Abstract
This study examined the ability of plumbagin to induce apoptosis in chronic myelogenous leukemia (CML). Plumbagin exposure led to a significant reduction in cell viability and the induction of apoptosis. Mechanistically, plumbagin treatment led to elevated levels of ROS. Plumbagin-induced apoptosis was inhibited by N-acetyl L-cysteine (NAC) and PEG-catalase. Furthermore, plumbagin exposure led to elevated expression of DR4 and DR5 and increased killing through soluble TRAIL. The plumbagin-induced increase in DR4 and DR5 was inhibited by treatment with NAC. Together, this study suggests that plumbagin may be an effective treatment of CML through increased sensitivity to TRAIL-mediated killing.
Keywords: TRAIL, Apoptosis, ROS, CML
Introduction
Initial studies exploring the use of death receptor signaling in tumors focused on signaling through Fas/Fas ligand. Although signaling through this pathway was effective at killing many tumor types, this treatment was unable to discriminate between normal and malignant cells. In many cases normal cells appeared to more sensitive to killing through Fas/Fas ligand when compared to their malignant counterparts. More recently, a new member of the death receptor family, known as TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors was identified. This signaling pathway has several potential advantages over other death receptor pathways, such as Fas/Fas ligand. Most importantly, induction of apoptosis through TRAIL receptors is selective for tumors, as most normal cells are resistant to TRAIL-induced apoptosis [1, 2]. In addition, signaling through TRAIL can be independent of p53 [3]. It is believed that as many as 12.5% of lymphoid malignancies have mutations in p53 which may lead to resistance to chemotherapy or radiotherapy [4–7] Therefore, signaling through TRAIL receptors may bypass any resistance conferred upon tumors with mutations in p53. Together these studies suggest that targeting TRAIL for the treatment of various lymphoid-derived tumors, including chronic myelogenous leukemia, has significant potential and treatments that can selectively sensitize tumors to TRAIL-induced apoptosis may lead to significantly improved treatments for various malignancies of the immune system.
Induction of apoptosis by TRAIL take place primarily through signaling through TRAILR1 (DR4) and TRAIL-R2 (DR5)[8]. Unfortunately, many tumor cells are or become resistant to TRAIL-induced apoptosis [9–12]. The mechanism by which normal and many transformed cells develop resistance remains unclear [13]. One possibility is low or altered expression of functional death receptors (DR4 and/or DR5). This possibility was the subject of a recent review and suggests that strategies to increase expression of functional DR4 and/or DR5 in hematologic malignancies may lead to enhanced TRAIL-based treatments [14]. TRAIL receptor expression is sensitive to ROS levels. More specifically, increases in ROS levels can lead to increase levels of TRAIL receptors [15–17]. Therefore, there is great interest in finding new compounds that can alter the expression of TRAIL receptors possibly through alteration in ROS levels.
Extracts from plants containing plumbagin have a long history of use in ayurvedic medicine in China and other Asian countries. More specifically, these plants have been used for the treatment of a number of diseases including cancer. However, most claims of plumbagin’s antitumor activity are supported only by anecdotal evidence and there are few scientific reports describing their mechanism of action or their efficacy in the treatment of cancer. Due to the fact that plumbagin is a quinone, increases in ROS levels have been proposed for its cytotoxic activity. In a recent study examining the cytotoxic effects of plumbagin, it was demonstrated that plumbagin-induced cytotoxicity in keratinocytes was mediated primarily through redox cycling, H2O2 production, and glutathione (GSH) oxidation [18]. Plumbagin has also been linked to cell cycle arrest in cancer cells. For example, exposure of the human nonsmall cell lung cancer cells, A549 to plumbagin led to cell cycle arrest and subsequent death of these cells [19]. Furthermore, it was shown that these effects were directly mediated through an increase in p21 levels [20]. Additionally, plumbagin exposure has been linked to an increase in ROS-mediated apoptosis signaling. More specifically, plumbagin exposure of the ME-180 human cervical cancer cell line led to an increase in intracellular ROS, activation of the caspase cascade and subsequent induction of apoptosis [21].
Although plant extracts containing plumbagin continue to be used for the treatment of cancer, little has been reported describing the effects of plumbagin exposure on TRAIL-induced signaling in hematological malignancies. Therefore, based on previous reports and preliminary data from our laboratory showing that plumbagin treatment leads to an increase in ROS levels [22], we further explored the mechanism of plumbagin induced apoptosis by examining the effect on TRAIL-induced killing. More specifically, we investigated the possibility that lumbagin led to elevated levels of TRAIL receptors increasing the sensitivity to TRAIL-induced killing in the TRAIL-resistant human leukemia cell line K562.
Material and Methods
Reagents
Plumbagin initially dissolved in DMSO was obtained from Sigma-Aldrich (St. Louis, MO). Plumbagin was further diluted with tissue culture medium for in vitro studies. N-acetylcysteine (NAC), and PEG-catalase were obtained from Sigma-Aldrich (St. Louis, MO). Recombinant human TRAIL was obtained from Peprotech (Rocky Hill, NJ).
Cell line
K562 cells were maintained in RPMI 1640 (Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal calf serum (FCS), 10 mM HEPES, 1mM glutamine, 40 µg/ml gentamicin sulfate, and 50 µM 2-mercaptoethanol.
Measurement of the effect of plumbagin on tumor cell viability in vitro
Tumor cells were adjusted to 0.5 × 106 cells/ml in complete medium. Next, the cells (0.5 × 106) were cultured for 24 hours in 24 well plates in 2 ml medium in the presence or absence of various concentrations of plumbagin (0.5, 1, 2, or 4 µM). Finally, the cells were harvested, washed twice in PBS and the viable cell count was determined by trypan blue dye exclusion or MTT assay. Absorbance for the MTT assay was quantified using a Tecan Infinite M1000 multimode plate reader (Männedorf, Switzerland).
Detection of plumbagin-induced apoptosis in vitro
Tumor cells (0.5 × 106 cells/well) were cultured in 24-well plates in the presence or absence of various concentrations of plumbagin (1, 2, or 4 µM) for 24 h. Next, the cells were harvested, washed twice in PBS and analyzed for the induction of apoptosis using the TUNEL and cell cycle analysis methods. To detect apoptosis using the TUNEL method the cells were washed twice with PBS and fixed with 4% p-formaldehyde for 30 minutes at room temperature. The cells were next washed with PBS, permeabilized on ice for 2 minutes and incubated with FITC-dUTP and TdT (Promega, Madison WI) for 1 hour at 37°C and 5% CO2. To detect apoptosis using cell cycle analysis the cells were fixed in 1 ml of cold (−20°C) 70% EtOH for 30 minutes. Next, the cells were washed twice with PBS containing 1% FBS. The cells were resuspended in 500 µl of PI solution containing 50 µg/ml propidium iodide, 200 µg/ml RNase A, 2% FBS, and 0.1% Igepal. The cells were then incubated for 30 minutes at 37°C and then transferred to 4°C for 1.5 hours. The samples were analyzed using a flow cytometer (FACSAria II, BD Biosciences, San Jose, CA). In all experiments, 10,000 cells were analyzed using forward/side-scatter gating.
Determination of Reactive Oxygen Species
K562 cells (0.5×106) were labeled for 1h with 5 µM carboxy-H2DCFDA (Molecular Probes, Eugene, OR). Next, excess carboxy-H2DCFDA was removed by washing the cells and then suspending them in serum-free, phenol red-free RPMI. The labeled cells were then exposed to vehicle control (DMS0) or various concentrations of plumbagin (1, 2, or 4 µM) for 1h and the levels of ROS were determined by flow cytometric analysis using a FACSAria II flow cytometer (BD Biosciences, San Jose, CA). In all experiments, 10,000 viable cells were analyzed using forward/side-scatter gating.
Flow cytometric analysis of cell surface protein expression
K562 cells were treated with various concentrations of PLB (0, 1, 2, and 4 µM). The cells were analyzed 24–48 hours later for altered expression of various cell surface markers using flow cytometry. Briefly, 1×106 K562 cell were incubated with 1 µg of anti-human DR4, or anti-human DR5 (R&D Systems, Minneapolis, MN) on ice for 30 minutes. The cells were washed with PBS three times and then stained on ice for 30 minutes with 0.5 µg/106 cells of DyLight 649–conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The cells were washed 3 times with PBS and analyzed for fluorescence using a FACSAria II flow cytometer (BD Biosciences, San Jose, CA) (11). In all experiments, 10,000 viable cells were analyzed using forward/side-scatter gating.
Statistical Analysis
Student’s t-test or Tukey Kramer tests were used to compare vehicle and plumbagin treated groups. P<0.05 was considered to be statistically significant.
Results
Treatment of the human chronic myelogenous leukemia cell line K562 with plumbagin leads to reduced cell viability through the induction of apoptosis
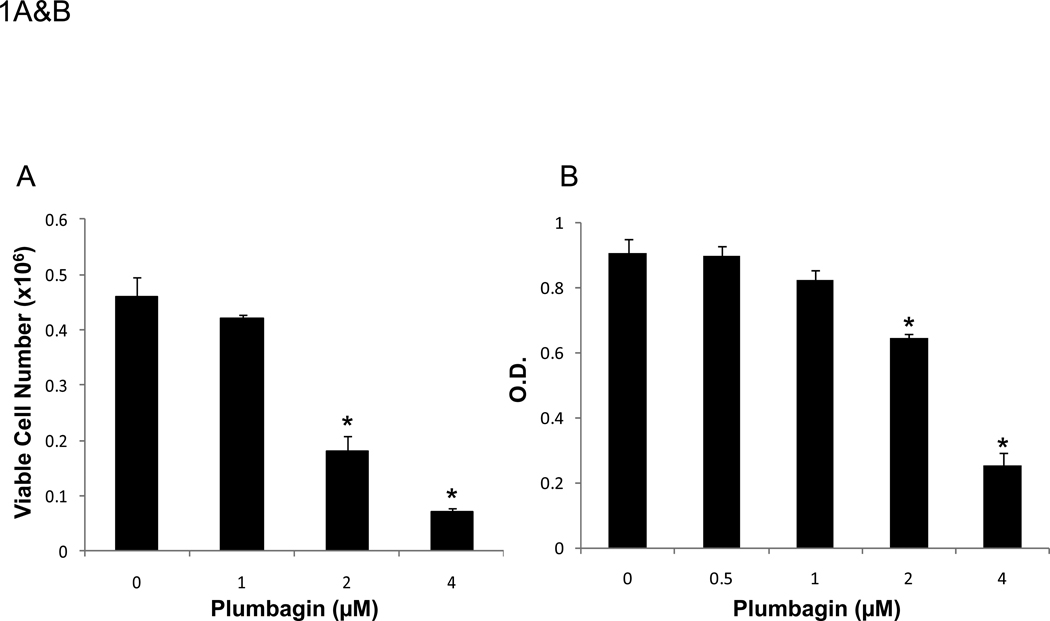
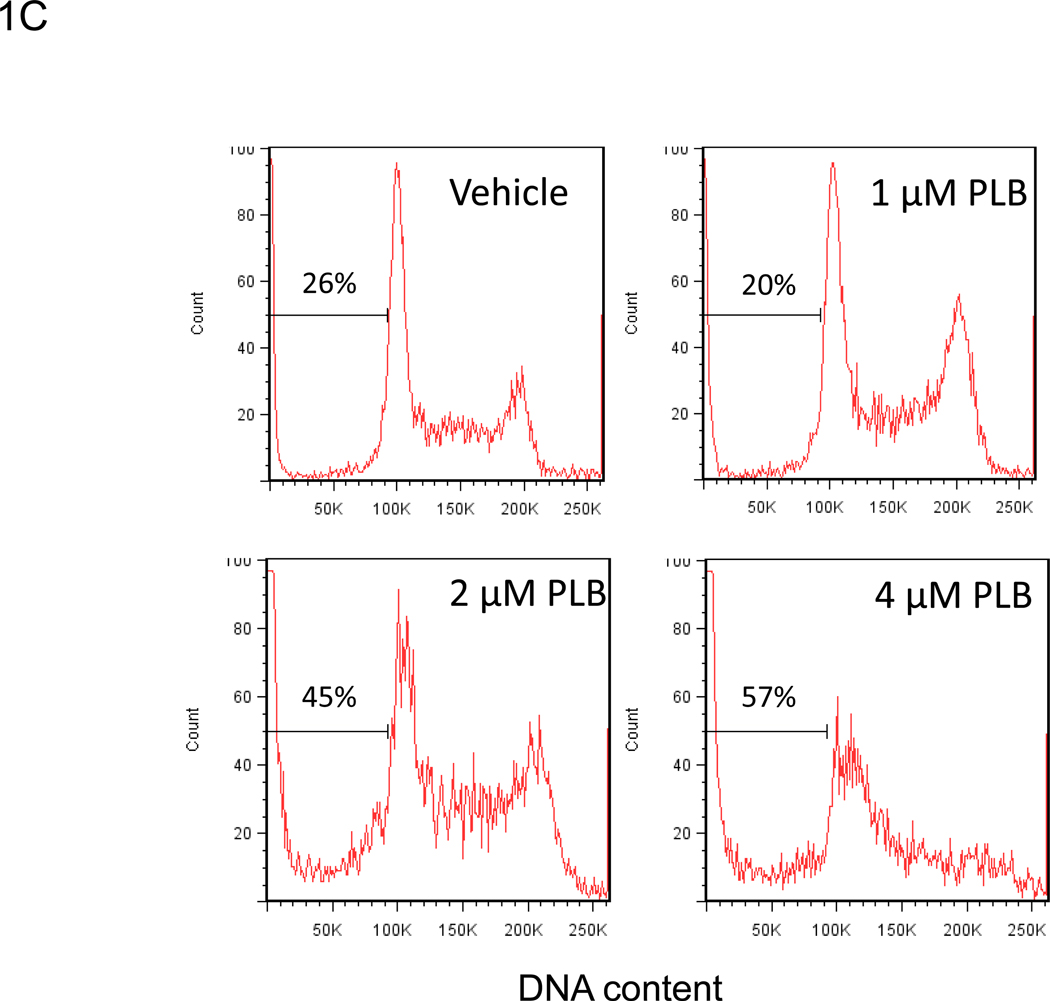
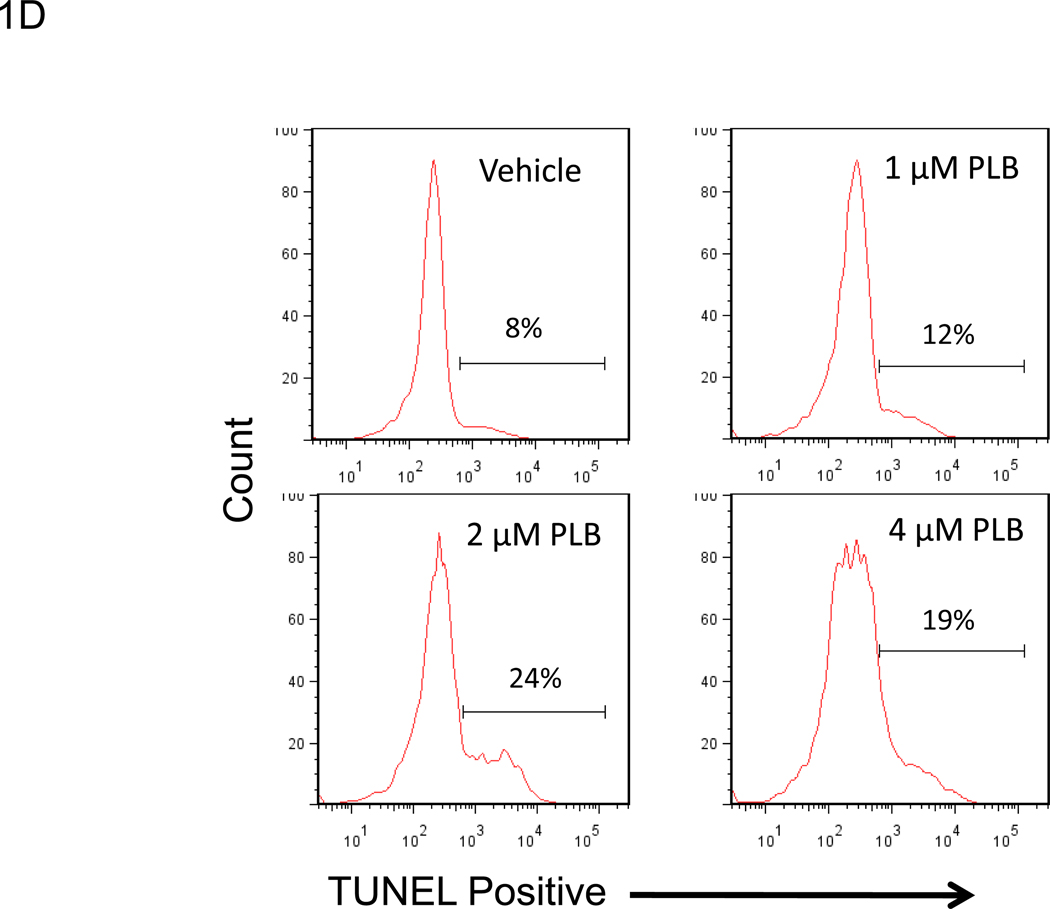
We examined whether plumbagin exposure had an effect on the viability of K562, tumor cells in vitro. To this end, the tumor cells were exposed to various concentrations of plumbagin (0, 0.5, 1, 2, and 4 µM) for 24 hours after which the viability was determined by Trypan blue dye exclusion and MTT assay (Figures 1A and 1B, respectively). The results showed that exposure to plumbagin at concentrations of ≥ 2 µM led to a significant reduction in the number of viable cells. Next, we analyzed the plumbagin-treated tumor cells for induction of apoptosis by cell cycle analysis and TUNEL staining (Figures 1C and 1D, respectively) and demonstrated that plumbagin induced significant apoptosis in K562 tumor cells in vitro. By cell cycle analysis, exposure of K562 cells to 2 and 4 µM of plumbagin resulted in 45% and 57% apoptosis, respectively, compared to 26% apoptosis in the Vehicle control treated cells (Figure 1C). Similar results were seen using the TUNEL assay in which 2 and 4 µM of plumbagin resulted in 24% and 19% apoptosis, respectively, compared to 8% apoptosis in the Vehicle control treated cells (Figure 1D). Together, these results suggested that exposure of K562 cells to ≥ 2 µM plumbagin in vitro led to tumor cell killing by induction of apoptosis.
Figure 1. Treatment of the human chronic myelogenous leukemia cell line K562 with plumbagin leads to reduced cell viability through the induction of apoptosis.
The effect of plumbagin on K562 viability was determined by treating cells with various concentrations of plumbagin (PLB). Cell viability was determined 24h later by Trypan blue dye exclusion (A) and MTT (B) assays. The data represent the mean ± SD. Asterisk denotes statistically significant difference (p<0.05) between vehicle (0 µM) and plumbagin treated cells. The effect of plumbagin treatment on the induction of apoptosis in K562 cells was determined by treating the cells with various concentrations of plumbagin for 24h and quantifying the percentage of sub G0 cells using cell cycle analysis (C) and the TUNEL assay (D).
Exposure to plumbagin leads to ROS-mediated apoptosis in K562 leukemia cells
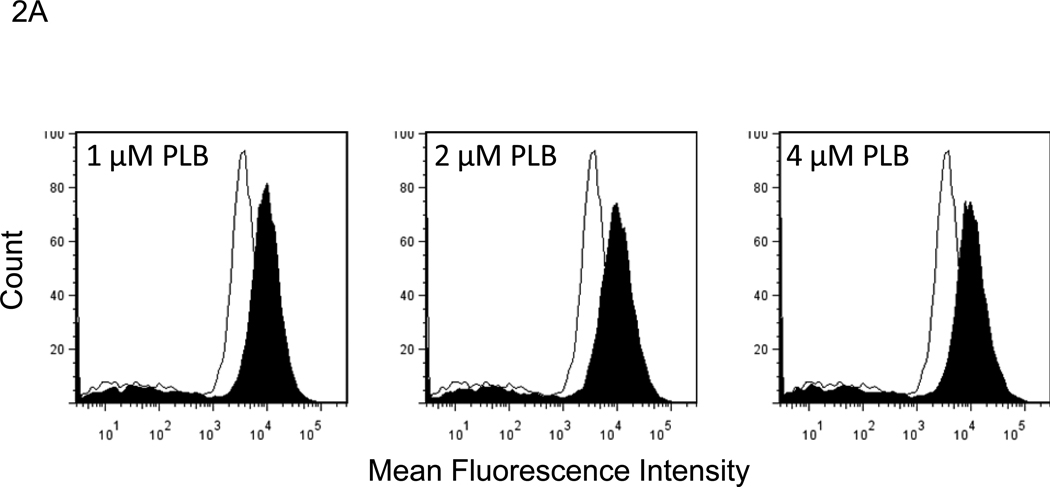
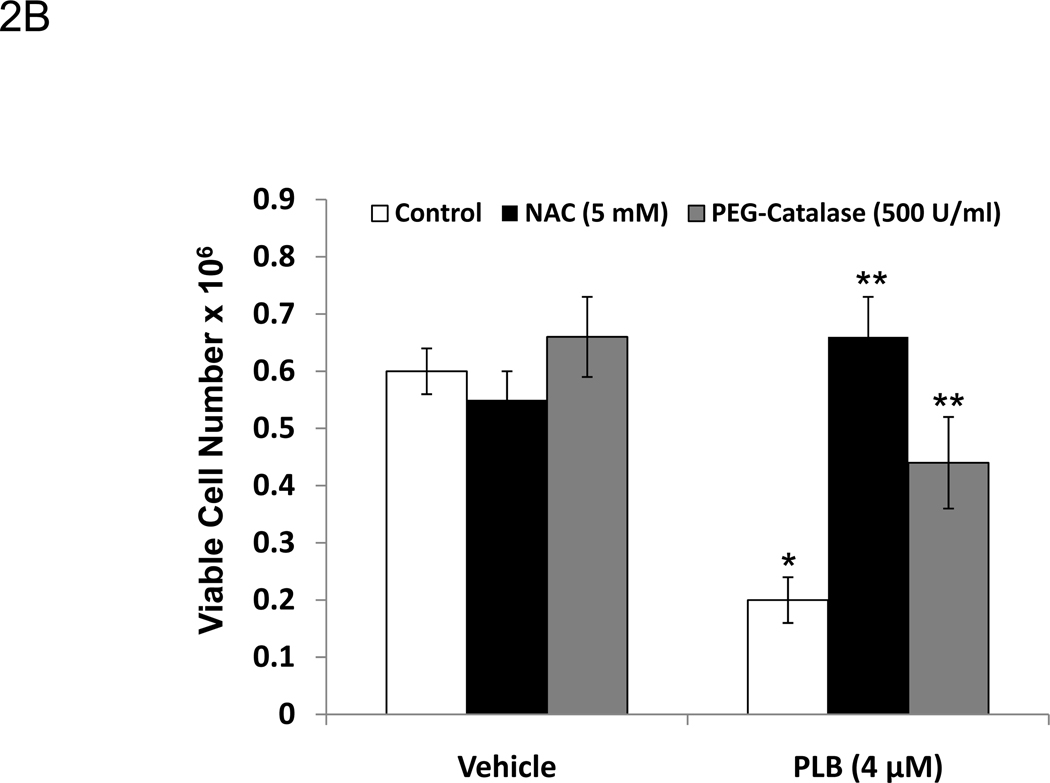
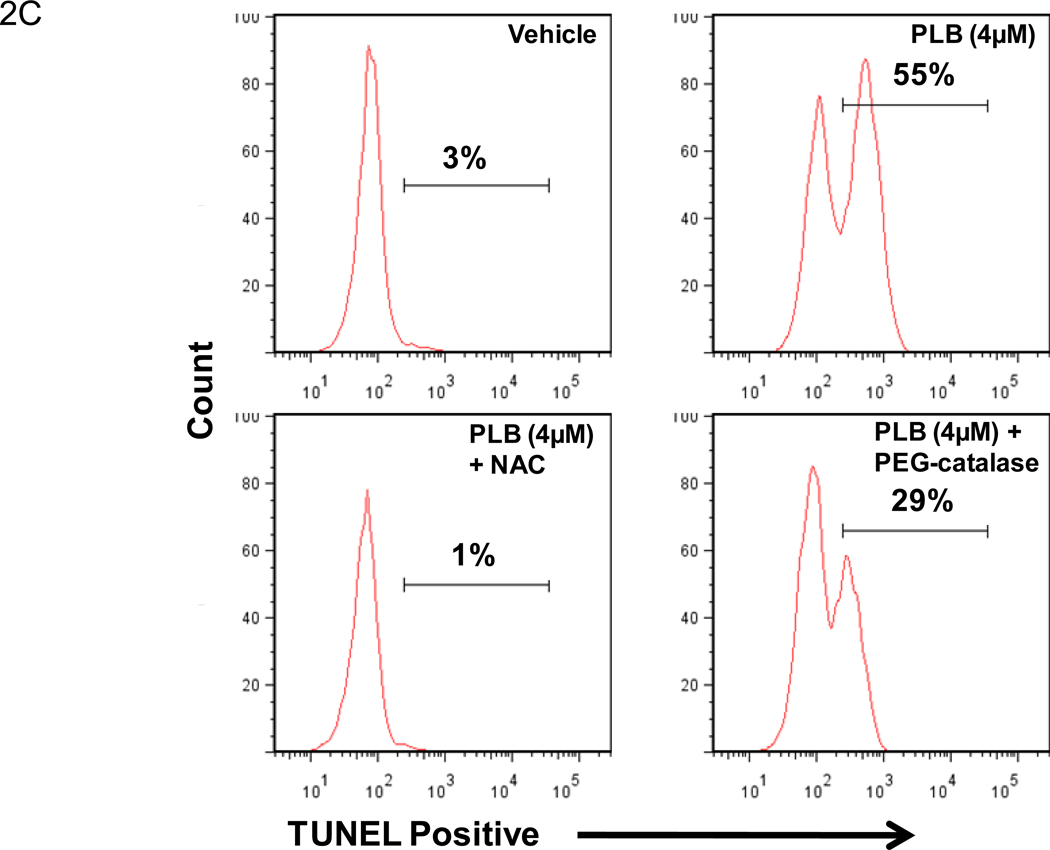
Plumbagin exposure has been associated with increased levels of ROS in a number of cancers including prostate, melanoma, cervical, and promyelocytic leukemia [21, 23–25]. Therefore, in the current study we examined the effect of plumbagin treatment on K562 ROS levels. To this end, K562 cells were pre-labeled with 5 µM carboxy-H2DCFDA and then exposed to various concentrations of plumbagin for 1h. Exposure of K562 cells to 1, 2, or 4 µM plumbagin led to an increase of over 2 fold in ROS-associated mean fluorescence intensity (MFI) when compared to vehicle treated cells (Figure 2A). Following exposure to 1, 2, or 4 µM plumbagin the MFI increased to from 3064 in the vehicle treated cells to 9276, 10662, and 10252 in cells treated with 1, 2, or 4 µM plumbagin, respectively. Together, these results suggest that exposure of K562 to 1 µM or greater plumbagin for 1h leads to a considerable increase in the levels of ROS. The involvement of the plumbagin-induced increase in ROS in K562 apoptosis was confirmed using NAC and PEG-catalase. More specifically, K562 cells were exposed for 24h to plumbagin ± NAC (5 mM) or plumbagin ± PEG-catalase (500 U/ml) and then analyzed for viable cell number (Figure 2B) and induction of apoptosis using the TUNEL assay (Figure 2C). The results demonstrated that treatment of K562 cells with NAC or PEG-catalase led to a significant reduction in plumbagin-induced cell killing and apoptosis. Together, these results suggest that plumbagin-induced increase in ROS levels plays an important role in mediating killing of K562 cells.
Figure 2. Exposure to plumbagin leads to ROS-mediated apoptosis in K562 leukemia cells.
The effect of plumbagin exposure on the intracellular levels of ROS in K562 cells was determined by exposing carboxy-H2DCFDA-labeled cells to various concentrations of plumbagin (A). ROS levels were determined 1h later by flow cytometric analysis. The data depict difference in the levels of intracellular ROS in control (open histograms) versus plumbagin (closed histograms) treated cells from a representative experiment. The role of ROS in plumbagin-mediated apoptosis was assessed using NAC and PEG-catalase. The effect of NAC and PEG-catalase on plumbagin-induced cytotoxicity was determined by trypan blue dye exclusion (B). The data represent the mean ± SD. Asterisk denotes statistically significant difference (p<0.05) between vehicle (0 µM PLB) and plumbagin treated cells. Double asterisk denotes statistically significant difference (p<0.05) between PLB and PLB + NAC-treated cells or between PLB and PLB + PEG-catalase-treated cells. Apoptosis was determined by TUNEL staining (C). Percentages depicted in Figure 2 C represent the percentage of apoptotic cells.
Plumbagin exposure leads to increased expression of TRAIL receptors in human chronic myelogenous leukemia cells
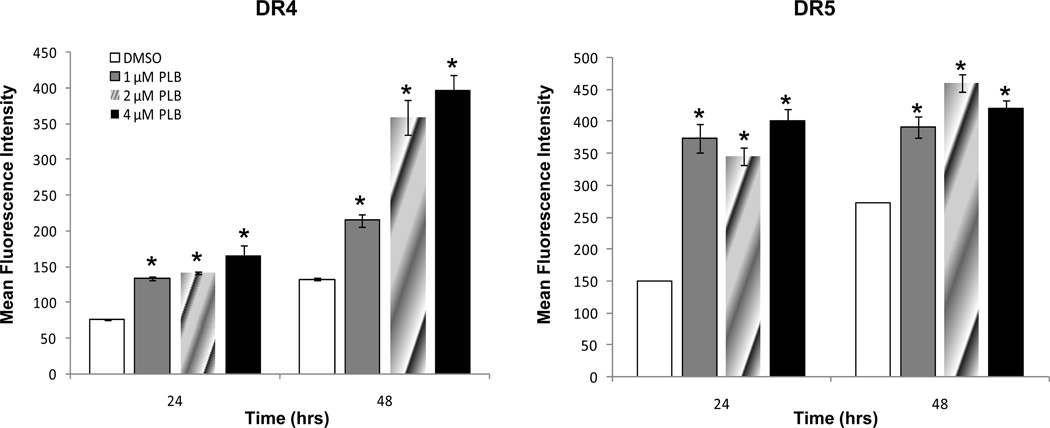
Approaches to increase TRAIL receptor-mediated tumor killing hold great promise. Therefore, experiments were conducted to determine whether plumbagin exposure had an effect on the expression of various death receptors in K562 cells. To this end, K562 cells were exposed for 24–48h to various concentrations of plumbagin (0, 1, 2, or 4 µM). Next the cells were harvested and the levels of DR4, and DR5 were determined using flow cytometric analysis (Figure 3). The results demonstrated that exposure of K562 cells to ≥ 1µM plumbagin led to a significant increase in the levels of DR4 and DR5 TRAIL receptors. In contrast, no significant alterations in the death receptor Fas were observed (data not shown). Together, these results suggest that plumbagin may be a unique regulator of TRAIL receptors DR4 and DR5 expression.
Figure 3. Plumbagin exposure leads to increased expression of TRAIL receptors in human chronic myelogenous leukemia cells.
The effect of plumbagin treatment on the expression of The TRAIL receptors, DR4 and DR5 was determined by exposing K562 cells to various concentrations of plumbagin for 24. Trail receptor expression was determined by flow cytometric analysis. The data represent the mean fluorescence intensity (MFI) from a representative experiment which was repeated at least three times with similar results.
NAC treatment prevents plumbagin induced increase in DR4 and DR5 expression in human chronic myelogenous leukemia cells
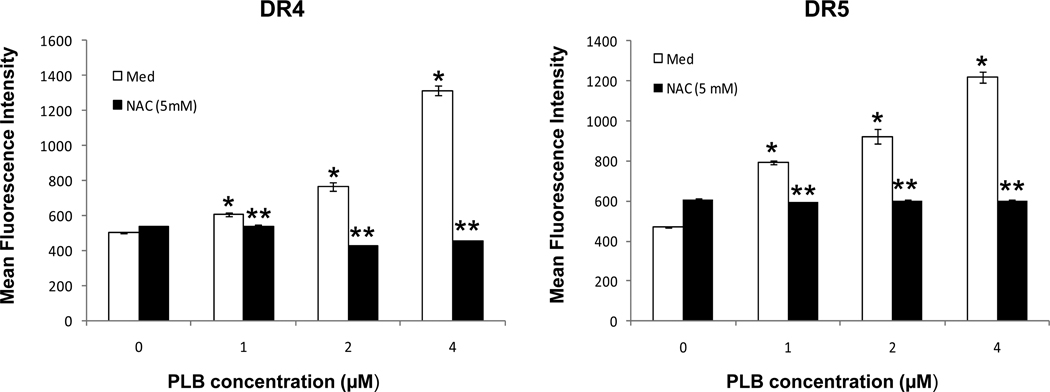
We examined whether the increased expression of DR4 and DR5 following plumbagin exposure were mediated by an increase in ROS. To this end, K562 cells were treated with or without NAC (5mM) and exposed to various concentration of plumbagin (0, 1.0, 2.0, or 4.0 µM). Following a 24h culture period the expression of DR4 and DR5 was determined by flow cytometric analysis (Figure 4). The results demonstrated that in the absence of NAC, plumbagin exposure led to a significant increase in DR4 and DR5 expression on K562 cells. However, if the cells were treated with NAC, the plumbagin-induced increase in DR4 and DR5 was inhibited. Together, these results suggest that plumbagin-mediated increase in DR4 and DR5 is ROS-dependent.
Figure 4. NAC treatment prevents plumbagin induced increase in DR4 and DR5 expression in human chronic myelogenous leukemia cells.
The role of ROS in the plumbagin-mediated increase in DR4 and DR5 expression was assessed using NAC. More specifically, K562 cells were cultured in medium or treated with NAC (5mM). The cells were exposed to various concentrations of plumbagin for 24h and the expression of DR4 and DR5 were determined by flow cytometric analysis. The data represent the mean fluorescence intensity (MFI) from a representative experiment which was repeated at least three times with similar results.
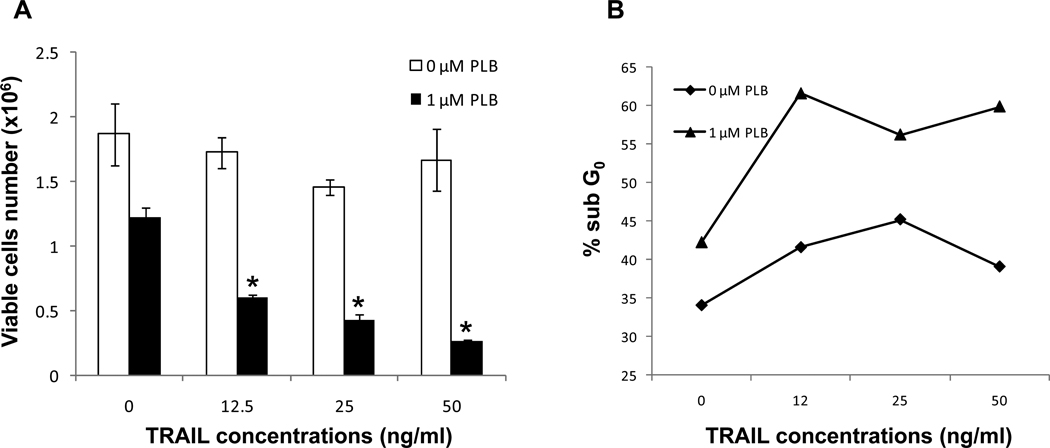
Plumbagin sensitizes human chronic myelogenous leukemia cells to TRAIL-induced apoptosis
Experiments were conducted to examine whether treatment with plumbagin led to increased sensitivity to TRAIL-mediated killing. To this end, K562 were treated with various concentrations of plumbagin (0 or 1 µM) in combination with various concentrations of soluble TRAIL (0–50 ng/ml). The tumor cells were harvested 24 hours later and the viable cell number and the induction of apoptosis were determined by Trypan blue dye exclusion (Figure 5A) and cell cycle analysis (Figure 5B), respectively. The results demonstrated that exposure of K562 cells to up to 50 ng/ml of soluble trail alone had little effect on cell viability or apoptosis. However, exposing K562 cell to soluble TRAIL at concentrations as low as 12.5 ng/ml, in combination with plumbagin (1 µM), led to a significant reduction in cell number and an increase in apoptosis. Together these results suggest that treatment with plumbagin may be a viable approach to increase the efficacy of TRAIL-mediated therapies for cancer.
Figure 5. Plumbagin sensitizes human chronic myelogenous leukemia cells to TRAIL-induced apoptosis.
The effect of plumbagin on soluble TRAIL-mediated apoptosis in K562 was examined. To this end, K562 were treated with various concentrations of plumbagin (0 or 1 µM) in combination with various concentrations of soluble TRAIL (0–50 ng/ml). The tumor cells were harvested 24 hours later and the viable cell number and the induction of apoptosis were determined by Trypan blue dye exclusion (Figure 5A) and cell cycle analysis (Figure 5B), respectively. The data represent the mean ± SD. Asterisk denotes statistically significant difference (p<0.05) between vehicle (0 µM PLB) and plumbagin treated cells. Cell cycle analysis data represent the percentage of apoptotic cells (Sub G0) from a representative experiment.
Discussion
A number of studies demonstrated the potential usefulness of plumbagin as an anti-cancer treatment. For example, dietary exposure of rats to plumbagin led to a significant decrease in the incidence in azoxymethane-induced intestinal carcinogenesis [26]. In a separate study, oral administration of plumbagin led to tumor regression in the 3-methyl-4-dimethyl aminoazobenzene model of hepatoma [27]. Furthermore, use of plumbagin was shown to be effective in the treatment of the sarcoma-180 solid tumor in BALB/c mice [28]. In addition, the potential use of plumbagin to treat hematologic malignancies has received some attention. For example, plumbagin treatment was effective at inducing apoptosis in human promyelocytic leukemia cells and well as various human myeloma cell lines [29]. In the current study we examined the potential use of plumbagin to treat chronic myelogenous leukemia. More specifically, we demonstrated that low doses of plumbagin were highly active at killing the K562 cell line. Previous studies examining the effect of plumbagin showed an effect at concentration ranging from an IC50 of less than 5 µM in certain gynecological malignances to an IC50 of more than 14 µM in lung cancer and melanoma. Here we demonstrated that plumbagin, at doses as low as 2 µM, led to significant reduction in K562 tumor cell proliferation and viability due to the induction of apoptosis, suggesting that leukemias such as CML might by highly sensitive to plumbagin treatment.
We propose that plumbagin exposure induces apoptosis through an increase in ROS. This was directly evidenced by our studies that demonstrated that plumbagin exposure led to increase ROS levels in K562 cells and indirectly in experiments that showed that treatment with NAC or PEG-catalase prevented plumbagin-mediated apoptosis. Other reports support our finding of ROS involvement in killing of leukemia cells. For example, plumbagin induced DNA damage in HL-60 was prevented by pretreatment with NAC [30]. Similar results were seen in plumbagin induced apoptosis in the NB4 human promylocytic leukemia cells where pretreatment of NB4 cells with NAC completely blocked plumbagin-induced apoptosis [25]. Furthermore, our results demonstrating a protective role of PEG-catalase suggest a role of H2O2 in plumbagin-mediated cell death. This is supported by a previous study in which catalase protected a human melanoma cell line from plumbagin-induced apoptosis [24].
There is increasing evidence to suggest that some naphthoquinones including plumbagin may act via an influence on the regulation of transcriptions factors. For example, PLB-exposure has been reported to lead to the suppression of NF-κB activation in a number of human tumor cell lines including the chronic myeloid leukemia, KBM-5, the human multiple myeloma, U266, the lung adenocarcinoma, H1299, the embryonic kidney carcinoma A293 and the squamous cell carcinoma, SCC-4 [31]. In addition, a study examining the effect of plumbagin treatment in human nonsmall cell lung cancer revealed that plumbagin exposure led to the induction of apoptosis through alterations in p53 activity [19]. Interestingly, leukemias have been shown to have alterations in many of these transcriptions factors, including NF-κB, JAK/STAT, PI3K/Akt and Ras [32, 33]. Therefore compounds, such as plumbagin, that target the expression and/or activity of these transcription factors may have a significant impact on the growth and/or survival of various leukemias.
In the current study we demonstrate that exposure of the human chronic myelogenous leukemia cell line K562 to plumbagin led to the induction of apoptosis. Exposure to plumbagin alone at dose of ≥ 2 µM led to cell killing through ROS-dependent apoptosis. However, elevated levels of ROS were detected at plumbagin levels as low as 1µM. In a recent study we demonstrated an effect of plumbagin on lymphocyte expression of death receptors. Specifically, we demonstrated an effect of plumbagin on lymphocyte expression of the Fas death receptor while no effects on TRAIL receptors were noted [22]. In an attempt to determine the possible involvement of death receptors in the killing of K562 by plumbagin we examined the expression of various death receptors including Fas, DR4 and DR5 following exposure to plumbagin. The results showed that exposure to sub-lethal levels of plumbagin significantly increased cell surface expression of DR4 and DR5. Furthermore, we demonstrated that the plumbagin-mediated increase in DR4 and DR5 increased the sensitivity of K562 cells to TRAIL-mediated killing. Therefore, use of TRAIL for the treatment of various tumors including CML has significant potential and treatments that can selectively sensitize tumors to TRAIL-induced apoptosis may lead to significantly improved treatments for CML.
The mechanism by which plumbagin leads to increased TRAIL receptor expression remains unclear. However our studies suggest that plumbagin exposure increases the sensitivity of CML to TRAIL-induced apoptosis through an ROS-mediated increase in DR4 and DR5 expression. This potential mechanism of tumor killing by plumbagin is further supported by a recent studies demonstrating the involvement of ROS in plumbagin-mediated killing of human promyelocytic leukemia and a study demonstrating that plumbagin enhanced killing of the human melanoma cell line A375 by upregulation of DR4 and DR5 [25, 34]. Furthermore, studies demonstrate that increase oxidative stress can directly affect tumor cell expression of TRAIL receptors. For example, hydrogen peroxide treatment led to increased DR5 in human astrocytic cells. In this study the ROS-mediated increase in DR5 was associated with an increase in NF-ΚB activity. In a second study, treatment of human colon cancer cells with zerumbone led to a ROS-mediated increase in DR4 and DR5 [16, 35]. Mechanistically, the zerumbone-mediated increase in DR4 and DR5 was linked to ROS-dependent increase in ERK1/2 and p38 activity. Together, with our studies, these results suggest that ROS plays an important role in plumbagin-mediated increase in TRAIL receptor expression. Additional, studies examining the possible role of NF-ΚB and MAPK pathways in plumbagin-mediated increase in TRAIL receptor expression are currently underway in our laboratory and should help to further define plumbagin’s mechanism of action.
Many of the current treatments for CML are designed to induce apoptosis. Unfortunately, many cancer cells are/or become resistant to the activity of these treatments. Signaling through death receptors plays an important role in the chemotherapy-induced apoptosis in a variety of tumor cells, including CML. In the current study we show that treatment with the natural plant product, plumbagin, leads to increased apoptosis as well as increased expression of DR4 and DR5 in the human K562 chronic myelogenous leukemia cell line. Therefore, it may be possible that plumbagin treatment can increase the sensitivity of a number of hematologic malignancies to chemotherapeutic as well as immunotherapeutic treatments. If successful, these findings may lead to new treatment regimens that include compounds designed to increase the sensitivity of tumor cells to current and future treatments by increasing the cancer cell’s expression of death receptors.
Acknowledgements
We thank Gabriela Paige Law for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
R.J.M was responsible for study design, experiment design, interpretation of the data, and writing of the manuscript. J.S. was responsible for conducting experiments and for analysis of data.
Conflicts of interest
The authors have no conflicts of interest to report
References
- 1.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996 May 31;271(22):12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 2.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995 Dec;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.El-Deiry WS. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 2001 Nov;8(11):1066–1075. doi: 10.1038/sj.cdd.4400943. [DOI] [PubMed] [Google Scholar]
- 4.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 5.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 6.Lowe SW, Ruley HE. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993 Apr;7(4):535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 7.Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, et al. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 8.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005 Feb;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Hylander BL, Baer MR, Chen X, Repasky EA. Multiple mechanisms underlie resistance of leukemia cells to Apo2 Ligand/TRAIL. Mol Cancer Ther. 2006 Jul;5(7):1844–1853. doi: 10.1158/1535-7163.MCT-06-0050. [DOI] [PubMed] [Google Scholar]
- 10.Kim MJ, Kim HB, Bae JH, Lee JW, Park SJ, Kim DW, et al. Sensitization of human K562 leukemic cells to TRAIL-induced apoptosis by inhibiting the DNA-PKcs/Akt-mediated cell survival pathway. Biochem Pharmacol. 2009 Sep 15;78(6):573–582. doi: 10.1016/j.bcp.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 11.MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, et al. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002 Oct 3;21(44):6809–6818. doi: 10.1038/sj.onc.1205853. [DOI] [PubMed] [Google Scholar]
- 12.Riccioni R, Pasquini L, Mariani G, Saulle E, Rossini A, Diverio D, et al. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica. 2005 May;90(5):612–624. [PubMed] [Google Scholar]
- 13.Hao XS, Hao JH, Liu FT, Newland AC, Jia L. Potential mechanisms of leukemia cell resistance to TRAIL-induced apopotosis. Apoptosis. 2003 Dec;8(6):601–607. doi: 10.1023/A:1026131425204. [DOI] [PubMed] [Google Scholar]
- 14.Testa U. TRAIL/TRAIL-R in hematologic malignancies. J Cell Biochem. 2010 May;110(1):21–34. doi: 10.1002/jcb.22549. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, Tai CJ, Cheng JT, Zheng JJ, Chen YZ, Liu TZ, et al. 6-dehydrogingerdione sensitizes human hepatoblastoma Hep G2 cells to TRAIL-induced apoptosis via reactive oxygen species-mediated increase of DR5. J Agric Food Chem. 2010 May 12;58(9):5604–5611. doi: 10.1021/jf904260b. [DOI] [PubMed] [Google Scholar]
- 16.Kwon D, Choi K, Choi C, Benveniste EN. Hydrogen peroxide enhances TRAIL-induced cell death through up-regulation of DR5 in human astrocytic cells. Biochem Biophys Res Commun. 2008 Aug 8;372(4):870–874. doi: 10.1016/j.bbrc.2008.05.148. [DOI] [PubMed] [Google Scholar]
- 17.Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010 Apr 9;285(15):11498–11507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem Res Toxicol. 2004 Jan;17(1):55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- 19.Hsu YL, Cho CY, Kuo PL, Huang YT, Lin CC. Plumbagin induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via JNK-mediated phosphorylation at Serine 15 in vitro and in vivo. J Pharmacol Exp Ther. 2006 Apr 21; doi: 10.1124/jpet.105.098863. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal AS, Bloom LB, Narayan S. Long-patch base excision repair of apurinic/apyrimidinic site DNA is decreased in mouse embryonic fibroblast cell lines treated with plumbagin: involvement of cyclin-dependent kinase inhibitor p21Waf-1/Cip-1. Oncogene. 2002 Aug 29;21(38):5912–5922. doi: 10.1038/sj.onc.1205789. [DOI] [PubMed] [Google Scholar]
- 21.Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog. 2004 Aug;40(4):201–211. doi: 10.1002/mc.20031. [DOI] [PubMed] [Google Scholar]
- 22.McKallip RJ, Lombard C, Sun J, Ramakrishnan R. Plumbagin-induced apoptosis in lymphocytes is mediated through increased reactive oxygen species production, upregulation of Fas, and activation of the caspase cascade. Toxicol Appl Pharmacol. 2010 Aug 15;247(1):41–52. doi: 10.1016/j.taap.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res. 2008 Sep;25(9):2171–2180. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008 Jan 18;259(1):82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Xu KH, Lu DP. Plumbagin induces ROS-mediated apoptosis in human promyelocytic leukemia cells in vivo. Leuk Res. 2009 May;34(5):658–665. doi: 10.1016/j.leukres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Sugie S, Okamoto K, Rahman KM, Tanaka T, Kawai K, Yamahara J, et al. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998 May 15;127(1–2):177–183. doi: 10.1016/s0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 27.Parimala R, Sachdanandam P. Effect of Plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol Cell Biochem. 1993 Aug 11;125(1):59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- 28.Naresh RA, Udupa N, Devi PU. Niosomal plumbagin with reduced toxicity and improved anticancer activity in BALB/C mice. J Pharm Pharmacol. 1996 Nov;48(11):1128–1132. doi: 10.1111/j.2042-7158.1996.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 29.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2010 Nov 9; doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- 30.Kawiak A, Piosik J, Stasilojc G, Gwizdek-Wisniewska A, Marczak L, Stobiecki M, et al. Induction of apoptosis by plumbagin through reactive oxygen species-mediated inhibition of topoisomerase II. Toxicol Appl Pharmacol. 2007 Sep 15;223(3):267–276. doi: 10.1016/j.taap.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Suppresses NF-{kappa}B Activation and NF-{kappa}B-regulated Gene Products Through Modulation of p65 and I{kappa}B{alpha} Kinase Activation, Leading to Potentiation of Apoptosis Induced by Cytokine and Chemotherapeutic Agents. J Biol Chem. 2006 Jun 23;281(25):17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 32.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008 Apr;22(4):708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 33.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008 Arp;22(4):686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Shen Q, Peng R, Chen R, Jiang P, Li Li, et al. Plumbagin enhances TRAIL-mediated apoptosis through up-regulation of death receptor in human melanoma A375 cells. J Huazhong Univ Sci Technolog Med Sci. 2010 Aug;30(4):458–463. doi: 10.1007/s11596-010-0449-x. [DOI] [PubMed] [Google Scholar]
- 35.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009 Aug 15;69(16):6581–6589. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]