Abstract
In this article, we briefly summarize the incidence and significant consequences of falls among older adults, the insufficient effectiveness of commonly used multifactorial interventions and the evidence linking falls and cognitive function. Recent pharmacologic and nonpharmacologic studies that evaluated the effects of cognitive therapy on fall risk are reviewed. The results of this article illustrate the potential utility of multiple, diverse forms of cognitive therapy for reducing fall risk. The article also indicates that large-scale, randomized controlled trials are warranted and that additional research is needed to better understand the pathophysiologic mechanisms underlying the interplay between human mobility, fall risk and cognitive function. Nonetheless, we suggest that multimodality interventions that combine motor and cognitive therapy should, eventually, be incorporated into clinical practice to enable older adults and patients to move safer and with a reduced fall risk.
Keywords: aging, attention, cognition, cognitive intervention, dual task, elderly, executive function, falls, gait, therapy
Gait impairments and falls are ubiquitous among the general elderly population, especially among patients with common neurological diseases [1–11]. Until recently, gait and balance were largely perceived as automated, biomechanical processes, and falls were viewed as a failure of these motor mechanisms. Age-associated declines in several systems including musculo-skeletal, cardiovascular, visual, vestibular and proprioception, coordination and slowed postural responses were seen as the key to changes in gait and balance with aging [5,8,10–13]. However, work over the past decade or so has underscored the connections between balance, gait and falls, on the one hand, and cognitive function on the other hand [5,14–26]. Here we briefly summarize the evidence linking cognitive and ‘motor’ function and the growing body of literature that suggests that not only is there an association between cognitive function, gait and falls, but that a cause and effect relationship may also exist. Apparently cognitive deficits exacerbate and may even cause gait impairment and increase fall risk, especially during more challenging situations. This background leads to the heart of the article: a summary of the intriguing studies in this emerging area that demonstrates that therapies designed to improve certain aspects of cognitive function may also enhance gait and reduce the risk of falls. Finally, we discuss the limitations of the extant findings, future directions and implications for clinical practice.
Background & significance: falls among older adults & patient populations
Approximately a third of community-living older adults fall at least once every year in developed countries [1–3]. The incidence increases with age; falls occur in approximately 50% of community-living older adults aged 85 years or older [27]. Patients with neurological disease, such as Parkinson’s disease (PD), multiple sclerosis, stroke or Alzheimer’s disease (AD), experience falls more frequently. Reports suggest that 80% of these patients experience at least one fall in a year and typically many experience multiple falls [3,4,6,7]. Falls profoundly impact the health and quality of life of older people [1,28,29]. Between 5 and 10% of falls result in serious injuries, such as head injuries or fractures [1]. Even in the absence of a history of falling [30] or when there is no physical injury after a fall, approximately a third of older adults develop a fear of falling that leads to self-imposed restrictions in mobility, reduced activity, depression, social isolation and subsequent increased fall risk [31–35]. It is not surprising, therefore, that falls and fall-related injuries are a huge medical concern representing an enormous burden to individuals, society and healthcare systems [36]. In 2008, nonfatal and fatal fall-related costs were estimated at US$23.3 billion in the USA and US$1.6 billion just in the UK [37]. National fall-related costs of prevalence-based studies are a dramatic 0.85–1.5% of total healthcare expenditures [38,201].
Traditionally, programs designed to reduce the risk of falls have identified intrinsic and extrinsic factors. Intrinsic factors include advanced age, chronic disease and potentially modifiable factors, such as sensory decline, muscle weakness and impaired balance [1,39,40]. Most falls occur during walking [31,41] and, not surprisingly, gait disturbances have been associated with an increased risk of falls [2,42]. So-called extrinsic factors generally include foot wear, environmental hazards and hazardous activities [1,40,43]. Given the multiple risk factors for falls, a multi-factorial assessment and intervention targeting the identified risk factors has become the recommended approach to reduce the rate of falling [39,40,44,45]. Components of these interventions include medical evaluation and treatment, balance and gait training, exercise, home-hazard modifications [46–49], building of balance confidence, appropriate care of poor vision and avoidance of the use of medications that increase fall risk, particularly psycho-tropic medications and hip protectors [27,40,50]. Some of the more successful single-mode (e.g., exercise) and multifactorial interventions reduce the occurrence of falling by 25–39% [44,51,52]. However, there is a wide range in reported effects on fall risk reduction [51,53]. In fact, some well-designed large-scale, controlled studies, reported that multifactorial interventions reduced falling by only 6% among community-dwelling older adults [52] or even had no significant benefit [54–59]. A number of factors may explain the range in the reported effectiveness of fall prevention programs (e.g., content fails to ensure progression and intensity; inadequate tailoring to target population; suboptimal uptake of the intervention; and insufficient compliance) [51,52,54,60]. An additional factor may explain the inconsistencies in the outcomes. While patients with dementia have often been excluded in fall prevention studies, this still leaves a large range in cognitive abilities among study participants. As described further later, we suggest that cognitive function is one factor that has not always received sufficient attention in the design of intervention studies. The effects of intervention programs on fall risk may be further improved if cognitive deficits, even among those that do not reach the level of clinical impairment, are addressed.
A brief review of the evidence linking cognitive function to falls
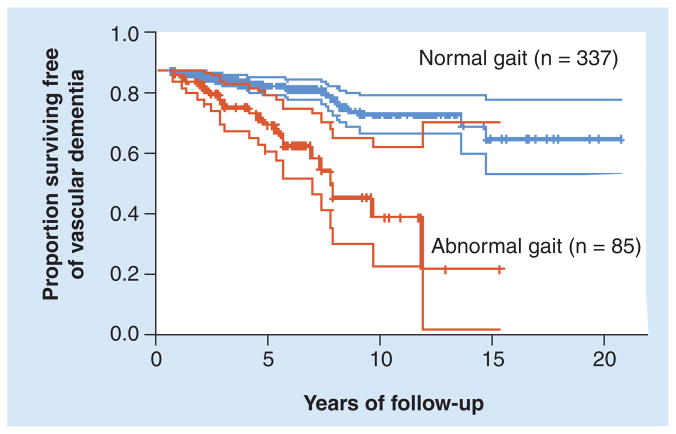
Approximately 60% of older people with cognitive impairment fall annually, approximately twofold more than that observed among cognitively intact peers [6,44,61]. Among patients with dementia, fall frequency can even reach as high as 80% [62]. The high prevalence of falls among patients with dementia, despite relatively intact motor function, highlights the idea that falls are often not just a motor problem [63]. From a slightly different perspective, several prospective studies have reported that subjects with ‘neurologic’ gait abnormalities had an increased risk of developing dementia and cognitive decline, with gait alterations predicting the development of dementia 6 to 10 years later (see, for example, Figure 1) [9,64,65]. These prospective studies support the notion that gait and cognitive function are connected, perhaps because they share common neural networks and/or because gait utilizes, and hence relies on, certain aspects of cognitive function. Furthermore, it has been suggested that gait changes may act as a biomarker for the future development of full-blown cognitive decline [18].
Figure 1. Kaplan–Meier curves for the cumulative risk of dementia, comparing older adults with and without gait alterations at baseline.
Those with gait alterations at baseline were much more likely to develop vascular dementia, as much as 6 years later. These findings highlight the connection between gait and cognitive function. One possible explanation is that these attributes of gait rely on, and are therefore sensitive to, subtle changes in executive function; these changes are precursors of the development of cognitive decline and dementia. Dotted lines represent the 95% CIs.
Adapted with permission from [65].
In early studies, the relationship between falls and cognitive function was generally examined using gross measures, such as the Mini Mental State Examination (MMSE) [66]. Often, the MMSE was used to screen out patients with cognitive impairment. Epidemiological studies reported significant associations [1,27,39], while others found that general measures of cognitive function (e.g., the MMSE) and long-term memory were not related to fall risk [67–69]. Several studies reported that scores on the MMSE were similar in older adult fallers and nonfallers, while fallers performed worse on specific common domains, for example, executive function (EF) and attention [15,67,68,70–78].
Attention contributes to gait & fall risk
Attention, by some accounts a specific type of EF, is a dynamic function driven by sensory perception and the need to select a preferred stimulus for a particular action while ignoring the unnecessary and the irrelevant [79]. Attention can be classified into four separate functions: selective, sustained, divided and alternating [80]. Selective attention enables the filtering of stimulus information and suppression of distracters. Sustained attention refers to the ability to maintain attention to a task over a period of time. Alternating attention refers to rapid shifting of attention from one task to another and divided attention refers to the ability to carry out more than one task at the same time – that is, dual tasking (DT) [18,80]. DT paradigms have been widely used to investigate the effects of cognitive abilities on balance, gait and fall risk [14,15]. If attentional resources are limited in capacity and if both gait and a secondary task are attention demanding, performance of at least one of the tasks will deteriorate when they are performed simultaneously [18]. Numerous studies have shown that DT effects are larger among elderly fallers and patients with neurological disease, such as stroke, AD or PD, compared with healthy older adults [15,18,68,69,81,82]. Since DT is a common part of daily living activities that routinely elevates the risk of falls, these findings have important implications to fall risk and suggest, as described later, that interventions that enhance DT abilities may reduce fall risk.
EF & fall risk
Executive function refers to a set of higher order cognitive processes that control, integrate, organize and maintain other cognitive abilities [83]. EF can be divided into a number of distinct subdomains. Task planning, problem solving, sensory integration, judgment and reasoning are EF components that are intuitively related to safe navigation and mobility in complex everyday environments [15,63,84]. EF also includes the ability to manipulate attention. EF may decline with aging [85] and is further reduced in elderly fallers [67]. For example, relatively healthy older adults who fell at least twice performed more poorly than nonfallers on computerized tests of EF and attention, even while long-term memory was similar in the fallers and nonfallers [67]. In addition, the awareness of self and one’s surroundings apparently may modulate postural control, gait and fall risk [18,86–88]. Reinforcement of this idea comes from a study that found that community-dwelling fallers (mean age: 79 ± 7 years) with poor working memory, a form of EF, overestimated their reach capacity by 16% compared with only a 2% error among older fallers with good working memory [89]. These results suggest that impaired EF may also increase fall risk by altering older adults’ judgment during motor planning for daily activities, such as reaching [89].
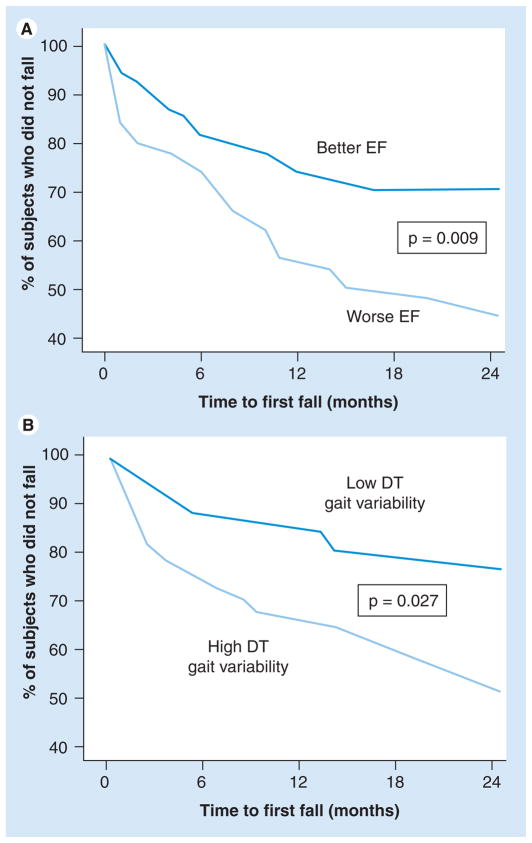
Mental flexibility, an important aspect of EF, was independently associated with fall-risk mediators, such as stride length variability and postural instability (medio-lateral trunk sway), while walking under DT circumstances in community-living elderly people (mean age: 80.6 ± 4.0 years) [69]. Similar associations between EF and gait variability, a measure of instability and fall risk [2,9,90], were also observed among older adult fallers, patients with Parkinson’s disease, patients with cognitive impairment and frail older adults. Generally, the association between EF and gait became tighter during DT, when there was greater reliance on attentional resources [70,91–93]. Furthermore, worse EF scores at baseline were associated with falls that occurred during a 2-year prospective follow-up among healthy participants (mean age: 76 ± 4 years) who reported no falls in the year prior to the study (Figure 2) [68].
Figure 2. Survival curves illustrating the percentage of participants who did not fall (the y-axes) as a function of time after the baseline testing and (A) executive function or (B) dual tasking gait variability.
Participants with worse EF (lowest quartile) or relatively increased DT gait variability were more likely to become fallers and recurrent fallers (not shown) sooner than those with better EF (highest quartile) or DT gait (highest quartile).
DT: Dual tasking; EF: Executive function.
Adapted from [68].
These findings have implications for the safe performance of everyday activities that require postural stability during ambulation. Older people with reduced EF walk slower, fall more often and have poorer performance on complex mobility tasks [21,68,94,95]. Deficits in EF probably diminish the ability to recruit compensatory mechanisms in response to age-associated changes in gait and balance, contribute to disruptions of gait and balance [15], and increase the risk of falls [68]. This may explain why EF measures are thought to be good predictors of falls [68,70]. EF might be considered to be a general biomarker of brain reserve or cognitive abilities and flexibility necessary to minimize fall frequency [68]. Moreover, the association between EF and falls suggests that improvement in the former will carry over to reduce fall risk.
Information processing & reaction time: additional cognitive factors linked to fall risk
In addition to EF and attention, other cognitive domains may also play a role in fall risk. Lord et al. suggested a multifactorial model of the primary contributors to stability and fall risk [96]. This model includes sensory–motor components (e.g., muscle strength, vision and sensation), as well as reaction time. In psychometric psychology, reaction time is considered to be an index of speed of information processing; generally, reaction times becomes longer with aging [97]. Several studies demonstrated that slow reactions – that is, increased reaction times – were associated with an increased risk of falls [96,98–100]. Using a neuropsychological test battery, Holtzer et al. built a cognitive index that reflects attention, EF and the speed of information processing [75]. They found that this index was associated with single and recurrent falls among older adults, while memory was not. Delbaere et al. reported that performance on simple reaction time test was one of three most important factors for identifying individuals at risk of falling [100]. Furthermore, Lajoie and Gallagher reported that older people in nursing homes who tend to fall had a significantly slower reaction time than those that did not fall [99]. A fall can be viewed as a loss of balance followed by insufficient or late recovery. If reaction time is slowed, initiation of the postural response will be delayed, decreasing the likelihood of successful recovery and increasing the likelihood that a fall will take place.
Neuroimaging evidence linking cognitive function & gait
Additional support that links cognition, gait and fall risk derives from brain neuroimaging findings. There is a fairly large amount of evidence linking white matter changes to postural instability, gait disturbances and falls in older adults [101–106]. A prospective study reported that increasing severity of ventricular enlargement or white matter hyperintensity and brain infarcts were associated with a higher risk for functional impairment and greater decline of gait speed [107]. Rosano et al. studied gait and its association with gray matter volume in 220 older adults. None of the gait measures were associated with the cerebellum or with regions related to memory or motor imagery domains. By contrast, shorter stride length and longer support times during gait were associated with diminished sensorimotor regions and with smaller fronto-parietal regions [106]. Furthermore, it appears that the dorsal lateral prefrontal cortex, a brain network associated with EF and attention, was related to specific gait features, further highlighting the relationships between prefrontal areas, cognition and gait.
Somewhat different findings were seen in a pilot functional MRI (fMRI) study among 83 community-dwelling women without cognitive impairment [108]. Study participants performed tasks that engaged selective attention and response inhibition during fMRI scanning. Among all participants, regions showing increases in the hemodynamic response included bilateral inferior and middle frontal gyri, anterior cingulate cortex, bilateral precuneus and the right cerebellum. However, among fallers (n = 14), the posterior lobe of the right cerebellum had a significantly lower hemodynamic response compared with nonfallers. The authors suggest that this finding may reflect deficits in EF and spatial navigation among fallers, both of which are integral to safe movement [108]. This is consistent with other findings that suggest, somewhat surprisingly, that certain regions of the cerebellum are related to EF and spatial navigation [109–111].
In an effort to sort out the relationship between memory, gait and fall risk, Zimmerman et al. examined hippocampal volume and neurochemistry as predictors of gait function in 48 non-demented older adults [112]. They found that increased stride length variability was associated with lower levels of hippocampal neuronal metabolism, but not with hippocampal volume. Conversely, decreased stride length was associated with smaller hippocampal volumes, but not hippocampal neurochemistry. These findings indicate that distinct neurobiological hippocampal substrates may support specific aspects of gait and fall risk in older adults.
Bohnen et al. used PET imaging studies to test the hypothesis that gait control depends on cholinergic system-mediated higher-level cortical and subcortical processing, including pedunculo-pontine nucleus (PPN) function among patients with PD [113]. Cortical and thalamic acetylcholinesterase (AChE) hydrolysis rates were significantly lower in patients with PD compared with healthy control subjects, and were significantly lower in the PD fallers compared with the PD nonfallers. Interestingly, no significant differences were found between PD fallers and PD non-fallers in dopaminergic nerve terminal density at the level of the basal ganglia [113]. These findings support the idea that fall status among PD is related to cholinergic hypofunction, perhaps representing changes in cholinergic output of the PPN. This idea is consistent with reports that gait slowing may be related to cholinergic activity [114] and that the PPN degeneration may be a major cause of impaired postural control and gait dysfunction in PD. Indeed, Karachi et al. recently wrote that “cholinergic neurons of the PPN play a central role in controlling gait and posture and represent a possible target for pharmacological treatment of gait disorders in PD” [115]. Another possibility is that the cholinergic changes reflect more general cognitive deficits and are not necessarily PPN-mediated; serum levels of cholinergic activity have also been related to reaction times and cognitive slowing [114,116].
Cognitive interventions for balance, gait & fall risk
If cognitive deficits are in the causal pathway and they exacerbate the risk of falls or prevent appropriate compensatory mechanisms, one would anticipate that improvement of cognitive abilities, at least certain aspects, would improve gait and reduce the risk of falls, especially in more challenging conditions that rely on EF. The studies that have explicitly examined this possibility are summarized in the following sections. We note from the onset that only a few studies have directly assessed the effects on fall risk per se. Thus, in our review of this emerging field, we also include investigations that examined the effects of cognitive interventions on gait and balance, recognized mediators of fall risk, as well as the few investigations that more directly evaluated the effects on falls.
Cognitive pharmacotherapy
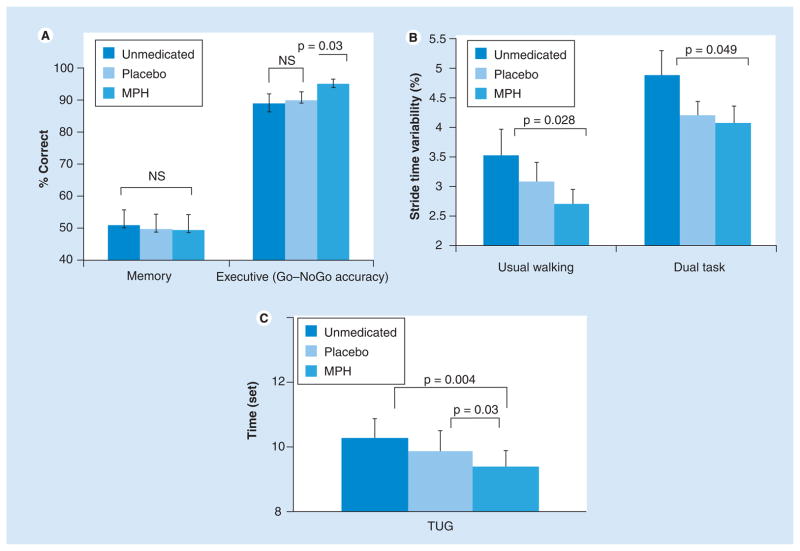
Studies that examined the possible effects of medications that augment cognitive function and gait and fall risk are summarized in Table 1. Methylphenidate (MPH), derived from amphetamine, and other drugs designed to enhance attention and EF are a natural target, given the importance of attention in DT. The effects of a single dose of 20 mg of MPH was studied in 21 patients with idiopathic PD [117]. In response to MPH, neuropsychological testing found no change in memory, while EF scores improved. At the same time, Timed Up and Go performance, gait speed and stride time variability all improved in response to MPH [117]. Consistent with this, 3 months of relatively high doses of MPH improved performance on the Stand–Walk–Sit test and the Tinetti Scale in an open-label study among 17 patients with advanced PD [118]. Similarly, a study among community-living older adults who complained of memory problems reported on the effects of a single dose of 20-mg MPH on markers of fall risk. Subjects were evaluated before and 2 h after taking MPH or a placebo. As illustrated in Figure 3, MPH significantly improved gait measures and EF (e.g., Go–NoGo accuracy), but did not affect memory or finger-tapping performance. These improvements were not observed after treatment with the placebo [119]. While alternative mechanisms may have caused the observed changes in motor function in response to the drug, one possibility is that MPH enhanced attention and this, in turn, improved gait [117,119,120].
Table 1.
Cognitive pharmacotherapy for balance, gait and fall risk.
| Study (year) | Type of intervention | Duration of intervention | Participants | Summary of findings | Study design | Ref. |
|---|---|---|---|---|---|---|
| Baezner et al. (2001) | A daily dose of 500 ml iv. amantadine vs placebo. Both groups were treated with physical therapy on a daily basis | 5 days | 40 patients (age not specified) diagnosed as SVE | Gait disorder score improved by 25% in the amantadine group, compared with only 11% in the placebo group (p = 0.08). In the study group, cadence, length of heel-to-toe movements in the single support phases, variability of double support times and double support time improved significantly (p < 0.05) | Randomized, double-blind placebo-controlled | [121] |
| Auriel et al. (2006) | Before and 2 h after taking a single dose of 20 mg of MPH | 2 h | 21 patients with idiopathic PD who receive l-DOPA, (mean age: 70.2 ± 9.2 years, MMSE: 28.8 ± 1.7) | Significant improvement in the Attention Index (p=0.025), whereas the EF Index showed only a trend (p = 0.099). The Timed Up and Go times (p = 0.001), gait speed (p = 0.005) and stride time variability (p = 0.013) showed significant improvements | Open-label, before–after design | [117] |
| Ben- Itzhak et al. (2008) | Before and 2 h after taking 20 mg MPH or a placebo | 2 h | 26 older adults without dementia with subjective complaints of ‘memory problems’ (mean age: 73.8 ± 1.2 years, mean MMSE: 27.8 ± 1.4) | MPH significantly improved Timed Up and Go times (p = 0.004), stride time variability (p = 0.03) and EF (p = 0.03), effects not observed after treatment with the placebo | Randomized, double- blind, placebo- controlled | [119] |
| Litvinenko et al. (2008) | Galantamine (ACEIn) up to a maximum dose of 16 mg/day was given by the following protocol: 4 mg twice-daily for the first 4 weeks, and then 8 mg twice-daily | 24 weeks | 41 patients with PD and galantamine treatment group (n=21; mean age: 68.6 ± 9.3years; MMSE: 17.6 ± 3.3) and a control group (n=20; mean age: 72.6±8.6years; MMSE: 18.1 ± 3.6) | The treatment group improved on cognitive scores (MMSE: 21.3 ± 1.9) compared with the control group (MMSE: 16.8±1.4; p<0.005). Improvements in scores of gait, freezing and falls<0.005; (p derived from the UPDRS items) were observed in the galantamine-treated group, while there was further decline in motor function in the control group | Open-label controlled trial | [125] |
| Montero- Odasso et al. (2009) | 5 mg/day of donepezil (ACEIn) for 1 month, and another 3 months with 10 mg/day. The MCI group with no treatment | Up to 4 months | Six patients with mild AD (mean age: 79.9±4.8 years; MMSE: 22.3±1.2; MoCA: 15 ± 1.4) compared with eight patients with MCI (mean age: 75.6±6.2 years; MMSE: 27.9±1.7; MoCA: 22.9 ± 1.7) | AD patients significantly increased their gait velocity after 1 month under single (p = 0.045) and dual tasking (p = 0.047). Gait variability decreased (improved) during follow-up. These increases were maintained for 4 months. Gait measures in the MCI group were worse than at baseline | Open-label study with controls | [122] |
| Assal et al. (2008) | Galantamine (ACEIn) mean dose of 17.8 ± 3.5 mg/day | 24 weeks | Nine patients with mild-to- moderate AD (mean age: 77.9±2.1 years; mean MMSE: 26.4 ± 5.2) compared with 18 no- treatment control subjects without dementia (mean age: 78.1±1.0 years; mean MMSE: 29.4 ± 0.8) | Stride time was significantly shorter under dual tasking after treatment (p = 0.01). There was no change in the controls | Before–after design | [126] |
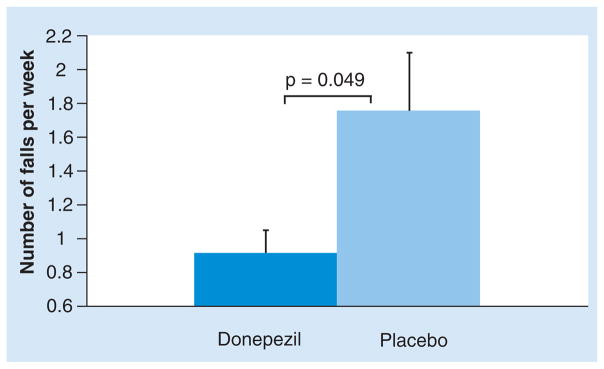
| Chung et al. (2010) | Donepezil compared with placebo. In each drug phase, subjects were instructed to take one tablet (5 mg of donepezil or placebo) for 3 weeks and to increase to two tablets (10 mg) for the remaining 3 weeks | 6 weeks, 3 weeks washout, 6 weeks of placebo | 23 patients with PD who reported falling or nearly falling (mean age: 68.3±10.8years; MMSE: 27.6 ± 4.5) | Less falls with donepezil than when taking placebo (p = 0.049). Subjects with the most falls at baseline tended to show the largest improvements. No differences in Activities of Balance Confidence Scale, Berg Balance Scale, UPDRS III or MMSE scores | Randomized, crossover, double-blind | [123] |
ACEIn: Acetylcholinesterase inhibitor; AD: Alzheimer’s disease; EF: Executive function; iv.: Intravenous; L-DOPA: Levodopa; MCI: Mild cognitive impairment; MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment; MPH: Methylphenidate; PD: Parkinson’s disease; SVE: Subcortical vacular encephalopathy; UPDRS: Unified Parkinson’s Disease Rating Scale.
Figure 3. Effects of methylphenidate or placebo on cognition and gait in older adults.
(A) Effects of a single dose of 20 mg of M or placebo on cognitive function (% correct or accuracy), (B) stride time variability and (C) TUG times in older adults. There was small time variability and TUG performance in response to MPH, but not in response but to placebo.
MPH: Methylphenidate; NS: Not significant; TUG: Timed Up and Go.
Data from [119].
Amantadine, typically used as an antiparkinsonian medication, is believed to enhance the release of endogenous brain dopamine. In a study among patients with a frontal gait disorder due to subcortical vascular encephalopathy (SVE) [121], 40 patients with SVE were given a daily dose of amantadine or placebo in addition to physical therapy. In response to amantadine, several measures of gait quality improved (e.g., single support time and variability). These findings suggest that amantadine may enhance gait steadiness in patients with a frontal gait disorder.
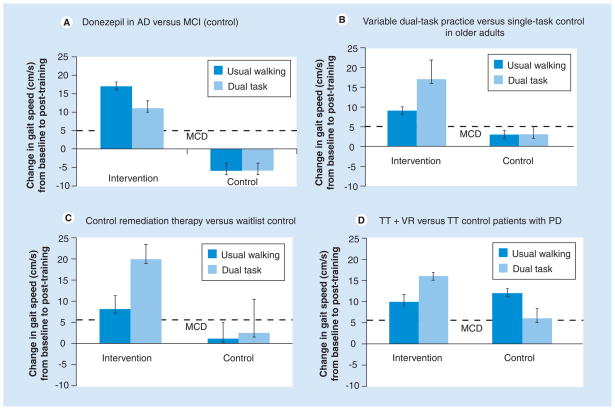
Acetylcholinesterase (AChE) inhibitors are widely used to treat cognitive impairment among patients with Alzheimer’s dementia. Several studies have assessed the effects of AChE inhibitors on gait and fall risk. The rationale is supported by the intriguing imaging study noted previously that described cortical cholinesterase deficits in PD fallers, compared with PD nonfallers [113], and by the general cognitive-enhancing properties of these drugs. The effects of donepezil, a cognitive-enhancing drug that works via cholinergic manipulation, was studied in a small, pilot study [122]. Gait was assessed over 4 months of treatment with donepezil among six individuals with AD and eight subjects with mild cognitive impairment (MCI) who received no treatment. No improvements in gait speed were seen in the control subjects. By contrast, the participants with AD who were taking donepezil improved their gait after 1 month under both single and DT walking conditions (Figure 4a). These increases in gait speed were sustained and continued to improve after 4 months.
Figure 4. Examples of the effects of four different forms of cognitive therapy on usual-walking gait speed and dual-tasking gait speed.
Values shown are change with respect to baseline. Note that 5 cm/s and 10 cm/s have been identified as the MCD and substantial difference [177]. (A) Effects of 4 months of donepezil use on gait speed in patients with Alzheimer’s disease and compared with control patients with mild cognitive impairment. Data from [122]. (B) Effects of dual-task training during walking on gait speed in older adults with balance impairment, compared with subjects who only practiced walking. Data from [138]. (C) Effects of 8 weeks of computerized cognitive training (while seated) in sedentary older adults, compared with wait list controls. Data from [147]. (D) Effects of 6 weeks of TT augmented with VR among patients with PD, compared with an active control comparison of 6 weeks of TT alone. Usual-walking gait speed increased in both the TT alone and TT + VR groups; however, DT gait speed only improved who participated in TT + VR. Data from [148].
AD: Alzheimer’s disease; DT: Dual tasking; MCD: Minimal clinically significant difference; MCI: Mild cognitive Parkinson’s impairment; disease; TT: Treadmill training; VR: Virtual reality.
Further reinforcement for the idea that cognitive-enhancing treatment reduces the risk of falls comes from a randomized, crossover, double-blind study with donepezil [123]. A total of 23 subjects with PD who reported falling or nearly falling (more than two times per week) were given donepezil or placebo and then crossed to receive placebo or donepezil. As illustrated in Figure 5, fall frequency was 0.25 ± 0.08 per day when patients were receiving placebo, while on donepezil, fall frequency was almost 50% lower – that is, 0.13 ± 0.03 per day (p < 0.05). The results of this trial need replication on a larger scale [123,124]; however, these findings provide important support for the idea that cortical cholinergic augmentation may reduce the risk of falls.
Figure 5. Mean fall frequency was almost 50% lower during the 6-week period when subjects with Parkinson’s disease were taking donepezil, compared with the 6-week period when they were taking placebo.
Note that those subjects who had relatively high fall frequencies on placebo seemed to be most responsive to donepezil.
Data from [123].
An open controlled trial with galantamine, another AChE inhibitor, at a maximum dose of 16 mg/day, included 41 patients with PD with dementia, randomized to a galantamine group (21 patients) or a control group (20 patients). Cognitive, neuro-psychiatric and motor symptoms were assessed clinically before the trial and at 4, 12 and 24 weeks. Patients treated with galantamine had better cognitive scores compared with the control group. In addition, significant improvements in gait, freezing of gait and falls (as derived from the Unified Parkinson’s Disease Rating Scale scores) were seen in the galantamine treatment group [125]. Similarly, after 24 weeks on galantamine, the dual-task effects on stride time tended to become smaller (better) in a pilot study among patients with mild-to-moderate AD [126]. These findings are consistent with the idea that central cholinergic stimulation via AChE inhibitors can improve gait quality and stability, and thereby reduce fall risk.
Recent studies, both cross-sectional and prospective, have demonstrated the potential of vitamin D to reduce fall risk [52,127–129]. Intuitively, vitamin D acts as mediator of fall risk because it modifies muscle strength, power and bone quality. However, several investigations have shown that vitamin D may also ameliorate fall risk by way of its effect on cognitive function [130,131]. Nonetheless, many questions remain about the role of vitamin D in fall risk and how and if it should be used clinically [132,133]. Future studies are needed to investigate the specific pathways that mediate between vitamin D supplementation and a reduced fall risk, to identify the specific cognitive function subdomains that respond to vitamin D, and to evaluate how vitamin D supplementation may be combined with other forms of cognitive and motor therapy to achieve optimal benefits.
Cognitive training
There is good evidence that cognitive training interventions can improve cognitive function in older adults, at least in certain cohorts [134–136]. The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was the first multicenter, randomized controlled trial (RCT) to examine the long-term outcomes of cognitive interventions on the daily functioning of older individuals living independently [134]. Subjects in the training groups received ten sessions of training focusing on different aspects of cognitive function. Each intervention improved the targeted cognitive ability and the results were retained for 2 years after the termination of the study (p < 0.001 for all) [134]. After 5 years, training gains persisted and reasoning training resulted in less functional decline in self-reported independent instrumental activities of daily living [135]. This intriguing finding supports the idea that specific cognitive training can have functional benefits. Table 2 describes and summarizes studies that specifically evaluate the effects of different forms of cognitive training on gait and fall risk. In general, these investigations demonstrate a transfer of training effects to physical outcomes related to mobility and fall risk. Although these studies are generally preliminary in nature, with a relatively small number of participants, they support the idea that cognitive training can improve postural control and gait, lower DT costs and perhaps even reduce the risk of falls.
Table 2.
Cognitive training interventions.
| Study (year) | Type of intervention | Duration of intervention | Participants | Summary of findings | Study design | Ref. |
|---|---|---|---|---|---|---|
| Yang et al. (2007) | Participants were randomized into a control group (n = 12) or experimental group (n=13). Subjects in the control group did not receive any rehabilitation training. Subjects in the experimental group practiced dual tasks while catching a ball | 12 training sessions; three times a week over 4 weeks | 25 subjects with chronic stroke (mean age: 59 ± 12 years) | The experimental group showed significant improvement in most gait measures under single- and dual-task conditions (p < 0.05), compared with the control group | RCT | [141] |
| Canning et al. (2008) | Each session consisted of walking alone, walking while performing various additional cognitive, manual and triple tasks ‘fast-as-possible’ | Three training sessions; 30min once a week during a 3-week training phase | Five adults aged 61 ± 8 years with mild-to-moderate PD | Multiple-task walking velocity increased by 0.09 m/s (95% CI: 0.02–0.16) and this increase was maintained in the retention phase | Open-label, intervention study | [142] |
| You et al. (2009) | Dual-task CGI: simultaneous motor (walking 30 m) and cognitive (memory recall) task (n = 8), whereas the control group (n = 5) received a placebo treatment (walking while listening to simple music) | 18 training sessions, 30 min per session over 6 weeks | 13 older adults (mean age: 68.3 ± 6.5 years) with a history of falls | Working memory performance under the dual-task condition improved (p < 0.05), but significant changes in gait velocity and stability were not observed | RCT | [137] |
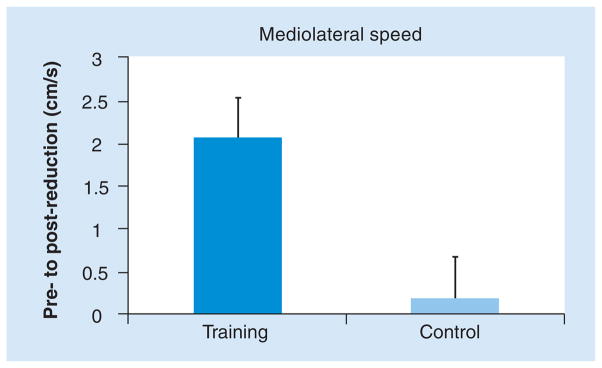
| Silsupadol et al. (2009) | Participants were randomly assigned to one of three interventions: single-task training (n = 7), dual-task training with fixed-priority instructions =8), (n and dual-task training with variable-priority instructions (n = 6) | 12 training sessions; 45-min individualized sessions, three times a week for 4 weeks | 21 older adults (aged 65 years and older) with balance impairment | Improvement in balance and gait speed was found in all groups. Only the DT training with variable-priority instructions group demonstrated a DT training effect at the second week maintained at 12-week follow-up | Double- blind RCT | [138] |
| Yogev- Seligmann et al. | A 4-week program of one-on- one training included walking while performing several distinct cognitive tasks | 12 training sessions; three times a week for 4 weeks | Seven patients with PD (mean age: 63.8 ± 8.4 years) | Gait speed and gait variability during DT significantly improved. Untrained DT also improved and was retained 1 month after the end of the training | Open-label, pilot | [143] |
| Li et al. (2010) | Computerized cognitive dual-task training (n = 10) consisted of computer exercises where two-choice decisions were visually presented compared with control group with no treatment (n = 10) | Five 1-h sessions, spaced at least 2 days apart | Healthy older adults: training group (mean age: 74.6 ± 5.7 years), control group (mean age: 77.7 ± 7.1 years) | Dual-task performances improved significantly across the first four training sessions (p < 0.01). Only the training group showed benefits in double-support standing balance and especially in single-support standing balance | RCT | [146] |
| Verghese et al. (2010) | Computerized ‘Mindfit’ program (n = 10). Each training session included a mixture of 21 visual, auditory and cross-modality tasks compared with wait-list (n = 10) | 24 training sessions; 45–60 min each session, three times a week for 8 weeks, compared with wait-list controls | Sedentary older adults (mean age: 77.4 ± 7.0 years, MMSE: 29 ± 0.3) at training, and wait-list (mean age: 79.9 ± 7.5 years; MMSE: 29.1 ± 0.4) | Gait velocity improved during normal walking (p = 0.05) and ‘walking while talking’ (p = 0.002) only in study group. Speed of processing improved significantly in the training group (p = 0.03) | RCT | [147] |
| Schwenk et al. (2010) | Intervention group (n = 20) underwent dual-task-based exercise training (motor: throwing or catching a ball and cognitive: arithmetic tasks, repeating names of animals). The control group (n = 29) performed low-intensity exercise | 24 training sessions over 12 weeks, 1 h twice a week | 49 geriatric patients (mean age: 81.9 ± 7.5 years) with confirmed mild-to- moderate dementia (MMSE 17–26: 21.4 ± 2.9) | The specific training significantly improved dual-task performance gait speed (p < 0.001), cadence (p = 0.007), stride length (p = 0.001), single support (p=0.003) under complex three-step backward calculation conditions compared with the control, but not under the less challenging DT two-step forward calculation conditions | RCT | [144] |
| Mirelman et al. (2010) | TT with virtual obstacles. The VR simulation required obstacle negotiation in two planes, while continuing to walk on the treadmill. Comparison was made to a historical active control group who followed similar protocol of TT but without VR [149] | 18 training sessions (three per week for 6 weeks) | 20 patients with PD (mean age: 67.1 ± 6.5 years) moderately impaired but were able to walk unassisted for at least 5 min | Similar improvements in gait speed and stride length under usual walking. However, under the dual-task condition, gait speed (p = 0.003) and stride length (p < 0.001) were significantly higher after training with TT + VR compared with TT alone. Dual-task gait variability decreased and Trail Making Test times improved after TT + VR training | Repeated measures design | [148] |
| Hiyamizu et al. (2010) | Experimental group (n=17) were given strength and balance training while performing cognitive tasks simultaneously. Control group (n = 19) were given strength and balance training only | 24 training sessions; twice a week for 3 months | 17 healthy older adults (mean age: 72.9 ± 5.1 years), who were free from any neurologic or musculoskeletal diagnosis were compared with a control group (mean age: 71.2 ± 4.4 years) | There were no significant differences in motor measures at baseline and after training between the two groups. However, the rate of Stroop task while maintaining a standing position was significantly higher (better) in the experimental group who practiced DT, than in the control group (p = 0.04) | RCT | [140] |
CGI: Cognitive-gait intervention; DT: Dual tasking; MMSE: Mini Mental State Examination; PD: Parkinson’s disease; RCT: Randomized controlled trial; TT: Treadmill training; VR: Virtual reality.
The effects of a dual-task cognitive-gait intervention (CGI) on working memory and gait were examined in 16 older adults with a history of falls [137]. The study demonstrated that the DT CGI was effective in improving working memory performance under the DT condition, but did not show significant changes in gait speed or stability [137]. The effect of the intervention on falls was not studied.
Silsupadol et al. studied 21 older adults with balance and gait impairment [138,139]. Participants were randomly assigned to one of three interventions: single-task (active control), fixed-prioritization DT training and DT training with variable-prioritization. Improvements in balance and gait speed were found in all groups after training (Figure 4b). However, when a cognitive, dual task was added during testing, only participants who received DT training exhibited significant improvements in gait speed (p < 0.001). Furthermore, the group that trained with variable-priority instructions demonstrated a DT training effect that was retained at the 12-week follow-up assessment. The authors concluded that training balance under single-task conditions may not generalize to balance control during DT contexts. They further suggest that a task-specific gait training program that specifically focuses on DT reduces the negative effects of DT while walking and that variable prioritization apparently has added benefits compared with fixed prioritization [138]. Another study among 36 healthy older adults compared the effects of strength and balance training while performing cognitive tasks to strength and balance training only [140]. There were no significant group differences in the effects of the training on the motor measures. However, performance on the Stroop task during standing was significantly better after training in the experimental group than in the control group (p = 0.04), suggesting that dual-task balance training in older adults improves dual-task performance while maintaining balance [140]. Similar findings were observed in studies with post-stroke individuals [141] and patients with PD [142,143], suggesting that even among patients with neurodegenerative disease, intensive and repetitive practicing of DT while walking can lower dual-task costs.
Perhaps the most striking example of the effects of DT training was observed in a RCT study in 49 patients with mild-to-moderate dementia [144]. DT-specific training significantly improved DT performance under complex gait conditions (e.g., serial 3 subtractions) compared with the controls, but not under the less challenging DT conditions [144]. While this RCT does suffer from some important limitations, it provides “the first Class II evidence that dual-task training improves walking under complex conditions in patients with mild-to-moderate dementia” [145].
In contrast to the studies where DT costs were targeted by practicing DT walking, two investigations examined the effects of cognitive training in the form of computer games that subjects ‘played’ while sitting in front of a computer. Li et al. examined the effects of a computerized cognitive training program designed to enhance DT abilities in 20 healthy older adults [146]. All participants were tested on cognitive function, balance and mobility under single and DT conditions. The training group demonstrated benefits in double-support postural control (i.e., sway) (Figure 6), especially in single-support standing balance, whereas the control group showed no specific improvements. These results demonstrate training-related benefits to gross motor performance that stem from a cognitive training protocol [146].
Figure 6. Effects of five sessions of computerized dual-task training, while seated, on postural control in older adults.
Sway magnitude during dual tasking was reduced (better) after training, while there was no effect in the controls.
Adapted with permission from [146].
Another demonstration of transfer from a computerized cognitive remediation program to a functional, untrained task such as mobility was shown in a pilot RCT among sedentary seniors [147]. Older adults were randomly assigned to an 8-week computerized ‘Mindfit’ program, designed to improve EF and working memory or wait-list control group. The ten participants who completed the cognitive remediation program demonstrated improved gait speed compared with baseline during normal walking and during DT (Figure 4C). All ten participants in the intervention group improved on DT walking, compared with only three controls [147]. This study illustrates how a cognitive training program that is designed to improve EF and attention may enhance both usual and DT walking.
Finally, another approach is exemplified by the work by Mirelman et al. [148]. They suggested using a program of treadmill training (TT) augmented with virtual reality (TT + VR) in patients with PD. The study aimed to promote the development of new motor and cognitive strategies for obstacle navigation and to implicitly teach the participants to walk while DT (e.g., planning and stepping over virtual obstacles). After 6 weeks of TT + VR, gait speed significantly improved in usual and DT walking conditions with significant improvements also observed in stride length and stride time in usual and DT gait conditions, as well as during over-ground obstacle negotiation. In addition, DT gait variability decreased (improved) and Trail Making Test times (parts A and B) also improved. After training, a significant association was found between the change in the Trail Making Test (parts A and B), a measure representing EF, and gait speed during DT and obstacle negotiation conditions. Comparison with a previous study of TT without virtual reality [149] showed that the virtual reality training provided added value for DT training beyond that observed with a treadmill intervention alone (Figure 4D) [148].
Expert commentary
Despite the now well-recognized relationship between cognitive function, gait and falls [14,15,18,19,22,65,67–69,71–75,84,89,92,150], there are still prevention interventions and guidelines that do not explicitly address the cognitive component of falls [54,56,59,151–155]. Tables 1 & 2 summarize promising results of the effects of cognitive therapy on gait and fall risk. This article should help to heighten the awareness of the essential role of cognitive function and the possibility of using cognitive therapy to reduce fall risk among older adults. At the same time, this article should motivate researchers to initiate the additional studies that are needed to consolidate this concept and enable transfer to clinical practice.
This article summarizes the emerging evidence that indicates that cognitive interventions have effects that carry over from the cognitive to the physical domain to enhance gait, reduce DT costs and may reduce fall frequency. The cognitive remediation study in which sedentary seniors played ‘computer games’ that improved usual-walking and DT gait speed [147] clearly illustrates the potential of cognitive therapy. The cross-over study of the effects of donepezil on fall frequency in patients with PD [123] and the RCT investigation of DT training in patients with dementia [144] are perhaps two of the more compelling studies. While the findings of the current literature are preliminary and do not yet reach the level of Class IA evidence – indeed, many of the studies have limited power owing to small sample sizes – the accumulating evidence is starting to substantiate the potential of cognitive therapy to reduce fall risk (see Figure 4).
For many years, fall risk has been associated with medication burden. Since more medications meant an increased risk of falls, geriatricians and other clinicians were encouraged to reduce the number of medications to lower fall risk [39]. Certainly, there is good evidence and rationale for this advice. Nonetheless, the initial studies of pharmacologic therapy for fall risk suggest that this negative view of all drugs needs to be reconsidered in the light of the putative positive effects of certain cognitive-enhancing medications. More recent recommendations [40,156] that advocate a review of only specific drug categories (e.g., psychoactive agents), rather than targeting the total number of medications, is more in line with the potential benefits of certain drugs on fall risk.
Questions regarding optimal dosing, form and content of delivery, retention, transfer and booster effects, availability of some of the drugs, and cost–effectiveness have not yet been fully addressed. In addition, several different – perhaps complementary, perhaps contradictory – theories have been proposed to explain the precise link between cognitive function and fall risk and the putative mode of action of cognitive therapy [18]. One possibility, as Liu-Ambrose et al. suggested, is that judgment in the planning stages of motor daily activities is impaired among older adults and this, in turn, leads to instability [89]. Judgment may also play an important role in the assessment, planning, response to a given situation and choice of the gait pattern (e.g., fast or cautious); however, only a few studies have looked into this issue [63]. Indeed, Delbaere et al. suggest that fear of falls and fall risk as assessed using performance-based measures of function were both independent predictors of future falls and should be included as part of a fall risk assessment to allow tailoring of interventions for preventing falls in elderly people [157]. Additional work is needed to further assess the importance of hazard perception and the potential of cognitive and/or behavioral therapy to improve this cognitive property and its role in fall risk.
Another plausible explanation is that reduced EF causes less effective compensation for age- or disease-associated changes in gait and balance, which in turn leads to an increased risk of falls [15,68]. In other words, what distinguishes an older adult who falls from one who does not is, in part, the ability to effectively compensate for deteriorated functions secondary to aging or disease. For example, compensatory abilities may enable an older adult to avoid a high-risk situation or to take appropriate steps to minimize risk [63].
During dual- or multi-task situations, the attention demand increases and the ability to divide attention may become critical [70,94,95,143]. Cognitive therapies improve divided attention abilities during walking, while some also apparently have the potential to augment usual-walking, restoring a degree of automaticity (see Tables 1 & 2). Nonetheless, further work is needed to determine the exact mechanism of action of cognitive training and other possible ‘cognitive-enhancing’ modalities on the cognitive and motor aspects of gait. This has ramifications for the understanding of pathophysiology and has important potential clinical implications. The combination of drug therapy with cognitive and/or behavioral therapy is another unexplored area of research that should be examined given the possibility of superiority of combining the modalities over each therapeutic mode of intervention alone. To move the field forward, additional work is needed to better understand the underlying pathology and more fully identify the mechanism(s) of actions of the different approaches, possibly by combining advanced neuroimaging with behavioral and biomechanical methods and advanced online neuropsychological and motor evaluations of brain function. Multiple neural networks and pathways (e.g., cholinergic and dopaminergic) and cognitive functions (e.g., EF, DT, obstacle negotiation and judgment) may connect cortical function to fall risk. Precise teasing out of these relationships will inform the design, evaluation and optimization of multimodality intervention programs for safer mobility and lower fall risk.
Five-year view
As the world’s population ages [158], the need to refine, promote and implement effective fall risk interventions for populations with differing characteristics and risk factors increases [36]. A key challenge for the future is to increase the awareness of the general public and the medical community to the importance of multifactorial fall prevention interventions to also include an emphasis on cognitive function. We suggest that this will improve the efficacy of these interventions and will add another dimension that will empower transfer of gains into everyday function. At this stage, one can only speculate about the overall effects that such an approach would have on cognitive decline, mobility impairment and functional decline among the elderly. Still, when thinking about the costs, cost–effectiveness and tradeoffs of a multimodality interventional program for fall risk, the potential impact on cognitive function itself should not be overlooked. In this regard, the bidirectional nature of the interaction between the training of cognitive and motor function should also be kept in mind. Although questions remain about the effects of physical activity and exercise on cognitive function [159], there is evidence suggesting that cognitive therapy may improve motor function and that the reverse may also be true (compare and contrast Figure 1 and Figure 2) [160,161]. Thus, the potential impact of a cognitive intervention in aging populations may be profound.
The work to date is promising, but largely preliminary and limited by small sample sizes. Tables 1 & 2 illustrate the potential of multiple, diverse forms of cognitive therapy. However, before such an approach can become part and parcel of evidence-based medicine and clinical practice, a number of issues must be resolved. Large-scale RCTs that examine the short- and long-term effects in various cohorts are needed. The implications and findings of many cross-sectional and pilot studies often could not be substantiated when they were translated into larger, prospective studies. Perhaps because of the multifactorial nature of fall risk, the cognitive and motor heterogeneity of study populations, and the difficulties in duplicating specifics of intervention content, even previously successful multifactorial RCT studies were not always successfully reproduced [54,60]. We anticipate that large-scale trials will, in the coming years, provide the necessary evidence to validate the clinical effectiveness of various forms of cognitive therapy for reducing fall risk. The study proposed by Montero-Odasso et al. is likely to be one example of RCT investigations that address this issue [162].
One size generally does not fit all. Thus, in the future, it may be helpful to tailor the cognitive therapy by defining subgroups (e.g., nonamnestic MCI) with specific cognitive, motor and personality profiles, perhaps according to comorbidity and medication load. For example, Anstey et al. suggest that “occasional falls in late life may be associated with subtle age-related changes in the prefrontal cortex leading to failures of executive control, whereas recurrent falling may result from more advanced brain aging that is associated with generalized cognitive decline” [163]. If an array of cognitive therapies are shown to be effective, the clinician or therapist will be able to optimize the match between the patient characteristics and therapy. For example, findings by Silsupadol et al. suggest that variable-prioritization DT training and fixed-prioritization training (e.g., giving equal weighting to the cognitive and motor task) are both effective, while the former apparently conveys additional long-term benefits among older adults with balance impairment [138].
One problem facing the clinical and investigational linkage between cognition, gait and falls is the neuropsychological measures and terminology used. The sensitivity of outcome measures varies widely and the nomenclature and definitions in the extant literature are not always clear [1,27,123,164]. For example, traditionally, attention refers to selective attention [80] and response inhibition [165], but it has also been viewed as a type of EF or an independent domain [166]. Similarly, working memory has been considered to be a part of short-term memory [165], but is also included as a measure of EF [166]. There is a need to develop a more refined vocabulary and start to carefully peel apart the attention–EF domain in order to further delineate and clarify the multiple associations with gait and fall risk. Perhaps such measures can provide researchers with more sensitive markers of fall risk that could potentially lead to the design of more effective multifactorial interventions that include both motor and specific cognitive aspects.
It is also important to develop a simple clinical screening tool for risk assessment that can be used in clinical practice to identify subjects who are most likely to benefit from a cognitive intervention. A small study (n = 30) did not find added value of conducting the Timed Up and Go test under DT conditions [167]. A larger study among 187 older adults living in senior housing facilities also failed to demonstrate any added value of testing gait under DT conditions [168]. Nonetheless, there is a fair amount of evidence suggesting that DT walking tests can be used in clinical settings to improve fall risk identification [68,167,169–171] and two recent reviews both concluded that DT tasks are apparently valuable for assessing fall risk [172,173]. Still, further work is needed to develop and validate an easy-to-use, quick, reliable and accurate test for identifying and evaluating the cognitive component of mobility and fall risk.
In healthy young adults, a variety of cognitive-enhancing options are increasingly becoming available. The positive effects of physical activity on cognitive function have long been recognized [174] and other options are gaining attention. A recent editorial even went so far as to discuss the ethical dilemmas that arise from the use of transmagnetic stimulation (TMS) for enhancing different aspects of cognitive function in healthy adults [175]. Among older adults, TMS has largely been used for the relief of depressive symptoms. It is, nonetheless, interesting to speculate about the possibility of using TMS and perhaps developing programs for deep brain stimulation to improve EF, attention, alertness (perhaps via the PPN) and cognition to decrease fall risk. As new options for improving cognitive function in young adults become available, it will be important to evaluate their potential utility in aging populations.
A key principle underlying many of the investigations that proposed using cognitive therapy is that the benefits of the cognitive intervention transfer to the motor domain. While this is a bit of a oversimplification since gait is no longer viewed simply as a motor task [14,15,18,21], it is still worthy of consideration. In this regard, it is important to account for the somewhat disappointing findings of Owen et al. [176]. They studied 11,430 adults who participated in a 6-week online cognitive training program designed to improve reasoning, memory, planning, visuospatial skills and attention. Encouragingly, improvements were observed in every one of the cognitive tasks that were trained. However, no evidence was found for transfer effects to untrained tasks, even when those tasks were cognitively closely related. In contrast to the findings of Owen et al., recall that the 5-year results of the relatively large, prospective ACTIVE study showed that certain types of computerized cognitive training produce effects that transfer to functional activities of daily living in older adults [135]. Thus, when considering how cognitive therapy affects and transfers to mobility and fall risk, we need to make sense of these apparently contradictory results, recognize the limits of the specificity of training and more fully understand what, when and why cognitive training benefits transfer to other cognitive and motor domains.
Many unanswered questions need to be addressed. We recognize the possibility that a single cognitive intervention might be sufficient if it targets the key to fall risk, but suggest that multifactorial interventions that ameliorate motor, cognitive and possibly behavioral and educational domains may be the optimal approach to maximize efficacy. Work in the coming years will probably provide evidence that such interventions are efficacious, practical and cost effective and that they should be integrated into the standard of care to promote successful, independent and thriving aging in society, as well as more optimal care of patients with neurodegenerative disease.
Key issues.
There are many cross-sectional and prospective observation studies that link cognitive function, especially attention, working memory and executive function, to gait and fall risk. Other cognitive abilities (e.g., hazard estimation and planning) may also connection between falls and cognitive function.
General measures of cognitive function, such as Mini Mental State Examination, are often not closely related to falls community-dwelling healthy seniors.
The varied and sometimes suboptimal results of multifactorial interventions for fall risk may be improved upon by taking into account cognitive function, both with respect to assessment and intervention.
Although strong randomized controlled trial-based evidence is currently lacking, the results of multiple studies support the idea that cognitive interventions are useful for enhancing gait and reducing fall risk.
Pharmacologic and nonpharmacologic therapies may be effective at augmenting cognitive function and reducing the risk of falls. The advantages and disadvantages of the different approaches need to be carefully weighed.
Certain cognitive therapies apparently only improve gait under complex walking conditions, such as dual tasking, while enhance usual-walking.
There are reasons to suggest that the optimal approach to fall risk should target cognitive, behavioral and motor function, perhaps with task-specific training. Although a few small studies provide evidence of this intuitive solution, questions about dosing, delivery and efficacy remain.
Footnotes
Financial & competing interests disclosure
This work was supported in part by the National Institute of Aging (AG14100) and the Israel Ministry of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La VC, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 2.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 4.Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:1274–1279. doi: 10.1016/j.apmr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 6.van Dijk PT, Meulenberg OG, van de Sande HJ, Habbema JD. Falls in dementia patients. Gerontologist. 1993;33:200–204. doi: 10.1093/geront/33.2.200. [DOI] [PubMed] [Google Scholar]
- 7.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander NB. Gait disorders in older adults. J Am Geriatr Soc. 1996;44:434–451. doi: 10.1111/j.1532-5415.1996.tb06417.x. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudarsky L. Geriatrics: gait disorders in the elderly. N Engl J Med. 1990;322:1441–1446. doi: 10.1056/NEJM199005173222007. [DOI] [PubMed] [Google Scholar]
- 11.Sudarsky L. Gait disorders: prevalence, morbidity, and etiology. Adv Neurol. 2001;87:111–117. [PubMed] [Google Scholar]
- 12.Horak FB, Shupert CL, Mirka A. Components of postural dyscontrol in the elderly: a review. Neurobiol Aging. 1989;10:727–738. doi: 10.1016/0197-4580(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff JM, Nelson ME, Kaliton D, et al. Etiology and modification of gait instability in older adults: a randomized controlled trial of exercise. J Appl Physiol. 2001;90:2117–2129. doi: 10.1152/jappl.2001.90.6.2117. [DOI] [PubMed] [Google Scholar]
- 14.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 15.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander NB, Hausdorff JM. Linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2008;63:1325–1328. doi: 10.1093/gerona/63.12.1325. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 20.Faulkner KA, Redfern MS, Cauley JA, et al. Multitasking: association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007;55:570–576. doi: 10.1111/j.1532-5415.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 21.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 22.Welmerink DB, Longstreth WT, Jr, Lyles MF, Fitzpatrick AL. Cognition and the risk of hospitalization for serious falls in the elderly: results from the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:1242–1249. doi: 10.1093/gerona/glq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 24.Alfaro-Acha A, Al SS, Raji MA, Markides KS, Ottenbacher KJ. Does 8-foot walk time predict cognitive decline in older Mexicans Americans? J Am Geriatr Soc. 2007;55:245–251. doi: 10.1111/j.1532-5415.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 25.Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1335–1343. doi: 10.1093/gerona/63.12.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srygley JM, Mirelman A, Herman T, Giladi N, Hausdorff JM. When does walking alter thinking? Age and task associated findings. Brain Res. 2009;1253:92–99. doi: 10.1016/j.brainres.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iinattiniemi S, Jokelainen J, Luukinen H. Falls risk among a very old home-dwelling population. Scand J Prim Health Care. 2009;27:25–30. doi: 10.1080/02813430802588683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NICE. Background to the Current Guideline. Royal College of Nursing; London, UK: 2004. [Google Scholar]
- 29.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 30.Legters K. Fear of falling. Phys Ther. 2002;82:264–272. [PubMed] [Google Scholar]
- 31.Mackenzei L, Byles J, Higginbotham N. A prospective community-based study of falls among older people in Australia: frequency, circumstance, and consequences. Fall. 2002;22:143–152. [Google Scholar]
- 32.Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing. 1997;26:189–193. doi: 10.1093/ageing/26.3.189. [DOI] [PubMed] [Google Scholar]
- 33.Arfken CL, Lach HW, Birge SJ, Miller JP. The prevalence and correlates of fear of falling in elderly persons living in the community. Am J Public Health. 1994;84:565–570. doi: 10.2105/ajph.84.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamberlin ME, Fulwider BD, Sanders SL, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci. 2005;60:1163–1167. doi: 10.1093/gerona/60.9.1163. [DOI] [PubMed] [Google Scholar]
- 35.Friedman SM, Munoz B, West SK, Rubin GS, Fried LP. Falls and fear of falling: which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc. 2002;50:1329–1335. doi: 10.1046/j.1532-5415.2002.50352.x. [DOI] [PubMed] [Google Scholar]
- 36.Stevens JA. Falls among older adults – risk factors and prevention strategies. J Safety Res. 2005;36:409–411. doi: 10.1016/j.jsr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Davis JC, Robertson MC, Ashe MC, Liu-Ambrose T, Khan KM, Marra CA. International comparison of cost of falls in older adults living in the community: a systematic review. Osteoporos Int. 2010;21:1295–1306. doi: 10.1007/s00198-009-1162-0. [DOI] [PubMed] [Google Scholar]
- 38.Heinrich S, Rapp K, Rissmann U, Becker C, Konig HH. Cost of falls in old age: a systematic review. Osteoporos Int. 2010;21:891–902. doi: 10.1007/s00198-009-1100-1. [DOI] [PubMed] [Google Scholar]
- 39.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 40.Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 41.Sartini M, Cristina ML, Spagnolo AM, et al. The epidemiology of domestic injurious falls in a community dwelling elderly population: an outgrowing economic burden. Eur J Public Health. 2010;20:604–606. doi: 10.1093/eurpub/ckp165. [DOI] [PubMed] [Google Scholar]
- 42.Axer H, Axer M, Sauer H, Witte OW, Hagemann G. Falls and gait disorders in geriatric neurology. Clin Neurol Neurosurg. 2010;112:265–274. doi: 10.1016/j.clineuro.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Connell BR. Role of the environment in falls prevention. Clin Geriatr Med. 1996;12:859–880. [PubMed] [Google Scholar]
- 44.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 45.Beauchet O, Dubost V, Revel DC, Berrut G, Belmin J. How to manage recurrent falls in clinical practice: guidelines of the French society of geriatrics and gerontology. J Nutr Health Aging. 2011;15:79–84. doi: 10.1007/s12603-011-0016-6. [DOI] [PubMed] [Google Scholar]
- 46.Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of Falls in the Elderly Trial (PROFET): a randomised controlled trial. Lancet. 1999;353:93–97. doi: 10.1016/S0140-6736(98)06119-4. [DOI] [PubMed] [Google Scholar]
- 47.NICE. Methods Used to Develop the Guideline. Royal College of Nursing; London, UK: 2004. [Google Scholar]
- 48.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 49.Wagner EH. The cost–quality relationship. Do we always get what we pay for? JAMA. 1994;272:1951–1952. doi: 10.1001/jama.272.24.1951. [DOI] [PubMed] [Google Scholar]
- 50.NICE. Aims of the Guideline. Royal College of Nursing; London, UK: 2004. [Google Scholar]
- 51.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009:CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Michael YL, Whitlock EP, Lin JS, Fu R, O’Connor EA, Gold R. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815–825. doi: 10.7326/0003-4819-153-12-201012210-00008. [DOI] [PubMed] [Google Scholar]
- 53.Evidence report and evidence-based recommendations: fall prevention interventions in the Medicare population. Southern California Evidence-Based Practice Center; CA, USA: 2003. Rand Report. [Google Scholar]
- 54.de Vries OJ, Peeters GM, Elders PJ, et al. Multifactorial intervention to reduce falls in older people at high risk of recurrent falls: a randomized controlled trial. Arch Intern Med. 2010;170:1110–1117. doi: 10.1001/archinternmed.2010.169. [DOI] [PubMed] [Google Scholar]
- 55.Peeters GM, Heymans MW, de Vries OJ, Bouter LM, Lips P, van Tulder MW. Multifactorial evaluation and treatment of persons with a high risk of recurrent falling was not cost-effective. Osteoporos Int. 2010;22(7):2187–2196. doi: 10.1007/s00198-010-1438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vind AB, Andersen HE, Pedersen KD, Jorgensen T, Schwarz P. An outpatient multifactorial falls prevention intervention does not reduce falls in high-risk elderly Danes. J Am Geriatr Soc. 2009;57:971–977. doi: 10.1111/j.1532-5415.2009.02270.x. [DOI] [PubMed] [Google Scholar]
- 57.Vind AB, Andersen HE, Pedersen KD, Joergensen T, Schwarz P. Effect of a program of multifactorial fall prevention on health-related quality of life, functional ability, fear of falling and psychological well-being. A randomized controlled trial. Aging Clin Exp Res. 2010;22:249–254. doi: 10.1007/BF03324804. [DOI] [PubMed] [Google Scholar]
- 58.Salminen M, Vahlberg T, Kivela SL. The long-term effect of a multifactorial fall prevention programme on the incidence of falls requiring medical treatment. Public Health. 2009;123:809–813. doi: 10.1016/j.puhe.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Salminen MJ, Vahlberg TJ, Salonoja MT, Aarnio PT, Kivela SL. Effect of a risk-based multifactorial fall prevention program on the incidence of falls. J Am Geriatr Soc. 2009;57:612–619. doi: 10.1111/j.1532-5415.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 60.Mahoney JE. Why multifactorial fall-prevention interventions may not work: Comment on “Multifactorial intervention to reduce falls in older people at high risk of recurrent falls”. Arch Intern Med. 2010;170:1117–1119. doi: 10.1001/archinternmed.2010.193. [DOI] [PubMed] [Google Scholar]
- 61.Eriksson S, Gustafson Y, Lundin-Olsson L. Risk factors for falls in people with and without a diagnose of dementia living in residential care facilities: a prospective study. Arch Gerontol Geriatr. 2008;46:293–306. doi: 10.1016/j.archger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Shaw FE, Bond J, Richardson DA, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ. 2003;326:73. doi: 10.1136/bmj.326.7380.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Iersel MB, Verbeek AL, Bloem BR, Munneke M, Esselink RA, Rikkert MG. Frail elderly patients with dementia go too fast. J Neurol Neurosurg Psychiatry. 2006;77:874–876. doi: 10.1136/jnnp.2005.084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 65.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 66.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 67.Hausdorff JM, Doniger GM, Springer S, Yogev G, Giladi N, Simon ES. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65:1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Olde Rikkert MG. Executive functions are associated with gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- 70.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 71.Hausdorff JM, Yogev G. Cognitive function may be important for fall injury prevention trials. J Am Geriatr Soc. 2006;54:865–866. doi: 10.1111/j.1532-5415.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 72.Liu-Ambrose T, Pang MY, Eng JJ. Executive function is independently associated with performances of balance and mobility in community-dwelling older adults after mild stroke: implications for falls prevention. Cerebrovasc Dis. 2007;23:203–210. doi: 10.1159/000097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu-Ambrose T, Katarynych LA, Ashe MC, Nagamatsu LS, Hsu CL. Dual-task gait performance among community-dwelling senior women: the role of balance confidence and executive functions. J Gerontol A Biol Sci Med Sci. 2009;64:975–982. doi: 10.1093/gerona/glp063. [DOI] [PubMed] [Google Scholar]
- 74.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 75.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:110–115. doi: 10.1016/j.parkreldis.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Lord S, Rochester L, Hetherington V, Allcock LM, Burn D. Executive dysfunction and attention contribute to gait interference in ‘off’ state Parkinson’s disease. Gait Posture. 2010;31:169–174. doi: 10.1016/j.gaitpost.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 78.Lord SE, Weatherall M, Rochester L. Community ambulation in older adults: which internal characteristics are important? Arch Phys Med Rehabil. 2010;91:378–383. doi: 10.1016/j.apmr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain. 1999;122(Pt 3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- 80.Rogers WA. Attention and aging. In: Park DC, Schwarz N, editors. Cognitive Aging. Psychology Press, Taylor and Francis Group; USA: 2006. pp. 57–71. [Google Scholar]
- 81.Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9:41. doi: 10.1186/1471-2318-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plotnik M, Giladi N, Dagan Y, Hausdorff JM. Postural instability and fall risk in Parkinson’s disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp Brain Res. 2011;210(3–4):529–538. doi: 10.1007/s00221-011-2551-0. [DOI] [PubMed] [Google Scholar]
- 83.Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- 84.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 86.Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JH. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol. 2004;92:3255–3265. doi: 10.1152/jn.01139.2003. [DOI] [PubMed] [Google Scholar]
- 87.Pasman EP, Murnaghan CD, Bloem BR, Carpenter MG. Balance problems with Parkinson’s disease: are they anxiety-dependent? Neuroscience. 2011;177:283–291. doi: 10.1016/j.neuroscience.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 88.Sanders AE, Holtzer R, Lipton RB, Hall C, Verghese J. Egocentric and exocentric navigation skills in older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1356–1363. doi: 10.1093/gerona/63.12.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu-Ambrose T, Ahamed Y, Graf P, Feldman F, Robinovitch SN. Older fallers with poor working memory overestimate their postural limits. Arch Phys Med Rehabil. 2008;89:1335–1340. doi: 10.1016/j.apmr.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 90.Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26:555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allali G, Assal F, Kressig RW, Dubost V, Herrmann FR, Beauchet O. Impact of impaired executive function on gait stability. Dement Geriatr Cogn Disord. 2008;26:364–369. doi: 10.1159/000162358. [DOI] [PubMed] [Google Scholar]
- 92.Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil. 2011;8:2. doi: 10.1186/1743-0003-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 94.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 95.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 97.Turgeon M, Wing AM, Taylor LW. Timing and aging: Slowing of fastest regular tapping rate with preserved timing error detection and correction. Psychol Aging. 2010;26(1):150–161. doi: 10.1037/a0020606. [DOI] [PubMed] [Google Scholar]
- 98.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 99.Lajoie Y, Gallagher SP. Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr. 2004;38:11–26. doi: 10.1016/s0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 100.Delbaere K, Close JC, Heim J, et al. A multifactorial approach to understanding fall risk in older people. J Am Geriatr Soc. 2010;58:1679–1685. doi: 10.1111/j.1532-5415.2010.03017.x. [DOI] [PubMed] [Google Scholar]
- 101.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 102.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 103.Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80:608–613. doi: 10.1136/jnnp.2008.154633. [DOI] [PubMed] [Google Scholar]
- 104.Masdeu JC, Wolfson L, Lantos G, et al. Brain white-matter changes in the elderly prone to falling. Arch Neurol. 1989;46:1292–1296. doi: 10.1001/archneur.1989.00520480034016. [DOI] [PubMed] [Google Scholar]
- 105.Novak V, Haertle M, Zhao P, et al. White matter hyperintensities and dynamics of postural control. Magn Reson Imaging. 2009;27:752–759. doi: 10.1016/j.mri.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 108.Liu-Ambrose TY, Nagamatsu LS, Handy TC, Leghari A. Does impaired cerebellar function contribute to risk of falls in seniors? A pilot study using functional magnetic resonance imaging. J Am Geriatr Soc. 2008;56:2153–2155. doi: 10.1111/j.1532-5415.2008.01984.x. [DOI] [PubMed] [Google Scholar]
- 109.Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 110.Peterburs J, Bellebaum C, Koch B, Schwarz M, Daum I. Working memory and verbal fluency deficits following cerebellar lesions: relation to interindividual differences in patient variables. Cerebellum. 2010;9:375–383. doi: 10.1007/s12311-010-0171-z. [DOI] [PubMed] [Google Scholar]
- 111.Rondi-Reig L, Burguiere E. Is the cerebellum ready for navigation? Prog Brain Res. 2005;148:199–212. doi: 10.1016/S0079-6123(04)48017-0. [DOI] [PubMed] [Google Scholar]
- 112.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113••.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. Imaging study that sets the stage for using cholinesterase inhibitors to reduce fall risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticholinergic activity and motor performance in elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:83–85. doi: 10.1093/gerona/62.1.83. [DOI] [PubMed] [Google Scholar]
- 115.Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nebes RD, Pollock BG, Halligan EM, Houck P, Saxton JA. Cognitive slowing associated with elevated serum anticholinergic activity in older individuals is decreased by caffeine use. Am J Geriatr Psychiatry. 2011;19:169–175. doi: 10.1097/JGP.0b013e3181e4490d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29:15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 118.Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119•.Ben-Itzhak R, Giladi N, Gruendlinger L, Hausdorff JM. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008;56:695–700. doi: 10.1111/j.1532-5415.2007.01623.x. The positive effects of an attention-enhancing drug are documented in this study among older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Auriel E, Hausdorff JM, Giladi N. Methylphenidate for the treatment of Parkinson disease and other neurological disorders. Clin Neuropharmacol. 2009;32:75–81. doi: 10.1097/WNF.0B013E318170576C. [DOI] [PubMed] [Google Scholar]
- 121.Baezner H, Oster M, Henning O, Cohen S, Hennerici MG. Amantadine increases gait steadiness in frontal gait disorder due to subcortical vascular encephalopathy: a double-blind randomized placebo-controlled trial based on quantitative gait analysis. Cerebrovasc Dis. 2001;11:235–244. doi: 10.1159/000047645. [DOI] [PubMed] [Google Scholar]
- 122.Montero-Odasso M, Wells J, Borrie M. Can cognitive enhancers reduce the risk of falls in people with dementia? An open-label study with controls. J Am Geriatr Soc. 2009;57:359–360. doi: 10.1111/j.1532-5415.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123••.Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–1269. doi: 10.1212/WNL.0b013e3181f6128c. Small cross-over design study that clearly demonstrates that a cognitive-enhancing drug can reduce fall frequency, at least in selected patients with Parkinson’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahlskog JE. Think before you leap: donepezil reduces falls? Neurology. 2010;75:1226–1227. doi: 10.1212/WNL.0b013e3181f5d507. [DOI] [PubMed] [Google Scholar]
- 125.Litvinenko IV, Odinak MM, Mogil’naya VI, Emelin AY. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial) Neurosci Behav Physiol. 2008;38:937–945. doi: 10.1007/s11055-008-9077-3. [DOI] [PubMed] [Google Scholar]
- 126.Assal F, Allali G, Kressig RW, Herrmann FR, Beauchet O. Galantamine improves gait performance in patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:946–947. doi: 10.1111/j.1532-5415.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 127.Annweiler C, Montero-Odasso M, Schott AM, Berrut G, Fantino B, Beauchet O. Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil. 2010;7:50. doi: 10.1186/1743-0003-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Annweiler C, Beauchet O. High-dose vitamin D repletion-related falls and fractures: an uncontrolled mobility gain? Biofactors. 2010;36:407. doi: 10.1002/biof.124. [DOI] [PubMed] [Google Scholar]
- 129.Annweiler C, Schott AM, Allali G, et al. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology. 2010;74:27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- 130.Annweiler C, Allali G, Allain P, et al. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol. 2009;16:1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 131.Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. 2010;75:1810–1816. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 132.Grey A, Bolland M. Vitamin D: a place in the sun? Arch Intern Med. 2010;170:1099–1100. doi: 10.1001/archinternmed.2010.174. [DOI] [PubMed] [Google Scholar]
- 133.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 134.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peretz C, Korczyn AD, Shatil E, Aharonson V, Birnboim S, Giladi N. Computer-based, personalized cognitive training versus classical computer games: a randomized double-blind prospective trial of cognitive stimulation. Neuroepidemiology. 2011;36:91–99. doi: 10.1159/000323950. [DOI] [PubMed] [Google Scholar]
- 137.You JH, Shetty A, Jones T, Shields K, Belay Y, Brown D. Effects of dual-task cognitive-gait intervention on memory and gait dynamics in older adults with a history of falls: a preliminary investigation. NeuroRehabilitation. 2009;24:193–198. doi: 10.3233/NRE-2009-0468. [DOI] [PubMed] [Google Scholar]
- 138.Silsupadol P, Shumway-Cook A, Lugade V, et al. Effects of single-task versus dual-task training on balance performance in older adults: a double-blind, randomized controlled trial. Arch Phys Med Rehabil. 2009;90:381–387. doi: 10.1016/j.apmr.2008.09.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silsupadol P, Lugade V, Shumway-Cook A, et al. Training-related changes in dual-task walking performance of elderly persons with balance impairment: a double-blind, randomized controlled trial. Gait Posture. 2009;29:634–639. doi: 10.1016/j.gaitpost.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hiyamizu M, Morioka S, Shomoto K, Shimada T. Effects of dual task balance training on dual task performance ability in elderly people: a randomized controlled trial. Clin Rehabil. 2011 doi: 10.1177/0269215510394222. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 141.Yang YR, Wang RY, Chen YC, Kao MJ. Dual-task exercise improves walking ability in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1236–1240. doi: 10.1016/j.apmr.2007.06.762. [DOI] [PubMed] [Google Scholar]
- 142.Canning CG, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson’s disease: a pilot study. Clin Rehabil. 2008;22:226–233. doi: 10.1177/0269215507082341. [DOI] [PubMed] [Google Scholar]
- 143.Yogev-Seligman G, Giladi N, Brozgol M, Hausdorff JM. A training program to improve gait while dual tasking in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil. 2011 doi: 10.1016/j.apmr.2011.06.005. In press. [DOI] [PubMed] [Google Scholar]
- 144••.Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improved in patients with dementia: a randomized controlled trial. Neurology. 2010;74:1961–1968. doi: 10.1212/WNL.0b013e3181e39696. Largest study to date that examined the effects of dual-task training on gait. The results provide Class II evidence of its utility among patients with mild-to-moderate dementia. [DOI] [PubMed] [Google Scholar]
- 145.Verghese J, Holtzer R. Walking the walk while talking: cognitive therapy for mobility in dementia? Neurology. 2010;74:1938–1939. doi: 10.1212/WNL.0b013e3181e3d97d. [DOI] [PubMed] [Google Scholar]
- 146.Li KZ, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley PA. Benefits of cognitive dual-task training on balance performance in healthy older adults. J Gerontol A Biol Sci Med Sci. 2010;65:1344–1352. doi: 10.1093/gerona/glq151. [DOI] [PubMed] [Google Scholar]
- 147.Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010;65:1338–1343. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- 148.Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66:234–240. doi: 10.1093/gerona/glq201. [DOI] [PubMed] [Google Scholar]
- 149.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study 13. Arch Phys Med Rehabil. 2007;88:1154–1158. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 150.Anstey KJ, von SC, Luszcz MA. An 8-year prospective study of the relationship between cognitive performance and falling in very old adults. J Am Geriatr Soc. 2006;54:1169–1176. doi: 10.1111/j.1532-5415.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 151.Hendriks MR, Bleijlevens MH, van Haastregt JC, et al. Lack of effectiveness of a multidisciplinary fall-prevention program in elderly people at risk: a randomized, controlled trial. J Am Geriatr Soc. 2008;56:1390–1397. doi: 10.1111/j.1532-5415.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 152.Sjosten NM, Salonoja M, Piirtola M, et al. A multifactorial fall prevention programme in home-dwelling elderly people: a randomized-controlled trial. Public Health. 2007;121:308–318. doi: 10.1016/j.puhe.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 153.Robertson MC, Campbell AJ. Final Report. University of Otago; Otago, New Zealand: 2008. Optimisation of ACC’s Fall Prevention Programmes for Older People. [Google Scholar]
- 154.McKay C, Anderson KE. How to manage falls in community dwelling older adults: a review of the evidence. Postgrad Med J. 2010;86:299–306. doi: 10.1136/pgmj.2009.093468. [DOI] [PubMed] [Google Scholar]
- 155.Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 156.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 157.Delbaere K, Close JC, Brodaty H, Sachdev P, Lord SR. Determinants of disparities between perceived and physiological risk of falling among elderly people: cohort study. BMJ. 2010;341:c4165. doi: 10.1136/bmj.c4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59:704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- 160.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 161.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Montero-Odasso M, Wells JL, Borrie MJ, Speechley M. Can cognitive enhancers reduce the risk of falls in older people with mild cognitive impairment? A protocol for a randomised controlled double blind trial. BMC Neurol. 2009;9:42. doi: 10.1186/1471-2377-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology. 2009;23:500–508. doi: 10.1037/a0015389. [DOI] [PubMed] [Google Scholar]
- 164.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levy L. Cognition and Occupation Across the Lifespan. 2. The American Occupational Therapy Association, Inc; USA: 2005. Cognitive aging in prespective: implication for occupational therapy practitioners; pp. 327–344. [Google Scholar]
- 166.Liu-Ambrose T, Davis JC, Nagamatsu LS, Hsu CL, Katarynych LA, Khan KM. Changes in executive functions and self-efficacy are independently associated with improved usual gait speed in older women. BMC Geriatr. 2010;10:25. doi: 10.1186/1471-2318-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 168.Beauchet O, Allali G, Annweiler C, et al. Does change in gait while counting backward predict the occurrence of a first fall in older adults? Gerontology. 2008;54:217–223. doi: 10.1159/000127318. [DOI] [PubMed] [Google Scholar]
- 169.Lundin-Olsson L, Nyberg L, Gustafson Y. ‘Stops walking when talking’ as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- 170.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 171.Beauchet O, Annweiler C, Allali G, Berrut G, Herrmann FR, Dubost V. Recurrent falls and dual task-related decrease in walking speed: is there a relationship? J Am Geriatr Soc. 2008;56:1265–1269. doi: 10.1111/j.1532-5415.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- 172.Zijlstra A, Ufkes T, Skelton DA, Lundin-Olsson L, Zijlstra W. Do dual tasks have an added value over single tasks for balance assessment in fall prevention programs? A mini-review. Gerontology. 2008;54:40–49. doi: 10.1159/000117808. [DOI] [PubMed] [Google Scholar]
- 173.Beauchet O, Annweiler C, Dubost V, et al. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16:786–795. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 174.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 175.Hamilton R, Messing S, Chatterjee A. Rethinking the thinking cap: ethics of neural enhancement using noninvasive brain stimulation. Neurology. 2011;76:187–193. doi: 10.1212/WNL.0b013e318205d50d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Owen AM, Hampshire A, Grahn JA, et al. Putting brain training to the test. Nature. 2010;465:775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
Website
- 201.Healthcare Quality Improvement Partnership. Patient and Public Involvement: Older People’s Experiences of Falls and Bone Health Services. 2008 http://tinyurl.com/bone-healthexperience.