Abstract
Noxa is a Bcl-2 homology domain-containing pro-apoptotic mitochondrial protein. Noxa mRNA and protein expression are upregulated by dsRNA or virus, and ectopic Noxa expression enhances cellular sensitivity to virus or dsRNA-induced apoptosis. Here we demonstrate that Noxa null baby mouse kidney (BMK) cells are deficient in normal cytopathic response to lytic viruses, and that reconstitution of the knockout cells with wild type Noxa restored normal cytopathic responses. Noxa regulation by virus mirrored its regulation by proteasome inhibitors or ER stress inducers and the ER stress response inhibitor salubrinal protected cells against viral cytopathic effects. Noxa mRNA and protein were synergistically upregulated by IFN or dsRNA when combined with ER stress inducers, leading to Noxa/Mcl-1 interaction, activation of Bax and pro-apoptotic caspases, degradation of Mcl-1, loss of mitochondrial membrane potential and initiation of apoptosis. These data highlight the importance of ER stress in augmenting the expression of Noxa following viral infection.
Keywords: Mcl-1, Apoptosis, Cytopathicity, Bax, Salubrinal, VSV, EMCV
INTRODUCTION
Activation of the innate and adaptive immune responses is critical to survival of the host during virus infection. Production of type I interferons (IFNs), including IFN-β and members of the IFN-α family, is an obligatory component of the antiviral response (Stark et al., 1998). Recognition of components of the viral genome or replicative intermediates, such as double-stranded RNA (dsRNA) and single-stranded RNA is carried out by a variety of intracellular receptors, which trigger signaling cascades that culminate in the induction of target genes, including type I IFNs and other inflammatory cytokines (Iwasaki and Medzhitov, 2004). IFNs induce many genes that mediate cellular antiviral responses by either directly inhibiting virus replication or stimulating the adaptive immune system (Leaman et al., 2006). dsRNA may also directly stimulate expression of many of the same genes involved in the innate immune response (Bandyopadhyay et al., 1995).
Effective mechanisms for preventing viral replication, spread, and persistence include inhibition of protein synthesis, protein transport, and induction of apoptosis. Several IFN-regulated genes encode proteins that are important components of the antiviral response, including the dsRNA-activated protein kinase R (PKR), RNase L, guanylate binding protein 1 (Gbp-1), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo2L), cig 5 (viperin), and XIAP-1 associated factor-1 (XAF1) (Reviewed in Leaman et al., 2006; Chawla-Sarkar et al., 2003). It is likely that these proteins function to block virus replication directly, as in the case of PKR, RNase L, Gbp-1, and viperin, and indirectly by promoting apoptosis of infected cells prior to completion of viral replication, effectively limiting further virus infection (Haller et al., 2007; Silverman, 2007; George et al., 2009). Upregulation of proteins involved in sensing viral nucleic acids and priming components of the adaptive immune response are also critical for a full antiviral response to IFNs (Takeuchi and Akira, 2009).
Virus infection and replication not only stimulate innate immune and inflammatory responses, but also cause stress to the endoplasmic reticulum (ER; He, 2006). The ER is sensitive to imbalances in cellular homeostasis, including massive protein production and misfolding, loss of calcium homeostasis, and inhibition of N-linked glycosylation (Szegezdi et al., 2006). Replication of many viruses requires the production of properly folded and heavily modified proteins, making the ER an essential organelle for proper maturation of nascent viruses (He, 2006). In response to ER stress, a complex adaptive process, termed the unfolded protein response (UPR), is initiated to help reduce the number of misfolded proteins, either by suppressing protein synthesis or by elevating levels of proteins involved in folding and degradation (Harding et al., 2002). A major component of the UPR is PKR-like ER kinase (PERK), which down-regulates global protein translation by phosphorylating eukaryotic translation initiating factor 2α (eIF2α). In addition to the PERK-dependent pathway, activation of ATF6 and Inositol-requiring enzyme 1 (IRE1) induces other mediators of the UPR, including X box binding protein 1 (XBP1). When the cell is unable to recover from extended ER stress, apoptotic signals, including upregulation of C/EBP homologous protein (CHOP/GADD153), are initiated to eliminate the stressed cell (Kaufman, 2002). Indeed, infection of cells by various viruses can activate a canonical ER stress response including induction of CHOP expression, eIF2a phosphorylation, XBP1 splicing and eventual induction of apoptosis in the infected cell (Yu et al., 2006; Medigeshi et al., 2007; Barry et al., 2010), although some viruses combat this process to allow production of large amounts of structural proteins (He, 2006).
A small molecule pharmacologic inhibitor of ER stress response, salubrinal, is able to block many of the cytotoxic responses to ER stress as a means to prevent cellular death (Boyce et al., 2005). Salubrinal inhibits PP1-GADD34 phosphatase activity, thereby blocking eIF2α dephosphorylation and extending translational attenuation. It may also block apoptotic responses downstream of the IRE1 and ATF6 transcription factors, depending on the cellular context (Wiseman and Balch, 2005). Salubrinal has been used to block cellular cytopathic responses to Dengue and Herpes Simplex viruses (Boyce et al., 2005; Umareddy et al., 2007), thereby implicating ER stress in the replication and cellular response to some lytic viruses.
We have previously shown that Noxa, a mitochondrial BH3-only member of the Bcl-2 family, is induced by virus infection or dsRNA and can augment virus-induced apoptosis (Sun and Leaman, 2005). Noxa was originally identified as a p53-regulated protein required for DNA damage-induced apoptosis (Oda et al., 2000; Yakovlev et al., 2004), but has subsequently been implicated in a variety of stress response pathways (Ploner et al., 2009). Noxa expression can be upregulated by dsRNA or virus infection in a p53-independent manner (Sun and Leaman, 2005; Goubau et al., 2009), as well as by the ER stressors tunicamycin and thapsigargin (Li et al., 2006). Noxa expression is also rapidly upregulated in a number of different tumor cell lines by the proteasome inhibitors bortezomib (velcade, PS-341) and MG132, again independently of p53 status (Jüllig et al., 2006; Fennell et al., 2007). Application of the synergistic upregulation of Noxa by proteasome inhibitors, and other chemotherapeutics, and restoration of apoptotic responsiveness has recently been the target of intense study in a number of refractory tumor types (Nikiforov et al., 2007; Zall et al., 2010; Kuroda et al., 2010; Ri et al., 2010; Weber et al., 2009). Because virus infection and proteasome inhibitors can both induce ER stress, we sought to examine the effects of lytic viruses and viral mimetics, including the combination of dsRNA and MG132 or other well-characterized ER stressors (Lee et al., 2003), on Noxa regulation and cytopathicity. We hypothesize that Noxa is a critical mediator of this response, which represents a mechanism by which cells detect and respond to virus infection via apoptosis.
In the present study, we show that lytic viruses can replicate in Noxa null cells, but that viral CPE is dramatically reduced. The relative roles of the dsRNA and ER stress-sensing pathways were examined and suggested that ER stress is a more potent regulator of cellular cytopathicity in response to lytic virus infection. We also show that dsRNA synergizes with MG132 to rapidly upregulate Noxa and augment apoptosis, in a manner similar to VSV or EMCV infection. We observed enhanced activation of caspase 3 and cell death in response to the combination as compared to each individual treatment alone. We also show activation of Bax, release of cytochrome c, degradation of Mcl-1, and loss of mitochondrial membrane potential in response to dsRNA and MG132. Knockdown of Noxa levels using siRNA attenuated the pro-apoptotic effects of dsRNA and MG132. Together, these data highlight the importance of Noxa as an intermediate in both dsRNA- and ER stress-dependent cellular cytopathic responses.
RESULTS
Noxa induction by virus is required for enhanced apoptosis
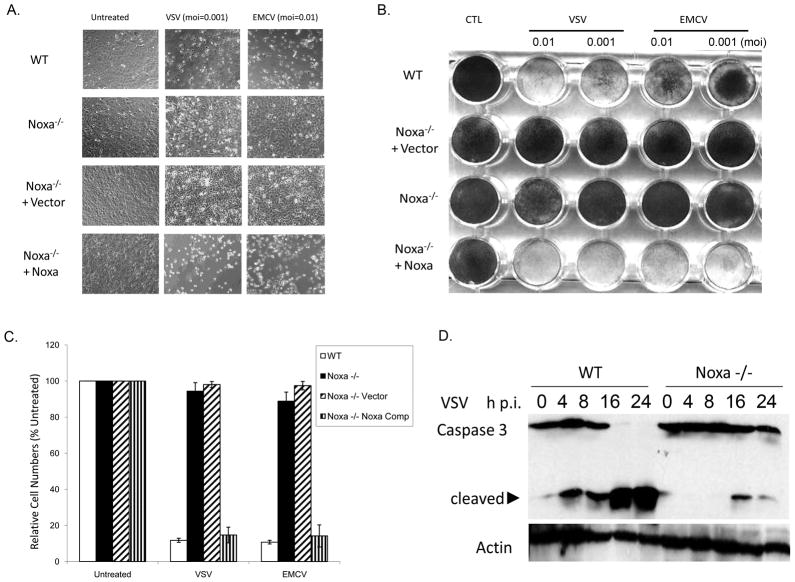
Upregulation of Noxa expression is observed in a variety of cell types in response to DNA damage, hypoxia and therapeutically relevant proteasome inhibitors (Oda et al., 2000; Yakovlev et al., 2004; Kim et al., 2004; Fernandez et al., 2005; Pérez-Galán et al., 2006). We have shown previously that Noxa is upregulated by virus or dsRNA in a p53-independent, IRF-3-dependent manner (Sun and Leaman, 2005; Goubau et al., 2009). To extend our analyses into Noxa’s function in cellular antiviral responses, immortalized wild-type (WT) and Noxa-null (−/−) baby mouse kidney (BMK) epithelial cells were used in virus replication and cytopathicity studies. To serve as reconstituted controls, the Noxa knockout cells were stably transfected with empty plasmid (−/− Vector) or wild type Noxa (−/− Noxa) (Goubau et al., 2009). Wild type, −/−, −/− Vector and −/− Noxa BMK cells were infected with VSV (moi 0.001) or EMCV (moi 0.01) for 16 h and assessed for cytopathicity. Both virus types undergo 2–3 rounds of replication in this timeframe in these cells (data not shown). Whereas WT and Noxa-complemented cells were sensitive to VSV- or EMCV-induced cytopathicity, Noxa-null cells (unmodified or vector transfected) were resistant (Fig. 1A, B). This phenotype was observed across multiple vector or Noxa complemented clonal lines (data not shown). The Noxa null cells (−/− and −/− Vector) also showed reduced loss in viable cell numbers after VSV or EMCV infection, as compared to the WT or Noxa complemented cells (Fig. 1C), and knockout cells exhibited less caspase 3 activation following VSV infection (Fig. 1D), consistent with our earlier siRNA studies (Sun and Leaman, 2005).
Fig. 1. Noxa null cells resist viral cytopathicity.
A) Wild type (WT) BMK cells, Noxa −/− cells, Noxa −/− cells transfected with empty vector and Noxa −/− cells stably expressing wild type Noxa were left untreated or infected with VSV (moi = 0.001) or EMCV (moi = 0.01) for 16 h. After that time, photomicrographs were taken to illustrate viral CPE in wild type and Noxa complemented, but not Noxa null or vector complemented cells. B) The same cells as in “A” were infected with VSV (moi = 0.01 or 0.001) or EMCV (moi = 0.1 or 0.01) for 16 h and then fixed and remaining cell monolayers stained with crystal violet. C) To quantify cellular viability, cells were left untreated or infected with VSV or EMCV as in “A” and after 16 h fixed in TCA and stained with SRB. To quantify relative cell numbers, incorporated dye was eluted and absorbance quantified (OD 550 nm). Cell viability for each sample was expressed as a percentage of the staining in the untreated cells. D) To assess apoptosis, cells were lysed at the indicate times after infection and caspase 3 cleavage was monitored by immunoblotting.
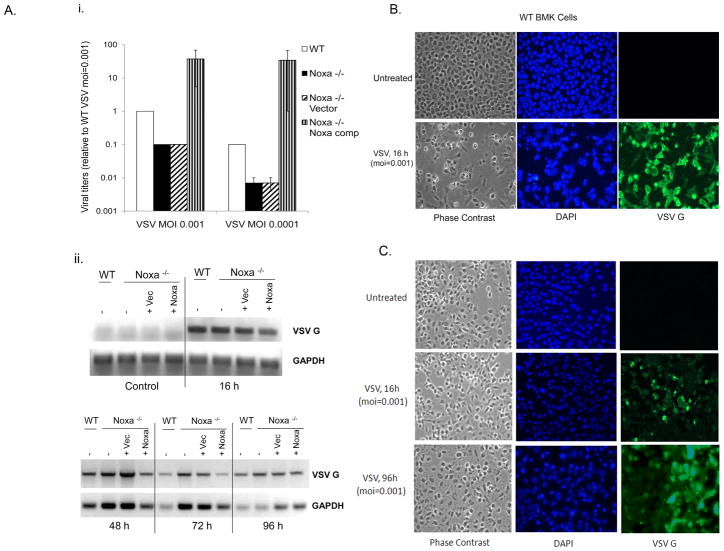
The lack of viral CPE in the knockout cells could reflect a difference in infectivity or defects in cellular response to the virus. To confirm that the −/− and −/− Vector cells were not deficient in VSV adsorption or uptake, viral replication was assessed in all cell lines by measuring viral yields and VSV G protein mRNA accumulation (Fig. 2A). Results indicated that viral production in the knockout cells was reduced by about 10-fold as compared to WT cells (Fig. 2Ai), but that virus was still produced in the knockout cells as indicated by VSV G mRNA accumulation in cells (Fig. 2Aii). Similar data were obtained for EMCV (Supplemental Fig. 1A–C). VSV G-protein expression was assessed by immunofluorescence in infected WT or Noxa null cells (Fig. 2B,C). After 16 h, staining in the WT and knockout BMK cells was observed (Fig. 2B,C), but unlike the WT BMK cells that died quickly thereafter, VSV G-protein expression persisted beyond 96 h in the Noxa null cells (Fig. 2C). Even infection with a high moi of VSV failed to induce death of the knockout cells (Supplemental Fig. 2D), indicating that it was the lack of a cellular death response, and not reduced viral replication rates, that lead to the differences in cytopathic responses between the wild type and Noxa −/− cells. Together, these data suggested that loss of Noxa blocked cellular apoptosis but did not prevent virus replication or persistence.
Fig. 2. Viral replication in WT and −/− cells.
A) i. The indicated BMK cell lines were infected with VSV (moi=0.001 or 0.0001) for 16 h, the conditioned medium was harvested and applied to indicator HT1080 cells in Log10 dilutions. After an additional 16–24 h, indicator cells were fixed and stained with crystal violet and viral titers quantified as the dilution providing 50% cytopathicity, and expressed as a value relative to that observed in WT BMK infected with moi=0.001. ii. To assess viral RNA accumulation, cells were infected with VSV (moi=0.001) for 0–96 h and then total cellular RNA was isolated and reverse transcribed into cDNA that was used as a template for RT-PCR analysis of VSV G-protein mRNA expression. GAPDH served as a cellular RNA control. B) WT BMK cells were infected with VSV (moi=0.001) for 16 h and then fixed and stained for immunofluorescent detection of VSV using a G-protein primary antibody and a FITC-conjugated goat anti-mouse secondary antibody. Cellular morphology was assessed by phase contrast microscopy, and cell nuclei were detected by DAPI staining. C) Noxa −/− BMK were infected with VSV for 16h or 96 h and then fixed and stained as in “B”.
Involvement of ER stress pathways in Noxa regulation and viral CPE
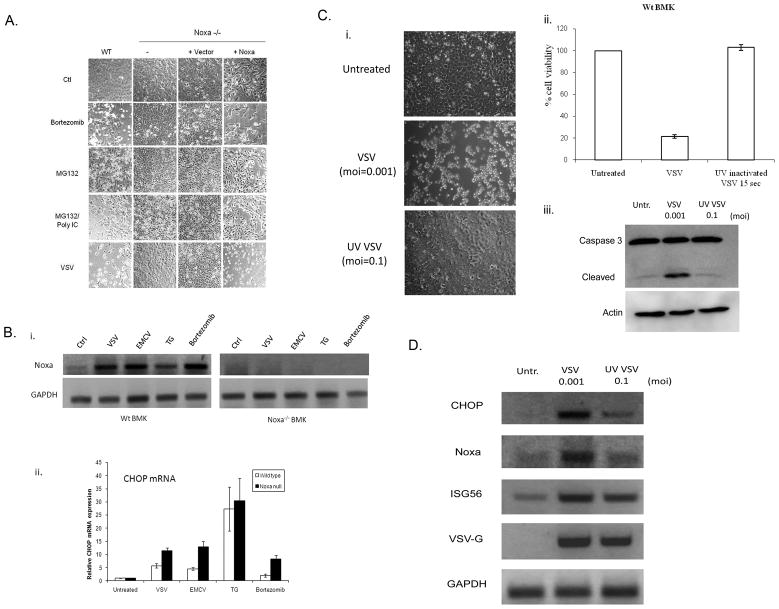
Although IRF3 is clearly involved in Noxa upregulation by dsRNA or virus (Goubau et al., 2009), the response of Noxa null cells infected with lytic viruses more closely mirrored the observed effects of proteasome inhibitors or ER stress inducers, which preferentially kill Noxa-intact versus knockout cells (Fig. 3A). Indeed, combined MG132/dsRNA treatment of cells promoted cytopathic responses that were nearly identical to those obtained with VSV infection (Fig. 3A). Thus, to investigate a role for ER stress responses in the resistance of Noxa null cells to lytic viruses, we treated WT and Noxa null cells with VSV, EMCV, thapsigargin (TG) or bortezomib and assessed Noxa mRNA expression. Viral infection or treatment with TG or bortezomib induced Noxa mRNA in WT BMK cells (Fig. 3Bi). CHOP/GADD153, a marker of ER stress that mediates specific transcriptional responses to ER perturbation, was also upregulated by virus infection, TG, and bortezomib treatment of WT BMK cells (Fig. 3Bii). Importantly, CHOP was also upregulated in the Noxa null cells (Fig. 3Bii), indicating that the upstream components of the ER stress response were intact in these cells. In addition to Noxa and CHOP, ATF4 was similarly induced by these treatments (Supplemental Fig. 2A,B). Other BH3-only proteins, including Bim and PUMA have been implicated in virus-induced apoptosis. We found that both PUMA and Bim were induced by dsRNA and weakly by virus, but not by ER stressors (Supplemental Fig. 2D). Furthermore, both were still induced in the Noxa null cells that resisted virus-induced cytopathicity (Supplemental Fig. 2D), suggesting that loss of Noxa is dominant to the pro-apoptotic effects that these proteins may exert in this cell type.
Fig. 3. Role of ER stress response in Noxa mode of action.
A) WT BMK cells, −/− cells, −/− cells transfected with empty vector and −/− cells stably expressing wild type Noxa were left untreated or treated with bortezomib (16 h, 20 nM), MG132 (6h, 10 μM), MG132 + poly IC (6h, 100 μg/ml) or infected with VSV (16 h, moi=0.001). After treatment, cells were photographed under phase contrast microscopy to illustrate morphological changes associated with those treatments. B) i. WT and −/− BMK cells were left untreated, infected with VSV (moi=0.001) or EMCV (moi=0.01) for 16 h, or treated with thapsigargin (TG; 1 μm) or bortezomib (20 nM) for 16h. RNA was isolated and RT-PCR studies were performed using primers specific for mouse Noxa and mouse GAPDH as a loading control. ii. CHOP (a marker of ER stress) mRNA levels were quantified by using real time SYBR green based qRT-PCR. C) i. WT BMK cells were left uninfected or were infected with replication competent VSV (moi=0.001) or UV-inactivated VSV (moi=0.1) for 16 h, after which time photomicrographs of the cells were taken under phase contrast microscopy. ii. Viability of cells treated as in “i” was determined by SRB staining, and results were presented as a percentage of the staining observed in uninfected controls. iii. Cells were infected as above and apoptosis demonstrated by immunoblot analysis of caspase 3 cleavage. D) Induction of ER stress-regulated and dsRNA-regulated genes was assessed by RT-PCR analysis of WT BMK cells infected with either wild type VSV (moi=0.001) or UV-inactivated virus (moi=0.1) 16 h after infection. Mouse GAPDH was used as an internal control.
To further investigate the importance of the ER stress versus the dsRNA sensing pathway in upregulating Noxa expression following virus infection, WT BMK cells were infected with replication active or UV-inactivated VSV. Activation of IRF3 upon viral entry, but in the absence of replication, is sufficient to induce an innate immune response (Collins et al., 2004). While infection with active VSV elicited a normal CPE response, including cell rounding, loss of viable cell numbers and activation of caspase 3 (Fig. 3C), infection with UV-inactivated VSV had no effect on cell viability (Fig. 3C). Nevertheless, the UV-inactivated virus did infect the cells as demonstrated by RT-PCR of VSV G mRNA (Fig. 3D), but it did not produce progeny or induce cell death. As expected, CHOP mRNA was only weakly induced by the UV-inactivated virus (Fig. 3D). Noxa similarly showed little or no upregulation, whereas the dsRNA-responsive ISG56 mRNA was upregulated strongly by both wild type and UV-inactivated VSV (Fig. 3D). These data suggest that ER stress pathways that are activated in response to virus infection play an important role in Noxa induction in infected cells.
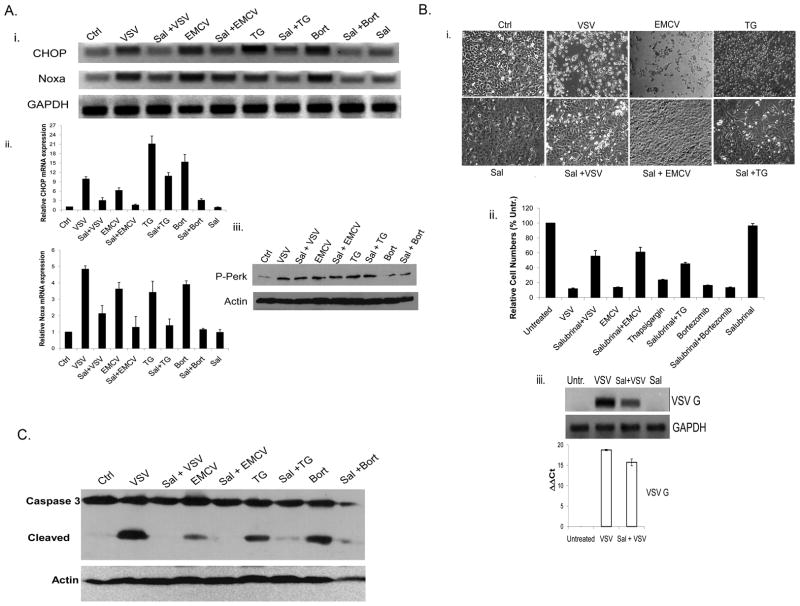
To determine whether the ER stress pathways that upregulate Noxa and CHOP are required to elicit virus-induced CPE responses in cells, the ER stress inhibitor salubrinal was employed. Salubrinal is proposed to inhibit ER stress signaling by blocking cellular phosphatase complexes that dephosphorylate eIF2α, preventing the ER stress response and subsequent cell death (Boyce et al., 2005). In WT BMK cells, salubrinal pretreatment was able to partially or fully block upregulation of CHOP and Noxa by all of the stimuli tested (Fig. 4Ai, ii). Salubrinal is proposed to act downstream of PERK phosphorylation, and immunoblot analysis confirmed that PERK phosphorylation was not affected in the salubrinal treated cells (Fig. 4Aiii). Importantly, salubrinal was able to protect cells from CPE (Fig. 4Bi, ii) and apoptosis (Fig. 4C) induced by the viruses and ER stress activators employed in these studies. As with loss of Noxa, salubrinal did not fully prevent virus infection or replication (Fig. 4Biii), and cells did eventually succumb to cytopathic effects (data not shown). Nevertheless, these data demonstrate a role for ER stress in the immediate cytopathic response to lytic viruses such as VSV or EMCV.
Fig. 4. Inhibition of ER stress response blocks Noxa upregulation and viral CPE.
A) i. WT BMK cells were left untreated (Ctrl) or were infected with VSV (16 h, moi=0.001), EMCV (16 h, moi=0.01), TG (16 h, 1 μM) or bortezomib (16 h, 20 nM) with or without prior treatment with the ER stress inhibitor salubrinal (Sal; 75 μM, 24 h). Total cellular RNA was then isolated and reverse transcribed for RT-PCR analysis of CHOP and Noxa mRNA induction. GAPDH was used as an internal control. ii. To quantify CHOP and Noxa mRNA expression, SYBR green-based qRT-PCR was used and the values were normalized to GAPDH. B) i. WT BMK cells were infected with VSV or EMCV or treated with TG, with or without Sal (75 μM) pretreatment. After 16 h, photomicrographs were taken to illustrate morphological changes associated with VSV, EMCV and TG cytopathicity.
Noxa upregulation correlates with enhanced apoptosis typified by mitochondrial dysfunction
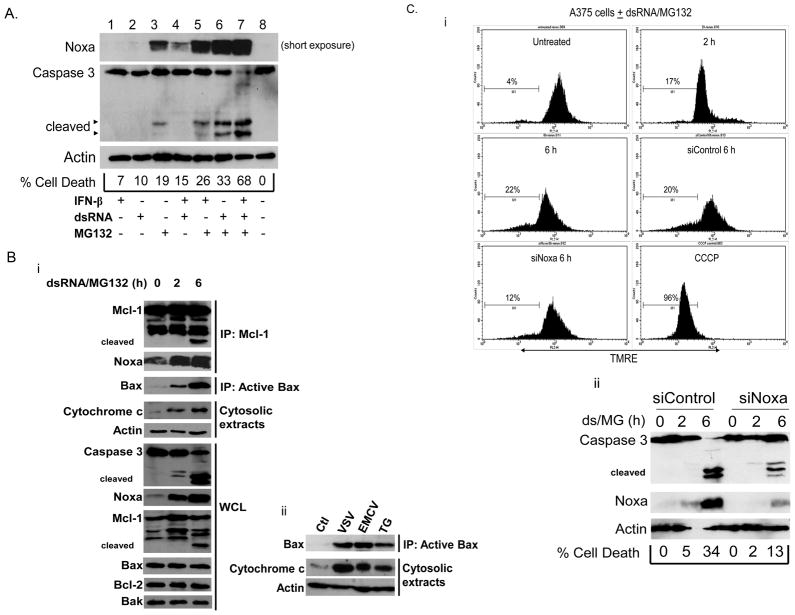
Our data suggest that ER stress is an important contributor to Noxa induction and virus-induced CPE. Previous studies had clearly indicated that dsRNA can directly stimulate Noxa expression (Sun and Leaman, 2005; Goubau et al., 2009). Although the studies conducted here suggest that ER stress is an obligatory component of virus-induced Noxa expression, we sought to determine which aspects of virus infection stimulated Noxa transcription. In particular, we investigated whether dsRNA plus ER stress stimuli could mimic a virus infection and combinatorially upregulate Noxa expression. Treatment of A375 melanoma cells with IFN-β, dsRNA, or MG132 alone or in combination led to Noxa upregulation (Fig. 5A, see also Supplemental Fig. 3A). The combination of dsRNA and MG132 synergistically upregulated Noxa and this corresponded to enhanced caspase 3 activation and cell death (Fig. 5A, lane 6) versus treatment with either stimulus alone. Similar results were obtained with Thapsigargin and dsRNA (data not shown). In all cells tested, upregulation of Noxa by virus, dsRNA, MG132, or TG occurred at the transcriptional level (Fig. 4A, Supplemental Fig. 3B, C) and was not simply due to MG132-mediated stabilization of protein. CHOP and GADD34 were assessed in parallel as additional markers of ER stress (Supplemental Fig. 3B).
Fig. 5. Noxa mechanism of action in MG132/dsRNA treated A375 cells.
A) Immunoblot analysis of Noxa upregulation by IFN-β (1000 U/mL, 16 h), dsRNA (poly IC, 100 μg/mL, 6 h), MG132 (10 μM, 6 h) and combinations thereof in A375 melanoma cells. Because of the significant synergistic increase in Noxa protein levels, as compared to single treatments, a short exposure was chosen for the clearest representation of all samples. Cell death was qualitatively assessed by immunoblotting for caspase 3 and quantified by assessing the number of trypan blue positive cells. B) i. Mcl-1 was immunoprecipitated from cells treated for the indicated times and Noxa association was assessed by immunoblotting. Noxa was associated with Mcl-1 basally and exhibited enhanced association with treatment, combined with Mcl-1 cleavage (ns=non-specific; arrowheads=cleaved forms). Replicate samples were used to immunoprecipitate Bax with the active conformation-specific 6A7 antibody, followed by immunoblotting to show Bax activation upon MG132/dsRNA treatment. Fractionation of cells to generate post-mitochondrial cytosolic extracts was used to assess the presence of released cytochrome c. A parallel study was done showing Noxa upregulation, caspase 3 activation and Mcl-1 cleavage in whole-cell lysates (WCL). Bax, Bcl-2, and Bak blots are provided to show lack of change in the levels of these proteins. ii. A375 melanoma cells were left untreated or infected with VSV (16 h, moi=0.001) or EMCV (16 h, moi=0.01), or treated with TG (16 h, 1 μM). As described in “i”, cells were harvested for either immunoprecipitating active Bax or fractionated to assess the presence of cytochrome c in post-mitochondrial cytosolic extracts. C) i. Cells were reverse transfected with control or Noxa-specific siRNAs and treated for up to 6 h with dsRNA and MG132. After treatment, cells were stained with TMRE (10 μM) and then analyzed by flow cytometry to detect changes in mitochondrial membrane potential. Treatment of a subset of control cells with the protonophore CCCP was used to set the marker for the TMRE-low population. The percentage of cells with depolarized mitochondrial membranes is indicated in each histogram. ii. A portion of cells not subjected to flow cytometry was also analyzed for cell death by immunoblotting for caspase 3 and staining with trypan blue. Successful knockdown of Noxa was also confirmed by immunoblotting.
The mechanism of Noxa-induced cell death has been well characterized (Shibue et al., 2003; Villunger et al., 2003; Seo et al., 2003). Noxa binds to the anti-apoptotic Bcl-2 family member Mcl-1 (Willis et al., 2005; Chen et al., 2005). This results in the displacement of sequestered Bax and Bak, which allows for their activation and the degradation of Mcl-1. Once activated, Bax and Bak oligomerize to form pores in the outer mitochondrial membrane that promote the release of cytochrome c and activation of caspases, which then cleave intracellular targets and promote the morphological changes associated with apoptosis. To investigate the mechanism of Noxa-induced cell death in response to virus infection and ER stress, A375 cells treated with dsRNA and MG132 were lysed to immunoprecipitate Mcl-1 to assess Noxa and Mcl-1 association. Interestingly, we found an interaction between Noxa and Mcl-1 in both untreated and treated cells. Over the 6 h time course, Noxa protein levels increased and we eventually observed cleavage of Mcl-1 (Fig. 5Bi). Replicate samples were used to immunoprecipitate active Bax using an active state-specific monoclonal antibody. Levels of active Bax increased significantly after 2 and 6 h of treatment with dsRNA and MG132 (Fig. 5Bi). We have been unable to detect an interaction between either Bak or inactive Bax and the Noxa-Mcl-1 complex (data not shown). An aliquot of the treated cells not used for immunoprecipitating active Bax or Mcl-1 was fractionated into mitochondrial and cytosolic extracts to monitor the release of cytochrome c. Not surprisingly, the activation of Bax and degradation of Mcl-1 also corresponded to an increase in cytosolic cytochrome c (Fig. 5Bi). We also analyzed the cleavage and activation of caspase 3 in whole-cell lysates of the same samples. At 6 h of treatment with dsRNA and MG132, where levels of active Bax, Mcl-1 cleavage, and cytochrome c release are highest, activation of caspase 3 is also seen (Fig. 5Bi). There was no change in total levels of Bax, Bak, or Bcl-2, underscoring the importance of the Noxa-Mcl-1 interaction and subsequent degradation of Mcl-1 in the setting of ER stress.
To confirm that the observed effects on Bax activation and cytochrome c release in response to ER stress appropriately mimicked virus infection, A375 cells were infected with either VSV or EMCV, or treated with TG. After 16h of treatment, cells were harvested and either lysed to immunoprecipitate active Bax or fractionated to detect cytosolic levels of cytochrome c. In agreement with dsRNA/MG132 treatment, all three stimuli promoted activation of Bax and concomitant release of cytochrome c from the mitochondria into the cytosol (Fig. 5Bii). The mitochondrial marker COX IV was assessed in parallel experiments, confirming the purity of cellular fractions employed in these studies (Supplemental Fig. 3D).
Activation of pro-apoptotic Bcl-2 family members leads to permeabilization of the outer mitochondrial membrane, release of key mediators for augmenting the apoptotic response, and, from a biochemical standpoint, leads to depolarization of the inner mitochondrial membrane. To extend our studies on the effects of Noxa induction on apoptosis, we used a potentiometric dye, TMRE, to measure mitochondrial membrane potential in A375 cells treated with dsRNA and MG132. In line with our previous mechanistic data, the increase in apoptosis also corresponded to a loss of mitochondrial membrane potential (Fig. 5Ci). To determine the relative contribution of Noxa to dsRNA/MG132-induced apoptosis, we transfected cells with either control or Noxa-specific siRNA to reduce levels of the endogenous protein. In accordance with previous observations, knocking down Noxa resulted in an approximately 50% reduction in cell death, loss of mitochondrial membrane potential, and caspase 3 activation (Fig. 5Ci and 5Cii; Jüllig et al., 2006). These data paralleled our earlier observations that Noxa knockdown reduced EMCV-induced apoptosis (Sun and Leaman, 2005).
Discussion
The role of cellular apoptosis in the dissemination of viral progeny within an infected host is complex. Many lytic viruses induce apoptosis as part of the normal replication process as a means to move beyond the infected cell. However, host factors, rather than virus-encoded proteins, appear to be the primary initiators of cellular apoptosis. This host innate immune response utilizes apoptotic mechanisms to minimize virus damage and prevent long term infections. A number of cellular factors have been implicated in modulating viral CPE (apoptotic) effects. These include PKR, TRAIL and PML, each of which are upregulated by virus infection directly or through IFN feedback and have been implicated in virus-induced apoptotic responses (Barber, 2001). However, others must certainly play a role in determining whether a cell ultimately undergoes a cytopathic response or survives the initial infection. Our data suggest that Noxa is another example of a host factor that is required for appropriate cellular apoptotic responsiveness to viral stresses.
Noxa is potently upregulated by dsRNA, IFN and virus within 3–6 h of treatment (Sun and Leaman, 2005, Goubau et al., 2009), and the upregulation by virus or dsRNA does not require IFN (Sun and Leaman, 2005) or p53 (Sun and Leaman, 2005, Lallemand et al., 2007) as an intermediate. Upregulation by virus or dsRNA clearly involved IRF3 regulated mechanisms: Noxa was robustly induced by adenovirally-expressed IRF3 5D, a constitutively active form of IRF3, and its induction by dsRNA was defective in IRF3 null MEFs (Goubau et al., 2009). Expression of a Noxa promoter/luciferase construct was upregulated by IRF3 5D, and IRF3 was recruited to the Noxa promoter following dsRNA treatment (Goubau et al., 2009). In contrast, IRF7 was only weakly able to induce Noxa expression (Goubau et al., 2009). Thus, IRF3 clearly can induce Noxa following virus infection. However, while IRF3-null cells are not deficient in virus-induced apoptosis (Sato et al., 2000), Noxa null cells were unable to mount a normal CPE response to VSV or EMCV (Fig. 1), suggesting that Noxa plays a prominent role in virus-induced apoptosis and that factors other than IRF3 are involved in its regulation. Studies conducted herein with UV-inactivated virus supported this observation and implicated other virus-induced pathways in the regulation of Noxa and viral CPE responses.
Although Noxa was originally implicated as an integral component of the p53-dependent response to DNA damage or hypoxia (Oda et al., 2000), a number of recent studies have demonstrated the significance of the p53-independent induction of Noxa downstream of proteasome inhibition and ER stress. Proteasome inhibitors were originally developed to counteract the aberrant activation of classical NF-κB signaling (Traenckner et al., 1994; Grivennikov et al., 2010) by preventing the proteasome-dependent degradation of IκBα. In addition to the NF-κB-inhibitory effects, treatment with proteasome inhibitors also promoted expression and activation of pro-apoptotic BH3-only proteins including Noxa, Bid, and Puma (Fennell et al., 2008). In particular, induction of Noxa in response to bortezomib treatment has been reported in a wide variety of tumor cell types and serves as a prognostic indicator for the efficacy of treatment (Pérez-Galán et al., 2006; Ri et al., 2009; Gomez-Bougie et al., 2007; Chen et al., 2010; Nikiforov et al., 2007). Rapid induction of Noxa by bortezomib correlated with a significant increase in apoptosis in melanoma, but not in normal keratinocytes (Fernandez et al., 2006), and shRNA-dependent knockdown of Noxa attenuated bortezomib-induced cytotoxicity (Pérez-Galán et al., 2006). Overall, proteasome inhibitor-dependent upregulation of Noxa has been used synergistically with other therapeutics to maximize the induction of tumor cell death (Kim et al., 2010; Ackler et al., 2010; Zall et al., 2010; Weber et al., 2009).
An important consequence of treating cells with proteasome inhibitors is the accumulation of improperly folded or modified proteins within the ER, leading to ER stress. A cytoprotective activity, known as the unfolded protein response (UPR), is initiated to promote refolding or degradation of the ER burden. However, the UPR can also lead to the induction of apoptosis if the aggregation of faulty proteins cannot be resolved. ER stress-induced apoptosis depends on pro-apoptotic Bcl-2 family members, including Noxa and Bax, and activation of caspases (Lai et al., 2006). Once activated Bax/Bak oligomerize and promote the release of cytochrome c and activation of caspases 9 and 3 through permeabilization of the outer mitochondrial membrane (Adams and Cory, 2007). Mcl-1 is then subject to degradation by caspases, enhancing the apoptotic signal. Indeed, Mcl-1 downregulation can promote activation of Bax and Bak (Adams and Cooper, 2007). Mcl-1 levels are regulated both transcriptionally and translationally, and Mcl-1 protein is subject to rapid turnover (Fritsch et al., 2007). Cells that express high levels of Mcl-1 are resistant to proteasome inhibitor-induced cell death (Jiang et al., 2008). However, concomitant upregulation of Noxa and downregulation of Mcl-1 using siRNA or BH3 mimetics promotes apoptosis in resistant cells, suggesting that a balance in favor of increased Noxa activity is required for optimal killing (Zall et al., 2010; Weber et al., 2009). Upregulation of Noxa in response to the ER stressors thapsigargin and tunicamycin correlates with the induction of apoptosis, while MEFs lacking both Bax and Bak are resistant to apoptosis induced by thapsigargin, tunicamycin, and brefeldin A (Wei et al., 2001). These data implicate Bax/Bak in Noxa-mediated effects on ER stress-induced apoptosis. Our results are consistent with this, and a recent report emphasizing the importance of Mcl-1 degradation in VSV-induced apoptosis (Pearce and Lyles, 2009), although that study suggested that Bid also played an important role in the process. Other BH3-only proteins, including Bim and PUMA have been implicated in viral cytopathic responses (Perfettini et al., 2004; Puthalakath et al., 2007). While our results were consistent with their upregulation by virus and dsRNA, this induction was also observed in Noxa null cells that were resistant to virus induced cytopathicity (Suppl. Fig. 2). While this does not rule out a role for these proteins in innate immune response to virus, it does imply that they are either less critical than Noxa, or at least insufficient to replace the loss of Noxa in these cells.
Because of the importance of Noxa in mediating ER stress responses, we assessed the role of virus-associated ER stress in promoting Noxa expression and cellular apoptosis. Virus infection and replication are well-known inducers of ER stress and apoptosis (He, 2006; Yu et al., 2006; Medigeshi et al., 2007; Barry et al., 2010). Viruses rely on the infected host cell machinery, namely the ER, to fold and modify proteins for productive replication. VSV, in particular, produces large amounts of G-protein that is modified through the ER/golgi apparatus. Our studies using UV-inactivated VSV demonstrate that, although the defective virus was still able to infect BMK cells, it was unable to induce Noxa expression, presumably due to its inability to replicate or induce ER stress. Furthermore, treatment with an ER stress signaling inhibitor, salubrinal, prevented not only the upregulation of Noxa in response to virus infection, proteasome inhibition, and direct ER stressors, but also blocked virus-induced cytopathic effects and ER stress-induced apoptosis. Although salubrinal exhibits some non-specific effects, particularly those that result from its ability to reduce protein synthesis, the results obtained with this inhibitor were consistent with those observed in the Noxa knockout cells, suggesting that least a portion of the resulting phenotypes (i.e. reduced CPE in Noxa −/− or salubrinal-treated cells) was due to reduced ER stress responses. Additional studies with future, more specific ER stress inhibitors will be required to confirm the relative contribution of the UPR in this process. Similarly, our studies demonstrated that cells lacking Noxa were also devoid of cytopathic effects after the induction of ER stress by virus infection and therapeutics.
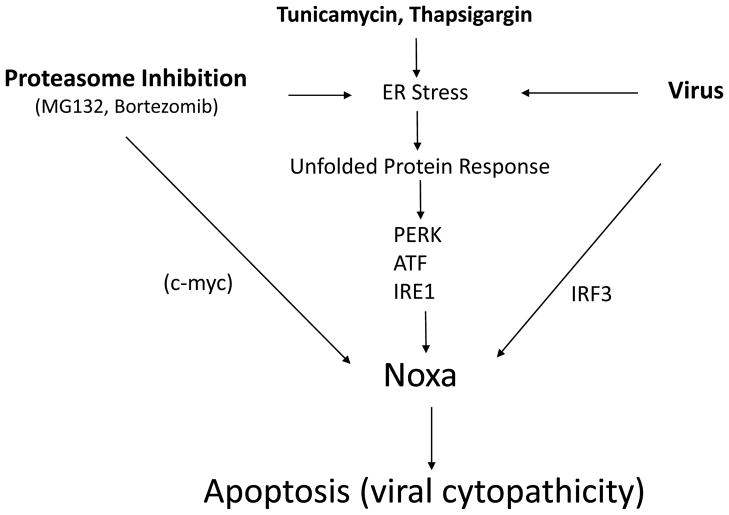
The data presented here suggest that both ER stress and dsRNA pathways are crucial for optimal Noxa induction by virus (Fig. 6). Noxa in turn promotes Mcl-1 degradation and, presumably, Bax or Bak oligomerization, which promotes mitochondrial outer membrane permeabilization leading to cytochrome c release and apoptosis. Although the precise transcriptional regulators downstream of the unfolded protein response that regulate Noxa transcriptional induction were not identified in this study, it is likely that transcription factors such as ATF3/ATF4 (Wang et al., 2009), p38 (Hassan et al., 2004) and other stress-activated regulators are involved. Although c-myc has been implicated in the regulation of Noxa by proteasome inhibitors (Nikiforov et al., 2007; Fig. 6), we did not observe a change in c-myc expression that would be consistent with a role in Noxa induction in the BMK cells examined here (data not shown). Thus, c-myc’s role in regulating Noxa may be most prominent in tumor cells, as suggested previously by others (Nikiforov et al., 2007). Regulation via these coordinated pathways ultimately promotes sufficient Noxa expression to displace pro-apoptotic factors from Mcl-1 and tip the balance in favor of cell death. Although we feel that Noxa plays a critical role in this determination, our model is not intended to imply that Noxa is therefore both necessary and sufficient for the process. Other factors, such as IRF-3, MAVS, caspase 8 and Bid, clearly must coordinate with Noxa to regulate cell death or survival, and different cell types may depend on Noxa to a greater or lesser extent (Barber, 2001; Yu et al., 2010; Chattopadhyay et al., 2010). Nevertheless, our data strongly connect Noxa to virus-induced cellular damage and, potentially, to viral persistence because failure to rid the body of infected cells could sustain the infection and/or reduce adaptive immune responses. These results are also relevant to the use of oncolytic viruses aimed at specifically targeting tumor cells (Barber, 2005). Since Noxa expression may be more strongly impacted in tumor cells as compared to normal cells, its analysis may provide a prognostic marker for anti-tumor effectiveness in lytic virus-based tumor therapies.
Fig. 6. Model for Noxa induction by virus and other stimuli: involvement of ER Stress responses.
The data presented in this and previous reports suggest that Noxa is regulated by virus through both IRF3-dependent regulation and ER stress induced pathways. Of these, our data suggest that ER stress response signaling is most critical for Noxa regulation. These same pathways are also known to mediate Noxa induction by other ER stressors, including proteasome inhibition, thapsigargin or tunicamycin. In tumor cells, it has been proposed that c-myc also regulates Noxa expression following proteasome inhibition (Nikiforov et al., 2007).
Conclusions
These studies provide evidence that Noxa is a critical component of ER stress-induced apoptosis and cytopathic responses in virally infected cells. Although ER stress is a known product of virus infection, the specific identities of genes activated through this response that regulate viral cytopathicity and dissemination are less well characterized. Our data for the first time clearly implicate Noxa as a downstream effector of the ER stress pathway in virally infected cells, and suggest that loss of this response could lead to a state of persistent infection.
MATERIALS AND METHODS
Cells and Reagents
A375 melanoma and HT1080 fibrosarcoma cells have been described before (Müller et al., 1993; Kohlhuber et al., 1997). Wild-type and Noxa-null baby mouse kidney cells (a generous gift from Eileen White, Rutgers University) were maintained in Dulbecco’s modified Eagles medium containing 8% fetal bovine serum. IFN-β (Serono), dsRNA (poly(I)-poly(C); GE Healthcare), MG132 (Z-Leu-Leu-Leu-H aldehyde; Peptides International), thapsigargin (Sigma Aldrich), Tuncamycin (Sigma Aldrich), Bortezomib (LC Laboratories) and salubrinal (Tocris Bioscience) were used at the concentrations described in the text. Noxa expression plasmids have been described previously (Sun and Leaman, 2005). In addition to the antibodies described in the following methods, we also used commercial antibodies to P-PERK (Cell Signaling), Cox IV (Molecular Probes) and alpha actin (Cell Signaling) in the reported studies.
RNAi
Control or Noxa-specific siRNAs were obtained as a pool of four targeting siRNA duplexes (On-Target plus SMART pool; Dharmacon). A375 cells were reverse transfected with control or Noxa siRNAs using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s recommendations. Knock-down of Noxa was confirmed by western blot using a Noxa monoclonal antibody (Alexis Biochemicals).
Assessment of cell viability and apoptosis induction
To determine cell viability, replicate plates were stained with sulforhodamine B (SRB) as described previously (Sun and Leaman, 2005). Briefly, cells were fixed with 10% TCA for 15 min, then stained with SRB solution (0.4% in 1% acetic acid) for 15–30 min and washed four times with 1% acetic acid. After drying, the dye was eluted in 10 mM unbuffered Tris and the absorbance was read on a plate reader at 550 nm.
For immunoblot analysis of caspase 3 cleavage, floating and adherent cells were lysed in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8.0, 1 mM PMSF), and proteins were separated on 10–15% SDS-PAGE, electrotransferred to Immobilon-P PVDF membrane (Millipore Corp.), and caspase 3 was detected by probing with an antibody for caspase 3 (Cell Signaling). Cytochrome c release from the mitochondria was assessed by fractionating adherent and floating cells into mitochondrial and cytoplasmic extracts. Briefly, the cell pellet was resuspended in 5 volumes of mitochondrial isolation buffer (MIB; 220 mM mannitol, 68 mM sucrose, 10 mM HEPES pH 7.4, 10 mM KCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2) then dounce homogenized. The homogenate was centrifuged at 1,000 g for 10 min. The low-speed pellet was washed once in MIB and spun again. Low-speed supernatants were pooled and centrifuged at 10,000 g for 10 min to generate the crude mitochondrial pellet and cytoplasmic extract (supernatant).
To assess changes in mitochondrial potential, A375 cells were treated with dsRNA and MG132 for up to 6 h, and then analyzed by FACS following tetramethylrhodamine ethyl ester (TMRE, Molecular Probes; 10 μM) staining. Treatment of cells with the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) was used as a control to set the marker for the TMRE-low population. TMRE fluorescence was quantified on a FACS Calibur flow cytometer (Becton Dickinson) using Cell Quest software.
Immunoprecipitations
For Bax activation and Mcl-1 immunoprecipitations, cells were treated as described above, harvested and lysed in CHAPS buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 1% CHAPS) by three rounds of freeze-thaw. Cleared lysates (500 μg total protein) were incubated with 2 μg of the Bax activation state-specific monoclonal antibody (6A7; Trevigen) or 1 μg of Mcl-1 monoclonal antibody (RC13; Lab Vision Corporation) for 2 h, after which protein G sepharose beads (GE Healthcare) were added and incubated for an additional 1 h. The beads were washed three times with CHAPS lysis buffer and SDS loading buffer was used to elute immune complexes. After SDS-PAGE and transfer, blots were probed with polyclonal antisera to Bax (BD Pharmingen), Mcl-1 (S-19; Santa Cruz Biotechnology) or with a monoclonal antibody to Noxa, where appropriate.
Immunofluorescence
For immunofluorescence detection of VSV, wild type and Noxa null BMK cells were seeded on poly-L–lysine coated coverslips for 24 h and then infected with VSV (moi=0.001) for 16 h (both BMK lines) or 96 h (Noxa null BMK only). Coverslips were then washed with PBS and fixed with 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.2% Triton X-100 for 3 min and then blocked with 5% bovine serum albumin (BSA) for 15 min. Cells were incubated with rabbit anti VSV-G (Sigma Aldrich) primary antibody (1:1000 dilution) for 2 h at room temperature, washed and then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit (Sigma Aldrich) secondary antibody (1:200 dilution) for 1 h at room temperature. Cover slips were mounted on to glass slides in Vectashield (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured on an Olympus 1×51 fluorescent microscope. Additional studies were performed with higher moi (Supplemental Fig. 1) following the above staining procedures.
Virus infection and yield assays
Wild type BMK cells, Noxa null cells, Noxa null cells stably transfected with empty vector, and Noxa null cells stably complemented with Noxa were plated on 6 cm dishes for 24 h and infected with laboratory stocks of VSV (Indiana strain) or EMCV at an moi of 0.001 and 0.01 for 1 h in serum free media. After 1 h, the cells were washed with PBS and 2 mL of complete media was added. On the subsequent day, the virus-infected media was harvested and centrifuged for 3–4 min at 4000 g. For viral yield assays, the clarified supernatants were used to infect an indicator cell line (HT1080 fibrosarcoma cells) at 1:10 serial dilutions. The next day, cells were fixed in 100% methanol for 10 min and stained with crystal violet for 5 min. Quantified results, plotted on a log scale, represented the dilution at which 50% cell lysis was observed. For UV inactivation of virus, high concentration stocks of VSV were exposed to UV light for 15 sec in a 6 cm dish using a Stratagene UV Stratalinker 1800 (6000 μJ/cm2). Cells were infected with the inactivated virus at a moi of 0.1.
RNA isolation and RT-PCR
Total cellular RNA was isolated using Trizol reagent (Invitrogen, CA) according to the manufacturer’s instructions and reverse transcribed (RT) with Moloney murine leukemia virus (MMLV) reverse transcriptase (Fisher scientific, NJ). For virus infected cells, both floating and attached cells were harvested for RNA isolation. RT reactions utilized 1 μg of total cellular RNA, which was incubated with 1 μg of random hexamer primers at 70°C for 10 min then quick chilled on ice. Reactions included dNTPs (1.25 mM), ribonuclease inhibitor (RNasin, 40U; Promega Corp., CA) and MMLV reverse transcriptase (200U, Fisher scientific, NJ) and were incubated at 42°C for 1 h. The resulting cDNA was used a template for semi-quantitative PCR amplification (25–30 cycles: 30 sec at 94°C, 30 sec at 52°C and 60 sec at 72°C). PCR products were analyzed on 1% agarose gels, visualized with ethidium bromide and digital images were inverted to give dark bands on a light background. Real-time RT-PCR studies were conducted with iQ SYBR Green supermix (BioRad, CA) using a two-step amplification (94°C, 30 sec; at 60°C for 60 sec) on an Eppendorf Real Plex thermocycler. Relative target gene expression was calculated by the ΔΔCt method using GAPDH as an internal control.
Supplementary Material
Acknowledgments
We would like to thank Delphine Goubau (Cancer Research UK London Research Institute, London, UK) and John Hiscott (McGill University, Montreal, Canada) for fruitful discussions about the role of Noxa in virus replication. We also wish to thank Dr. Eileen White (Rutgers University, Rutgers, NJ) for the wild type and Noxa null BMK cells. Supported by grants R01AI068133 and R21AI063014 (to D.W.L.).
ABBREVIATIONS
- ATF
Activation transcription factor
- BH3
Bcl-2 homology domain
- CHOP
C/EBP homologous protein
- dsRNA
double-stranded RNA
- eIF2α
Eukaryotic translation initiating factor 2α
- EMCV
Encephalomyocarditis virus
- ER
Endoplasmic reticulum
- GADD
Growth arrest and DNA damage-inducible protein
- IFN
Interferon
- IRE1
Inositol-requiring enzyme 1
- PERK
PKR-like ER Kinase
- UPR
Unfolded protein response
- VSV
Vesicular stomatitis virus
- XBP1
X box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, Xiao Y, Tse C, Frost DJ, Fesik SW, Rosenberg SH, Elmore SW, Shoemaker AR. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-009-1232-1. In press. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–96. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–6200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay SK, Leonard GT, Jr, Bandyopadhyay T, Stark GR, Sen GC. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J Biol Chem. 1995;270:19624–29. doi: 10.1074/jbc.270.33.19624. [DOI] [PubMed] [Google Scholar]
- Barber GN. Host defense, viruses, and apoptosis. Cell Death Differ. 2001;8:113–26. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Barry G, Fragkoudis R, Ferguson MC, Lulla A, Merits A, Kohl A, Fazakerley JK. Semliki forest virus-induced endoplasmic reticulum stress accelerates apoptotic death of mammalian cells. J Virol. 2010;84:7369–77. doi: 10.1128/JVI.02310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–9. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Yamashita M, Zhang Y, Sen GC. The IRF-3/Bax-Mediated Apoptotic Pathway, Activated by Viral Cytoplasmic RNA and DNA, Inhibits Virus Replication. J Virol. 2011;85:3708–16. doi: 10.1128/JVI.02133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–49. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chen S, Blank JL, Peters T, Liu XJ, Rappoli DM, Pickard MD. Genome-wide siRNA screen for modulators of cell death induced by proteasome inhibitor bortezomib. Cancer Res. 2010;70:4318–26. doi: 10.1158/0008-5472.CAN-09-4428. [DOI] [PubMed] [Google Scholar]
- Collins SE, Noyce RS, Mossman KL. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J Virol. 2004;78:1706–17. doi: 10.1128/JVI.78.4.1706-1717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2007;27:1189–97. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, Lowe SW, Soengas MS. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- Fritsch RM, Schneider G, Saur D, Scheibel M, Schmid RM. Translational repression of MCL-1 couples stress-induced eIF2 alpha phosphorylation to mitochondrial apoptosis initiation. J Biol Chem. 2007;282:22551–62. doi: 10.1074/jbc.M702673200. [DOI] [PubMed] [Google Scholar]
- George CX, Li Z, Okonski KM, Toth AM, Wang Y, Samuel CE. Tipping the balance: antagonism of PKR kinase and ADAR1 deaminase functions by virus gene products. J Interferon Cytokine Res. 2009;29:477–87. doi: 10.1089/jir.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Bougie P, Wuillème-Toumi S, Ménoret E, Trichet V, Robillard N, Philippe M, Bataille R, Amiot M. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67:5418–24. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- Goubau D, Romieu-Mourez R, Solis M, Hernandez E, Mesplède T, Lin R, Leaman DW, Hiscott J. Transcriptional re-programming of primary macrophages reveals distinct apoptotic and anti-tumoral functions of IRF-3 and IRF-7. Eur J Immunol. 2009;39:527–40. doi: 10.1002/eji.200838832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Staeheli P, Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007;89:812–8. doi: 10.1016/j.biochi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–99. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Hassan M, Alaoui A, Feyen O, Mirmohammadsadegh A, Essmann F, Tannapfel A, Gulbins E, Schulze-Osthoff K, Hengge UR. The BH3-only member Noxa causes apoptosis in melanoma cells by multiple pathways. Oncogene. 2008;27:4557–68. doi: 10.1038/onc.2008.90. [DOI] [PubMed] [Google Scholar]
- He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, de Bock C, Thorne RF, Allen J, Hersey P, Zhang XD. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68:6708–17. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- Jüllig M, Zhang WV, Ferreira A, Stott NS. MG132 induced apoptosis is associated with p53-independent induction of pro-apoptotic Noxa and transcriptional activity of beta-catenin. Apoptosis. 2006;11:627–41. doi: 10.1007/s10495-006-4990-9. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–98. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199:113–24. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Guan J, Chang I, Chen X, Han D, Wang CY. PS-341 and histone deacetylase inhibitor synergistically induce apoptosis in head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2010;9:1977–84. doi: 10.1158/1535-7163.MCT-10-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhuber F, Rogers NC, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn BA, Kotenko SV, Pestka S, Stark GR, Ihle JN, Kerr IM. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol Cell Biol. 1997;17:695–706. doi: 10.1128/mcb.17.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, Yamamoto M, Nagoshi H, Kobayashi T, Sasaki N, Shimura Y. Targeting activating transcription factor 3 by galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol Cancer Res. 2010;8:994–1001. doi: 10.1158/1541-7786.MCR-10-0040. [DOI] [PubMed] [Google Scholar]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 2006;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Lallemand C, Blanchard B, Palmieri M, Lebon P, May E, Tovey MG. Single-stranded RNA viruses inactivate the transcriptional activity of p53 but induce Noxa-dependent apoptosis via post-translational modifications of IRF-1, IRF-3 and CREB. Oncogene. 2007;26:328–38. doi: 10.1038/sj.onc.1209795. [DOI] [PubMed] [Google Scholar]
- Lee A-H, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. PNAS. 100:9946–51. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaman DW, Rosebeck S, Borden EC. Biological and clinical properties of type I interferons. In: Disis ML, editor. Immunotherapy of Cancer. Humana Press; USA: 2006. [Google Scholar]
- Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–70. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- Medigeshi GR, Lancaster AM, Hirsch AJ, Briese T, Lipkin WI, Defilippis V, Früh K, Mason PW, Nikolich-Zugich J, Nelson JA. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol. 2007;81:10849–60. doi: 10.1128/JVI.01151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur AG, Barbieri G, Witthuhn BA, Schindler C, Pellegrini S, Wilks AF, Ihle JN, Stark GR, Kerr IM. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and –gamma signal transduction. Nature. 1993;366:129–35. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, Verhaegen M, Varambally S, Chinnaiyan AM, Jakubowiak AJ, Soengas MS. Tumor cell-selective regulation of Noxa b c-myc in response to proteasome inhibition. PNAS. 2007;104:19488–93. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Pearce AF, Lyles DS. Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J Virol. 2009;83:9102–12. doi: 10.1128/JVI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- Perfettini J-L, Roumier T, Castedo M, Larochette N, Boya P, Raynal B, Lazar V, Ciccosanti F, Nardacci R, Penninger J, Piacentini M, Kroemer G. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med. 2004;199:629–640. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner C, Kofler R, Villunger A. Noxa: at the tip of the balance between life and death. Oncogene. 2009;27:S84–92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Ri M, Iida S, Ishida T, Ito A, Yano H, Inagaki A. Bortezomib-induced apoptosis in mature T-cell lymphoma cells partially depends on upregulation of Noxa and functional repression of Mcl-1. Cancer Sci. 2009;100:341–8. doi: 10.1111/j.1349-7006.2008.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–48. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Seo YW, Shin JN, Ko KH, Cha JH, Park JY, Lee BR, Yun CW, Kim YM, Seol DW, Kim DW, Yin XM, Kim TH. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J Biol Chem. 2003;278:48292–9. doi: 10.1074/jbc.M308785200. [DOI] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N. Integral role of Noxa in p53-mediate apoptotic response. Genes Dev. 2003;17:2233–8. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81:12720–9. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Sun Y, Leaman DW. Involvement of Noxa in cellular apoptotic responses to interferons, double-stranded RNA, and virus infection. J Biol Chem. 2005;280:15561–8. doi: 10.1074/jbc.M412630200. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of IkappaB-alpha that is still bound to NF-kappa B. EMBO J. 1994;13:5433–41. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umareddy I, Pluquet O, Wang QY, Vasudevan SG, Chevet E, Gu F. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virology J. 2007;4:91. doi: 10.1186/1743-422X-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mora-Jensen H, Weniger MA, Pérez-Galán P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein Noxa in cancer cells. PNAS. 2009;106:2200–5. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Kirejczyk Z, Potthoff S, Ploner C, Häcker G. Endogenous Noxa determines the strong proapoptotic synergism of the BH3-mimetic ABT-737 with chemotherapeutic agents in human melanoma cells. Trans Onc. 2009;2:73–83. doi: 10.1593/tlo.08223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic Bax and Bak: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-XL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RL, Balch WE. A new pharmacology-drugging stresses folding pathways. Trends Mol Med. 2005;11:347–50. doi: 10.1016/j.molmed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Di Giovanni S, Wang G, Liu W, Stoica B, Faden AI. Bok and Noxa are essential mediators of p53-dependent apoptosis. J Biol Chem. 2004;279:28367–74. doi: 10.1074/jbc.M313526200. [DOI] [PubMed] [Google Scholar]
- Yu CY, Chiang RL, Chang TH, Liao CL, Lin YL. The interferon stimulator mitochondrial antiviral signaling protein facilitates cell death by disrupting the mitochondrial membrane potential and by activating caspases. J Virol. 2010;84:2421–31. doi: 10.1128/JVI.02174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CY, Hsu YW, Liao CL, Lin YL. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. J Virol. 2006;80:11868–80. doi: 10.1128/JVI.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zall H, Weber A, Besch R, Zantl N, Häcker G. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol Cancer. 2010;9:164–75. doi: 10.1186/1476-4598-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.