80°C. Whole tissue extracts were isolated and one hundred and fifty μg of each extract was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western Blot analysis. The detection system was HRP conjugated secondary antibodies and enhanced chemiluminescence.
Discussion
These studies demonstrate that ectopic STAT5A expression can confer the adipogenic capabilities of Swiss 3T3 fibroblasts in athymic mice. A variety of in vitro studies have indicated that STAT5A expression and/or activity is associated with murine (1, 4–10) and human (2) adipogenesis in vitro. In addition, transgenic mice lacking either STAT5A or STAT5B have reduced fat pad size, and mice lacking both STATs 5A and 5B have fat pads one-fifth the size of normal (11). However, the observed difference in fat pad size in these transgenic mice models could be an indirect result of alterations in growth or development and do not specifically demonstrate a role of STAT5 protein in adipose tissue formation. However, the athymic mice experiments described above indicates that STAT5A plays a role in adipogenesis in vivo. To our knowledge, these are also the first studies to indicate that ectopic fat pads express adiponectin. It should be noted that there is remote possibility that the 3T3 F442As and Swiss 3T3 cells expressing STAT5A injected in these mice did not undergo adipogenesis, but the resulting ectopic fad was formed by endogenous preadipocytes whose adipogenesis was promoted by the injected cells. We do not think this is a likely scenario, however, since ectopic fat pads were only found at the site of injection. It should be noted that Swiss 3T3 fibroblasts are aneuploid immortalized cells and the role of STAT5A in promoting adipogenesis in these cells may not be representative of normal adipogenesis in vivo. An interesting and consistent observation in our studies was the increased expression of adiponectin in ectopic fat pads generated from Swiss 3T3 STAT5A containing cells as compared to 3T3-F442A cells. The relevance of this observation is not clear and we are conducting studies to determine if STAT5A plays a direct role in the modulation of adiponectin expression in adipocytes. In addition to demonstrating a direct role of STAT5A in preadipocytes differentiation in vivo in this model system, these studies also demonstrate the usefulness of athymic mice in studying the role of transcription factors in adipose tissue development.
Acknowledgments
This work was supported by an MTSU Summer Research and Academic year grant to W.C.S grant and R01 DK052968-10 from the National Institutes of Health to J.M.S.
We are grateful to Judith D. Shardo from MTSU for her participation in this project and Ursula A. White for technical assistance.
References
- 1.Stephens JM, Morrison RF, Pilch PF. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem. 1996;271(18):10441–4. doi: 10.1074/jbc.271.18.10441. [DOI] [PubMed] [Google Scholar]
- 2.Harp JB, Franklin D, Vanderpuije AA, Gimble JM. Differential expression of signal transducers and activators of transcription during human adipogenesis. Biochem Biophys Res Commun. 2001;281(4):907–12. doi: 10.1006/bbrc.2001.4460. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16(8):4128–36. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens JM, Morrison RF, Wu Z, Farmer SR. PPARgamma ligand-dependent induction of STAT1, STAT5A, and STAT5B during adipogenesis. Biochem Biophys Res Commun. 1999;262(1):216–22. doi: 10.1006/bbrc.1999.0889. [DOI] [PubMed] [Google Scholar]
- 5.Stewart WC, Morrison RF, Young SL, Stephens JM. Regulation of signal transducers and activators of transcription (STATs) by effectors of adipogenesis: coordinate regulation of STATs 1, 5A, and 5B with peroxisome proliferator-activated receptor-gamma and C/AAAT enhancer binding protein-alpha. Biochim Biophys Acta. 1999;1452(2):188–96. doi: 10.1016/s0167-4889(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 6.Yarwood SJ, Sale EM, Sale GJ, Houslay MD, Kilgour E, Anderson NG. Growth hormone-dependent differentiation of 3T3-F442A preadipocytes requires Janus kinase/signal transducer and activator of transcription but not mitogen-activated protein kinase or p70 S6 kinase signaling. J Biol Chem. 1999;274(13):8662–8. doi: 10.1074/jbc.274.13.8662. [DOI] [PubMed] [Google Scholar]
- 7.Shang CA, Waters MJ. Constitutively active signal transducer and activator of transcription 5 can replace the requirement for growth hormone in adipogenesis of 3T3-F442A preadipocytes. Mol Endocrinol. 2003;17(12):2494–508. doi: 10.1210/me.2003-0139. [DOI] [PubMed] [Google Scholar]
- 8.Nanbu-Wakao R, Morikawa Y, Matsumura I, et al. Stimulation of 3T3-L1 adipogenesis by signal transducer and activator of transcription 5. Mol Endocrinol. 2002;16(7):1565–76. doi: 10.1210/mend.16.7.0862. [DOI] [PubMed] [Google Scholar]
- 9.Floyd ZE, Stephens JM. STAT5A promotes adipogenesis in nonprecursor cells and associates with the glucocorticoid receptor during adipocyte differentiation. Diabetes. 2003;52(2):308–14. doi: 10.2337/diabetes.52.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Baugh JE, Jr, Floyd ZE, Stephens JM. The modulation of STAT5A/GR complexes during fat cell differentiation and in mature adipocytes. Obesity (Silver Spring) 2007;15(3):583–90. doi: 10.1038/oby.2007.500. [DOI] [PubMed] [Google Scholar]
- 11.Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93(5):841–50. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 12.Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101(1):169–71. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- 13.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci U S A. 1997;94(9):4300–5. doi: 10.1073/pnas.94.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing W, Lin Y, Wu L, et al. Ectopic adipogenesis of preconditioned adipose-derived stromal cells in an alginate system. Cell Tissue Res. 2007;330(3):567–72. doi: 10.1007/s00441-007-0493-4. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18(4):1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90(18):8392–6. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]