Abstract
Romidepsin (Istodax®), a selective inhibitor of histone deacetylases (HDACs), was approved for the treatment of cutaneous T-cell lymphoma in November 2009 by the U.S. FDA. This unique natural product was discovered from cultures of Chromobacterium violaceum, a Gram-negative bacterium isolated from a Japanese soil sample. This bicyclic compound acts as a prodrug, its disulfide bridge being reduced by glutathione upon uptake into the cell, allowing the free thiol groups to interact with Zn ions in the active site of class I and II histone deacetylase enzymes. Due to the synthetic complexity of the compound, as well as the low yield from the producing organism, analogs are sought to create synthetically accessible alternatives. As a T-cell lymphoma drug, romidepsin offers a valuable new treatment for diseases with few effective therapies.
Keywords: romidepsin, depsipeptide, cutaneous T-cell lymphoma, Chromobacterium violaceum, Istodax, histone deacetylase inhibitor
Introduction to Histone Deacetylase (HDAC) Inhibitors
Alteration of gene expression due to epigenetic modification of the chromatin structure has been implicated as an important factor in tumorigenesis.1,2 In chromatin, DNA is tightly coiled around core histone proteins, forming nucleosomes; linker histones bind nucleosomes together as chromatin. Methylation, acetylation, phosphorylation, ADP-ribosylation, or ubiquitination of the histone tail regions affect the binding of the histone proteins to DNA, changing the structure of the chromatin and subsequently the accessibility of transcription factors to DNA regions.3 Such epigenetic processes alter levels of gene expression without changing the nucleotide sequence.4 Of these modifications, histone acetylation is the most widely studied, due to its role in the development and progression of tumors.2
Histone acetylation is controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs direct the addition of acetyl groups to lysine residues in the amino-terminal histone tails, neutralizing that portion of the protein and relaxing the chromatin structure, thereby increasing accessibility of transcription complexes as well as recruiting transcription cofactors.2,3,5,6 Conversely, HDACs remove acetyl groups from the histone tails, resulting in a more compact and inaccessible form of chromatin, consequently silencing transcription.2,3,5 These effects are highly localized, and only 2–5% of encoding genes are transcriptionally regulated by the histone acetylation state.2 Importantly, this selection includes genes that control the cell cycle and apoptosis, and HDACs have been associated with several well characterized oncogenes and tumor suppressor genes.7,8 Certain tumors have overexpressed HDACs and downregulated or mutated HATs.9 It has been suggested that an imbalance of HDAC relative to HAT activity can lead to a diminished expression of such regulatory genes and ensuing tumorigenesis.9
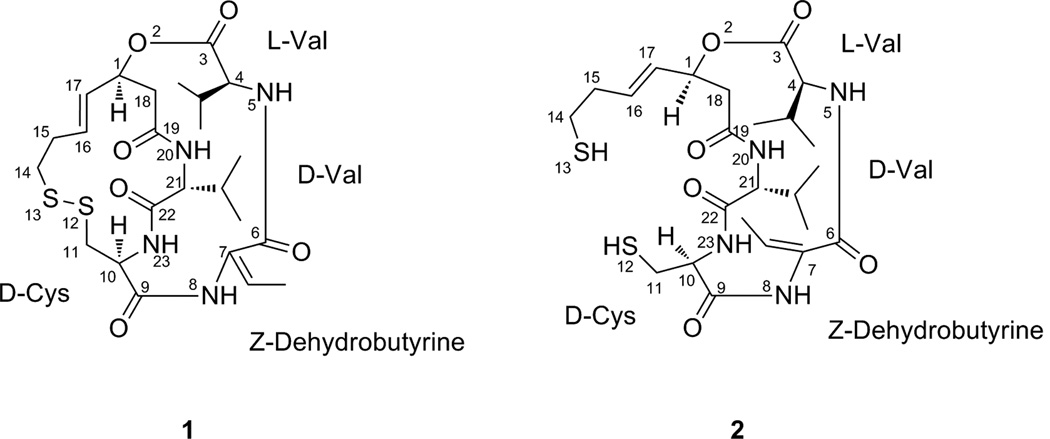
HDAC inhibitors have been found to have therapeutic properties in many different human tumor cell lines, including those derived from the bladder, breast, prostate, lung, ovary, and colon, among others,10 indicating that the inhibition of HDAC activity may be a viable strategy for the treatment of cancers.11,12,13 It is also likely that HDAC inhibitors affect a number of subcellular pathways, and time will tell what impact these other mechanisms have on treatment of cancer and/or other diseases. HDAC inhibitors can be divided into four classes, namely hydroximates, cyclic peptides, aliphatic acids, and benzamides.14 A new HDAC inhibitor, romidepsin, which has been referred to by several different names in the literature, including FK228, FR 901228, NSC 630176, depsipeptide, and Istodax®, is a pentapeptide isolated from cultures of Chromobacterium violaceum.15 The bicyclic depsipeptide structure of romidepsin (1) is composed of four amino acids (D-valine, D-cysteine, Z-dehydrobutyrine, L-valine) and (3S,4E)-3-hydroxy-7-mercapto-4-heptenoic acid, and the numbering of 1 is based on Xiao et al.16,17 Romidepsin was approved by the U.S. FDA in 2009 for use in patients with cutaneous T-cell lymphoma (CTCL).
Background on Cutaneous T-Cell Lymphoma (CTCL)
The incidence of cutaneous T-cell lymphoma (CTCL) in the general population is estimated to be 1–4/100 000,18,19 with approximately 1500 new cases and 500 deaths per year in the U.S.20 CTCL is twice as prevalent in males as in females, and the median age of presentation is 57 years. In the U.S., the disease is also more common among African-Americans than in other race categories.19 Approximately 16 000 to 20 000 individuals in the US are affected by CTCL.20 One study from 1973 to 1984 found a 3.2-fold increase in the incidence of CTCL over that time period;21 a similar study from 1973 to 2002 reported a 3.4-fold increase in incidence over the course of the study.22
The most common types of CTCL are mycosis fungoides (MF), so called because early physicians believed it to be a fungal infection, although it is not, and Sézary syndrome. The latter, the leukemic variant, is characterized by pruritus, generalized exfoliative erythroderma, and abnormal lymphoid cells in the blood. On the other hand, the blood is usually not affected in MF, especially in the early stages. Skin lesions are characterized as patches, plaques, or tumors that have a “bathing trunk” distribution. The lymphoma may extend to cover a large surface area, lymph nodes, and, in later stages, viscera.
MF is the most common type of CTCL, and is characterized by pink or erythematous scaly patches and plaques, with varying degrees of scaling and pruritus.23 At the time of diagnosis, the majority of patients with MF have limited plaques on ≤10% of their total body surface. However, approximately one-third of them have extensive plaques. Relatively small proportions of patients present with cutaneous tumors (16%) or generalized erythroderma (12%). Non-skin lesions are more likely to be seen in later stage disease, with 8% seen in stage T2, 30% in stage T3, and 42% in stage T4. The primary non-skin and non-lymph node sites are lung, gastrointestinal, liver, and central nervous system. Bone marrow involvement is rare in early stage disease.
CTCL is uniformly fatal. The ultimate cause of death for patients with CTCL is commonly infection, particularly sepsis, from Pseudomonas aeruginosa or Staphylococcus aureus, caused by chronic skin infection and subsequent systemic infections.24 Prognosis is dependent on age as well as disease stage at diagnosis,25 with earlier stage disease associated with higher 5-year overall survival and disease-free survival rates than later stage disease. The overall 5-year survival rate for patients with MF is 91%, while it is only 40% for those with Sézary syndrome.26 Typically, those presenting with early stages of CTCL have a favorable prognosis, with around 95% 5-year survival;25,27,28 more advanced stages have lower survival rates ranging from 30% to 84%,25,27,28 again depending on form and stage of the cancer. According to one study, the 10-year survival rate drops from around 100% for those with stage T1 CTCL to 67% for T2; by stage T3, this number drops to 40%.29 While most patients present with the early-stage disease, approximately 10–20% of these will progress to a later and often fatal disease.25,27,30 Patients that undergo transformation to large-cell lymphoma have a mean survival ranging from 2 to 19 months.30
DISCOVERY AND DEVELOPMENT OF ROMIDEPSIN
Romidepsin was discovered in the early 1990s through a program that was evaluating fermentation products for antimicrobial and antitumor activities. The producing organism, Chromobacterium violaceum, is a Gram-negative, rod-shaped bacterium with a single polar flagellum. The strain was isolated from a soil sample collected in the Yamagata-prefecture, Japan.15 Presently, the commercial supply of romidepsin is generated via fermentation, although the yield and exact conditions are proprietary.
C. violaceum was cultured in nutrient broth with additional glucose and incubated at 30°C aerobically with agitation. The organism reached stationary phase after 72 h, providing a maximal yield of romidepsin at this time of 19 µg/mL, which was present in the aqueous part of the culture. The fermentation was filtered, and the filtrate extracted twice with EtOAc. The extract was evaporated in vacuo to give an oily residue, which was then purified via normal phase chromatography to yield a yellow powder.
This powder was dissolved in CH2Cl2:CH3OH:CH3CN (10:1:20), and romidepsin crystallized as colorless prisms. It displayed weak antifungal but no antibacterial activity, but it showed potent cytotoxicity against several human lung carcinoma cell lines, as well as human stomach, breast, and colon adenocarcinoma cell lines.15 The structure of romidepsin (1), which is a cage-shaped bicyclic depsipeptide, was determined using a combination of spectroscopic techniques, primarily NMR and x-ray crystallography.31
Romidepsin demonstrated the ability to reverse the effects of the ras oncogene in vitro, which has been shown to play a role in tumor development; expression of the Ha-ras oncogene appears to be directly correlated with tumorigenic potential.32 Because of romidepsin’s ability to reverse the ras-transformed phenotype to normal, as well as its cytotoxic effects, it was developed initially by the U.S. National Cancer Institute as an anti-ras compound,33,34 but recently it has been shown to be an HDAC inhibitor.35,36 A program screening a number of known microbial metabolites for transcriptional activation of the SV40 promoter identified romidepsin as an antitumor compound.35 Comparison of its activity to that of trichostatin A (TSA; a known HDAC inhibitor) revealed that romidepsin was a new HDAC inhibitor.35
Defining a clear path for the development of romidepsin was complicated somewhat due to the many different parties involved. Fujisawa Corporation (now Astellas Inc.) discovered the compound in the early 1990s via a search for molecules that would revert the ras-driven oncogenic phenotype, and then, under a Cooperative Research and Development Agreement (CRADA), further oncology investigations were initiated by the U.S. National Cancer Institute (NCI) in 1998. When the NCI confirmed that romidepsin was a powerful anticancer agent, Fujisawa Corporation initiated their own, separate clinical trials program in 2002. Romidepsin was licensed to Gloucester Pharmaceuticals in 2004; the company was later acquired by Celgene Corporation in 2010. Gloucester approached the U.S. FDA (beginning in 2004), obtained both Orphan Drug Status and Fast Track Status, and were able to come to an agreement, through the FDA Special Protocol Assessment (SPA) process, on a path to approval, which was a pivotal single arm (Phase II) design, although data from the NCI’s ongoing study was also to be part of the New Drug Application (NDA) filing. Essentially, the activity of romidepsin, coupled with the unmet medical need in cutaneous T-cell lymphoma, were persuasive enough arguments to move the drug forward.
MECHANISMS OF ACTION
Romidepsin is converted in cells to its active form by the reduction of the disulfide bond by glutathione, resulting in a monocyclic dithiol (2),16,37 and thus romidepsin serves as a prodrug that is activated only after uptake into cells. This reduced form of romidepsin (2) is inactivated rapidly in serum, perhaps due to sequestration by serum proteins.37 A cysteine in the active site pocket (Cys-151 in HDAC1) was thought to covalently bind with the reduced sulfur atom at position 13, but a mutant HDAC1 in which Cys-151 was replaced by a serine was still sensitive to romidepsin, though a higher concentration (~eight fold) of the drug was necessary for inhibition.37 This fact, combined with the reversibility of the inhibition, suggests that while the cysteine may play a role in the affinity of the drug, it most likely does not bind covalently to the sulfur atom.37 Crystallographic and computer modeling studies using a HDAC-trichostatin A (TSA) complex suggested that one of the thiol groups of the reduced depsipeptide interacts with zinc ions in the active site pocket of certain enzymes, preventing access of the substrate.38 A computer modeling study by Furumai et al.37 concluded that when interacting with HDAC1 the sulfur atom of the reduced romidepsin was located at a position that allowed interaction with the zinc ion via a water molecule. They also suggested that, based on a similar mechanism in protease inhibitors, the sulfur may tetrahedrally coordinate with the zinc, displacing the water molecule.37 The interaction between the thiol group and the active site of the enzyme prevents other substrates from binding.
The reduced form more strongly inhibits HDAC1 and HDAC2 enzymes (class I) than HDAC4 and HDAC6 enzymes (class II).37 By inhibiting class I HDAC, romidepsin inhibits the removal of acetyl groups from the lysine residues of N-terminal histone tails, maintaining a more open and transcriptionally active chromatin state.5 In addition, romidepsin also results in altered acetylation of other nuclear and cytoplasmic proteins, though the precise pathways by which it affects the cell cycle, apoptosis, and angiogenesis have not been defined completely.39
Cell Cycle Arrest
The induction of growth arrest and/or apoptosis by romidepsin depends on the cell line tested and the concentration of drug applied.39 Growth arrest, as opposed to apoptosis, is the predominant response in cell lines in which the application of romidepsin induces the expression of the p21 tumor-suppressor gene.40 Cell lines with reduced p21 expression preferentially undergo apoptosis.41 Tumor cells are more susceptible to romidepsin than normal cells,15 a fact that leads to a viable therapeutic index in the clinic.
Direct acetylation of non-histone proteins may also trigger growth arrest or apoptosis, and several pathways have been studied, though the complete role of HDAC inhibitors is not fully understood. Exposure of whole cells to romidepsin results in a decrease of cyclin D1 and c-Myc, accompanied by an increase in p53-independent p21 WAF1/Cip 1 induction. The p21 induction leads to inhibition of cyclin-dependent kinase (CDK) and dephosphorylation of the retinoblastoma protein (Rb), which results in early G1 phase cell cycle arrest.42,43 A different mechanism involves altered expression of cyclin A and D, and p27/Kip1, again resulting in reduced CDK activity and cell cycle arrest.43
Apoptosis
There are two mechanisms leading to apoptosis: the death receptor pathway and the intrinsic pathway.44 While exposure to romidepsin and other HDAC inhibitors can lead to hyperacetylation of death receptor promoters, including TRAIL (tumor-necrosis factor-related apoptosis-inducing ligand), death receptor 5, Fas ligand and Fas,45 the significance of this mechanism does not seem to be universally acknowledged. Some leukemia cells do not show induction of the TRAIL or Fas pathways, or undergo apoptosis after exposure to HDAC inhibitors.46 The intrinsic pathway involves the perturbation of mictochondrial membranes resulting in the generation of reactive oxygen species (ROS). In normal cells exposed to HDAC inhibitors, ROS do not accumulate due to breakdown by thioredoxin; some tumor cells that do not express the thioredoxin gene accumulate ROS and undergo apoptosis.44
Romidepsin has also been shown to increase acetylation of the HSP 90 chaperone protein, causing proteasomal degradation and inducing apoptosis.47 The proteasome inhibitor bortezomib appears to work synergistically with HDAC inhibitors,48 but it is interesting that the HSP 90 inhibitor, geldanamycin, antagonizes HDAC inhibitor activity.49
Angiogenesis Inhibition
Romidepsin inhibits hypoxia-induced angiogenesis of endothelieal cells in vitro, but does not cause cytotoxicity. It decreased angiogenesis in a chick embryo model, with no signs of thrombosis or hemorrhage, and inhibited angiogenesis strongly in mouse model tumors.50 Romidepsin appears to reduce angiogenic-stimulating factors such as VEGFs, VEGF receptor, FLT1 and FLK1, and increases induction of angiogenic inhibitory factors VHL and neurofibromin2;50 however, the correlation between anti-VEGF activity and in vivo efficacy has not been established.
PHARMACOLOGY
Pharmacokinetics
A two-compartment model with linear kinetics can be used in the pharmacokinetic analysis of romidepsin.51,52 Two dosing schedules were studied in separate Phase I trials. One schedule involved a 4-h infusion on days 1 and 5 of a 21-day cycle, where the maximum tolerated dose (MTD) was determined as 17.8 mg/m2,52 while the second involved a 4-h infusion on days 1, 8, and 15 of a 28-day cycle, where the MTD was determined as 13.3 mg/m2.53 With both schedules, romidepsin exhibited linear pharmacokinetics up to the MTD.52,53 Total clearance volumes in adults have been reported as 4.8 L/h/m2 at a 13 mg/m2 dose,54 and 10.5 L/h/m2 at a 17.8 mg/m2 dose.52 A Phase I study in pediatric patients (2–21 years, median age 13) with refractory solid tumors who received romidepsin in 4-h infusions of 17 mg/m2 reported a clearance volume of 6.8 L/h/m2.55 The elimination half-life has been reported as 3.5 h51 and 3.67 h54 at 13 mg/m2, and 8.1 h52 at 17.8 mg/m2.
Pharmacodynamics
A typical assay for pharmacodynamic studies is to monitor histone acetylation of peripheral blood mononuclear cells (PBMCs).52,55,56 Increased acetylation is observed in PBMCs of patients after treatment with romidepsin, with maximal accumulation of acetyl H3 histones in PBMCs occurring at 4 h after the end of an infusion.55A study in patients with acute myelogenous leukemia (AML) and chronic lymphocytic leukemia (CLL) using a 4 h 13mg/m2 infusion on days 1, 8, and 15 of a 28-day cycle reported 100% histone acetylation of H3 and H4 histones for all CLL patients after 4 h, and for 6 out of 7 CLL patients after 24 h.54 This study also reported an increase in p21 protein expression concurrent with H4 acetylation of the p21 promoter gene.54
A Phase II study of T-cell lymphoma patients monitored several biomarkers: PBMCs histone acetylation, ABCB1 gene expression in PBMCs, ABCB1 gene expression in biopsy samples, and blood fetal hemoglobin (HbF) levels.57 A global increase in PBMCs histone acetylation was reported in 73% of patients within 4 h of treatment, and in 40% of patients after 24 to 48 h.57 The number of patients having a twofold or higher than baseline ABCB1 expression in PBMCs was 56% at 4 h, and 30% at 48 h.57 A fourfold or greater increase in circulating HbF was reported in 60% of the patients. The histone H3 acetylation in PBMCs at the 24-h time point appeared to correlate with response; there was no correlation between the levels of ABCB1 induction and pharmacokinetic parameters, or between ABCB1 induction in biopsy samples and clinical disease reponse.57 These data suggested that peak drug concentration (Cmax) and overall exposure were important in determining response.57,58
T-Cell Lymphoma Clinical Trials
In preclinical studies, greater antitumor activity was observed with intermittent administration than with daily administration.52 Short infusions (>30 s to 4 min) and prolonged infusions (>24 h) caused greater toxicity than infusions of 1–4 h.52 Therefore, in Phase I trials, romidepsin was tested using a 4-h intravenous infusion. Patients who consent to be treated in Phase I trials have cancers for which no known standard therapy exists, or such therapy has already failed, so that patients are not denied any curative or definitely life-extending options. One Phase I study52 administered a 4-h infusion on days 1 and 5 of a 21-day cycle; the maximum tolerated dose (MTD) was defined at 17.8 mg/m2 with dose-limiting toxicity (DLT) manifest as grade-3 fatigue, grade-3 nausea and vomiting, grade-4 thrombocytopenia (low platelet levels), and grade-4 cardiac arrhythmia. Another Phase I study53 administered romidepsin in a 4-h infusion on days 1, 8, and 15 of a 28-day cycle; the MTD in this trial was defined at 13.3 mg/m2 with DLT manifesting as grade 3 thrombocytopenia and fatigue. A partial response was seen in a patient with renal cell carcinoma.52 Several patients with T-cell lymphoma (cutaneous or peripheral) exhibited significant reductions in skin lesions and tumor size after treatment with romidepsin.53,59
Due to the dramatic responses seen in Phase I trials, a Phase II trial to define the response rate and toxicity profile in patients with T-cell lymphoma was initiated.40 Romidepsin was administered via a 4-h infusion on days 1, 8, and 15 of a 28-day cycle. Complete responses were observed in 4 patients (6%), and partial responses were observed in 20 patients (28%).60 The toxicities observed were consistent with those reported previously, including extreme fatigue, nausea, and vomiting.52 Granulocytopenia (failure of bone marrow to make white blood cells) and thrombocytopenia (low platelet counts) were observed as well, with values returning to baseline by the next cycle of treatment.60
Clinical Trials of Romidepsin
Table 1 highlights the published clinical trial data on romidepsin; all of the Phase I trials were on patients who had non-responsive cancers. The favorable results of the NCI-sponsored multi-institutional Phase II trial,51,57,60,61 followed by consistent results by the pivotal Phase II trial conducted by Gloucester Pharmaceuticals62 led to the approval by the U.S. FDA in November of 2009.
Table 1.
Clinical trials of romidepsin
| Phase | Target Disease | Number of Patients | Dose and Schedule | Outcome | References |
|---|---|---|---|---|---|
| I | Advanced or refractory neoplasms | 37 | 17.8 mg/m2 Day 1 and 5 of a 21-day cycle |
Dramatic response in two patients with T-cell lymphoma. | 52,59 |
| II | Cutaneous and Peripheral T-cell lymphoma | 98 | 14mg/m2 Day 1, 8, and 15 of a 28-day cycle |
34% response rate, complete response in 4 patients. Cardiac pharmacokinetic, and pharmacodynamic data acquired. | 51,57,60,61 |
| II | Refractory cutaneous T-cell lymphoma | 96 | 14mg/m2 Day 1, 8, and 15 of a 28-day cycle |
34% response rate, 6 patients with complete response. | 62 |
| I | Advanced cancers | 33 | 13.3 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
No demonstrated activity. | 53 |
| I | Chronic lymphocytic leukemia and acute myeloid leukemia | 10 (CLL), 10 (AML) | 13 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
Some clinical activity; more evident anti-leukemic activity in CLL patients. | 54 |
| I | Children with refractory or recurrent neoplasms | 18 | 13 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
No objective responses. | 55 |
| I–II | Acute myelogenous leukemia and advanced myelodysplastic syndromes | 9 (AML), 3 (MDS) | 18 mg/m2 Day 1 and 5 of a 21-day cycle |
Limited clinical activity, though results indicated genetics may play a role in patient response to the drug. | 56 |
| II | Acute myeloid leukemia | 20 | 13 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
Limited antileukemic activity, possibility for combination therapy. | 74 |
| II | Colorectal cancer | 25 | 13 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
No objective responses. | 75 |
| II | Lung cancer | 19 | 17.8 mg/m2 Day 1 and 7 of a 21-day cycle |
Limited clinical activity, possibility for combination therapy. | 76 |
| II | Refractory metastatic renal cell cancer | 26 | 13 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
Limited clinical activity. | 63 |
| II | Metastatic castration-resistant prostate cancer | 35 | 13 mg/m2 Day 1, 8, and 15 of a 28-day cycle |
Limited antitumor activity, possibility for combination therapy. | 77 |
Toxicity and Side Effects
Cardiac toxicities were a primary concern in the early clinical trials due to preclinical results, leading to electrocardiograms (ECGs) and other measures related to cardiac function being taken before and after every treatment.52 Many of the early toxicities were abnormalities of the ECG waveform, which represent repolarisation abnormalities in the electric functioning of the heart; grade 1 T-wave flattening or grade 2 ST segment depression was observed in more than half of the ECGs, but although these abnormalities can also be indicative of cardiac ischemia, in the case of romidepsin this was not so, since they were not associated with elevation of cardiac troponin or with altered left ventricular function.61 Further follow up over three years with a larger body of clinical data confirms that the ECG abnormalities are not clinically significant nor indicative of any real cardiac toxicity.39 Therefore, this observation is no longer a clinical concern, except that patients are screened with an ECG to check for pre-existing cardiac rhythm abnormalities, including the very rare congenital long QT syndrome, which is a condition where the interval between the Q-wave and the T-wave on the ECG is prolonged, and this can lead to further, even fatal, problems if the electrical circuitry of the heart is further affected in these patients.
The majority of patients receiving romidepsin experience nausea, vomiting, and anorexia,52,54,58,60,63 and Grant et al.58 note that the antiemetic routine followed at the NCI was to administer 1 mg granisetron intravenously prior to romidepsin, followed by 1 mg orally every 12 h for 3 days.58 Progressive fatigue and occasional fever were also noted with romidepsin.52,53,54,60,63 Also of concern are the hematological side effects; regular blood tests are administered to patients on romidepsin to monitor these symptoms.64 Leukopenia, granulocytopenia, and thrombocytopenia were all observed; in each case the effect is quickly reversed after cessation of the treatment.58,60
SYNTHETIC STUDIES
The main challenges in romidepsin synthesis include the asymmetric construction of the hydroxy mercapto heptenoic acid unit, the 16-membered cyclic depsipeptide ring itself (i.e. four amino acids and hydroxy mercapto heptenoic acid), and the intramolecular oxidative coupling of the thiol moieties to produce the stable prodrug form of the molecule. The total synthesis was first completed in 1996 in 14 steps with an 18% overall yield.65 An improved synthesis utilizing nine steps with a 13% overall yield was published in 2007,66 using, as in the first scheme, an asymmetric acetate aldol reaction and a lactonization step for macrolization. In 2008, Wen et al.67 utilized a lactamization as an alternative route to cyclization, reporting increased cyclization efficiency. However, due both to the chemical complexity of synthesis and the low yield of the producing organism, development was hindered mainly by shortages of the product.68 Therefore, attempts have been made to develop active analogs.
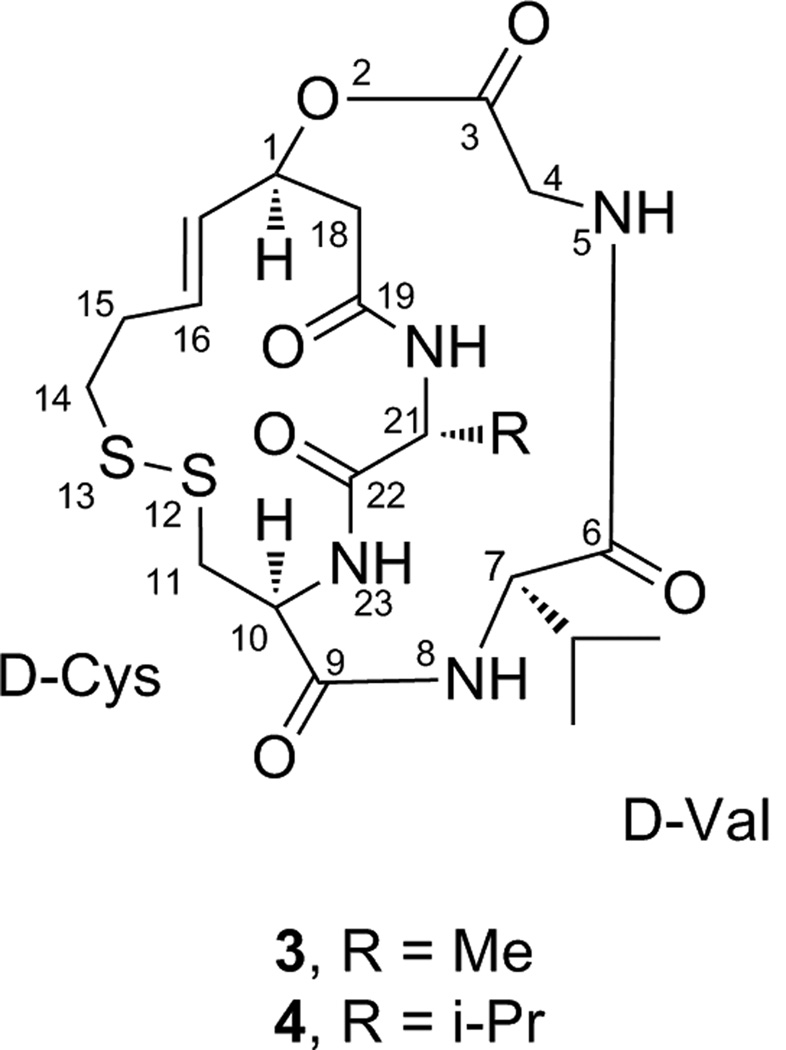
Using a series of synthetic analogs, Yurek-George et al.69 were able to examine the importance of several features of the romidepsin structure. They concluded that while the thiol group at position 13 does indeed function as a Zn-binding group within the HDAC active site, the cysteine residue is non-essential, provided that the Zn-binding thiol remains protected as in the oxidized pro-drug form.69 This assertion is supported by work done with linear compounds in which the thiol was masked as a thioester, undergoing enzymatic hydrolysis in the cell to produce the free thiol.70,71 Yurek-George et al. also found that the unsaturated Z-dehydrobutyrine (Z-Dhb) residue, which could be susceptible to a Michael addition, could be replaced by less reactive side chains (e.g. 3 and 4, Figure 2) with no loss in in vitro potency, but that the macrocyclic scaffold itself was an essential component of HDAC inhibition.69 However, several small methyl ester structures without the macrocyclic backbone have proved to induce hyperacetylation in Drosophila S2 cells.72 These structures retain the disulfide link of romidepsin, thus preserving its prodrug quality. The analogs studied consisted of several small cyclic disulfides; the number of methylene units in the ring was varied to provide a range of lengths for the linker from the ester to the reduced thiol group.72 Studies with HDAC inhibitors TSA and SAHA showed important interactions between the drugs and several phenylalanine residues surrounding the enzyme active site pocket,38 so the methyl ester was used to attach various lipophilic caps, in an effort to improve enzyme interaction.72
Figure 2.
Structures of two romidepsin analogs (compounds 3 and 4) where the Z-dehydrobutyrine (Z-Dhb) residue, which may be susceptible to a Michael addition, has been replaced with a valine moiety.69
Despite these interesting medicinal chemistry studies, to the best of our knowledge, no analogs of romidepsin are in advanced stages of development.
CONCLUSIONS
Romidepsin, a potent HDAC inhibitor, offers a promising new treatment for a disease with few existing therapies. This natural product was approved by the U.S. FDA in November of 2009 for use in patients with cutaneous T-cell lymphoma (CTLC) under the trade name Istodax® marketed by Celgene Corporation (Summit, NJ). The dosing schedule was approved as 14 mg/m2 on days 1, 8 and 15 of a 28-day cycle.64 After a year of approval as an antineoplastic agent, preliminary information suggests that romidepsin is finding a real place in the treatment of CTCL, and the activity and manageable toxicity are helpful for both patients receiving treatment and doctors prescribing it (personal communication by several medical doctors to W.M.). At the time of preparation of this review, a supplemental new drug application (NDA) was being prepared for peripheral T-cell lymphoma (PTCL),73 and only time will tell if romidepsin’s use expands to the treatment of other cancers.
Figure 1.
Structures of romidepsin (1) and the reduced counterpart (2).17 The numbering of 1 is based on Xiao et al.16
Acknowledgement
This review was prepared in support of grant P01-CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD. Some details were added (by WM) with permission from Celgene Corporation. The authors thank Drs. Sloan Ayers, Mario Figueroa, James McAlpine, and Arlene Sy-Cordero for helpful comments.
References and Notes
- 1.Kurdistani SK. Histone modifications as markers of cancer prognosis: a cellular view. Br. J. Cancer. 2007;97:1–5. doi: 10.1038/sj.bjc.6603844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emanuele S, Lauricella M, Tesoriere G. Histone deacetylase inhibitors: apoptotic effects and clinical implications (Review) Int. J. Oncol. 2008;33:637–646. [PubMed] [Google Scholar]
- 3.Glaser KB. HDAC inhibitors: Clinical update and mechanism-based potential. Biochem. Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Zheng YG, Wu J, Chen Z, Goodman M. Chemical regulation of epigenetic modifications: opportunities for new cancer therapy. Med. Res. Rev. 2008;28:645–687. doi: 10.1002/med.20120. [DOI] [PubMed] [Google Scholar]
- 5.Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda H, Sano N, Muto S, Horikoshi M. Simple histone acetylation plays a complex role in the regulation of gene expression. Brief. Funct. Genomic. Proteomic. 2006;5:190–208. doi: 10.1093/bfgp/ell032. [DOI] [PubMed] [Google Scholar]
- 7.Mitsiades CS, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc. Natl. Acad. Sci. USA. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melnick A, Licht JD. Histone deacetylases as therapeutic targets in hematologic malignancies. Curr. Opin. Hematol. 2002;9:322–332. doi: 10.1097/00062752-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Mahlknecht U, Hoelzer D. Histone acetylation modifiers in the pathogenesis of malignant disease. Mol. Med. 2000;6:623–644. [PMC free article] [PubMed] [Google Scholar]
- 10.Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr. Opin. Oncol. 2001;13:477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Klisovic MI, et al. Depsipeptide (FR 901228) promotes histone acetylation, gene transcription, apoptosis and its activity is enhanced by DNA methyltransferase inhibitors in AML1/ETO-positive leukemic cells. Leukemia. 2003;17:350–358. doi: 10.1038/sj.leu.2402776. [DOI] [PubMed] [Google Scholar]
- 12.Bieliauskas AV, Pflum MK. Isoform-selective histone deacetylase inhibitors. Chem. Soc. Rev. 2008;37:1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papeleu P, et al. Differential effects of histone deacetylase inhibitors in tumor and normal cells-What is the toxicological relevance? Crit. Rev. Toxicol. 2005;35:363–378. doi: 10.1080/10408440590935639. [DOI] [PubMed] [Google Scholar]
- 14.Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J. Cell. Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- 15.Ueda H, et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physicochemical and biological properties, and antitumor activity. J. Antibiot. 1994;47:301–310. doi: 10.7164/antibiotics.47.301. [DOI] [PubMed] [Google Scholar]
- 16.Xiao JJ, Byrd J, Marcucci G, Grever M, Chan KK. Identification of thiols and glutathione conjugates of depsipeptide FK228 (FR901228), a novel histone protein deacetylase inhibitor, in the blood. Rapid Commun. Mass Spectrom. 2003;17:757–766. doi: 10.1002/rcm.976. [DOI] [PubMed] [Google Scholar]
- 17.Note that the structures of romidepsin (1) and its reduced counterpart (2) were published incorrectly in the review by Grant et al.58
- 18.Willemze R, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 19.Whittaker SJ, Marsden JR, Spittle M, Russell Jones R. Joint British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. Br. J. Dermatol. 2003;149:1095–1107. doi: 10.1111/j.1365-2133.2003.05698.x. [DOI] [PubMed] [Google Scholar]
- 20.Litvinov IV, Jones DA, Sasseville D, Kupper TS. Transcriptional profiles predict disease outcome in patients with cutaneous T-cell lymphoma. Clin. Cancer Res. 2010;16:2106–2114. doi: 10.1158/1078-0432.CCR-09-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstock MA, Horm JW. Mycosis fungoides in the United States: increasing incidence and descriptive epidemiology. JAMA. 1988;260:42–46. [PubMed] [Google Scholar]
- 22.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch. Dermatol. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 23.Kim EJ, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J. Clin. Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorincz AL. Cutaneous T-cell lymphoma (mycosis fungoides) Lancet. 1996;347:871–876. doi: 10.1016/s0140-6736(96)91350-1. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch. Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 26.Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064–5073. doi: 10.1182/blood-2008-10-184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YH, Chow S, Varghese A, Hoppe RT. Clinical characteristics and long-term outcome of patients with generalized patch and/or plaque (T2) mycosis fungoides. Arch. Dermatol. 1999;135:26–32. doi: 10.1001/archderm.135.1.26. [DOI] [PubMed] [Google Scholar]
- 28.Duvic M, et al. Analysis of long-term outcomes of combined modality therapy for cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2003;49:35–49. doi: 10.1067/mjd.2003.449. [DOI] [PubMed] [Google Scholar]
- 29.Zackheim HS, Amin S, Kashani-Sabet M, McMillan A. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. J. Am. Acad. Dermatol. 1999;40:418–425. doi: 10.1016/s0190-9622(99)70491-3. [DOI] [PubMed] [Google Scholar]
- 30.Siegel RS, Pandolfino T, Guitart J, Rosen S, Kuzel TM. Primary cutaneous T-cell lymphoma: review and current concepts. J. Clin. Oncol. 2000;18:2908–2925. doi: 10.1200/JCO.2000.18.15.2908. [DOI] [PubMed] [Google Scholar]
- 31.Shigematsu N, et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. II. Structure determination. J. Antibiot. 1994;47:311–314. doi: 10.7164/antibiotics.47.311. [DOI] [PubMed] [Google Scholar]
- 32.Manoharan TH, Burgess JA, Ho D, Newell CL, Fahl WE. Integration of a mutant c-Ha-ras oncogene into C3H/10T1/2 cells and its relationship to tumorigenic transformation. Carcinogenesis. 1985;6:1295–1301. doi: 10.1093/carcin/6.9.1295. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Brunner T, Zhang L, Shi Y. Fungal metabolite FR901228 inhibits c-Myc and Fas ligand expression. Oncogene. 1998;17:1503–1508. doi: 10.1038/sj.onc.1202059. [DOI] [PubMed] [Google Scholar]
- 34.Rajgolikar G, Chan KK, Wang HC. Effects of a novel antitumor depsipeptide, FR901228, on human breast cancer cells. Breast Cancer Res. Treat. 1998;51:29–38. doi: 10.1023/a:1006091014092. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res. 1998;241:126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, et al. Histone deacetylase as a new target for cancer chemotherapy. Cancer Chemother. Pharmacol. 2001;48 Suppl 1:S20–S26. doi: 10.1007/s002800100300. [DOI] [PubMed] [Google Scholar]
- 37.Furumai R, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 38.Finnin MS, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 39.Piekarz R, Bates S. A review of depsipeptide and other histone deacetylase inhibitors in clinical trials. Curr. Pharm. Des. 2004;10:2289–2298. doi: 10.2174/1381612043383980. [DOI] [PubMed] [Google Scholar]
- 40.Archer SY, Meng S, Shei A, Hodin RA. p21WAF1 is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc. Natl. Acad. Sci. USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgess AJ, et al. Up-regulation of p21WAF1/CIP1 by histone deacetylase inhibitors reduces their cytotoxicity. Mol. Pharmacol. 2001;60:828–837. [PubMed] [Google Scholar]
- 42.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63:3637–3645. [PubMed] [Google Scholar]
- 43.Sandor V, et al. p21-dependent G1 arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br. J. Cancer. 2000;83:817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peart MJ, et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003;63:4460–4471. [PubMed] [Google Scholar]
- 45.Insinga A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 46.Nebbioso A, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 47.Yu X, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J. Natl. Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 48.Yu C, et al. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003;102:3765–3774. doi: 10.1182/blood-2003-03-0737. [DOI] [PubMed] [Google Scholar]
- 49.Huang HC, Liu YC, Liu SH, Tzang BS, Lee WC. Geldanamycin inhibits trichostatin A-induced cell death and histone H4 hyperacetylation in COS-7 cells. Life Sci. 2002;70:1763–1775. doi: 10.1016/s0024-3205(01)01558-2. [DOI] [PubMed] [Google Scholar]
- 50.Kwon HJ, Kim MS, Kim MJ, Nakajima H, Kim KW. Histone deacetylase inhibitor FK228 inhibits tumor angiogenesis. Int. J. Cancer. 2002;97:290–296. doi: 10.1002/ijc.1602. [DOI] [PubMed] [Google Scholar]
- 51.Woo S, et al. Population pharmacokinetics of romidepsin in patients with cutaneous T-cell lymphoma and relapsed peripheral T-cell lymphoma. Clin. Cancer Res. 2009;15:1496–1503. doi: 10.1158/1078-0432.CCR-08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandor V, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 53.Marshall JL, et al. A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J. Exp. Ther. Oncol. 2002;2:325–332. doi: 10.1046/j.1359-4117.2002.01039.x. [DOI] [PubMed] [Google Scholar]
- 54.Byrd JC, et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105:959–967. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 55.Fouladi M, et al. Phase I study of depsipeptide in pediatric patients with refractory solid tumors: a Children's Oncology Group report. J. Clin. Oncol. 2006;24:3678–3685. doi: 10.1200/JCO.2006.06.4964. [DOI] [PubMed] [Google Scholar]
- 56.Klimek VM, et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin. Cancer Res. 2008;14:826–832. doi: 10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 57.Bates SE, et al. Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br. J. Haematol. 2009;148:256–267. doi: 10.1111/j.1365-2141.2009.07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant C, et al. Romidepsin: A new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev. Anticancer. Ther. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piekarz RL, et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 60.Piekarz RL, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piekarz RL, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin. Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 62.Whittaker SJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 63.Stadler WM, Margolin K, Ferber S, McCulloch W, Thompson JA. A phase II study of depsipeptide in refractory metastatic renal cell cancer. Clin. Genitourin. Cancer. 2006;5:57–60. doi: 10.3816/CGC.2006.n.018. [DOI] [PubMed] [Google Scholar]
- 64.This information comes from the package insert.
- 65.Li KW, Wu J, Xing WN, Simon JA. Total synthesis of the antitumor depsipeptide FR-901,228. J. Am. Chem. Soc. 1996;118:7237–7238. [Google Scholar]
- 66.Greshock TJ, Johns DM, Noguchi Y, Williams RM. Improved total synthesis of the potent HDAC inhibitor FK228 (FR-901228) Org. Lett. 2008;10:613–616. doi: 10.1021/ol702957z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen S, Packham G, Ganesan A. Macrolactamization versus macrolactonization: total synthesis of FK228, the depsipeptide histone deacetylase inhibitor. J. Org. Chem. 2008;73:9353–9361. doi: 10.1021/jo801866z. [DOI] [PubMed] [Google Scholar]
- 68.Jones P, Steinkuhler C. From natural products to small molecule ketone histone deacetylase inhibitors: development of new class specific agents. Curr. Pharm. Des. 2008;14:545–561. doi: 10.2174/138161208783885317. [DOI] [PubMed] [Google Scholar]
- 69.Yurek-George A, et al. The first biologically active synthetic analogues of FK228, the depsipeptide histone deacetylase inhibitor. J. Med. Chem. 2007;50:5720–5726. doi: 10.1021/jm0703800. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki T, et al. Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxamates. J. Med. Chem. 2005;48:1019–1032. doi: 10.1021/jm049207j. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki T, et al. Identification of a potent and stable antiproliferative agent by the prodrug formation of a thiolate histone deacetylase inhibitor. Bioorg. Med. Chem. Lett. 2007;17:1558–1561. doi: 10.1016/j.bmcl.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 72.Mays JR, Restituyo JA, Katzenberger RJ, Wassarman DA, Rajski SR. Cyclic disulfides as functional mimics of the histone deacetylase inhibitor FK-228. Tetrahedron Lett. 2007;48:4579–4583. doi: 10.1016/j.tetlet.2007.04.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pro B, et al. Final Results From a Pivotal, Multicenter, International, Open-Label, Phase 2 Study of Romidepsin In Progressive or Relapsed Peripheral T-Cell Lymphoma (PTCL) Following Prior Systemic Therapy; 52nd American Society of Hematology Annual Meeting and Exposition; 2010. Abstract 114. [Google Scholar]
- 74.Odenike OM, et al. Histone deacetylase inhibitor romidepsin has differential activity in core binding factor acute myeloid leukemia. Clin. Cancer Res. 2008;14:7095–7101. doi: 10.1158/1078-0432.CCR-08-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitehead RP, et al. Phase II trial of romidepsin (NSC-630176) in previously treated colorectal cancer patients with advanced disease: a Southwest Oncology Group study (S0336) Invest. New Drugs. 2009;27:469–475. doi: 10.1007/s10637-008-9190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schrump DS, et al. Clinical and molecular responses in lung cancer patients receiving romidepsin. Clin. Cancer Res. 2008;14:188–198. doi: 10.1158/1078-0432.CCR-07-0135. [DOI] [PubMed] [Google Scholar]
- 77.Molife LR, et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC) Ann. Oncol. 2010;21:109–113. doi: 10.1093/annonc/mdp270. [DOI] [PubMed] [Google Scholar]