Abstract
Background
Advancements in gerontology have revealed key insights into the molecular and biochemical aspects of the aging process. The sirtuin pathway, most notable for its association with the anti-aging effects of calorie restriction, has received particular attention, and pharmacologic or transgenic upregulation of the sirtuin pathway has demonstrated some very promising results in laboratory models of aging. Alzheimer disease (AD), the leading cause of senile dementia, is a devastating neurodegenerative condition that is imposing an increasing burden on society. The lack of therapeutics currently available for the disease provides strong incentive for the development of an effective treatment strategy and, interestingly, research has uncovered a novel mechanism of action of the sirtuin pathway that offers significant potential as such.
Recent Developments
Sirt1, one of the seven mammalian homologues of the sirtuin family of NAD+-dependent deacetylases, has recently been demonstrated to attenuate amyloidogenic processing of amyloid-β protein precursor (AβPP) in cell culture studies in vitro and transgenic mouse models of AD. Mechanistically, Sirt1 increases α-secretase production and activity through activation of the α-secretase gene ADAM10. Since α-secretase is the critical enzyme responsible for the non-amyloidogenic cleavage of AβPP, upregulation of α-secretase shifts AβPP processing to reduce the pathological accumulation of the presumptive toxic Aβ species that results from β- and γ-secretase activity. Interestingly, a recent study of the spatial patterns of Aβ deposition in the brain indicates a strong correlation with an increased utilization of aerobic glycolysis in those regions. Aerobic glycolysis depletes cellular levels of NAD+ (via decreased NAD+/NADH ratio), and it is possible that a corresponding downregulation of the NAD+-dependent sirtuin pathway is partly responsible for the amyloidogenic processing of AβPP.
Where Next?
The specific inhibition of Aβ generation by Sirt1 coupled with the link between aerobic glycolysis, NAD+ depletion, and amyloidogenesis via the sirtuin pathway has translational implications. On the one hand, the likely underlying role of the sirtuin pathway in AD onset and development may enlighten our understanding of this devastating condition. On the other, therapeutic upregulation of Sirt1 may provide opportunities for the amelioration of AD-type neuropathology through an inhibition of amyloidogenesis, among other things (i.e., regulation of cellular metabolism or inhibition of tau pathology — see below). Ultimately, further analysis into both aspects is necessary if any progress is to be made.
Introduction
Aging is considered the manifestation of stochastic damages to the cell that accumulate over many years, and the generation of reactive oxidative species (ROS), which naturally coincides with respiration,1, 2 is believed to be significantly involved. ROS are produced as unwanted byproducts in electron transport during oxidative phosphorylation. Despite the presence of endogenous antioxidants, a significant portion of ROS go unsequestered within mitochondria and eventually damage macromolecules, such as membrane phospholipids, proteins, DNA, and RNA, rendering them impaired or entirely dysfunctional.3 Furthermore, accumulating ROS within the mitochondria can trigger apoptosis through the release of cytochrome c into the cytoplasm. The latter forms an apoptosomal complex with procaspase, ATP, and Apaf-1 (an apoptotic, protease activating factor).4 Over time, biological tissue succumbs to ROS-mediated damages; aging is thus traditionally viewed as a disorganized and inevitable process.
Calorie restriction (CR) has provided an interesting glimpse into the mechanisms regulating aging. Animals undergoing CR, a dietary regimen involving strictly reduced caloric intake (approximately 30–40% compared to normal intake), exhibit lifespan extension and reduced morbidity development with aging.5–8 These benefits have been demonstrated to be partly mediated by a family of NAD+-dependent histone/protein deacetylases, called the sirtuins.9 Histone deacetylases (HDACs) function as epigenetic regulators of gene and protein activity that act via catalytic cleavage of acetyl groups from lysine residues (Figure 1), and the HDAC superfamily is comprised of over 45 enzymes identified in a wide variety of eurkaryotes.10 HDACs are generally divided into three classes based on sequence homologies of the catalytic domain, with the sirtuin family belonging to the class III, NAD+-dependent HDACs.9
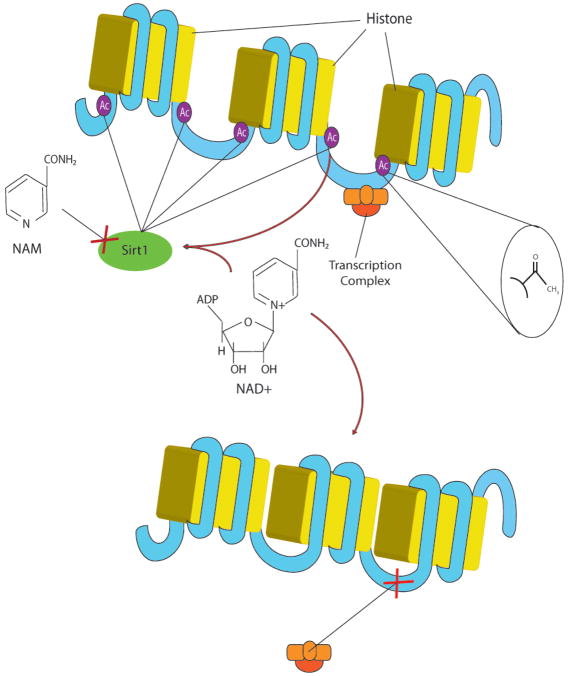
Figure 1.
NAD+-dependent histone deacetylase (HDAC) activity in relation to gene transcription. DNA is typically inaccessible to transcription complexes as it is tightly wound around histone proteins. Once acetylated (usually by an acetylase enzyme that attaches an acetyl group to a lysine residue on the histone protein), the DNA becomes physically available for transcription, as noted in the figure by the larger gaps between the histones. NAD+-dependent HDAC enzymes (Sirt1 shown here) effectively reverse this process by catalytically removing acetyl groups from lysine residues, thereby tightening the DNA/histone complexes. These enzymes require NAD+ and an acetylated lysine as cofactors for activation. Therefore, the presence of NAD+ promotes enzyme activity, while the presence of the cleavage product of the deacetylation reaction, nicotinamide (NAM), inhibits it. In the case of protein activation, the NAD+-dependent HDAC acts by removing acetyl groups from lysine residues directly from the protein. This may activate or inhibit the protein, depending on the particular context (see Figure 2).
Sirt1, one of the seven mammalian homologues belonging to the sirtuin family, is a regulator of proteins and genes involved in antioxidant response (FOXO3),11, 12 anti-inflammatory response (NFκB),13 anti-apoptotic response (p5314 and FOXO3), insulin response (IGF-1),15 and gene transcription (PGC-1).15 Sirt1 has also been demonstrated to be a regulator of mitochondrial biogenesis,16 as well as an indispensible agent in synaptic plasticity, learning, and memory.17, 18 The sirtuin pathway is thus important in cell survival under stressful conditions, such as CR. Notably, laboratory investigations support the role of Sirt1 in the proliferative, anti-aging effects of CR in a variety of mammals, and Sirt1 transgenic mice demonstrate phenotypes resembling those induced by CR.19 The remaining members of the sirtuin family (i.e., Sirt2–7) are less well characterized, although studies also indicate their relevance to cell survival and stress response. The seven sirtuin proteins and their corresponding localization and activities are summarized in Table 1.
Table 1.
Established biochemical and functional features of human sirtuins. (Adapted from Albani et al., 2010)9 and reprinted with permission from IOS Press.
| Sirtuin | Cell Localization | Activity/Key Targets |
|---|---|---|
| Sirt1 | Nucleus | Deacetylase/p53, FOXO3, RARβ, PGC1-α, PPAR-γ, NFκB, IGF-1, Histone H1, H3, H4 |
| Sirt2 | Cytoplasm | Deacetylase/Histone H4, α-tubulin |
| Sirt3 | Mitochondria | Deacetylase and ART*/Ku70, Acetyl-CoA synthetase, Glutamate dehydrogenase, Isocitrate dehydrogenase |
| Sirt4 | Mitochondria | ART/Glutamate dehydrogenase |
| Sirt5 | Mitochondria | Deacetylase/Cytochrome-c |
| Sirt6 | Nucleus | Deacetylase and ART/Histone H3 |
| Sirt7 | Nucleus | Deacetylase (?)/RNA PolI complex (?) |
ART = ADP-ribosyltransferase activity
Laboratory upregulation of the sirtuin pathway, via sirtuin-activating compounds like resveratrol, has effectively reversed the aging process and its comorbidities in non-stressed animals (i.e., those not experiencing environmental stressors that typically activate the pathway, like CR).20 Even more, the correlation between aging and neurodegeneration has led researchers to investigate the sirtuin pathway as it pertains to Alzheimer disease (AD), and recent reports demonstrate that Sirt1 hyperactivity is capable of reducing AD pathologies both in vitro and in vivo through upregulation of the ADAM10 gene. We here discuss these results and their implications to the field.
Alzheimer disease
AD is an age-related neurodegenerative condition that affects 35 million people worldwide and is the leading cause of dementia among the elderly.21 Specifically, the incidence of AD is 15% among those 65 and older and close to 50% for those above age 85.21 The strong correlation between AD and age is demonstrated by the similar phenotypes of the two “conditions.” Most notably, normal aging involves the gradual decline in memory and cognitive functions that are associated with neuronal networks of the mediotemporal lobe. The neurons of the parahippocampal region and hippocampal formation are particularly affected in the normally aged brain,22 and it is precisely these regions that are first affected in AD.23 Moreover, AD is largely the result of years of accumulated oxidative damage and mitochondrial malfunction21 and, as stated above, aging involves similar perturbations. Importantly, our intention here is not to render AD and aging as a single, equal entity, but to allude to the distinct similarities between the two.
At present, AD is therapeutically untreatable. It is pathologically characterized by widespread oxidative stress, mitochondrial damage and altered distribution, neuroinflammation, calcium dysregulation, metal dishomeostasis, neurofibrillary tangle formation, and amyloid-β (Aβ) oligomerization and fibrillation.21 Although there are several differing theories describing the molecular and temporal instigation of AD, the latter phenomena (i.e., Aβ oligomerization/fibrillation), are certainly the most studied.
Aβ, the product of the proteolytic cleavage of the amyloid-β protein precursor (AβPP) into 38, 40, or 42 amino acid peptides, is generally viewed as a toxic mediator of AD,24 although this is still debated.25 The generation of Aβ is orchestrated by the β- and γ-secretases in a sequential manner such that inhibition of either secretase prevents Aβ generation. Although Aβ has no known function within the cell, its oligomerization is toxic to neurons in vitro and in vivo and produces synaptic dysfunction, calcium dysregulation, oxidative stress, and neuroinflammation.26 Additionally, Aβ (specifically Aβ42) is the primary component of the senile plaques that become deposited in the brains of AD and are a hallmark feature of the disease.27
Alternate processing of AβPP, through α-secretase cleavage (followed by that of γ-secretase), produces a soluble segment of AβPP that may be neuroprotective.28 This “non-amyloidogenic” pathway deters the processing of AβPP through the β- and γ-secretases and thus inhibits the formation of toxic Aβ species. Interestingly, recent evidence indicates that Sirt1, along with its varied role in the regulation of aging, is also a key regulator of α-secretase, and thus of amyloidogenic processing (see below).29 Sirt1 upregulation may therefore provide an excellent therapeutic approach to a disease that is hereto untreatable.
Sirt1 and AD: Biomolecular Amelioration of the AD Phenotype
Evidence for the benefits of the sirtuin pathway in AD has chiefly stemmed from a focus on the effects of Sirt1. In vitro Sirt1 overexpression, mediated by NAD+ or resveratrol, leads to a reduction of oligomerized Aβ peptides in a concentration-dependent manner15 and ameliorates oxidative stress.30, 31 Of note, the effects of the former were mediated by a switch to the non-amyloidogenic processing of AβPP due to increased α-secretase activation.32 Overexpression of Sirt1 is also reported to prevent microglia-dependent Aβ toxicity through an inhibition of NFκB signaling.33
Recent in vivo data has corroborated these findings, such that mutant mice overexpressing both AβPP and Sirt1 (APPswe/PSEN1dE9 double transgenic crossed with Sirt1 transgenic) exhibit a markedly reduced production of toxic Aβ species.29 Furthermore, the brain pathology and behavioral deficits of the APPswe/PSEN1dE9 mice were exacerbated when crossed with Sirt1 knockout mice.29 The anti-amyloidogenic effects of Sirt1 were demonstrated to be mediated through upregulation of α-secretase transcription and expression. Specifically, Sirt1 was shown to deacetylate and thereby activate the retinoic acid receptor-β (RARβ) protein (i.e., Sirt1 removes an acetyl group from lysine residue(s) of RARβ). RARβ stimulates transcription of the ADAM10 gene (which encodes α-secretase) by binding to the ADAM10 promoter;34 therefore, Sirt1-induced activation of RARβ increased ADAM10 transcription and α-secretase production. Notably, ADAM10 is also known to initiate the Notch pathway via cleavage of the membrane-bound Notch receptor.35 This liberates an intracellular Notch domain that forms a transcription complex and upregulates transcription of genes involved in neurogenesis.35, 36 The protective effects of Sirt1 overexpression in these models may therefore result from a beneficial double effect of Notch activation and α-secretase-induced non-amyloidogenic processing.
In p25 transgenic mice, which overexpress the cdk5-activating human p25 protein and exhibit tau hyperphosphorylation and neurodegeneration,37 injection of the Sirt1-activating polyphenol resveratrol resulted in less hippocampal degeneration, less cognitive deficit, and reduced acetylation of Sirt1 substrates PGC-1α and p53.38 Similarly, when Sirt1 was directly overexpressed in the hippocampus of these mice using a lentiviral vector, the effects of resveratrol injections were reproduced. The AD-attenuating effects of Sirt1 in the mammalian brain are summarized in Figure 2.
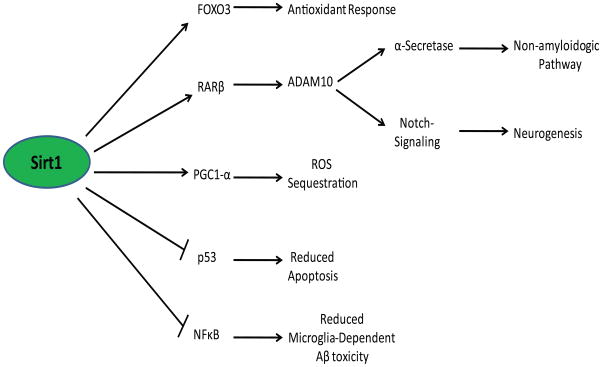
Figure 2.
Sirt1 activity as it relates to AD etiology. Activation of Sirt1 enables the deacetylation of a variety of proteins, resulting in a robust, protective cellular response. Of particular note is the activation of RARβ by Sirt1 that confers the non-amyloidogenic processing of AβPP as well as the instigation of notch signaling (both through ADAM10 promotion). Sirt1 is also a regulator of proteins and genes involved in antioxidant response (FOXO3),11, 12 anti-inflammatory response (NFκB),13 anti-apoptotic response (p53),14 and mitochondrial biogenesis/ROS sequestration (PGC1-α).15 As noted in the figure, Sirt1 inhibits p53 and NFκB, and activates FOXO3, RARβ, and PGC1- α. The net phenotype of a Sirt1-activated cell displays reduced amyloid oligomerization, reduced oxidative stress, and increased resistance to apoptosis/inflammation-induced toxicity.
Sirt1 and AD: Possible Mechanistic Link to Disease Pathogenesis
As mentioned above, AD is a tremendously complex disorder characterized by numerous biomolecular, genetic, and environmental aberrations. Despite our detailed knowledge of a number specific cascades of neurodegeneration in AD, there has yet to be a global understanding of its underlying causation or of its predictable and specific anatomic distribution of pathology and cell death. As to the latter, AD is known to be principally a disease of the parahippocampal region and the hippocampal formation of the mediotemporal lobe.39 Specifically, neurons of the perforant path, connecting the entorhinal cortex to the dentate gyrus, are first to be pathologically affected, and degeneration, thereafter, tends to spread cortico-cortically, such that the subsequently affected neurons are those of the CA1 region of the hippocampal formation (which are innervated by the dentate gyrus),40 followed by the CA2/3 neurons, and ultimately those of the neocortex and beyond.23 Normally aged brains demonstrate similar distribution of neuronal degredation,22 and as of yet, there is no explanation for this consistency. A recent study, however, has shed light on a potential link between mediotemporal degeneration, glucose metabolism, and the sirtuin pathway.
Using PET imaging, it was demonstrated that regions typically prone to Aβ plaque deposition and neurodegeneration in AD are spatially similar to the regions that metabolize glucose via aerobic glycolysis in normal, young brains.41 Utilization of aerobic glycolysis involves the metabolism of glucose-6-phosphate to pyruvate and lactic acid despite the presence of oxygen, and is important for proliferating cells as a quick source of ATP.42 Incidentally, aerobic glycolysis also involves a gradual depletion of NAD+ reserves within the cell via increased NADH production and decreased NAD+ regeneration (i.e., through oxidative phosphorylation). The NAD+-dependent histone deacetylase Sirt1 is inhibited by concomitant decreases in NAD+.43, 44 We therefore suggest that there may be a mechanistic link between the use of aerobic glycolysis in the mediotemporal lobe of the brain and the coincident spatial distribution of amyloid pathology in AD. That is, in the aforementioned brain region, the reliance of neurons on aerobic glycolysis likely inhibits sirtuin activity through the depletion of NAD+ pools and thus results in a shift in AβPP processing toward the amyloidogenic pathway. Aβ oligomerization and fibrillation thus occur strictly in that region and induce AD-type pathological cascades, neuronal dysfunction, and ultimately cognitive decline (Figure 3). While theses notions have not been empirically tested, they certainly merit some investigation.
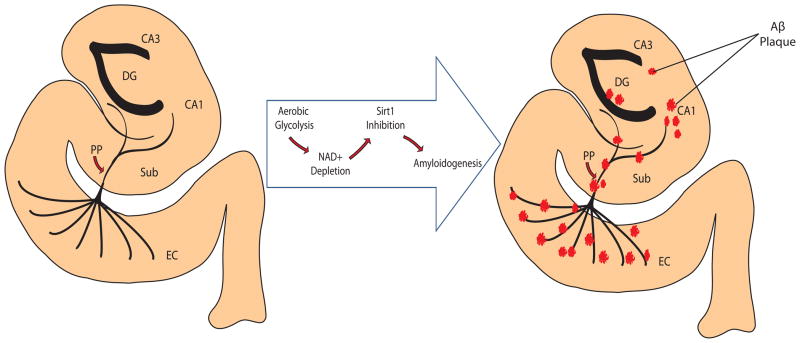
Figure 3.
Progression of amyloidogenesis in parahippocampal region and hippocampal formation of mediotemporal lobe. The energy-demanding regions of the brain, most notably the entorhinal cortex (EC) of the parahippocampal region, resort to aerobic glycolysis as an alternate means of generating ATP. As a result, the NAD+ pool is reduced and the NAD+-dependent deacetylase Sirt1 becomes hypoactive. As Sirt1 has been recently demonstrated to increase α-secretase production (through RARβ deacetylation and subsequent ADAM10 transcription), the amyloidogenic phenotype becomes upregulated. That is, the reduced processing of AβPP through α-secretase (due to the reduction in α-secretase synthesis) is complemented by an increase in AβPP processing by β- and γ-secretases to yield Aβ peptides. An eventual accumulation Aβ into senile plaques occurs along the network of innervation as the degeneration spreads cortico-cortically.
*DG = Dentate Gyrus, EC = Entorhinal Cortex, Sub = Subiculum, PP = Perforant Path
Conclusions and future directions
The dysregulation of the sirtuin pathway likely has a key underlying role in the pathogenesis of AD, although whether or not that role is causal is still uncertain. Nevertheless, the therapeutic potential of Sirt1 upregulation has been repeatedly demonstrated in cell culture and animal models of the disease, and its relevance to human dementia merits guarded optimism. Current clinical trials monitoring the beneficial effects of resveratrol administration to AD patients are underway,45–47 although it is not yet known whether late-stage, oral doses of the Sirt1-activating compound will be sufficient to protect patients from further neuronal decay. Ultimately, an efficient harnessing of the beneficial effects of the sirtuin pathway will certainly be of great therapeutic importance in the general population, where AD and aging both suffer a lack of preventative measures.
Search Strategy and Selection Criteria
References for this review were compiled through searches of PubMed. Search terms were as follows: “Aging”, “Alzheimer’s disease”, “amyloid”, “amyloidogenesis”, “glycolysis”, “longevity”, “perforant path”, “secretase”, and “sirtuin”. Only papers published in English, and those published between 2005 through December 2010, were considered. The final reference list was generated based on the relevance of the topics covered in each manuscript to those of this review.
Acknowledgments
Sources of support: Work in the authors’ laboratories was supported by the National Institutes of Health (AG028679 to MAS and AG031852 to XWZ). The funding source had no involvement in data collection, analysis or interpretation of the study.
Footnotes
Authors’ contributions
DJB conceptualized and wrote the manuscript. MAS assisted in the preparation of the manuscript. XWZ edited the final version of the manuscript. The remaining authors (HGL, AC, MP, GC) reviewed citations and assisted in editing the manuscript.
Conflicts of Interest
Dr. Zhu was a consultant for and received grant support from Medivation. Dr. Smith was a consultant for Medivation and Neurotez; received grant support from Neurotez; owned stock options in Curaxis, Neuropharm, and Neurotez; and received lecture fees from Medivation, and Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11 (3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132(2):171–6. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, et al. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59(4–5):290–4. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1(2):120–9. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 5.Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14(14):2135–7. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- 6.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35(3):299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 7.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17(3):313–21. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair DA. Paradigms and pitfalls of yeast longevity research. Mech Ageing Dev. 2002;123(8):857–67. doi: 10.1016/s0047-6374(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 9.Albani D, Polito L, Forloni G. Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: experimental and genetic evidence. J Alzheimers Dis. 2010;19(1):11–26. doi: 10.3233/JAD-2010-1215. [DOI] [PubMed] [Google Scholar]
- 10.Marmorstein R. Structure of histone deacetylases: insights into substrate recognition and catalysis. Structure. 2001;9(12):1127–33. doi: 10.1016/s0969-2126(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 11.Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9(4):371–8. [PubMed] [Google Scholar]
- 12.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 13.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23 (12):2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21(10):2383–96. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Fivecoat H, Ho L, Pan Y, Ling E, Pasinetti GM. The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochim Biophys Acta. 2010;1804(8):1690–4. doi: 10.1016/j.bbapap.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444(7121):868–74. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 17.Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–9. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6(6):759–67. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 20.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nature communications. 2010;1(1):1–8. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon. 2010;56(9):484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A. 2010;107(28):12687–91. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, et al. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain. 2009;132(Pt 5):1324–34. doi: 10.1093/brain/awp059. [DOI] [PubMed] [Google Scholar]
- 24.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 25.Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, Nakamura M, et al. Reexamining Alzheimer’s disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis. 2009;18(2):447–52. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101(5):1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 27.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 28.Kojro E, Fahrenholz F. The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell Biochem. 2005;38:105–27. doi: 10.1007/0-387-23226-5_5. [DOI] [PubMed] [Google Scholar]
- 29.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142(2):320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem. 2009;110(5):1445–56. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 31.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8(5):347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745–54. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280(48):40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 34.Tippmann F, Hundt J, Schneider A, Endres K, Fahrenholz F. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. Faseb J. 2009;23(6):1643–54. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- 35.Costa RM, Drew C, Silva AJ. Notch to remember. Trends Neurosci. 2005;28(8):429–35. doi: 10.1016/j.tins.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Xiao MJ, Han Z, Shao B, Jin K. Notch Signaling and Neurogenesis in Normal and Stroke Brain. Int J Physiol Pathophysiol Pharmacol. 2009;1(2):192–202. [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40 (3):471–83. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol. 1986;20(4):472–81. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- 40.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–82. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 41.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A. 2010;107(41):17763–7. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Wang SY, Fleuriel C, Leprince D, Rocheleau JV, Piston DW, et al. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci U S A. 2007;104(3):829–33. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Clinicaltrials.gov. Pilot Study of the Effects of Resveratrol Supplement in Mild-to-Moderate Alzheimer’s Disease. 2008 August 28; [cited December 15, 2010]; Available from: http://clinicaltrials.gov/ct2/show/NCT00743743?term=NCT00743743.
- 46.Clinicaltrials.gov. Randomized Trial of a Nutritional Supplement in Alzheimer’s Disease. 2008 March 24; [cited December 15, 2010]; Available from: http://clinicaltrials.gov/ct2/show/NCT00678431.
- 47.Clinicaltrials.gov. Effects of Dietary Interventions on the Brain in Mild Cognitive Impairment (MCI) 2010 [cited December 15, 2010]; Available from: http://clinicaltrials.gov/ct2/show/NCT01219244?term=NCT01219244.