Abstract
Context
The optimal treatment of acute myeloid leukemia (AML) in first complete remission (CR1) is uncertain. Current consensus, based on cytogenetic risk, recommends myeloablative allogeneic stem cell transplantation (alloSCT) for poor-risk but not for good-risk AML. AlloSCT, autologous transplant and consolidation chemotherapy are considered of equivalent benefit for intermediate-risk AML. We undertook a systematic review and meta-analysis of prospective trials evaluating alloSCT versus non-alloSCT therapies for AML-CR1.
Objective
To quantify relapse-free survival (RFS) and overall survival (OS) benefit of alloSCT for AML in CR1. In subgroup analyses, RFS and OS benefit of alloSCT was determined for good-, intermediate- and poor-risk AML.
Methods
Combining the search terms: ‘allogeneic’; ‘acut*’ and ‘leukem*/leukaem*/leucem*/leucaem*/aml’; ‘myelo*’ or ‘nonlympho*’, we searched the PubMed, Embase and Cochrane Registry of Controlled Trials databases in March 2009. 1712 articles were accessed.
Study Selection
Prospective trials assigning adult AML-CR1 patients to alloSCT versus non-alloSCT treatment(s) based on donor availability, and reporting RFS and/or OS outcomes on intent-to-treat, donor versus no-donor basis were identified.
Data Extraction
Two reviewers independently extracted study characteristics, interventions, and outcomes. Hazard ratios (HR) (with 95% CI) were determined.
Data Synthesis
24 trials and 6,007 patients were analyzed. Inter-study heterogeneity was not significant. Fixed effects meta-analysis was performed. HR of relapse or death with alloSCT for AML-CR1 was 0.80 (0.74–0.86). Significant RFS benefit of alloSCT was documented for poor-risk (HR 0.69 (0.57–0.84)) and intermediate-risk AML (HR 0.76 (0.68–0.85)); but not for good-risk AML (HR 1.06 (0.80–1.42)). HR of death with alloSCT for AML-CR1 was 0.90 (0.82–0.97). Significant OS benefit of alloSCT was documented for poor-risk (HR 0.73 (0.59–0.90)) and intermediate-risk AML (HR 0.83 (0.74–0.93)); but not for good-risk AML (HR 1.07 (0.83–1.38)).
Conclusion
AlloSCT has significant RFS and OS benefit for intermediate- and for poor-risk AML, but not for good-risk AML in CR1.
Keywords: acute myeloid leukemia, allogeneic transplantation, meta-analysis
Introduction
Achieving a cure, even for younger adult patients with de-novo acute myeloid leukemia (AML), remains a challenge. While over 70% of such patients will enter a first complete remission (CR1) after induction chemotherapy, a substantial number experience disease relapse. 1 Allogeneic stem cell transplantation (alloSCT) after myeloablative conditioning is a curative treatment option for younger AML patients in CR1. However, concerns regarding alloSCT related toxicity and questions regarding its benefit limit its utilization for patients who have attained an initial remission. Alternative therapies include intensive consolidation chemotherapy or autologous stem cell transplant (autoSCT). The current consensus, reflected in treatment guidelines of the National Comprehensive Cancer Network (NCCN) (V1.2009: www.nccn.org), is based on cytogenetic stratification into good-, intermediate-, and poor-risk AML. Patients with good-risk AML in CR1 are recommended chemotherapy, with autoSCT considered an acceptable alternative. Patients with poor-risk AML in CR1 are recommended alloSCT. There is no preferred therapy for patients with intermediate-risk AML in CR1: alloSCT, consolidation chemotherapy and autoSCT are considered of equivalent benefit.
Multiple prospective trials have been undertaken to clarify the role of alloSCT for AML in CR1. In the context of alloSCT trial design, treatment assignment has typically been based on donor availability: patients with HLA matched siblings are assigned to alloSCT (donor arm), and those without matched siblings (or without siblings) are assigned to non-alloSCT therapy (no-donor arm). Although not randomized comparisons, these studies have nevertheless been widely accepted as providing good quality evidence of treatment-effect since no evidence of major bias arising from differences in biological and socioeconomic factors has been identified.
Various prospective clinical trials, retrospective studies and systematic reviews have helped determine the current treatment consensus for AML in CR1. Retrospective analyses are prone to errors of bias and confounding, and may therefore provide inaccurate estimates of effect. Prospective biologic assignment trials offer a means of reducing such errors. However, their results have thus far not provided definitive evidence to support treatment recommendations. While some individual trials have documented superior relapse-free survival (RFS), none has documented an overall survival (OS) benefit for alloSCT across all cytogenetic risk groups. Within cytogenetic risk groups, the evidence regarding alloSCT impact is also limited (discussed below).
In order to arrive at comprehensive estimates of OS and RFS benefit from the totality of the clinical trial data available, we undertook a systematic literature review and meta-analysis of all prospective biologic assignment clinical trials of alloSCT versus consolidation chemotherapyand/or autoSCT for AML in CR1, on an intent-to-treat (ITT) donor versus no-donor basis.
Materials and Methods
Data Sources
We undertook searches of the Medline (PubMed), Embase and Cochrane Registry of Controlled Trials (updated March 2009), combining the search terms: ‘allogeneic’; ‘acut*’ and ‘leukem*/leukaem*/leucem*/leucaem*/aml’; ‘myelo*’ or ‘nonlympho*’. Medline (PubMed) and Embase searches were restricted to human studies.. Studies identified underwent title/abstract review (JK and CC), and clearly non-relevant articles were discarded. Text review of the remainder was performed to assess their suitability. The bibliographies of retained articles were examined to identify additional studies. The abstracts of relevant scientific meetings were examined similarly to ensure complete review of the available data. International expert input was obtained to identify additional relevant trials, including those in non-English speaking countries. Recent reviews and meta-analyses were also accessed to identify additional studies that met inclusion criteria. 2–7
Study Selection
Studies included were prospective trials of adults (wholly or predominantly) with AML in CR1 that assigned alloSCT versus a comparator of consolidation chemotherapy and/or autoSCT. Eligible trials reported hazard ratios (HR) (95% CI) for OS and/or RFS benefit on an ITT donor no-donor basis (or provided data to estimate HR by the method of Parmar et al). 8 When multiple publications reported on the study, the most updated data was analyzed. Unadjusted HR was preferred in the analysis, since adjusted HR, reported in a minority of studies, was considered likely to adjust for different covariates per study, potentially impeding analysis across studies. Further, prospective biological treatment assignment was considered likely to equalize covariates over the large number of patients analyzed. Adjusted HR was utilized in sensitivity analyses.
Data Extraction
The data was abstracted in a standardized format by two independent reviewers. The data collected for each study included: study name, study first author, publication year, year of initial enrollment, total number allocated to therapy, number assigned to donor and no-donor arm on an ITT basis, median patient age (years), median duration of follow-up (months), number of events (death, relapse) in each arm, study endpoints of OS and/or RFS benefit. We used OS and RFS (also reported as disease-free survival) as per the individual studies. Data on treatment related mortality (TRM) (also reported as non-relapse mortality) was also collected. We also collected data on therapy: induction therapy regimen, interim therapy regimen (if any), stem cell source (bone marrow (BM) or peripheral blood (PB)), alloSCT conditioning, autoSCT conditioning and consolidation chemotherapy regimen. Discrepancies in data extraction were resolved by consensus, referring back to the original article, and by contacting the study authors if necessary. When missing data were encountered, the primary authors were contacted to complete the data analysis.
Quality Assessment
We assessed for quality based on the requirement for prospective treatment assignment, the reporting of outcomes on an ITT basis, the study size, the number of participating centers, the adequacy of induction chemotherapy (treatment regimen; percentage of patients entering CR1), and the proportion of patients allocated to alloSCT who underwent assigned therapy (Tables 1–3). Given the unambiguous endpoints (OS, RFS) and study treatments, we did not anticipate any impact of lack of blinding on outcomes. We did not explicitly score the methodologic quality of the included trials since the value of doing so is controversial. Ad-hoc scores may lack demonstrated validity, and results may not be associated with quality. 9–12 Instead, we performed subgroup and sensitivity analyses, and undertook tests of interaction, as is widely recommended. 11–13
Table 1. Summary of clinical trials evaluating alloSCT benefit for AML in CR1.
The study ID, first author, year of publication, enrollment period, median patient age (years) at enrollment in the donor and no-donor arms (with range), median duration of follow-up (months) (with range), and study conclusions regarding overall survival (OS) and relapse-free survival (RFS) benefit are also shown for each study.
| Author (Pub Yr) | Study ID | Enrollment Period | Donor arm Median age Yr (range) | No-donor arm Median age Yr (range) | Median follow-up Mo (range) | AlloSCT benefit in AML-CR1? Study Conclusions |
|---|---|---|---|---|---|---|
| Ferrant17 (1991) | NR | 1985–1990 | NR | NR | 42 (4-NR) | Overall: RFS-Yes; OS-NR Cyto stratified: NR |
| Schiller18 (1992) | ALP3-4 | 1982–1990 | 33 (16–43) | 33 (18–45) | 48 (4.3–93.6) | Overall: RFS-No; OS-No Cyto stratfied: NR |
| Cassileth19 (1992) | E3483* | NR | NR (16–41) | NR (16–41) | 48 (NR) | Overall: RFS-No; OS-NR Cyto stratified: NR |
| Archimbaud20 (1994) | LYLAM85 | 1985–1990 | 31 (19–39) | 31 (17–39) | 63 (NR) | Overall: RFS-No; OS-No Cyto stratfied: NR |
| Hewlett21 (1995) | S8125 | 1982–1986 | 28 (16–49) | 32 (13δ–39) | 111 (83–142) | Overall: RFS-No; OS-No Cyto stratified: NR |
| Sierra22 (1996) | CETLAM88 | 1988–1993 | 31 (1–50) | 29.5 (1–50) | 98 (5–106) | Overall: RFS-No; OS-No Cyto stratified: NR |
| Harousseau23 (1997) | GOELAM1 | 1987–1994 | NRχ(15–40) | NRχ(15–40) | 62 (23–103) | Overall: RFS-No; OS-NR Cyto stratified: NR |
| Keating24 (1998) | EORTC/GIMEMA-AML8A | 1986–1993 | 32 (13α–45) | 31 (11α–45) | 69.6 (NR) | Overall: RFS-Yes; OS-No Cyto stratified: NR |
| Slovak25 (2000) | E3489/S9034 | 1990–1995 | 34 (18–54) | 39 (16–55) | 57.6 (8–90) | Overall: RFS-No; OS-No Cyto stratified: RFS-No; OS-Yes (poor-risk) |
| Suciu26 (2003) | EORTC/GIMEMA-AML10 | 1993–1999 | 35 (15–45) | 33 (15–45) | 48 (NR) | Overall: RFS-Yes; OS-No Cyto stratified: RFS-Yes;OS-Yes (poor-risk) |
| Brunet27 (2004) | CETLAM94 | 1994–1999 | 37 (16–50) | 37 (16–50) | 54 (1–85) | Overall: NA (alloSCT option for non-fav risk) Cyto stratified: RFS-No; OS-No |
| Jourdan28 (2005) | BGMT 84/87/91/95 | 1984–2001 | 34 (15–45) | 33.5 (15–45) | 114 (29–222) | Overall: RFS-Yes; OS-No Cyto stratified: RFS-NR; OS-NR |
| Burnett29 (2006) | MRC AML10 | 1988–1995 | NR (0–45+) | NR (0–45+) | 142 (26–193) | Overall: RFS-Yes; OS-No Cyto stratified: RFS-Yes; OS-Yes (intermed-risk) |
| Cornelissen30 (2007) | HOVON/SAKK AML4/29/42 | 1987–2003 | 39 (15–55) | 39 (16–55) | 63 (NR) | Overall: RFS-Yes; OS-No Cyto stratified: RFS-NRε; OS-NRε |
| Schlenk31 (2008) | AMLHD93 AMLHD98A |
1993–1998 1998–2004 |
44 (17–59) 48 (16–60) |
47 (16–60) 47 (18–60) |
106 (74–178) 73 (11–118) |
Overall: NA (alloSCT option for non-fav risk) Cyto stratified: RFS-Yes; OS-Yes (intermed-risk)φ |
| Basara32 (2009) | OSHO AML96/02 | 1996–2006 | 42 (17–59) | 51 (16–60) | 19 (4–94) | Overall: NA (alloSCT option for poor-risk) Cyto Stratified: RFS-Yes; OS-Yes (poor-risk) |
| Sakamaki33 (2009)β | JALSG AML97 | 1997–2001 | 37 (16–50) | 36.5 (15–50) | 74 (19–104) | Overall: NA (alloSCT for non-fav JALSG risk) Cyto stratifiedγ: RFS-No; OS-No |
this study, reported solely in abstract form, was considered marginal. Its removal did not impact conclusions regarding alloSCT survival benefit.
very few patients under 15 were enrolled, per the study report
manuscript submitted for publication
no significant difference between donor and no-donor groups, per the study report
described as a study of adult AML
pooled cytogenetic risk stratified results from four cooperative groups are presented, indicating RFS and OS alloSCT benefit in intermediate-risk AML-CR1, and RFS but not OS benefit in poor-risk AML-CR1. See text for details.
alloSCT benefit for intermed-risk AML-CR1 documented in AMLHD98A study
JALSG risk stratified outcomes were restratified by cytogenetic risk
Table 3. Summary of clinical trials evaluating alloSCT benefit for AML in CR1: Additional information regarding Trial Design, Entry Criteria, Response and Toxicity.
Additional information on the number of participating institutions, initial complete remission (CR1) rates, trial entry criteria, source of allogeneic stem cells, proportion of patients who underwent scheduled alloSCT, and treatment related mortality (TRM), for the studies listed in Table 1 is provided, when available from the original publications. The TRM data was variably reported, and had to be inferred for some studies.
| Study Author | Multi-center | CR1 % | Study Entry Criteria | Allogeneic Stem Cells | Underwent AlloSCT % | TRM-Donor % | TRM No-donor % | Risk stratified Treatment? |
|---|---|---|---|---|---|---|---|---|
| Ferrant17 | Yes | 89 | de-novo AML; <56y incl pediatric; no prior MDS; Cr<3 | BMSC | 83 | 5 | 27 | No |
| Schiller18 | No | 80 | newly dx AML; 16–45y; no renal/hep/card dz | BMSC (some TCD) | 89 | 32 | 4 | No |
| Cassileth19 | Yes | 71 | AML; 16–41y | BMSC | NR | NR | NR | No |
| Archimbaud20 | No | 74 | newly dx AML; 15–40y; | BMSC | 74% | 30 | 13 | No |
| Hewlett21 | Yes | 70 | AML; no prior Rx; <50y adult; no CNS dz | BMSC | 64 | 35 | 5 | No |
| Sierra22 | Yes | 75 | AML; <51y includes pediatric; no prior Rx/MDS; no severe concomitant dz | BMSC | 55 | 42 | 0 | No |
| Harousseau23 | Yes | 73 | de-novo AML; 15–40y; no prior Rx/MDS; no renal/hep/card dz | BMSC | 83 | 22 | 5 | No |
| Keating24 | Yes | 67 | newly dx AML; 10–45y; no prior Rx/MDS; no renal/hep/card/neuro dz | BMSC (some TCD) | 61 | 21 | 11 | No |
| Slovak25 | Yes | 71 | AML; 16–55y; no prior Rx; no infxn/renal/hep/card/dz | BMSC | 70 | 21 | 7 | No |
| Suciu26 | Yes | 72 | AML; 15–46y; no prior Rx/MDS/APL; no renal/hep/card/pulm/neuro dz | BMSC (some TCD) | 69 | 20 | 5 | No |
| Brunet27 | Yes | 72 | AML; 16–50y; no prior Rx/MDS/APL/CBF; no severe concomitant dz | BMSC/PBSC (some TCD) | 85 | 18 | 3 | Yes: non-favorable risk |
| Jourdan28 | Yes | 80 | AML; 15–45y; no prior MDS/APL (post ‘95) | BMSC/PBSC (some TCD) | 91 | 25 | 7 | No (few good-risk enrolled post ‘95) |
| Burnett29 | Yes | 83 | AML; ≤55y includes pediatric; few ‘good risk cytogenetics’ (post ‘94) | BMSC | 63 | 23 | 12 | No (few good-risk enrolled post ‘94) |
| Cornelissen30 | Yes | 85 | newly dx AML; 15–50 or 55y; no APL; no severe metab/card/pulm/neuro dz | BMSC | 82 | 25 | 5 | No |
| Schlenk31 | Yes | 73 70 |
primary AML; 16–60y; no MDS/APL/CBF; no renal/hep/card dz | BMSC/PBSC | 83 80 |
19 23 |
10 8 |
Yes: intermed-/poor-risk |
| Basara32 | Yes | 56 | AML; 16–60y; no APL/CBF/intermed-risk; no hep/card/pulm dz | PBSC (some TCD) | 72 | 15 | 4 | Yes: poor-risk |
| Sakamaki33 | Yes | 75 | untreated AML; 15–50y; no JALSG good-risk; no prior Rx/MDS; no severe concomitant dz | BMSC/PBSC | 76 | 16 | 17 | Yes: intermed-/poor-JALSG risk |
Acronyms/Abbreviations: APL: Acute promyelocytic leukemia
BMSC: Bone marrow stem cell
Card: Cardiac
CBF: Core binding factor
CNS: Central nervous system
Hep: Hepatic
Infxn: Infection
MDS: Myelodysplastic syndrome
Metab: Metabolic
Neuro: Neurologic
NR: Not reported
PBSC: Peripheral blood stem cell
Pulm: Pulmonary
TCD: T-cell depleted
Data Synthesis
Data analysis was done using STATA (version 7) software (STATA Corp, College Station, TX). Begg’s funnel plot and P value were used to investigate publication bias. 14 Heterogeneity was assessed by a Q statistic. 15 A Forrest plot with combined HR (with 95% CI) for OS and RFS benefit of alloSCT (donor) versus non-alloSCT (no-donor) was constructed using fixed-effects meta-analysis, given lack of significant inter-study heterogeneity. In sensitivity analyses, random-effects meta-analysis of DerSimonian and Laird was undertaken. 16 The threshold of significance was p≤0.05.
We explored our findings further by additional sensitivity analyses. To assess the potential impact of missing OS data from studies reporting only RFS outcomes, versus those reporting both OS and RFS endpoints, we looked for systematic differences in RFS outcomes between the two groups. We also evaluated the impact of including additional trials that stratified treatment options by cytogenetic risk (i.e. restricting alloSCT option to intermediate- and/or poor-risk AML) to the initial analysis. In subgroup analyses, we assessed OS and RFS benefit for the cytogenetic risk subgroups: poor-, intermediate-, and good-risk AML. Tests of interaction across the subgroups were performed to assess whether benefit of alloSCT varied significantly between the cytogenetic risk categories. Random effects meta-analysis was also undertaken to assess the robustness of all survival endpoints. Adjusted HR was utilized in additional sensitivity analyses.
This work was performed in accordance with the QUOROM guidelines for meta-analysis of randomized clinical trials. 11
Results
Systematic Review
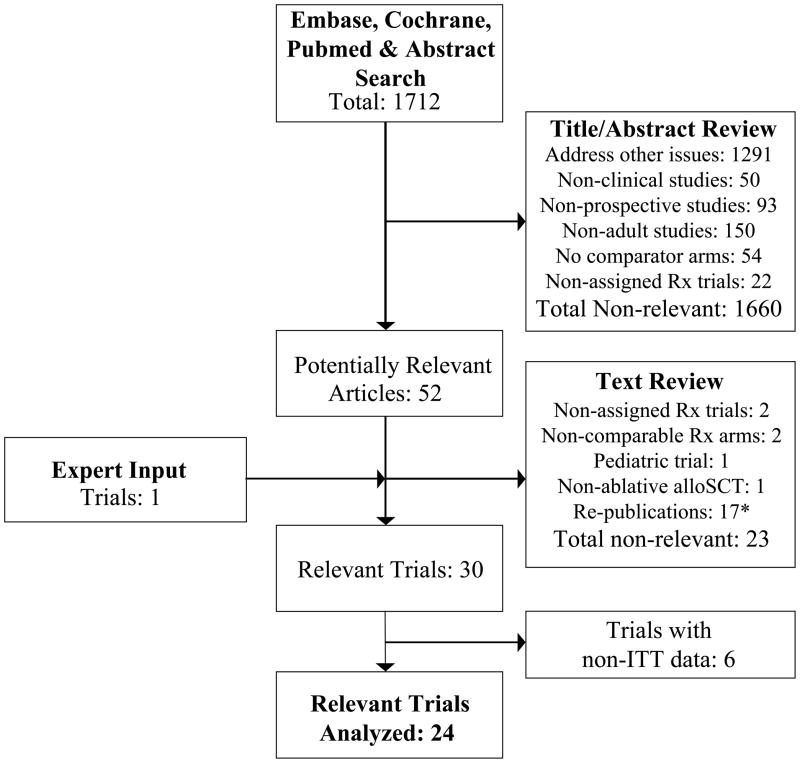
1712 articles were identified in the initial online databases’ and abstract search, delineated in Figure 1. After screening titles/abstracts, 1660 non-relevant articles were excluded. The remaining 52 articles were retrieved for further review. They were reviewed independently in a structured format, and 23 articles were discarded as they did not prospectively compare myeloablative alloSCT versus non-alloSCT options for adult patients with AML in CR1 on an ITT basis, were not assigned treatment trials, reported non-comparable patient cohorts, or represented repeat publications of the same trial. An additional relevant trial was identified by expert input.. Recent review articles and meta-analyses were also retrieved. 2–4 These did not yield additional relevant trials.
Figure 1. Search strategy flow chart.
The Embase, Pubmed, Cochrane and Abstract search, and the process of identifying relevant clinical trials for inclusion in the meta-analysis are shown.
* the most updated report was included
The search identified 30 potentially relevant trials that evaluated alloSCT versus non-alloSCT therapies (consolidation chemotherapy and/or autoSCT) for AML in CR1. 6 trials did not report outcomes based on treatment assigned, and their non-ITT data was not further evaluated. 24 trials provided prospective data on OS and/or RFS outcomes that was extractable on an ITT donor no-donor basis. 17–33 They are included in the analysis, as detailed in Tables 1–3. 18 trials reported RFS outcomes across all AML cytogenetic risk categories. 15 trials reported OS outcomes across all AML cytogenetic risk categories. 6 trials restricted the alloSCT option to intermediate- and/or poor-cytogenetic risk AML in CR1; their cytogenetic risk stratified OS/RFS outcomes are included in sensitivity and subgroup analyses. No significant discrepancies between reviewers were noted regarding trial inclusion or data extraction.
Qualitative Assessment
Overall, the studies in the analysis were considered of good quality, typically being prospective multi-center trials, reporting outcomes on a donor no-donor basis analyzed on an ITT basis, performed at the national level in the US, Europe and Japan, and published in well respected peer-reviewed journals. They enrolled patients between 1982–2006. Numbers of patients in the alloSCT and non-alloSCT arms ranged from 58 to 1305. Some studies combined individual patient data across multiple trials and reported aggregate survival endpoints. Eligible patients typically comprised adults with newly diagnosed AML who were <40–60 years of age, with adequate organ function and absence of significant concomitant disease (Table 3). Two trials included a minority population of pediatric patients, at 16% and 21% respectively. 22, 29 AlloSCT treatment adherence was reasonable for most studies, with only one trial reporting <60% compliance. One study (ECOG EST3483), reported solely in summary form, with missing data on several parameters, was considered marginal.19 In a sensitivity analysis, removal of this study did not impact combined estimates of alloSCT benefit. A subset of the studies reported survival outcomes stratified by AML cytogenetic risk. The cytogenetic criteria used in different studies (e.g. SWOG/ECOG, MRC, EORTC/GIMEMA cytogenetic risk classification) are substantially similar, but differ in some minor respects. 34 Studies utilizing non-standard risk stratification were also analyzed by cytogenetic risk (SWOG/ECOG; MRC). 31, 33, 35
The studies typically assigned patients on the basis of the availability of a HLA matched sibling donor for treatment allocation to the alloSCT arm. The comparator arm typically comprised autoSCT and/or consolidation chemotherapy. If both non-alloSCT alternatives were offered, randomization between the non-alloSCT arms was often performed at a later time point, introducing potential bias, as higher risk patients experiencing early relapse may not get randomized between their non-alloSCT therapies. To address this potential bias a donor no-donor comparison was undertaken, based solely on initial assignment to the alloSCT or non-alloSCT arm(s).
We could not assess the quality or completeness of sibling HLA testing, or exclude patients listed as having no siblings from this analysis, understanding that the inclusion of such patients may introduce a bias into the treatment comparison. 36 However, the trials that expressly permitted alloSCT for patients lacking HLA matched sibling donors did not report significant differences between sibling and non-sibling donor outcomes. 32 This suggests a lack of systematic bias between outcomes in transplanted AML patients with-versus those without- an HLA matched sibling.
The clinical trials also varied with respect to trial design and therapeutic interventions, with differences in induction chemotherapy (and the possibility of re-induction), interim chemotherapy, and consolidation chemotherapy (where applicable). AutoSCT commonly involved myeloablative conditioning (usually identical to that used for alloSCT) and autologous bone marrow (BM) infusion (some studies used peripheral blood (PB) stem cells). AlloSCT comprised myeloablative conditioning (with or without radiation) followed by infusion of allogeneic donor BM or PB stem cells (unmanipulated or variably T cell depleted), with graft versus host disease prophylaxis often comprising cyclosporine and methotrexate. Importantly however, despite the variability in patient eligibility, trial design, and study interventions, inter-study heterogeneity for the OS or RFS endpoints was not significant.
Quantitative Assessment
We subsequently undertook detailed quantitative assessments of the relevant studies, as discussed below. Additional sensitivity and subgroup analysis were also undertaken, and are described in detail.
Publication Bias
We constructed Begg’s funnel plots to evaluate for publication bias. For RFS benefit, the plots tended to maintain a symmetric distribution, both for both the primary analysis of 18 trials reporting RFS outcomes across all cytogenetic risk AML patients (p=0.50), and for the analysis that included 6 additional trials with alloSCT restricted to intermediate- and/or poor-risk AML patients (p>0.99). For OS benefit, the plots also tended to maintain a symmetric distribution, both for the primary analysis of 15 trials reporting OS outcomes for all cytogenetic risk AML patients (p=0.28), and for the analysis that included 6 additional trials with alloSCT restricted to intermediate- and/or poor-risk AML patients (p=0.62).
AlloSCT and RFS benefit
18 clinical trials reported endpoints of overall RFS across all cytogenetic risk groups. The summary hazard estimate for overall RFS benefit also varied between studies, ranging from 0.50 (donor better) to 1.56 (no-donor better). Inter-study heterogeneity was non-significant (p=0.45). A fixed-effects Forrest plot of the individual and combined HR (95% CI) for overall RFS benefit with alloSCT was 0.80 (0.74–0.86) (Figure 2). The overall estimate indicates statistically significant reduction in hazard of death or AML relapse with alloSCT in CR1, across all cytogenetic risk groups (p<0.01).
Figure 2. Forrest plot of relapse-free survival (RFS) benefit of alloSCT in AML-CR1.
The individual reports are indicated on the Y-axis. The summary effect estimate (HR) for individual study reports are indicated by black rectangles (the size of the rectangle is proportional to the study weight), with the lines representing 95% CI. The overall summary effect estimate (HR) and 95% CI are indicated by the diamond. Overall estimates after additional sensitivity and sub-group analyses are shown below. The corresponding values for number of patients at-risk in the donor versus no-donor arm and HR (95% CI) are indicated alongside. The number trials combined per pooled estimate are also indicated.
* studies only reporting RFS endpoints
We further evaluated the studies with sensitivity and subgroup analyses, summarized in Figure 2. In a sensitivity analysis, we repeated the initial RFS analysis, including 6 additional trials that restricted alloSCT option for intermediate- and/or poor-risk AML patients. The combined HR (95% CI) was 0.78 (0.73–0.84), also indicating a significant RFS benefit with alloSCT (p<0.01). We also assessed for systematic differences in effect estimates between the 15 trials that reported on both overall OS and RFS endpoints (group 1) versus the 3 trials that only reported on RFS endpoints (group 2). The combined HR (95% CI) for the 15 trials in group 1 was 0.80 (0.74–0.87); and for the 3 trials in group 2 was 0.79 (0.61–1.02). The near identical summary effect estimate (HR~0.80) of group 1 and 2 indicates a lack of systematic difference in survival outcomes between the groups. A test of interaction between the two groups was not significant, as anticipated. We subsequently evaluated RFS outcomes by cytogenetic risk category of good-, intermediate-, and poor-risk AML. 16 trials reported RFS outcomes by cytogenetic risk. Good-risk AML had a combined HR of 1.06 (0.80–1.42) across 10 trials, indicating a lack of RFS benefit (p=0.68). Intermediate-risk AML had a combined HR of 0.76 (0.68–0.85) across 14 trials, indicating significant RFS benefit with alloSCT in CR1 (p<0.01). Poor-risk AML had a combined HR of 0.69 (0.57–0.84) across 14 trials, indicating significant RFS benefit (p<0.01). Tests of interaction between the 3 cytogenetic risk groups were statistically significant (p<0.05), notably between good-risk versus poor-risk (p=0.02) and intermediate-risk AML (p=0.03), but not between poor-risk and intermediate-risk AML (p=0.40).
Two studies reported adjusted HR(95% CI) for RFS endpoints. Use of adjusted HR did not change our findings regarding alloSCT RFS benefit (Figure 2). Similarly, use of random-effects meta-analysis did not alter any conclusions regarding RFS benefit. In the 18 trials that reported endpoints of overall RFS across all cytogenetic risk groups, the overall random-effects RFS benefit with alloSCT was 0.80 (0.74–0.86). This overall estimate indicates statistically significant reduction in hazard of death or AML relapse with alloSCT in CR1, across all cytogenetic risk groups (p<0.01). Including 6 additional trials that restricted alloSCT option for intermediate- and/or poor-risk AML patients, the combined random-effects HR (95% CI) was 0.78 (0.71–0.85), also indicating a significant RFS benefit with alloSCT (p<0.01). We also evaluated RFS outcomes by cytogenetic risk category of good-, intermediate-, and poor-risk AML. Good-risk AML had a combined random-effects HR of 1.06 (0.80–1.42) across 10 trials, indicating a lack of RFS benefit (p=0.68). Intermediate-risk AML had a combined random-effects HR of 0.76 (0.64–0.92) across 14 trials, indicating significant RFS benefit with alloSCT in CR1 (p<0.01). Poor-risk AML had a combined random-effects HR of 0.67 (0.52–0.85) across 14 trials, indicating significant RFS benefit of alloSCT in CR1 (p<0.01).
AlloSCT and OS benefit
15 trials reported endpoints of OS across all cytogenetic risk groups, and are included in the primary analysis. The summary hazard estimate for overall OS benefit varied between studies, ranging from 0.81 (donor better) to 1.91 (no-donor better). Inter-study heterogeneity was non-significant (p=0.27). A fixed-effects Forrest plot of the individual and combined HR (95% CI) for overall OS benefit with alloSCT was 0.90 (0.82–0.97) (Figure 3). The overall estimate indicates statistically significant reduction in hazard of death with alloSCT across all cytogenetic risk AML in CR1 (p<0.01).
Figure 3. Forrest plot of overall survival (OS) benefit of alloSCT in AML-CR1.
The individual reports are indicated on the Y-axis. The summary effect estimate (HR) for individual study reports are indicated by black rectangles (the size of the rectangle is proportional to the study weight), with the lines representing 95% CI. The overall summary effect estimate (HR) and 95% CI are indicated by the diamond below. Overall estimates after sensitivity and sub-group analyses are shown below. The corresponding values for number of patients at-risk in the donor versus no-donor arm and HR (95% CI) are indicated alongside. The number trials combined per pooled estimate are also indicated.
We further evaluated the studies with sensitivity and subgroup analyses, summarized in Figure 3. In a sensitivity analysis, we repeated the initial analysis with 6 additional trials that provided OS alloSCT outcomes restricted to intermediate- and/or poor-risk AML patients. The combined HR (95% CI) was 0.87 (0.80–0.94), also indicating a significant OS benefit with alloSCT (p<0.01). We also evaluated OS outcomes by cytogenetic risk category of good-, intermediate-, and poor-risk AML. 16 trials reported OS outcomes stratified by cytogenetic risk. Good-risk AML had a combined HR of 1.07 (0.83–1.38) across 10 trials, indicating a lack of significant OS benefit (p=0.59). Intermediate-risk AML had a combined HR of 0.83 (0.74–0.93) across 14 trials, indicating significant OS benefit with alloSCT (p<0.01). Poor-risk AML had a combined HR of 0.73 (0.59–0.90) across 14 trials, indicating significant OS benefit with alloSCT (p<0.01). Tests of interaction were borderline statistically significant across the subgroups (p=0.07), primarily between good- versus poor-risk (p=0.02) and likely intermediate-risk AML (p=0.07), but not between poor-risk and intermediate-risk AML (p=0.30).
Three studies reported adjusted HR(95% CI) for OS endpoints. Use of adjusted HR did not change our findings regarding alloSCT OS benefit (Figure 3). Similarly, use of random-effects meta-analysis did not alter conclusions regarding OS benefit. In the 15 trials that reported OS endpoints across all cytogenetic risk groups, the combined random-effects OS benefit with alloSCT was 0.90 (0.82–1.00). This indicates statistically significant reduction in hazard of death with alloSCT for AML in CR1 across all cytogenetic risk groups (p=0.04). Including 6 additional trials that restricted alloSCT option for intermediate- and/or poor-risk AML patients, the combined random-effects HR (95% CI) was 0.87 (0.78–0.98), also indicating significant alloSCT OS benefit (p=0.02). We also evaluated OS outcomes by cytogenetic risk category of good-, intermediate-, and poor-risk AML. Good-risk AML had a combined random-effects HR of 1.06 (0.64–1.76) across 10 trials, indicating a lack of OS benefit (p=0.81). Intermediate-risk AML had a combined random-effects HR of 0.84 (0.71–0.99) across 14 trials, indicating significant OS benefit with alloSCT in CR1 (p=0.03). Poor-risk AML had a combined random-effects HR of 0.60 (0.40–0.90) across 14 trials, also indicating significant OS benefit of alloSCT in CR1 (p=0.01).
Discussion
Despite multiple prospective studies over the past two decades, the role of alloSCT for adult AML-CR1 patients remains ill-defined. A meta-analysis of 5 prospective trials by Yanada et. al. indicated an overall OS benefit with alloSCT (p=0.04), and meta-regression suggested that the OS benefit may be restricted to poor-risk AML (p=0.12). 5 In addition to the limited number of trials and the use of indirect evidence (meta-regression) to indicate possible cytogenetic subgroup benefit, double counting of alloSCT data from individual studies that reported alloSCT versus autoSCT and consolidation chemotherapy outcomes separately remains an unaddressed source of bias. An ITT donor no-donor analysis offers a better means to address such concerns. Cornelissen et. al. combined donor no-donor data from four cooperative groups (BGMT, HOVON/SAKK, MRC and EORTC) in a meta-analysis to demonstrate a statistically significant survival benefit to alloSCT; and in cytogenetic subgroup analyses, an OS benefit was documented for intermediate-risk, but not poor-risk AML. 30 The limited number of trials assessed (e.g. omission of EORTC AML8A study) has likely precluded general acceptance of their meta-analysis.
Thus, current recommendations from NCCN, and from the American Society of Blood and Marrow Transplantation (ASBMT), based on literature review and expert consensus, stratify treatment by cytogenetic risk, and state that there is a survival advantage for alloSCT in patients <55 years with poor-risk AML-CR1; that there is insufficient evidence to routinely recommend alloSCT for patients with intermediate-risk AML-CR1; and that there is no survival advantage for alloSCT in good-risk AML-CR1. 4 The direct evidence supporting these recommendations remains limited, as previously discussed. In part, this may be because all clinical trial data has not been systematically assessed. Quantitatively integrating data from all available trials will likely enhance our understanding of the role of alloSCT for AML-CR1. The robustness of any conclusions can be systematically assessed in secondary analyses.
To comprehensively assess the utility of upfront alloSCT for AML-CR1, we therefore undertook a systematic literature review and meta-analysis of published data from clinical trials allocating alloSCT versus non-alloSCT options (consolidation chemotherapy and/or autoSCT) for such patients. We focused on an ITT analysis based on donor availability in order to capture information from all AML patients who were evaluated for upfront alloSCT with a donor search as part of a prospective trial. Prior meta-analyses have shown that survival after autoSCT is equivalent to that with consolidation chemotherapy for AML-CR1 patients, supporting the decision to combine the non-alloSCT treatment options in a single no-donor category. 6, 7, 37
The systematic literature search identified 24 relevant trials comparing alloSCT versus non-alloSCT treatment for AML-CR1, none of which individually reported an alloSCT OS benefit across all cytogenetic risk groups (Table 1), possibly owing to limited sample size (power calculations were not routinely described in the study reports). Enrolling patients between 1982 and 2006, the trials are all mature, and further long-term follow-up is unlikely to yield substantially different results. The trials varied with regards to patient eligibility, study trial design, cytogenetic risk classification and specific interventions used (Table 2). Importantly however, inter-study heterogeneity was not significant for OS or RFS endpoints, indicating that the impact of study differences was limited.
Table 2. Summary of clinical trials evaluating alloSCT benefit for AML in CR1: Therapies utilized.
Further information on the studies listed in Table 1 is provided, to better describe study interventions. Information on the donor vs. no-donor treatment arms is provided.
| Study Author | Donor v No-donor Arms | Induction Therapy (optional) | Interim Therapy | Ablative Allogeneic Conditioning* | Consolidation Chemotherapy |
|---|---|---|---|---|---|
| Ferrant17 | Allo v Auto | Dnr+AraC->(hiDAC) | Dnr+AraC | Cy+AraC+TBI(8Gy) | NA |
| Schiller18 | Allo v CC | Dnr+AraC±Tg | None | Cy/AraC+TBI(11Gy)/TLI±AraC/Mito | hiDAC+Dnr/Mito/VP x1-3 |
| Cassileth19 | Allo v CC | Dnr+AraC+Tg x1-2 | None | NR | hiDAC+Amsa x1 |
| Archimbaud20 | Allo v CC | Dnr+AraC+Tg | hiDAC+Amsa | Cy+Bu;Cy+TBI(10-12Gy) | Dnr+AraC+Tg v midDAC+Dnr |
| Hewlett21 | Allo v CC | Dnr+AraC+Tg+Vcr+Pred x1-2 | None | Cy+TBI(12Gy) | Dnr+AraC+Tg+Vcr+Pred x2->maint v late intensification |
| Sierra22 | Allo v Auto | Dnr+AraC+VP x1-2 | hiDAC+Mito-> hiDAC+Amsa | Cy+TBI(12-14Gy); Bu+Mel; Bu+Cy±VP;Bu+VP | NA |
| Harousseau23 | Allo v Auto v CC | Ida/Rbz+AraC x1-2 | D:Amsa+AraC; ND:hiDAC+Ida/Rbz | Bu+Cy;Cy+TBI;Various+TBI | Amsa+VP |
| Keating24 | Allo v Auto v CC | Dnr+AraC±GMCSF x1-2 | midDAC+Amsa | Cy+TBI(10-12Gy);Bu+Gy | hiDAC |
| Slovak25 | Allo v Auto v CC | Ida+AraC x1-2 | Ida+AraC | Bu+Cy | hiDAC |
| Suciu26 | Allo v Auto | Dnr/Ida/Mito+AraC+VP x1-2 | Dnr/Ida/Mito+midDAC | Cy+TBI(12Gy);Bu+Cy | NA |
| Brunet27 | Allo v Auto(Non favorable risk) | Ida+AraC+VP x1-2 | midDAC+Mito | Cy+TBI(12-14Gy); Bu+Cy | NA |
| Jourdan28 | Allo v Auto ± v CC | Dnr+AraC x1-2 | Dnr+AraC | Cy+TBI(12Gy); Bu+Cy | hiDAC |
| Burnett29 | Allo v Auto v Obs | Dnr+AraC+Tg/VP x2 | Amsa+AraC+VP-> midDAC+Mito | Cy+TBI(7.5-14Gy); Bu+Cy | NA |
| Cornelissen30 | Allo v Auto v Obs | Dnr/Ida+AraC-> Amsa+midDAC | ND:Mito+VP | NR | NA |
| Schlenk31 | Allo v CC (int-risk) (HD93, 98A) or Auto (poor-risk) (HD93) | Ida+AraC+VP x2 | hiDAC+Mito | Cy+TBI(12-14Gy); Bu+Cy | hiDAC+Mito |
| Basara32 | Allo v CC/Auto (poor risk) | Ida+midDAC-> (midDAC+Mito/FLAG+Mito) | AML96: Ida+midDAC/Mito | Cy+TBI(12Gy) | AML96:midDAC; AML02:Ida+midDAC |
| Sakamaki33 | Allo v CC (int/poor JALSG risk) | Ida+AraC x1-2 | None | Per Institutional Standard | AraC+Anthracycline ± maint |
autologous conditioning was essentially identical
Acronyms/Abbreviations: Allo: Myeloablative allogeneic stem cell transplantation
AraC: Cytarabine
Amsa: Amsacrine
Auto: Autologous stem cell transplantation
Bu: Busulfan
CC: Consolidation chemotherapy
Cy: Cyclophosphamide
Dnr: Daunorubicin
FLAG: Fludarabine; AraC; GCSF
GMCSF: Granulocyte/Macrophage growth factor
hiDAC: high dose AraC
Ida: Idarubicin
Mel: Melphalan
midDAC: intermediate-dose AraC
Mito: Mitoxantrone
NA: Not applicable
NR: Not reported
Pred: Prednisone
Rbz: Rubidazone
TBI/TLI: Total body irradiation/Total lymphoid irradiation (Gray)
Tg: Thioguanine
Vcr: Vincristine
VP: VP16/Etoposide
We considered the effect, if any, of differences in cytogenetic risk classification between studies. Such differences, if significant, may be anticipated to increase the between-studies heterogeneity for each cytogenetic risk group’s endpoints, which was not observed. This is likely because the various cytogenetic risk classification schemes are fairly similar, although not identical. 30 While we could not assess the impact of such differences directly, individual prospective studies that directly compared cytogenetic risk classifications (SWOG/ECOG, EORTC/GIMEMA, MRC) documented highly concordant effect estimates, independent of the classification schema used. 25, 26 It is therefore unlikely that variability between cytogenetic risk classifications significantly impacted our analysis.
We also considered the role of treatment compliance. This likely disproportionately impacts the alloSCT (donor) arm, as a significant fraction of patients with donors did not receive alloSCT. Such crossover, analyzed on an ITT basis, is anticipated to reduce the observable survival benefit of alloSCT. Typically, the studies reported an alloSCT compliance rate of >60%, which is considered reasonable for such prospective trials (one trial reported an alloSCT compliance rate of 55%, and in a sensitivity analysis, its removal did not impact the overall conclusions). In addition, the impact of salvage alloSCT after AML relapse cannot be estimated, but likely diminishes any observable OS benefit of upfront alloSCT. Further, the inclusion of older trials, some over two decades old, likely also biases against alloSCT, since advances in supportive care (e.g. growth factors; improved anti-infective strategies; better prophylaxis/therapy of graft-versus-host disease) and transplantation methodology (e.g. PBSC) are considered responsible for improvement in alloSCT outcomes.
Our primary finding is that the totality of the prospective trial data indicates statistically significant RFS and OS benefit for alloSCT in adult AML-CR1. This conclusion is supported by a variety of sensitivity and subgroup analyses as reported above. Additionally, our analyses indicate that alloSCT benefit likely varies by AML cytogenetic risk. We document significant RFS and OS benefit for alloSCT in intermediate- and poor-risk AML, and a lack of significant RFS or OS benefit for good-risk AML. With regards comparative absolute survival, anticipating 5-year OS rates in the control (non-alloSCT) arm of 45% and 20% for intermediate- and poor-risk AML respectively, patients assigned alloSCT in CR1 would likely experience OS rates of 54% and 42% for intermediate- and poor-risk AML respectively.
There are limitations to our analysis. We are aware of relevant studies that have not yet been reported (e.g. UK MRC AML 12/15; GOELAM2). 38–40 As a meta-analysis of the published literature, we extracted summary statistics (HR) from individual studies to determine combined estimates. Dependence on published articles limits the level of detail that can be captured regarding sub-groups that may have greater or lesser benefit from alloSCT. We could not assess outcomes for clinically relevant subgroups other than cytogenetic risk. For instance, patient age is a likely relevant factor, and some, though not all, studies have indicated improved alloSCT outcomes in younger adults. 26, 29, 30 The median patient age in most trials in this report is in the 30s, and while the age eligibility was up to age 60 years in individual studies, it remains unclear if older eligible patients obtained an equivalent benefit.
With regards treatment toxicity, we have summarized available TRM data for individual studies (Table 3). However, the variable and limited data reported precluded a more formal analysis, and highlights the need for more systematic reporting of this important endpoint in the future. We also note that while patients in this analysis predominantly had de-novo AML, eligibility criteria in some studies permitted enrollment of patients with prior MDS or therapy-related AML. Finally, the impact of comorbidities could not be assessed, since trial eligibility criteria disbarred entry to such patients. Nonetheless, for treatment outside of the research setting, it has significant impact on alloSCT outcomes in AML. 41, 42
A meta-analysis of individual patient data from the relevant clinical trials is a way to obtain more complete estimates of OS and RFS benefit with alloSCT; and to assess the impact of additional factors like patient age. A broad overview of transplant for AML-CR1 utilizing individual patient data is currently being conducted by the Acute Leukemia Stem Cell Transplant Trialists’ Collaborative Group. Nonetheless, our quantitative analysis of data from 24 trials comprising 6,007 prospectively assigned patients provides the most complete estimate of alloSCT benefit available. It enables an informed assessment of the role of upfront alloSCT for adult AML patients in CR1.
Cytogenetic and molecular risk profiling in AML is an evolving field, and can further stratify outcomes within a known cytogenetic risk group. For instance, Schlenk et. al. from the German Austrian AML Study Group (AMLSG) reported that for patients with cytogenetically normal AML (who would be classified as intermediate-risk), alloSCT was beneficial for those with either a FLT3 internal tandem duplication (FLT3-ITD), or in the absence of FLT3-ITD, for those without mutations in NPM1 and CEBPA; while for the subgroup with mutations in NPM1 and without FLT3-ITD there was no apparent benefit to having a matched sibling. 31 However, such novel genetic lesions, as well as whole genome analyses, RNA and microRNA profiles that have the potential to further refine AML risk, are not in routine clinical use. 43, 44
The dilemma of how to best treat adult AML-CR1 patients with a known cytogenetic risk profile therefore remains. While enrollment in therapeutic trials is to be encouraged, our findings provide evidence to guide clinical decision-making and future trial design. We find evidence to support treatment based on AML cytogenetic risk. We conclude that alloSCT does not provide significant benefit for good-risk AML in CR1; and that alloSCT offers significant RFS and OS benefits for intermediate- and poor-risk AML in CR1. However, within these general guidelines, there remains a need to further individualize the alloSCT decision, based on factors like patient age, comorbidity, and the presence of additional molecular lesions.
Acknowledgments
We thank the authors of the clinical trials analyzed for their responses to our requests for clarification. In particular we would like to thank Didier Blaise MD, Benjamin Esterni PhD, Jan J. Cornelissen MD PhD, Bob Löwenberg MD PhD and Wim L. J. van Putten MSc for their input and critical feedback. J.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. No contributor was compensated for their participation.
C.C. is supported by the Stem Cell Cyclists of the Pan-Massachusetts Challenge.
Footnotes
J.K. designed the study, performed the literature search, extracted and analyzed data, and wrote the paper. R.S. provided data and wrote the paper. K.J.K. provided data and wrote the paper. S.H. provided data and wrote the paper. J.S. provided data and wrote the paper. B.J.D. analyzed data and wrote the paper. M.W. wrote the paper. D.J.D. wrote the paper. R.M.S. wrote the paper. H.S. provided data and wrote the paper. F.R.A. provided data and wrote the paper. H.D. provided data and wrote the paper. J.H.A. wrote the paper. R.J.S. wrote the paper. C.C. designed the study, extracted data and wrote the paper.
J.K., R.S., K.J.K, S.H., J.S., B.J.D., M.W., D.J.D., R.M.S., F.R.A., H.D., J.H.A., R.J.S. and C.C. report no disclosures. H.S. reports membership of the advisory board of Bristol Myers Squibb Co. and Wyeth Inc.
Contributor Information
Richard Schlenk, Email: Richard.Schlenk@uniklinik-ulm.de.
Kenneth J. Kopecky, Email: kkopecky@fhcrc.org.
Sumihisa Honda, Email: honda@nagasaki-u.ac.jp.
Jorge Sierra, Email: jsierra@santpau.cat.
Benjamin J. Djulbegovic, Email: DjulbeBM@moffitt.usf.edu.
Martha Wadleigh, Email: Martha_Wadleigh@dfci.harvard.edu.
Daniel J. DeAngelo, Email: Daniel_Deangelo@dfci.harvard.edu.
Richard M. Stone, Email: Richard_Stone@dfci.harvard.edu.
Hisashi Sakamaki, Email: sakamaki-h@cick.jp.
Frederick R. Appelbaum, Email: fappelba@fhcrc.org.
Hartmut Döhner, Email: hartmut.doehner@uniklinik-ulm.de.
Joseph H. Antin, Email: Joseph_Antin@dfci.harvard.edu.
Robert J. Soiffer, Email: Robert_Soiffer@dfci.harvard.edu.
Corey Cutler, Email: Corey_Cutler@dfci.harvard.edu.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999 Sep 30;341(14):1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Visani G, Olivieri A, Malagola M, et al. Consolidation therapy for adult acute myeloid leukemia: a systematic analysis according to evidence based medicine. Leuk Lymphoma. 2006 Jun;47(6):1091–1102. doi: 10.1080/10428190500513595. [DOI] [PubMed] [Google Scholar]
- 3.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006 Nov 25;368(9550):1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 4.Oliansky DM, Appelbaum F, Cassileth PA, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant. 2008 Feb;14(2):137–180. doi: 10.1016/j.bbmt.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Yanada M, Matsuo K, Emi N, Naoe T. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer. 2005 Apr 15;103(8):1652–1658. doi: 10.1002/cncr.20945. [DOI] [PubMed] [Google Scholar]
- 6.Nathan PC, Sung L, Crump M, Beyene J. Consolidation therapy with autologous bone marrow transplantation in adults with acute myeloid leukemia: a meta-analysis. J Natl Cancer Inst. 2004 Jan 7;96(1):38–45. doi: 10.1093/jnci/djh003. [DOI] [PubMed] [Google Scholar]
- 7.Levi I, Grotto I, Yerushalmi R, Ben-Bassat I, Shpilberg O. Meta-analysis of autologous bone marrow transplantation versus chemotherapy in adult patients with acute myeloid leukemia in first remission. Leuk Res. 2004 Jun;28(6):605–612. doi: 10.1016/j.leukres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998 Dec 30;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994 Aug 1;140(3):290–296. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 10.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. Jama. 1999 Sep 15;282(11):1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999 Nov 27;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000 Apr 19;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Hasselblad V, Eddy DM, Kotchmar DJ. Synthesis of environmental evidence: nitrogen dioxide epidemiology studies. J Air Waste Manage Assoc. 1992 May;42(5):662–671. doi: 10.1080/10473289.1992.10467018. [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994 Dec;50(4):1088–1101. [PubMed] [Google Scholar]
- 15.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;8:101–129. [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Ferrant A, Doyen C, Delannoy A, et al. Allogeneic or autologous bone marrow transplantation for acute non-lymphocytic leukemia in first remission. Bone Marrow Transplant. 1991 Apr;7(4):303–309. [PubMed] [Google Scholar]
- 18.Schiller GJ, Nimer SD, Territo MC, Ho WG, Champlin RE, Gajewski JL. Bone marrow transplantation versus high-dose cytarabine-based consolidation chemotherapy for acute myelogenous leukemia in first remission. J Clin Oncol. 1992 Jan;10(1):41–46. doi: 10.1200/JCO.1992.10.1.41. [DOI] [PubMed] [Google Scholar]
- 19.Cassileth PA, Andersen JW, Bennett JM, et al. Escalating the intensity of post-remission therapy improves the outcome in acute myeloid leukemia: the ECOG experience. The Eastern Cooperative Oncology Group. Leukemia. 1992;6( Suppl 2):116–119. [PubMed] [Google Scholar]
- 20.Archimbaud E, Thomas X, Michallet M, et al. Prospective genetically randomized comparison between intensive postinduction chemotherapy and bone marrow transplantation in adults with newly diagnosed acute myeloid leukemia. Journal of Clinical Oncology. 1994;12:262–267. doi: 10.1200/JCO.1994.12.2.262. [DOI] [PubMed] [Google Scholar]
- 21.Hewlett J, Kopecky KJ, Head D, et al. A prospective evaluation of the roles of allogeneic marrow transplantation and low-dose monthly maintenance chemotherapy in the treatment of adult acute myelogenous leukemia (AML): a Southwest Oncology Group study. Leukemia. 1995 Apr;9(4):562–569. [PubMed] [Google Scholar]
- 22.Sierra J, Brunet S, Granena A, et al. Feasibility and results of bone marrow transplantation after remission induction and intensification chemotherapy in de novo acute myeloid leukemia. Catalan Group for Bone Marrow Transplantation. J Clin Oncol. 1996 Apr;14(4):1353–1363. doi: 10.1200/JCO.1996.14.4.1353. [DOI] [PubMed] [Google Scholar]
- 23.Harousseau JL, Cahn JY, Pignon B, et al. Comparison of autologous bone marrow transplantation and intensive chemotherapy as postremission therapy in adult acute myeloid leukemia. The Groupe Ouest Est Leucemies Aigues Myeloblastiques (GOELAM) Blood. 1997 Oct 15;90(8):2978–2986. [PubMed] [Google Scholar]
- 24.Keating S, de Witte T, Suciu S, et al. The influence of HLA-matched sibling donor availability on treatment outcome for patients with AML: an analysis of the AML 8A study of the EORTC Leukaemia Cooperative Group and GIMEMA. European Organization for Research and Treatment of Cancer. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol. 1998 Sep;102(5):1344–1353. doi: 10.1111/j.1365-2141.1998.896hm3674.x. [DOI] [PubMed] [Google Scholar]
- 25.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000 Dec 15;96(13):4075–4083. [PubMed] [Google Scholar]
- 26.Suciu S, Mandelli F, de Witte T, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003 Aug 15;102(4):1232–1240. doi: 10.1182/blood-2002-12-3714. [DOI] [PubMed] [Google Scholar]
- 27.Brunet S, Esteve J, Berlanga J, et al. Treatment of primary acute myeloid leukemia: results of a prospective multicenter trial including high-dose cytarabine or stem cell transplantation as post-remission strategy. Haematologica. 2004 Aug;89(8):940–949. [PubMed] [Google Scholar]
- 28.Jourdan E, Boiron JM, Dastugue N, et al. Early allogeneic stem-cell transplantation for young adults with acute myeloblastic leukemia in first complete remission: an intent-to-treat long-term analysis of the BGMT experience. J Clin Oncol. 2005 Oct 20;23(30):7676–7684. doi: 10.1200/JCO.2005.02.5940. [DOI] [PubMed] [Google Scholar]
- 29.Burnett AK, Wheatley K, Goldstone AH, Stevens R, Hann I, Hills RK. Long-term results of the MRC AML10 trial. Clin Adv Hematol Oncol. 2006 Jun;4(6):445–451. [PubMed] [Google Scholar]
- 30.Cornelissen JJ, van Putten WL, Verdonck LF, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007 May 1;109(9):3658–3666. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 31.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008 May 1;358(18):1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 32.Basara N, Schulze A, Wedding U, et al. Early related or unrelated haematopoietic cell transplantation results in higher overall survival and leukaemia-free survival compared with conventional chemotherapy in high-risk acute myeloid leukaemia patients in first complete remission. Leukemia. 2009 Jan 8; doi: 10.1038/leu.2008.352. [DOI] [PubMed] [Google Scholar]
- 33.Sakamaki H, Miyawaki S, Ohtake S, et al. Allogeneic stem cell transplantation (Allo-SCT) for adults with acute myelogenous leukemia (AML). Final analysis of the JALSG AML97 study. Blood. 2008 November 16;112(11):a347. [Google Scholar]
- 34.Hamadani M, Awan FT, Copelan EA. Hematopoietic stem cell transplantation in adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2008 May;14(5):556–567. doi: 10.1016/j.bbmt.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Schlenk RF, Benner A, Hartmann F, et al. Risk-adapted postremission therapy in acute myeloid leukemia: results of the German multicenter AML HD93 treatment trial. Leukemia. 2003 Aug;17(8):1521–1528. doi: 10.1038/sj.leu.2403009. [DOI] [PubMed] [Google Scholar]
- 36.Gray R, Wheatley K. How to avoid bias when comparing bone marrow transplantation with chemotherapy. Bone Marrow Transplant. 1991;7( Suppl 3):9–12. [PubMed] [Google Scholar]
- 37.Breems DA, Boogaerts MA, Dekker AW, et al. Autologous bone marrow transplantation as consolidation therapy in the treatment of adult patients under 60 years with acute myeloid leukaemia in first complete remission: a prospective randomized Dutch-Belgian Haemato-Oncology Co-operative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) trial. Br J Haematol. 2005 Jan;128(1):59–65. doi: 10.1111/j.1365-2141.2004.05282.x. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen S, Leblanc T, Fenaux P, et al. A white blood cell index as the main prognostic factor in t(8;21) acute myeloid leukemia (AML): a survey of 161 cases from the French AML Intergroup. Blood. 2002 May 15;99(10):3517–3523. doi: 10.1182/blood.v99.10.3517. [DOI] [PubMed] [Google Scholar]
- 39.Delaunay J, Vey N, Leblanc T, et al. Prognosis of inv(16)/t(16;16) acute myeloid leukemia (AML): a survey of 110 cases from the French AML Intergroup. Blood. 2003 Jul 15;102(2):462–469. doi: 10.1182/blood-2002-11-3527. [DOI] [PubMed] [Google Scholar]
- 40.Burnett AK, Hills R, Goldstone A, et al. The MRC Transplantation Strategies. Ann Hematol. 2004 Feb;83(S1):S135. [Google Scholar]
- 41.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct 15;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007 Dec 15;110(13):4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcucci G, Radmacher MD, Maharry K, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008 May 1;358(18):1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 44.Dohner K, Dohner H. Molecular characterization of acute myeloid leukemia. Haematologica. 2008 Jul;93(7):976–982. doi: 10.3324/haematol.13345. [DOI] [PubMed] [Google Scholar]