Abstract
Introduction
Human orofacial bone mesenchymal stem cells (OFMSCs) from maxilla and mandible have robust osteogenic regenerative properties based on our previous reports that demonstrate phenotypic and functional differences between jaw and axial bone mesenchymal stem cells in same individuals. Furthermore, a combination of OFMSCs with bioactive calcium-releasing cements can potentially improve OFMSC multi-lineage differentiation capacity, but biocompatibility of calcium silicate cements with OFMSCs is still unclear. We tested the hypothesis that material extracts of calcium-releasing calcium-silicate cements support biomimetic microenvironment for survival and differentiation of human OFMSCs.
Methods
Two experimental calcium-silicate cements 1) calcium-silicate mineral powder (wTC) containing di- and tricalcium-silicate, calcium sulphate, and calcium chloride and 2) wTC doped with alpha-tricalcium phosphate (wTC-αTCP) were designed and prepared. Cement setting times were assessed by Gilmore needles, ability to release calcium and hydroxyl ions was assessed by potentiometric methods and OFMSC attachment to calcium-silicate discs was assessed. Calcium-silicate material extracts were tested for ability to support OFMSCs survival and in vitro/in vivo differentiation.
Results
Fewer OFMSCs attached to calcium-silicate discs relative to tissue culture plastic (p=0.001). Extracts of calcium-silicate cements sustained OFMSC survival, maintained steady state levels of vascular cell adhesion molecule-1, alkaline phosphatase and bone sialoprotein while upregulating their respective gene transcripts. Adipogenic and in vivo bone regenerative capacities of OFMSCs were also unaffected by calcium-silicate extracts.
Conclusions
Ion-releasing calcium-silicate cements support a biomimetic microenvironment conducive to survival and differentiation of OFMSCs. Combination of OFMSCs and calcium-silicate cement can potentially promote tissue regeneration in periapical bone defects.
Keywords: orofacial stem cells, calcium-silicate, alpha-tricalcium phosphate, endodontic, bone cements, bone regeneration
Introduction
Orofacial bone mesenchymal stem cells (OFMSCs) from maxilla and mandible are postnatal mesenchymal stem cells with inherently higher survival and osteogenic properties than bone mesenchymal stem cells (BMSCs) from non-oral bones (1, 2). We have previously shown that in same individuals, BMSCs in the jaw (i.e. OFMSCs) are skeletally site-specific based on proliferation, survival, differentiation and in vivo regenerative properties (1–3). A combination of the biology of the local microenvironment and circulating levels of soluble calcium and inorganic phosphates have been correlated with bone regeneration(4, 5); therefore calcium-silicate cements also known as Mineral Trioxide Aggregates (MTA) cements are used clinically in oral surgery and root canal therapy. Calcium-silicate cements have attractive chemical, physical and biological properties that include ability to set in the presence of moisture, the release of calcium ions, and ability to form apatite in the presence of phosphate-containing simulated-body fluids (6–11).
The bioactive properties of calcium-silicate cements combined with bio-interactivity (12) with local microenvironment may be conducive to cell survival and bone regeneration (13), but there is paucity of information on effects of calcium-silicate cements on jaw specific mesenchymal stem cells (14). Alpha-tricalcium phosphate (α-TCP) is a reactive compound that can serve as a source of phosphate (15) and calcium ions (16) for apatite formation (17) and tissue regeneration. Although calcium-silicate cements are more resorbable than hydroxyapatite-tricalcium phosphate commonly used to promote bone regeneration, they can potentially stimulate appropriate biomimetic microenvironments for OFMSC differentiation because they have been shown to promote attachment and proliferation of osteoblast-like cells and dental pulp stem cells (DPSCs) (5, 18). DPSCs share similar embryological origin with OFMSCs (1, 19) but OFMSCs display higher osteogenic differentiation (2). A combination of superior osteogenesis of OFMSCs with biomimetic properties of calcium-silicate cements is attractive for bone regeneration, but biocompatibility of calcium-silicate material extracts with survival and regenerative properties of OFMSCs are still unclear.
The aim of this study was to determine the biocompatibility of OFMSCs with biomimetic properties of two calcium-silicate preparations: 1) calcium-silicate mineral powder (wTC) containing di- and tricalcium-silicate, calcium sulphate and calcium chloride; and 2) wTC doped with alpha-tricalcium phosphate (wTC-αTCP). We hypothesized that calcium ions and other minerals (silicon and phosphorous) in material extracts released by calcium-silicate cements will provide supportive biomimetic microenvironment for survival and differentiation of human OFMSCs.
Materials and Methods
Preparation of calcium silicate cements
Experimental calcium-silicate mineral powder (wTC) containing di- and tricalcium-silicate, calcium sulphate, calcium chloride; and another wTC doped with alpha-tricalcium phosphate (wTC-αTCP) were designed and produced. The grinded mineral powders were mixed using 1 g of cement per 0.300 ml of calcium and magnesium free Dulbecco s Phosphate Buffered Saline (DPBS) pH 7.4 (Lonza Walkersville Inc, Walkersville, MD, USA, Cat #17–512) on a glass plate with stainless-steel spatula. A homogeneous paste was achieved within 30 seconds and packed into a circular polyvinyl chloride (PVC) mold (8 mm internal diameter and 1.6 mm thickness) to obtain a smooth surface.
Setting times of cements
Calcium-silicate cement (wTC and wTC-αTCP) discs covered on upper and lower surfaces with wet gauze to prevent dehydration were placed in a hermetically sealed curing chamber (37°C and 98% relative humidity) before evaluating setting times. Gilmore needles were used to assess setting times according to ASTM C266-03 (American Society for Testing and Materials International) and ISO 9917-1 (International Organization for Standardization) (20, 21). Briefly described, Gilmore initial setting time was the elapsed time (in minutes) between the mixing of the cement with liquid and the first penetration measurement that does not mark the specimen surface with a complete circular impression. As either the initial or final setting times approached (i.e. no indentation), the specimens were tested every minute to determine the exact Gilmore setting time. Following initial setting time, the specimens were assessed every 5 minutes until final stetting time was determined. Weight/diameter of Gilmore needle used were 113.4 g/2.12 mm and 453.6-g/1.06 mm to test initial and final setting times respectively (20). Setting time testing was performed on three replicates for each material and each sample was used for only one indentation test.
Calcium release and alkalinizing activity of cements
Calcium-silicate cement (wTC and wTC-αTCP) discs (n=10 for each material) were immediately immersed in 10 ml of deionized water (pH 6.8) in polypropylene sealed containers at 37°C. Soaking water was collected at 3 and 24 hours and after 7, 14 and 28 days to assess pH and calcium content. Calcium released in soaking water was measured using a calcium probe (Calcium ion electrode, Eutech instruments Pte Ldt, Singapore) after addition of 0.200 ml (2%) of ionic strength adjuster (ISA, 4 mol/L KCl, WTW, Weilheim, Germany). The pH of soaking water was measured using a selective temperature compensated electrode (Sen Tix Sur WTW, Weilheim, Germany) and a previously calibrated multi-parameter laboratory meter (inoLab 750, WTW Weilheim, Germany). The probes were inserted into the soaking media at room temperature (24°C) with continuous stirring. Each measurement was repeated three times.
Calcium-silicate disc and extract preparations for cell culture
Calcium-silicate cement (wTC and wTC-αTCP) discs for OFMSC culture were prepared as above using a circular PVC mold (12 mm internal diameter and 1.6 mm thickness). To obtain partial setting, cements were kept for 1 hour at 37°C and 95% relative humidity to allow for hydration and setting reactions. Cements discs were de-molded and sterilized for 2 hours in antibiotic medium consisting of α-modified minimum essential medium (α-MEM), 100 U/ml penicillin, 100 mg/ml streptomycin sulfate and 25 μg/ml amphotericin B. Discs were washed free of antibiotic medium with sterile PBS before using for cell attachment assay. Cement discs prepared in the similar manner as above were allowed to set for 1 hour at room temperature instead of 37°C, transferred to a 24-well culture plate and sterilized with antibiotic medium as above. Discs were incubated in 1.5 ml fresh α-MEM containing 100 U/ml penicillin, 100 mg/ml streptomycin sulfate and 2 mM glutamine at 37°C in humidified atmosphere for 24 hours to allow for release of calcium and silicate ions into the medium. The supernatant collected was referred to as material extract. wTC and wTC-αTCP material extracts were passed through separate 0.22 μm filters, pH recorded with colorimetric strips, and kept at 4°C until used for cell culture.
Culture of orofacial bone mesenchymal stem cells
Human OFMSCs were expanded from primary cultures of human maxilla and mandible primary bone mesenchymal stem cells previously isolated under an existing protocol approved by the University of Pennsylvania Institutional Review Board. The cells were cultured in growth medium consisting of α-MEM supplemented with 10% fetal bovine serum (FBS) (Equitech Bio Inc., Kerville TX), 100 U/ml penicillin, 100 mg/ml streptomycin sulfate and 2 mM glutamine (BioSource International Camarillo CA), incubated at 37ºC in a humidified atmosphere of 5% CO2 and air as previously described (1). Passage 3 OFMSCs were used for all experiments.
Attachment of OFMSCs to calcium-silicate discs
Sterile 12 mm diameter wTC and wTC-αTCP discs prepared as above were transferred into separate wells of a 24-well culture plate containing α-MEM growth medium. 2.0 × 104 OFMSCs were seeded on triplicate wTC and wTC-αTCP discs and in blank wells so that tissue culture plastic served as control. After 24 hours, the discs were transferred to another 24-well plate. Attached cells were released with trypsin/EDTA and cell scraper before counting with a hemocytometer.
OFMSC survival in calcium-silicate extracts
OFMSCs were seeded in 96-well plate at 3.2 × 104 cells per well. After 24 hours, α-MEM growth medium was changed so that three sets of 11 wells received extract of wTC or wTC-αTCP or fresh α-MEM growth medium. After another 72 hours in culture, surviving cells were assessed using colorimetric WST-1 cell proliferation assay (Roche Applied Science, Indianapolis, IN, USA) according to manufacturer s instructions.
Lineage differentiation of OFMSCs in calcium-silicate extracts
Osteogenesis
OFMSCs seeded at 9.5 × 104 cells per well in duplicate wells of a 6-well plate were kept in culture for 24 hours before exposure to either wTC or wTC- αTCP material extract prepared as above. Control cells received fresh α-MEM growth medium. After 48 hours, the cells were replenished with osteogenic medium consisting of α-MEM, 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin sulfate, 2 mM glutamine, 10−8 M dexamethasone and 100 μM L-ascorbic acid phosphate magnesium-hydrate. After another 48 hours, total protein was collected using Mammalian Protein Extraction Reagent (M-PER, Pierce Biotechnology, Rockford, IL). In a similar set up of parallel experiment, total RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA).
Western blotting
Protein amount was determined with BCA protein assay (Pierce Biotechnology, Rockford, IL). 50 μg protein was separated in 4–12% Bis–Tris gel (Invitrogen, Carlsbad, CA) under reducing conditions and western blotting was performed as previously described (3). Membranes were probed with the following primary antibodies: 1:200 dilution of mouse monoclonal anti-vascular cell adhesion marker-1 (VCAM-1, ab19264, AbCam, Cambridge MA), 1:100 dilution of mouse monoclonal anti-bone sialoprotein (BSP, LFMb-25, kindly provided by Dr. Larry Fisher, National Institute of Dental and Craniofacial Research/National Institutes of Health [NIDCR/NIH], Bethesda MD) and 1:200 rabbit anti-alkaline phosphatase (ALP, LF47, gift from Dr. Larry Fisher, NIDCR/NIH). Mouse anti-α-tubulin (12G10, Developmental Studies Hybridoma Bank, Iowa City, IA) at 1:200 dilution served as protein loading control. After probing with 1:5000–1:10000 dilution of specie-specific secondary antibody conjugated to horseradish peroxidase, the immunoreactive bands were detected with chemiluminescent reagents (GE Healthcare, UK) and analyzed digitally with Kodak Image Station 4000MM (Molecular Imaging Systems, Carestream Health, Rochester, NY). Relative abundance of adhesion and osteogenic markers were normalized to α-tubulin.
Real time-PCR
cDNA was prepared from 2 mg of total RNA using oligo(dT) and SuperScript First-Strand Synthesis System for reverse transcriptase PCR (Invitrogen, Carlsbad, CA). Real-time PCR reaction was performed in ABI 7300 Real-Time PCR System using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and primer sets designed with Primer Express software (Applied Biosystems, Foster City, CA) and GenBank sequences for: human VCAM-1 - forward, 5′-GAAGGAATTAACCAGGCTGGAA-3′, reverse, 5′-AGTTTTATGTCTTTTGGAGTAACTTG-3′; human ALP - forward, 5′-CCGTGGCAACTCTATCTTTGG-3′, reverse, 5′-GATGGCAGTGAAGGGCTTCTT-3′; human BSP - forward, 5′-AACGAAGAAAGCGAAGCAGAA-3′, reverse, 5′-TCTGCCTCTGTGCTGTTGGT-3′ and human TATA binding protein (TBP) - forward, 5′-GGAGCTGTGATGTGAAGTTTCCTA-3′, reverse, 5′-CCAGGAAATAACTCTGGCTCATAAC-3′ used as endogenous control. A negative PCR control without template was included in each assay. Gene expression levels normalized to TBP and presented as relative fold change using the ΔΔCt method (Applied Biosystems, Foster City, CA) was used to quantify changes in mRNA levels in response to material extracts.
Adipogenesis
2 × 104 OFMSCs plated in duplicate 60 mm dishes were treated with material extracts as above. They were further cultured for 4 weeks in adipogenic medium consisting of α-MEM supplemented with 10−8 M dexamethasone, insulin (1 μg/ml), 1-methyl-3-isobutylxanthine (IBMX, 5 × 10−8 M) and indomethacin (10−4 M); medium was changed twice weekly. The cells were fixed with 4% paraformaldehyde, stained with 0.3% Oil Red O and counterstained with 1% Fast green dye. Lipid droplets were assessed microscopically.
In vivo bone regeneration by OFMSCs exposed to calcium-silicate extracts
Using both extract-exposed and control OFMSCs, 2 × 106 cells attached to 40 mg of spheroidal hydroxyapatite tricalcium phosphate (particle size 0.5–1.0 mm; Zimmer, Warsaw, IN, USA) were transplanted aseptically into separate subcutaneous pockets of 8-week-old immunocompromised female nude mice (NIH-III-nu; Charles River Laboratories, Wilmington, MA, USA) as previously described (1, 3) and in accordance with animal protocol approved by University of Pennsylvania Institutional Animal Care and Use Committee. Transplants were harvested at 6 weeks, fixed with 4% paraformaldehyde, decalcified in 10% ethylenediaminetetraacetic acid (EDTA) (pH 8.0) and embedded in paraffin. Hematoxylin eosin-stained 5 μm sections were evaluated and quantified microscopically as previously described (1, 3).
Statistical analysis
Results were expressed as mean ± standard deviation (SD). Differences between groups were analysed with one-way analysis of variance (ANOVA) followed by post hoc comparisons with Tukey-Kramer test using SigmaStat 3.1, Systat Software, Inc., Chicago, IL). Statistical significance was set at P < 0.05.
Results
Setting reaction initiated earlier and hardness was completed faster in wTC-αTCP discs relative to wTC (Table 1). Similarly, release of calcium ions in unset freshly prepared cement was also faster in wTC-αTCP within the first 3 hours, but wTC displayed a more gradual calcium release within the first 24 hours in spite of relatively similar pH of soaking solution (Table 2). Discs formed from partially and fully set wTC and wTC-αTCP cements maintained their rigidity and shape irrespective of setting parameters (Figure 1A). Attachment of OFMSCs to cement discs was much lower than tissue culture plastic (p =0.001) but wTC discs apparently supported slightly more cell attachment than wTC-αTCP (Figure 1B). Exposure of sub-confluent monolayer of OFMSCs to cement extracts reduced cell-to-cell contact and plastic adherence (Figures 1C–E). There were evidences of cement sediments and free floating cells in the wTC and wTC-αTCP culture environments while control cells in α-MEM continued proliferation to 100% cell confluence (Figures 1C–E). Despite the sub-optimal culture conditions in material extracts, OFMSCs exposed to cement extracts displayed 80–98% survival relative to control cells in α-MEM but there was no statistically significant difference between wTC and wTC-αTCP extracts (p = 0.07) (Figure 1F). OFMSC levels of VCAM-1, a marker of cell attachment was unaffected by cement extracts based on western blot analysis (Figures 2A and B), but mRNA expression was upregulated as shown by real time PCR (Figure 2C). Similarly, protein levels of ALP and BSP remained at baseline level in OFMSCs exposed to cement extracts (Figure 2B) but mRNA levels of ALP and not BSP were distinctly upregulated by extracts of both wTC and wTC-αTCP (Figure 2C). There was no significant difference between wTC and wTC-αTCP. Interestingly, aggregates of fat droplets were fewer numerically but structurally larger in OFMCs pre-treated with material extracts relative to control cells (Figures 3A–C). Similarly OFMSCs pre-treated with material extracts before transplantation into immunocompromised mice regenerated appreciable in vivo bone structurally (Figures 3D–F) and quantitatively (Figure 3G) similar to those of control cells.
Table 1. Duration of setting reactions of calcium-silicate cements.
Initiation and completion of cement setting reaction
| Setting reaction (minutes) | ||
|---|---|---|
| Initiation | Completion | |
| wTC | 72.0 ± 4.1 | 135.5 ± 7.9 |
| wTC-TCP | 47.6 ± 5.6 | 79.3 ± 7.9 |
n =10 samples tested per cement type
Table 2. Quantity of calcium ions released by calcium-silicate cements and pH of soaking water.
Release of calicum and pH of soaking solution
| Calcium release (ppm)* | ||||
|---|---|---|---|---|
| 3 hours | 1 day | 3 days | 7 days | |
| wTC | 250 2 ± 12.1 | 129 2 ± 14 1 | 120.6 ± 3.5 | 19.8 ± 3.0 |
| wTC-TCP | 368.9 ± 9.6 | 88.8 ± 4.2 | 14.2 ± 4.7 | 16.2 ± 2.9 |
| pH soaking solution | ||||
| wTC | 12.0 ± 0.1 | 11.3 ± 0.4 | 10.2 ± 0.3 | 9.6 ± 0.3 |
| wTC-TCP | 11.6 ± 9.6 | 11.2 ± 0.4 | 9.6 ± 0.4 | 9.1 ± 03 |
n =10 samples tested per cement type
ppm = parts per million
Figure 1.
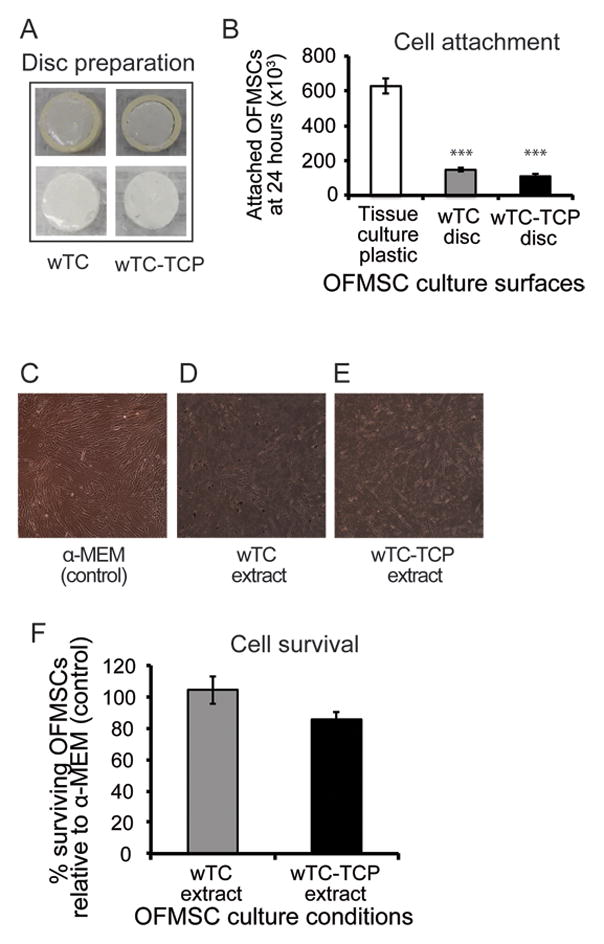
Effect of calcium silicate cements on attachment and survival of OFMSCs. Representative images of calcium silicate cements within the PVC mold (A, top panel) and after recovery from the mold (A, lower panel) indicate rigidity of the calcium silicate discs was maintained irrespective of setting parameters. OFMSC attachment to silicate discs was significantly lower (p = 0.001) than cells attached to tissue culture plastic (B). Plastic adherence in α-MEM culture medium (C) was reduced when OFMSCs were introduced to cement extracts (D, E) as demonstrated by floating cells and partial loss of cell-to-cell contact. Metabolically active cells in calcium silicate extracts and α-MEM were similar based on WST-1 survival assay (F) and there were no significant differences between surviving cells in wTC and wTC-αTCP extracts (p = 0.074). [*** = p ≤ 0.001].
Figure 2.
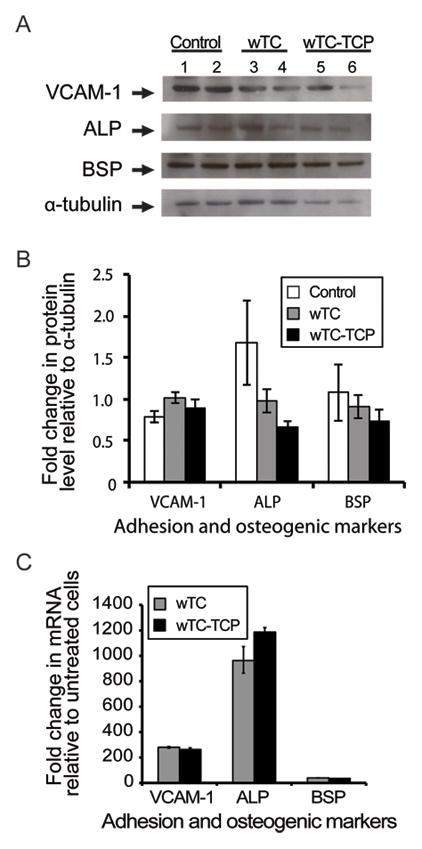
Effects of calcium silicate cements on in vitro differentiation of OFMSCs.
Representative Western blot bands of control (α-MEM), wTC and wTC-αTCP extract-treated OFMSCs proteins showed immunoreativity to vascular cell adhesion marker-1 (VCAM-1), alkaline phosphatase (ALP), bone sialoprotein (BSP) and α-tubulin [protein loading control] (A). Densitometric analysis of immunoreactive bands (B) showed minimal changes in VCAM-1 and BSP levels relative to control cells but ALP level was slightly more reduced in OFMCS exposed to wTC-αTCP extracts. Real time PCR amplification of specific primers for the same markers demonstrated significant upregulation of their gene transcripts with ALP > VCAM-1 > BSP (C) [PCR data normalized to non-osteogenic controls and TATA binding protein as housekeeping gene]. There were no significant differences between the two cement types.
Figure 3.
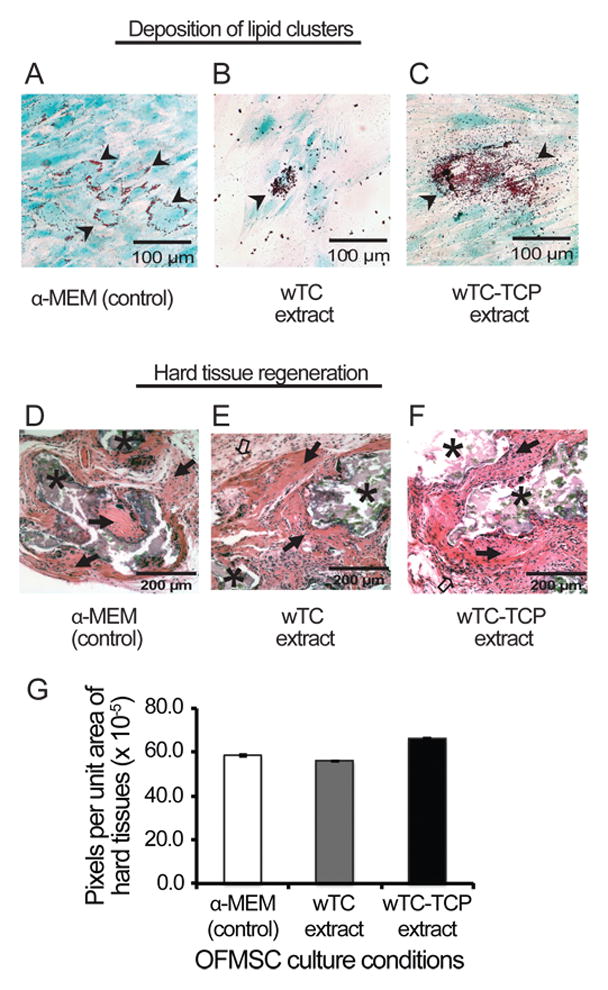
Effect of calcium silicate cements on adipogenesis and in vivo bone regeneration. Lipid aggregates (black arrow heads) were randomly distributed around majority of α-MEM-cultured (control) OFMSCs (A), while wTC and wTC-αTCP extract-treated cells displayed fewer but structurally larger lipid aggregates (B, C) than control cells. Similarly OFMSCs pre-treated with material extracts regenerated appreciable in vivo bone (black solid arrows) structurally similar to those of control cells (D, E, F); and there were no differences in amount of bone regenerated by extract-treated and α-MEM-treated OFMSCs (G) [* = hydroxyapatite carrier; clear arrow = fibrous tissues].
Discussion
Large periapical bone defects pose a surgical challenge in endodontic practice. They often require the use of bioactive materials like calcium silicate to promote apical bone healing (22–24). Calcium-silicate cements release calcium ions and the presence of Si-OH groups triggers the formation of a layer of apatite on the cement surface (6). Both wTC and wTC-αTCP calcium-silicate cements tested in this study set rapidly and released appreciably high calcium ions within the first 24 hours of fluid exposure. The ion-releasing effect that gradually tapered down within 7 days can therefore counteract potential tissue accumulation and cytotoxicity. Calcium and silicon ions released from bioactive glasses have been shown to stimulate osteoblast proliferation and gene expression (25–27). It is possible that calcium ion-releasing ability of cements tested in this study also favor OFMSC differentiation and jaw bone healing (28). We found that OFMSCs attachment to cements was low relative to tissue culture plastic, but they maintained proliferation and survival in the calcium-rich extract generated by the cements. While both types of calcium-silicate cement set rapidly and maintained their rigidity to allow for cell attachment, the reduced OFMSC attachment observed is similar to previously described attachment to oxidized titanium commonly used to promote osseointegration of dental implants (19). The relatively non-suppressible protein levels of VCAM-1, ALP and BSP and upregulation of their respective transcripts support ability of OFMSCs to differentiate in a calcium rich extract. Ability to induce adipogenesis and in vivo bone formation despite pre-treatment with cement extracts also support previous reports that osteoprogenitor cells respond to critical concentrations of calcium and silicate released by fresh cement surfaces (14, 28) and sol-gel bioactive glasses (29). In spite of reduced OFMSC attachment and survival, the residual OFMSCs demonstrated enhanced adipogenesis and comparatively similar in vivo bone formation as control cells. Therefore, gradual release of calcium and silicate ions from calcium-silicate cements may represent another stimulatory signal for OFMSC differentiation. Apart from larger aggregates of lipid droplets, there were no significant differences between wTC and wTC-αTCP on OFMSCs differentiation. Previous reports have demonstrated that different α-TCP doped calcium-silicate cements have enhanced bioactivity i.e. faster/higher apatite-forming ability (30, 31) but they also slightly reduced the proliferation of human BMSCs seeded on their surface (13).
Calcium-silicate cements have been proposed as root-end filling materials and root-canal sealers in endodontic therapy (23). Specifically, wTC and wTC-αTCP formulations can be used in contact with bone to seal defects and apices of resected teeth after apicoectomy; hence they can promote periapical bone regeneration and healing. Our study indicates the compatibility of wTC and wTC-αTCP calcium-silicate cements with OFMSCs, so if cements leach into surrounding bone, survival of resident osteoprogenitor cells will be relatively unaffected. Although calcium-silicate is more resorbable and less osteoconductive than hydroxyapatite-tricalcium phosphate commonly used to stimulate MSCs to form bone (1, 19), the present study shows that calcium-silicate cements have the potential to stimulate appropriate biomimetic microenvironments for OFMSC differentiation. Clinically, this property can ultimately promote rapid bone healing of large periapical bone defects following endodontic surgery. Additionally, we have previously reported that OFMSCs support hard tissue regeneration whether induced or non-induced (1); therefore, superior osteoregenerative and survival properties of OFMSCs in the milieu of ion-releasing calcium-silicates make OFMSCs attractive graft materials in endodontic surgery. Further studies are still warranted on the clinical applications of OFMSC-calcium silicate composite as donor grafts in endodontic surgery.
Acknowledgments
This project was supported in part with funds from United States Public Health Service/National Institutes of Health/National Cancer Institute (USPHS NIH NCI) research grant 5K08CA120875-05.
Footnotes
The authors deny any conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Stefanik D, Sarin J, Lam T, Levin L, Leboy PS, Akintoye SO. Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Dis. 2008;14:465–471. doi: 10.1111/j.1601-0825.2007.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damek-Poprawa M, Stefanik D, Levin LM, Akintoye SO. Human bone marrow stromal cells display variable anatomic site-dependent response and recovery from irradiation. Arch Oral Biol. 2010;55:358–364. doi: 10.1016/j.archoralbio.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tziafas D, Pantelidou O, Alvanou A, Belibasakis G, Papadimitriou S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int Endod J. 2002;35:245–254. doi: 10.1046/j.1365-2591.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 5.Trubiani O, D’Arcangelo C, Di Iorio D, Di Nardo Di Maio F, Caputi S. Dental pulp stem cells bioadhesivity: evaluation on mineral-trioxide-aggregate. Int J Immunopathol Pharmacol. 2007;20:81–86. doi: 10.1177/039463200702001s16. [DOI] [PubMed] [Google Scholar]
- 6.Gandolfi MG, Taddei P, Tinti A, Prati C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int Endod J. 2010;43:917–929. doi: 10.1111/j.1365-2591.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 7.Gandolfi MG, Iacono F, Agee K, Siboni F, Tay F, Pashley DH, et al. Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e39–45. doi: 10.1016/j.tripleo.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 8.de Vasconcelos BC, Bernardes RA, Cruz SM, Duarte MA, Padilha Pde M, Bernardineli N, et al. Evaluation of pH and calcium ion release of new root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:135–139. doi: 10.1016/j.tripleo.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, Fraga Sde C. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:345–347. doi: 10.1067/moe.2003.12. [DOI] [PubMed] [Google Scholar]
- 10.Gandolfi MG, Van Landuyt K, Taddei P, Modena E, Van Meerbeek B, Prati C. Environmental scanning electron microscopy connected with energy dispersive x-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time. J Endod. 2010;36:851–857. doi: 10.1016/j.joen.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Gandolfi MG, Taddei P, Tinti A, De Stefano Dorigo E, Rossi PL, Prati C. Kinetics of apatite formation on a calcium-silicate cement for root-end filling during ageing in physiological-like phosphate solutions. Clin Oral Investig. 2010;14:659–668. doi: 10.1007/s00784-009-0356-3. [DOI] [PubMed] [Google Scholar]
- 12.BSI. Terminology for the bio-nano interface. PAS; 2007. p. 132. [Google Scholar]
- 13.Gandolfi MG, Ciapetti G, Taddei P, Perut F, Tinti A, Cardoso MV, et al. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent Mater. 2010;26:974–992. doi: 10.1016/j.dental.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rabeah E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod. 2006;32:872–875. doi: 10.1016/j.joen.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Merten HA, Wiltfang J, Grohmann U, Hoenig JF. Intraindividual comparative animal study of alpha- and beta-tricalcium phosphate degradation in conjunction with simultaneous insertion of dental implants. J Craniofac Surg. 2001;12:59–68. doi: 10.1097/00001665-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Zhang X, de Groot K. Hydrolysis and phase transition of alpha-tricalcium phosphate. Biomaterials. 1997;18:737–741. doi: 10.1016/s0142-9612(96)00203-7. [DOI] [PubMed] [Google Scholar]
- 17.Ginebra MP, Driessens FC, Planell JA. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials. 2004;25:3453–3462. doi: 10.1016/j.biomaterials.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC, Ho CC, David Chen CH, Wang WC, Ding SJ. In vitro bioactivity and biocompatibility of dicalcium silicate cements for endodontic use. J Endod. 2009;35:1554–1557. doi: 10.1016/j.joen.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Akintoye SO, Giavis P, Stefanik D, Levin L, Mante FK. Comparative osteogenesis of maxilla and iliac crest human bone marrow stromal cells attached to oxidized titanium: a pilot study. Clin Oral Implants Res. 2008;19:1197–1201. doi: 10.1111/j.1600-0501.2008.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ANSI. ISO 9917-1. Part 1: Powder/liquid acid-base cements. 1. 2003. Dentistry – Water-based cements. [Google Scholar]
- 21.ASTM. Standard Test Method for time of setting of hydraulic cement paste by Gillmore needles. ASTM International; 2007. pp. 266–270. [Google Scholar]
- 22.Gandolfi MG, Perut F, Ciapetti G, Mongiorgi R, Prati C. New Portland cement-based materials for endodontics mixed with articaine solution: a study of cellular response. J Endod. 2008;34:39–44. doi: 10.1016/j.joen.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 24.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. Journal of Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Ding S-J, Shie M-Y, Wang C-Y. Novel fast-setting calcium silicate bone cements with high bioactivity and enhanced osteogenesis in vitro. Journal of Materials Chemistry. 2009;19:1183–1190. [Google Scholar]
- 26.Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26:4847–4855. doi: 10.1016/j.biomaterials.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Wei L, Liu X, Li J, Li B, Wang G, et al. Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater. 2009;5:1284–1293. doi: 10.1016/j.actbio.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Best SM, Bonfield W, Gibson IR, Hing KA, Damien E, et al. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med. 2002;13:1199–1206. doi: 10.1023/a:1021114710076. [DOI] [PubMed] [Google Scholar]
- 29.Jones JR, Tsigkou O, Coates EE, Stevens MM, Polak JM, Hench LL. Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials. 2007;28:1653–1663. doi: 10.1016/j.biomaterials.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Taddei P, Tinti A, Gandolfi MG, Rossi PL, Prati C. Ageing of calcium silicate cements for endodontic use in simulated body fluids: a micro-Raman study. Journal of Raman Spectroscopy. 2009;40:1858–1866. [Google Scholar]
- 31.Souza NJ, Justo GZ, Oliveira CR, Haun M, Bincoletto C. Cytotoxicity of materials used in perforation repair tested using the V79 fibroblast cell line and the granulocyte-macrophage progenitor cells. Int Endod J. 2006;39:40–47. doi: 10.1111/j.1365-2591.2005.01045.x. [DOI] [PubMed] [Google Scholar]


