Summary
Background
As trials of 5 years of tamoxifen in early breast cancer mature, the relevance of hormone receptor measurements (and other patient characteristics) to long-term outcome can be assessed increasingly reliably. We report updated meta-analyses of the trials of 5 years of adjuvant tamoxifen.
Methods
We undertook a collaborative meta-analysis of individual patient data from 20 trials (n=21 457) in early breast cancer of about 5 years of tamoxifen versus no adjuvant tamoxifen, with about 80% compliance. Recurrence and death rate ratios (RRs) were from log-rank analyses by allocated treatment.
Findings
In oestrogen receptor (ER)-positive disease (n=10 645), allocation to about 5 years of tamoxifen substantially reduced recurrence rates throughout the first 10 years (RR 0·53 [SE 0·03] during years 0–4 and RR 0·68 [0·06] during years 5–9 [both 2p<0·00001]; but RR 0·97 [0·10] during years 10–14, suggesting no further gain or loss after year 10). Even in marginally ER-positive disease (10–19 fmol/mg cytosol protein) the recurrence reduction was substantial (RR 0·67 [0·08]). In ER-positive disease, the RR was approximately independent of progesterone receptor status (or level), age, nodal status, or use of chemotherapy. Breast cancer mortality was reduced by about a third throughout the first 15 years (RR 0·71 [0·05] during years 0–4, 0·66 [0·05] during years 5–9, and 0·68 [0·08] during years 10–14; p<0·0001 for extra mortality reduction during each separate time period). Overall non-breast-cancer mortality was little affected, despite small absolute increases in thromboembolic and uterine cancer mortality (both only in women older than 55 years), so all-cause mortality was substantially reduced. In ER-negative disease, tamoxifen had little or no effect on breast cancer recurrence or mortality.
Interpretation
5 years of adjuvant tamoxifen safely reduces 15-year risks of breast cancer recurrence and death. ER status was the only recorded factor importantly predictive of the proportional reductions. Hence, the absolute risk reductions produced by tamoxifen depend on the absolute breast cancer risks (after any chemotherapy) without tamoxifen.
Funding
Cancer Research UK, British Heart Foundation, and Medical Research Council.
Introduction
In oestrogen receptor (ER)-positive early breast cancer, endocrine treatment reduces the recurrence and mortality rates, whether or not chemotherapy is also given.1 Adjuvant tamoxifen is a major endocrine treatment option, particularly for women who still have significant ovarian oestrogenic activity that cannot be controlled by aromatase inhibitors. In trials of about 5 years of adjuvant tamoxifen versus no tamoxifen for early breast cancer, follow-up now extends well into the second decade since randomisation. This extended follow-up allows improved assessment of long-term effects on breast cancer mortality and other mortality, and of the effects of endocrine therapy in disease that is only weakly hormone-receptor positive. We report updated meta-analyses of data for individual women in these trials, relating the effects of tamoxifen to quantitative measurements of hormone receptor levels, use of chemotherapy, and other factors.
Methods
Data collection
Trial identification and data handling procedures have been described previously.1–3 We sought updated data from each randomised trial in women with early breast cancer of adjuvant tamoxifen versus not, in which only tamoxifen differed (ie, unconfounded trials). Trials in women with ductal carcinoma in situ were excluded. Results of only 1–2 years of adjuvant tamoxifen (n=33 000 women randomly assigned) are essentially unchanged since previously reported,1 and are given only in webappendix p 2. In this Article, we report the trials of longer tamoxifen durations (described as about 5 years of tamoxifen).4–26 Most trials were of exactly 5 years of tamoxifen,4–16 four were of only 3 years,17–21 one re-randomised some participants at year 2 to stop or continue to year 5 (with all re-randomised patients remaining in the analyses),22 and two re-randomised some at year 5 to stop or continue to year 1023–26 (webappendix pp 18–36).
As in previous meta-analyses from the Early Breast Cancer Trialists' Collaborative Group (EBCTCG), information was sought for each patient on date of randomisation, allocated treatment, age, menopausal status, tumour diameter, grade, spread to locoregional lymph nodes, and any ER or progesterone receptor (PR) measurements, mostly in femtomoles of receptor protein per mg cytosol protein (fmol/mg). Values of 10 fmol/mg or greater were, as before,1 described as receptor positive, with lower values described interchangeably as receptor negative or receptor poor. Other receptor-positive or receptor-poor measurements (including the few measured by immunohistochemistry) were those given only qualitatively. Information was generally unavailable on assay methods and on whether assays were done centrally or at local hospitals. Within-trial receptor measurement distribution (0, 1–3, 4–9, 10–19, 20–29, 30–49, 50–99, 100–199, and ≥200 fmol/mg) was inspected to help to assess assay quality, with results showing no obvious anomalies (data not shown).
Follow-up was updated on dates of first recurrence of any breast cancer (locoregional, contralateral [either could include new onset], or distant), other second primary cancer, and death. Summary information on a whole-trial basis (rather than an individual basis) was sought on approximate levels of compliance with the treatment allocation 2–3 years after randomisation.
Statistical analysis
Methods of analysis were as previously described,1–3 except that analyses were stratified by trial, age at entry (<45, 45–54, 55–69, and ≥70 years), nodal status (node-negative by local criteria, 1–3 nodes positive after axillary clearance, ≥4 nodes positive, other or unknown), and ER status (poor, positive, unknown), defining 4×4×3 strata. Log-rank statistics and their variances were calculated separately in each stratum and summed, yielding the stratified result. To avoid over-stratification, subgroup analyses of tumour grade or diameter were stratified by only two categories of age (≥50 years, other [or unknown]) and nodal status (negative, other [or unknown]) and three of ER status, defining 2×2×3 strata.
Survival curves show time to recurrence, breast cancer mortality, and any mortality. Yearly rates of breast cancer mortality assess the excess mortality when the mortality rate in women without recurrence is subtracted from the overall mortality rate in all women. Correspondingly, rate ratios (RRs) for breast cancer mortality are estimated from log-rank analyses of mortality with recurrence, obtained by subtraction of the log-rank analyses of mortality without recurrence (ie, censored at recurrence) from those of all mortality.
If a log-rank statistic (O–E) has variance V, then, defining z=(O–E)/√V and b=(O–E)/V, RR=exp(b), the event rate ratio, is taken to have SE=(RR–1)/z and 95% CI exp(b±1·96/√V). Results cite RR (and its SE). p values (two sided) are obtained by comparing z with a standard normal distribution, so z=1·96 yields p=0·05 (described as 2p, for consistency with previous reports).
Role of the funding sources
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The secretariat had full access to all the data and analyses, and accepts responsibility for this report. Final analyses and a draft report were presented and discussed at a meeting of many trialists, after which a revised report was circulated to all trialists for written comment and revised again. The writing committee prepared the report and was responsible for the decision to submit for publication.
Results
Information was available for 99% (21 457/21 712) of all women known to have been randomly assigned into trials of about 5 years of adjuvant tamoxifen (webappendix pp 18–35). Although 21 trials started, one6 with 255 women was terminated early and the records were never analysed, and were lost. All women were randomly assigned evenly between tamoxifen and control. Six major trials described compliance with the tamoxifen allocation (75% in NSABP25,26 completed ≥3 years; and 89% in GROCTA,15 78% in IBCSG,13 82% in ICCG,10 69% in NCIC,11 and 86% in SWOG7 [weighted mean 82%] completed ≥2 years). Compliance with allocation to control was unavailable, but should have been good in early trials (although perhaps less good for women with ER-positive disease in later trials, undertaken when treatment guidelines recommended tamoxifen).
In ER-positive disease, allocation to tamoxifen halved the recurrence rate during years 0–4 and reduced it by a third during years 5–9 (with little further effect after year 10), so over all time periods the recurrence rate reduction averaged 39% (RR 0·61 [SE 0·03; 2p<0·00001] for any recurrence, and RR 0·62 [0·07; 2p<0·00001] for contralateral disease incidence). In ER-poor disease, however, there was no apparent effect on recurrence (RR 0·97 [0·05] for any recurrence, 95% CI 0·88–1·07; RR 0·94 [0·12] for contralateral disease, 95% CI 0·73–1·20) (webappendix p 9). Although the overall prognosis for ER-poor disease seemed (somewhat misleadingly) about as good as that for tamoxifen-treated ER-positive disease, this comparison was confounded by nodal status and, particularly, by widespread use of chemotherapy in ER-poor disease (figure 1).
Figure 1.
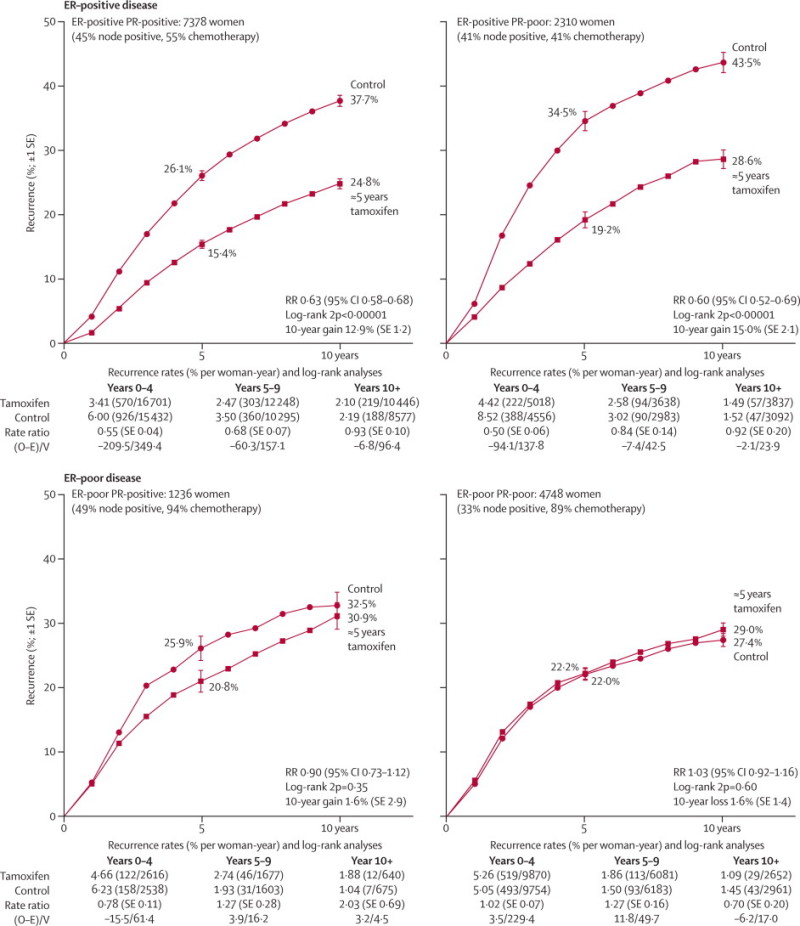
Relevance of measured ER and PR status to the effects of about 5 years of tamoxifen on the 10-year probability of recurrence
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. Event rate ratio (RR) is from summed log-rank statistics for all time periods. Gain (and its SE) is absolute difference between ends of graphs. ER=oestrogen receptor. PR=progesterone receptor. O–E=observed minus expected, with variance V.
ER and PR status were strongly associated; PR (when measured) was positive in 76% (7378 of 9688) of ER-positive and only 21% (1236 of 5984) of ER-negative (strictly, ER-poor) disease. Given ER status, however, PR status was not significantly predictive of response. The RR was 0·63 (SE 0·03) for ER-positive PR-positive disease and 0·60 (0·05) for ER-positive PR-negative disease (both 2p<0·00001). The RR was 0·90 (0·10) for ER-negative PR-positive disease (2p=0·35) and 1·03 (0·06) for ER-negative PR-negative disease (2p=0·60; figure 1).
Analyses of quantitative ER and PR measurements did not materially change these findings (figure 2). If the ER measurement was less than 10 fmol/mg cytosol protein (ie, ER-poor disease) there was no apparent benefit from addition of tamoxifen. Even for weakly positive ER, however, there was substantial benefit (RR 0·67 [SE 0·08] for ER 10–19 fmol/mg), and the proportional effect at much higher ER was only slightly better (RR 0·52 [0·07] for ER ≥200 fmol/mg, trend in RR with ER [if ER ≥10 fmol/mg] p=0·002). In ER-positive disease, the PR measurements were not predictive of who would respond to tamoxifen, so subsequent analyses ignore PR and are limited to the 10 645 women with ER-positive disease, with median follow-up in survivors of 13 years (IQR 9–18).
Figure 2.
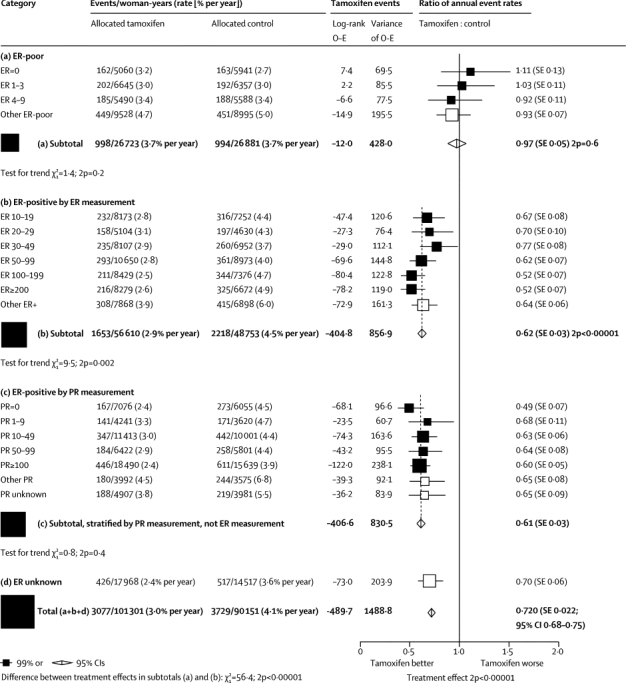
Relevance of quantitative ER and PR measurement (fmol/mg cytosol protein) to the tamoxifen versus control recurrence rate ratio
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. Other ER poor includes ER-negative by immunohistochemistry and ER unspecified, but less than 10 fmol/mg. ER=oestrogen receptor. PR=progesterone receptor. O–E=observed minus expected.
Figure 3 shows the 10-year recurrence risks for women with node-negative and node-positive ER-positive disease, subdivided by use of chemotherapy. Even if chemotherapy was given, tamoxifen was of substantial further benefit (ie, chemotherapy plus tamoxifen was better than chemotherapy alone), producing a further reduction of about a quarter in 10-year recurrence risk (figure 3).
Figure 3.
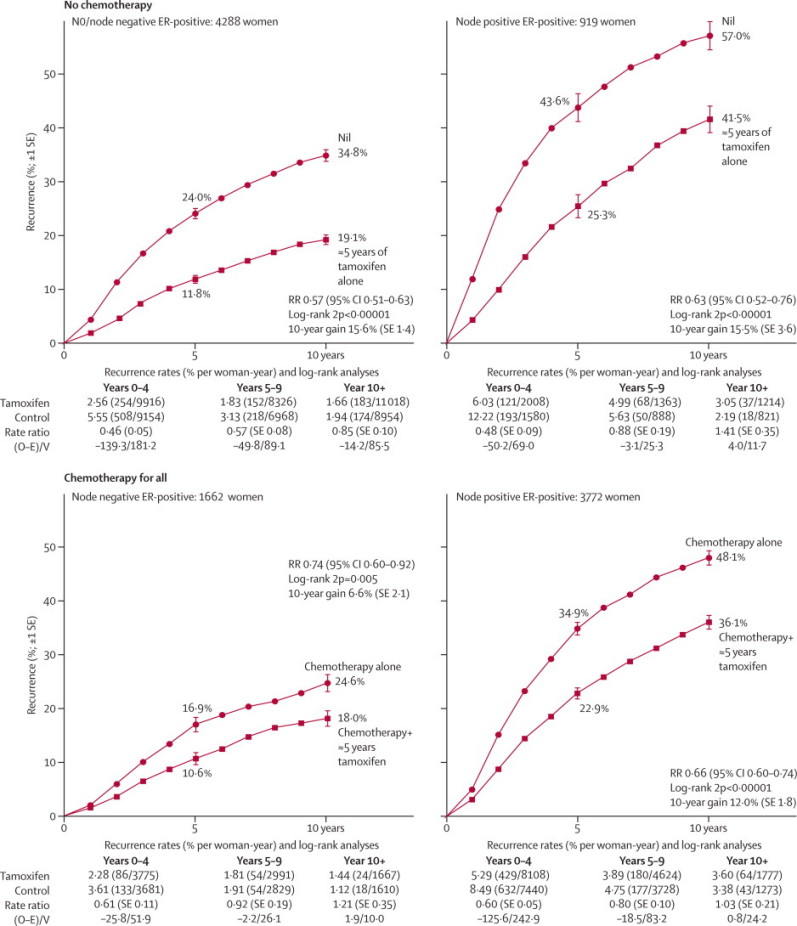
Relevance of nodal status and of background chemotherapy to the effects of tamoxifen on the 10-year probability of recurrence, for ER-positive disease
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. Event rate ratio (RR) is from summed log-rank statistics for all time periods. Gain (and its SE) is absolute difference between ends of graphs. ER=oestrogen receptor. PR=progesterone receptor. O–E=observed minus expected, with variance V.
Figure 4 subdivides the results for ER-positive disease according to daily tamoxifen dose tested, use of background chemotherapy (present or absent, and if present, concurrent or sequential), entry age, nodal status, tumour grade (poorly differentiated or moderately or well differentiated), diameter (1–20, 21–50, or >50 mm), site of first recurrence (isolated locoregional, contralateral, or distant), and time since randomisation (0–1, 2–4, 5–9, or ≥10 years). Substantial and highly significant recurrence reductions were recorded in every subgroup (apart from the period ≥10 years after entry). Corresponding subgroup analyses for breast cancer mortality (ie, mortality rate in all women less that in women without recurrence) yielded generally similar findings (webappendix p 4), except that a substantial mortality reduction continued well beyond year 10 (RR during years ≥10 after entry 0·73 [SE 0·07], p<0·00001). Thus, the recurrence reduction during years 0–9 caused a highly significant reduction in breast cancer mortality both during and after years 0–9.
Figure 4.
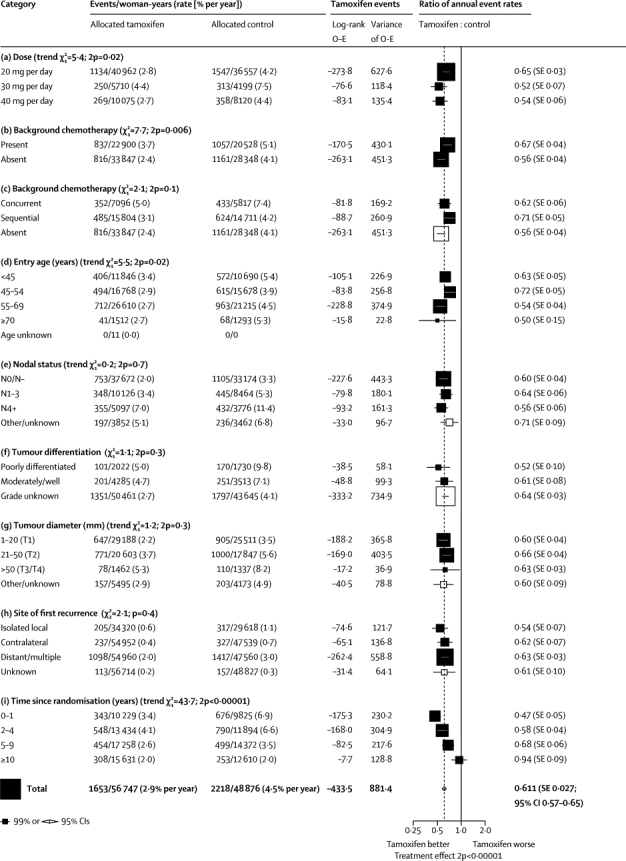
Subgroup analyses of the tamoxifen versus control recurrence rate ratio, for ER-positive disease
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. ER=oestrogen receptor. O–E=observed minus expected, with variance V.
The recurrence reduction seemed somewhat greater in trials of higher daily tamoxifen doses (p=0·02 for trend between RRs for 20, 30, and 40 mg per day), but we found no such dose effect for breast cancer mortality (webappendix p 4) or endometrial cancer incidence (data not shown). There were highly significant recurrence reductions both in the six trials with no chemotherapy (RR 0·56 [0·04]) and in the 14 trials of chemotherapy plus tamoxifen versus the same chemotherapy alone (RR 0·67 [0·04]), with a slightly greater effect of tamoxifen in those with greater degrees of ER positivity in both trial categories (data not shown). For patients receiving chemotherapy, tamoxifen was of further benefit whether it started concurrently with the chemotherapy (RR 0·62 [0·06]) or after it (RR 0·71 [0·05]). The slight superiority of starting concurrently was, however, not significant, and these tamoxifen trials did not randomise timing. In all regimens, tamoxifen had a substantial effect (figure 4).
The proportional risk reductions were slightly, but not significantly, greater at older ages, but benefits were substantial and consistent for women in each age range (including the many with entry age <45 years [and the few with entry age ≥70 years: 41 recurrences vs 68 recurrences, 2p=0·001]). Nodal status, tumour grade, and diameter did not materially affect proportional risk reductions. They were, however, importantly predictive of the absolute risk without tamoxifen, and hence of the absolute benefit of giving tamoxifen. Local recurrence, contralateral breast cancer (generally new primary), and distant recurrence were all substantially reduced by tamoxifen (each p<0·00001).
The proportional effects on recurrence rates differed between different time periods (figure 4). Recurrence was reduced by more than half during the first 2 years (when almost all those allocated treatment would have been partially or fully treated) and by almost half during the next 3 years. During years 5–9 after randomisation there was (in all but two trials23–26) no difference in adjuvant tamoxifen use between the treatment and control groups, yet the recurrence rate was still almost a third lower in those originally allocated tamoxifen (RR 0·68 [0·06], p<0·0001). After year 10, recurrence rates were similar (RR 0·97 [0·10]) in the two groups, indicating no loss after year 10 of the gains during years 0–9.
Figure 5 shows 15-year results for recurrence and breast cancer mortality in all women with ER-positive disease. Remarkably, the yearly rate of breast cancer mortality was reduced by about a third (RR 0·70 [0·05], p<0·00001) throughout the first 15 years after randomisation, with highly significant extra benefit during each of years 0–4 (RR 0·71 [0·05], 95% CI 0·62–0·80), years 5–9 (0·66 [0·05], 95% CI 0·58–0·75), and years 10–14 (0·68 [0·08], 95% CI 0·56–0·83), each p<0·00001 (figure 5, webappendix p 4). The absolute mortality difference was only 3% (9% vs 12%) at year 5, by which time trial treatment had ended (in all except the few patients re-randomised to continue after year 5), but it was three times as great (24% vs 33%) by year 15.
Figure 5.
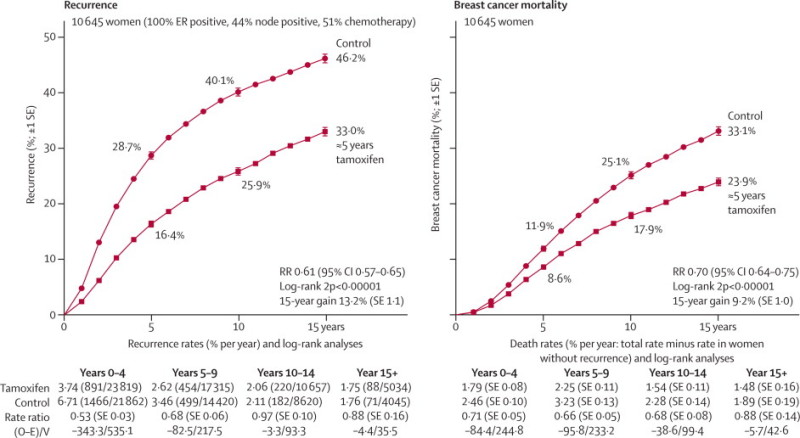
Effects of about 5 years of tamoxifen on the 15-year probabilities of recurrence and of breast cancer mortality, for ER-positive disease
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. Event rate ratio (RR) is from summed log-rank statistics for all time periods. Gain (and its SE) is absolute difference between ends of graphs. ER=oestrogen receptor. O–E=observed minus expected, with variance V.
In ER-positive disease, the reductions in recurrence and mortality during years 0–4 were almost as great in trials of only 1–2 years as in trials of about 5 years of tamoxifen (webappendix pp 2, 18–23). However, the reductions in recurrence during years 5–9 were greater in trials of about 5 years of tamoxifen than in trials of only 1–2 years of tamoxifen. Although 1–2 years of tamoxifen had little further effect on recurrence it had some further effect on mortality after year 5, although smaller than that of about 5 years of tamoxifen (webappendix p 2).
The table shows, for women with ER-positive disease, effects on cause-specific mortality and on second cancer incidence before any recurrence of the original breast cancer. (Effects on diseases other than breast cancer were not materially affected by ER status; webappendix pp 11–17.) Because tamoxifen delayed or prevented recurrence, those in the tamoxifen groups spent longer than controls at risk of death without recurrence (56 747 vs 48 876 woman-years). Hence, absolute numbers of deaths before recurrence in treatment and control groups are not directly comparable, but log-rank analyses make due allowance for this imbalance.
The main life-threatening side-effects of tamoxifen are uterine cancer and thromboembolic disease.1,27 In ER-positive disease (mean 10 years of follow-up) there were nine deaths in the tamoxifen group versus one in the control group from uterus (excluding cervix) cancer and six versus no deaths from pulmonary embolus during the first 5 years (but no apparent excess afterwards). These included one versus zero deaths in 2962 versus 3007 women younger than 55 years at entry, suggesting a 10-year mortality of less than 0·1%, but 14 versus one death in 2386 versus 2289 in older women, suggesting a 10-year mortality of 0·6% from these two side-effects. Otherwise, we recorded no definite differences in mortality without recurrence. A non-significant excess of stroke deaths (three extra per 1000 women during the first 15 years, none of which occurred during the treatment period) was balanced by a non-significant shortfall in cardiac deaths (three fewer per 1000 women during the first 15 years), so we recorded little net effect on overall vascular mortality (webappendix p 14).
Tamoxifen increased uterine cancer incidence (excluding cervix cancer, RR 2·40 [0·32], p=0·00002), reduced contralateral breast cancer incidence by, in each age range, a larger absolute amount, and had no significant effect on other types of cancer (table 1 and webappendix pp 16–17). These adverse and protective effects persisted for some years after treatment ended (webappendix pp 9–13). The uterine cancer risk was strongly correlated with age, with little absolute risk for entry age younger than 45 years or 45–54 years, but for entry age 55–69 years 15-year incidence was 3·8% in the tamoxifen group vs 1·1% in the control group (absolute increase 2·6% [SE 0·6], 95% CI 1·4–3·8). By contrast, the absolute (and proportional) decrease in contralateral breast cancer was independent of age, with 15-year incidence of 6·5% vs 9·8% in ER-positive disease (absolute reduction 3·2% [0·8]). In ER-poor disease, the 15-year incidence of contralateral disease was 7·1% in both treatment groups (absolute reduction 0·1% [1·1]).
Table.
Mortality by cause and incidence of second cancers, for ER-positive disease only
| Number of events (both groups) | O–E | Variance of O–E | Event RR (SE) | p value* | |||
|---|---|---|---|---|---|---|---|
| Death with or without recurrence | |||||||
| Death without recurrence | 1117 | 4·9 | 258·6 | 1·02 (0·06) | 0·79 | ||
| Death with recurrence | 2694 | −224·5 | 620·2 | 0·70 (0·03) | <0·00001 | ||
| Any death | 3811 | −219·6 | 878·8 | 0·78 (0·03) | <0·00001 | ||
| Death without recurrence (selected groups of causes) | |||||||
| Vascular disease | |||||||
| Stroke | 64 | 4·8 | 15·2 | 1·37 (0·30) | 0·27 | ||
| Pulmonary embolus† | 12 | 2·5 | 3·0 | 2·30 (0·90) | 0·25 | ||
| Heart and other vascular | 212 | −6·1 | 50·1 | 0·89 (0·13) | 0·43 | ||
| Neoplastic disease | |||||||
| Uterus, excluding cervix‡ | 10 | 3·2 | 2·2 | 4·28 (1·52) | 0·07 | ||
| Other neoplastic | 187 | −0·1 | 44·2 | 1·00 (0·15) | 1·00 | ||
| Other specified cause | 312 | 4·6 | 71·0 | 1·07 (0·12) | 0·63 | ||
| Unspecified cause | 320 | −4·0 | 72·9 | 0·95 (0·11) | 0·68 | ||
| Second cancer incidence without previous recurrence (selected sites) | |||||||
| Contralateral breast, by age at entry (years) | |||||||
| <45 | 110 | −17·7 | 27·2 | 0·52 (0·14) | 0·001 | ||
| 45–54 | 169 | −18·8 | 41·5 | 0·64 (0·12) | 0·004 | ||
| 55–69 | 268 | −28·7 | 64·0 | 0·64 (0·10) | 0·0001 | ||
| ≥70 | 17 | 0·1 | 4·1 | .. | .. | ||
| All ages | 564 | −65·1 | 136·7 | 0·62 (0·07) | <0·00001 | ||
| Uterus, excluding cervix‡, by age at entry (years) | |||||||
| <45 | 11 | 0·1 | 2·7 | 1·04 (0·62) | 1·00 | ||
| 45–54 | 25 | 3·3 | 5·9 | 1·75 (0·55) | 0·25 | ||
| 55–69 | 71 | 18·0 | 16·6 | 2·96 (0·44) | 0·00002 | ||
| ≥70 | 1 | 0·8 | 0·2 | .. | .. | ||
| All ages | 108 | 22·2 | 25·4 | 2·40 (0·32) | 0·00002 | ||
| Other or unknown site | 606 | 2·6 | 143·6 | 1·02 (0·08) | 0·86 | ||
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. Webappendix p 17 shows results for all women, irrespective of ER status. O–E=observed minus expected, with variance V. RR=rate ratio. ER=oestrogen receptor.
Two-sided p value, calculated with correction for continuity.
Six deaths in the tamoxifen group versus no deaths in the control group from pulmonary embolus during years 0–4, two deaths in each group at years 5–9, and one death in each group at years 10+.
Nine versus one death (age at entry: 45–54 years, one vs none; 55–69 years, seven vs one; ≥70 years, one vs none), and 83 versus 25 incident cases of uterine cancer, excluding cervix, as first event after entry.
In the hypothetical absence of breast cancer mortality, 15-year probabilities of death from other causes in these trials were about 3% for entry ages younger than 45 years, 6% for ages 45–54 years, and 20% for ages 55–69 years (similar to population mortality rates). Because this risk of a fifth for ages 55–69 years applied similarly to the tamoxifen and to the control group, in both groups 15-year overall survival is a fifth smaller than 15-year breast cancer survival, so the 15-year gain is a fifth smaller for overall mortality than for breast cancer mortality (figure 6); however, this finding does not suggest any adverse effect on mortality from causes other than breast cancer and the known side-effects of tamoxifen. For entry age younger than 45 years, intercurrent mortality was low, there were no deaths from uterine cancer or pulmonary embolus in either group, and 15-year gains in overall mortality and in breast cancer mortality were similar (figure 6).
Figure 6.
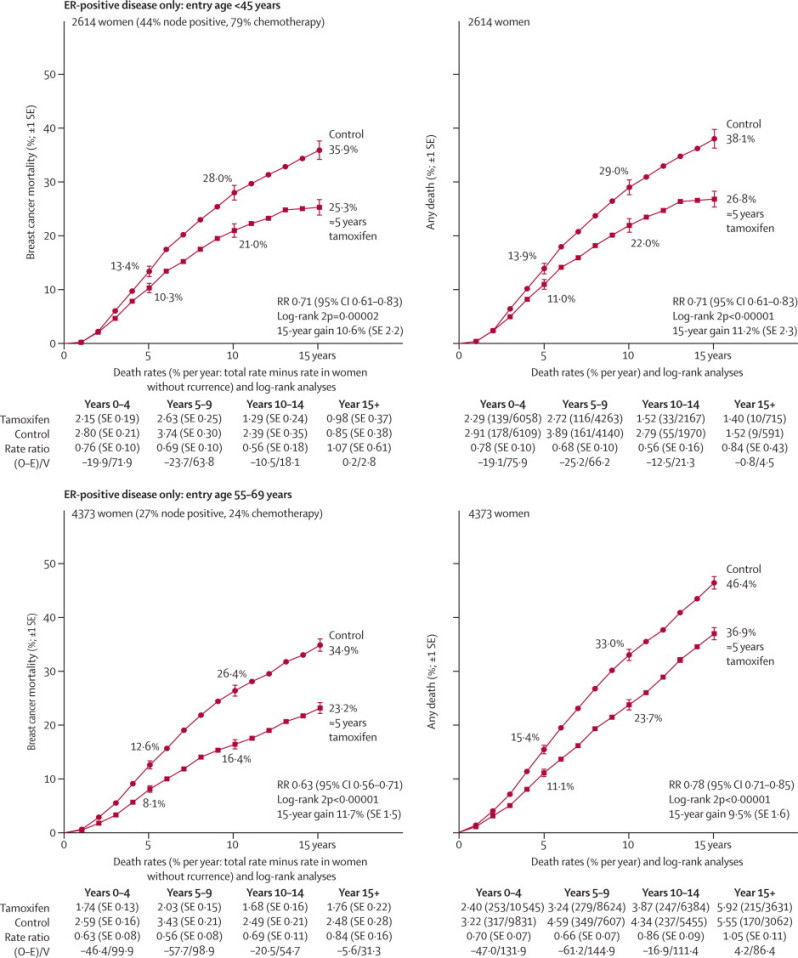
Relevance of intercurrent mortality in women younger than 45 years and 55–69 years of age to the absolute effects of tamoxifen on 15-year mortality, for ER-positive disease
Outcome by allocated treatment in trials of about 5 years of adjuvant tamoxifen. Event rate ratio (RR) is from summed log-rank statistics for all time periods. Gain (and its SE) is absolute difference between ends of graphs. ER=oestrogen receptor. O–E=observed minus expected, with variance V.
Discussion
Longer follow-up of the trials of about 5 years of tamoxifen has greatly strengthened the evidence that substantially reduced mortality rates for breast cancer continue well beyond year 10, as a delayed effect of the greatly reduced recurrence rates during years 0–9. It has also produced strong evidence of a substantial effect even in disease that was only weakly ER positive (10–19 fmol/mg), although not in disease that was wholly ER negative.
If all trials had been of exactly 5 years of tamoxifen versus no adjuvant tamoxifen, with full compliance in both groups, the net benefit would have been somewhat greater. A sixth of the treated patients in these trials of about 5 years of tamoxifen were allocated only 2–3 years of tamoxifen. In addition, of the patients allocated at least 5 years of tamoxifen about 18% discontinued adjuvant treatment within 2 years. Both direct comparisons1 and indirect comparisons (webappendix p 2) show greater mortality reduction with about 5 years than with only about 2 years of tamoxifen. Moreover, particularly in the later trials, some controls with ER-positive disease might eventually have started adjuvant hormonal therapy anyway.28,29 Although the combined effects of patient drop-out and drop-in cannot be quantified exactly, the rate ratio for breast cancer death of 0·70 (SE 0·06) in the present meta-analyses of outcome by allocated treatment means that in ER-positive disease full compliance with 5 years of tamoxifen would reduce 15-year breast cancer mortality rates by at least a third, and probably somewhat more.
Measured ER status of the original primary tumour was the only patient or tumour characteristic recorded that strongly predicted tamoxifen efficacy (ie, the proportional RR). In women with ER-poor primary breast cancers, tamoxifen did not significantly reduce the overall recurrence rate, and did not seem to reduce the incidence of contralateral breast cancer. This apparently null result is, however, still consistent with the hypothesis that the proportional reduction produced by tamoxifen in the incidence of ER-positive contralateral disease is unaffected by the ER status of the original primary. (In the US SEER cancer registries30 only about half of the contralateral tumours arising more than a year after ER-negative primary cancers are ER positive, compared with 80% after ER-positive primary tumours.)
There appeared to be a fairly sharp cutoff in tamoxifen efficacy with respect to the quantitative ER measurement, with little effect at 4–9 fmol/mg and substantial benefit at 10–19 fmol/mg. (Reassuringly, ≥10 fmol/mg has been the criterion for ER positivity used in most trials, and by the EBCTCG.1) However, in view of the limitations of the ligand-binding ER assay method used in these trials,31,32 a sharp efficacy cutoff at a particular assay value is not plausible. Although the evidence of substantial benefit from tamoxifen at ER measurements of only 10–19 fmol/mg is robust, the evidence of zero benefit at 4–9 fmol/mg is not, because the CI for tamoxifen efficacy in this subgroup is wide, despite more than 10 000 woman-years of follow-up.
If there is a continuous relation between the measured ER and the efficacy of tamoxifen, and the sharp cutoff was attributable mainly to chance, then detailed re-examination of these trial results is unlikely to provide clarification. The most appropriate use of the trial findings might be to conclude from them the remarkable importance of prevention of any stimulation of breast cancer cells by any functional ER in those cells, and the need to use sensitive and reliable ER assay methods in future patients.
Contemporary assessment of ER status is generally by immunohistochemistry (percentage of tumour cells stained by anti-ER antibody). However, because there is good concordance between immunohistochemistry and ligand-binding assays of ER positivity,31–35 the present finding of a substantial effect of tamoxifen even at relatively low levels of ER positivity is relevant to present practice. Guidelines for immunohistochemistry assays36 recommend definition of ER positivity as 1% or more cells staining, but with some uncertainty about whether to include the range 1–10%. However, few patients if tested properly have 1–10% cells staining,31,32 and a low cutoff minimises life-threatening false negative ER results due to technical error. Interpretation of marginally positive ER assays could in future be helped by ER gene expression assays. (Preliminary studies of new assay methods could, however, engender false negative claims about endocrine effects in some ER-positive subgroup.37)
Given the ER status, the PR measurement did not seem to be importantly predictive of efficacy. In disease recorded as ER positive there was substantial and highly significant benefit even if the sample was recorded as PR poor. The absolute recurrence reduction at 15 years seemed, if anything, somewhat greater in ER-positive PR-poor disease than in ER-positive PR-positive disease, perhaps because of the somewhat higher background risk of recurrence without treatment. Conversely, in disease reported to be ER poor, positive PR measurements did not identify a subgroup with significant benefit. There did seem to be some slight early benefit from tamoxifen in disease that was measured to be ER poor and PR positive but this finding was not significant, and might be attributable to inclusion in this category of a few patients with false-negative ER assays. As assays improve, fewer breast cancers are reported as ER negative PR positive (4% in the early 1990s but only 1% in recent years in the SEER cancer registry data30). For the few patients still reported as ER negative PR positive, repeat testing on another tissue sample has been recommended34,36 to rule out a false-negative ER assay in a patient who could benefit from endocrine treatment.
Although age is not a strong independent correlate of distant recurrence or of tamoxifen efficacy, being young is a major determinant of the gain in life expectancy from avoidance of distant recurrence. Worldwide, half of all patients with breast cancer are younger than 55 years when diagnosed.38 For premenopausal or perimenopausal women with ER-positive breast cancer, tamoxifen is a major hormonal treatment option (because ovarian function cannot be controlled by aromatase inhibitors), and there is little uterine cancer risk or excess risk of fatal pulmonary embolus from administration of tamoxifen before age 45 years or at ages 45–54 years.27 By contrast, for older women with an intact uterus the excess risk of death from endometrial cancer or pulmonary embolus could well be about 1%.
The key quantitative finding that is likely to be generalisable to future patients37 is the proportional risk reduction produced by about 5 years of tamoxifen in ER-positive disease, which is roughly independent of age, nodal status, tumour grade, diameter, chemotherapy use, and timing of chemotherapy (concurrent or sequential). This finding suggests that if chemotherapy was being given then the additional therapeutic effects of giving tamoxifen were approximately independent of any therapeutic effects of that chemotherapy (a conclusion strongly reinforced by meta-analyses1 of the trials of chemotherapy, which found that the proportional risk reduction produced by chemotherapy was unaffected by whether tamoxifen was being given).
Insofar as any of these factors substantially affect absolute risk in women without tamoxifen, they substantially affect the absolute reduction in risk produced by tamoxifen. Many treatment guidelines recommend endocrine treatment for disease with any degree of ER positivity.39 In accordance with this recommendation, our meta-analyses show a definite and substantial protective effect even at ER measurements of only 10–19 fmol/mg, and show that on average in all women with ER-positive disease full compliance with 5 years of adjuvant tamoxifen would reduce the breast cancer mortality rate during the first 15 years after the start of treatment by at least a third, compared with no adjuvant endocrine therapy.
Acknowledgments
Acknowledgments
We thank the 54 000 participants and their carers; those undertaking the trials and sharing the data; and the CTSU, which has long hosted this collaboration.
Contributors
The EBCTCG secretariat, including C Davies, J Godwin, R Gray, M Clarke, S Darby, P McGale, Y C Wang, and R Peto, identified trials, obtained datasets and had full access to them. J Godwin, C Davies, R Gray, H C Pan, and R Peto generated analyses and drafted the report, with M Dowsett and J Ingle as external advisers. All writing committee members contributed to revising the report.
Writing Committee
C Davies (Clinical Trial Service Unit [CTSU], Oxford, UK), J Godwin (CTSU), R Gray (CTSU), M Clarke (CTSU), D Cutter (CTSU), S Darby (CTSU), P McGale (CTSU), H C Pan (CTSU), C Taylor (CTSU), Y C Wang (CTSU), M Dowsett (Breakthrough Breast Cancer Centre, Institute of Cancer Research, Royal Marsden Hospital, London, UK), J Ingle (Mayo Clinic, Rochester, MN, USA), R Peto (CTSU).
Attendees at EBCTCG Steering Committee meetings
K Albain, S Anderson, R Arriagada, W Barlow, J Bergh, J Bliss, *M Buyse, D Cameron, E Carrasco, *† M Clarke, C Correa, A Coates, *† R Collins, J Costantino, † D Cutter, J Cuzick, *† S Darby, N Davidson, *† C Davies, † K Davies, † A Delmestri, A Di Leo, M Dowsett, † P Elphinstone, † V Evans, *M Ewertz, R Gelber, † L Gettins, C Geyer, A Goldhirsch, † J Godwin, † R Gray, † C Gregory, D Hayes, C Hill, J Ingle, R Jakesz, † S James, M Kaufmann, † A Kerr, † E MacKinnon, † P McGale, † T McHugh, L Norton, Y Ohashi, S Paik, † H C Pan, E Perez, *† R Peto, *M Piccart (co-chair), L Pierce, G Pruneri, *K Pritchard (co-chair), V Raina, P Ravdin, J Robertson, E Rutgers, YF Shao, S Swain, † C Taylor, P Valagussa, G Viale, T Whelan, *E Winer, † Y Wang, *W Wood. *Executive Group. † Secretariat.
EBCTCG collaborators, listed alphabetically by institution and then alphabetically by name
ACETBC, Tokyo, Japan O Abe, R Abe, K Enomoto, K Kikuchi, H Koyama, H Masuda, Y Nomura, Y Ohashi, K Sakai, K Sugimachi, M Toi, T Tominaga, J Uchino, M Yoshida. Addenbrooke's Hospital, Cambridge, UK J L Haybittle. Anglo-Celtic Cooperative Oncology Group, UK C F Leonard. ARCOSEIN Group, France G Calais, P Geraud. ATLAS Trial Collaborative Study Group, Oxford, UK V Collett, C Davies, A Delmestri, J Sayer. Auckland Breast Cancer Study Group, New Zealand V J Harvey, I M Holdaway, R G Kay, B H Mason. Australian New Zealand Breast Cancer Trials Group, Sydney, Australia J F Forbes, N Wilcken. Austrian Breast Cancer Study Group, Vienna, Austria R Bartsch, P Dubsky, C Fesl, H Fohler, M Gnant, R Greil, R Jakesz, A Lang, G Luschin-Ebengreuth, C Marth, B Mlineritsch, H Samonigg, C F Singer, G G Steger, H Stöger. Beatson Oncology Centre, Glasgow, UK P Canney, H M A Yosef. Belgian Adjuvant Breast Cancer Project, Liège, Belgium C Focan. Berlin-Buch Akademie der Wissenschaften, Germany U Peek. Birmingham General Hospital, UK G D Oates, J Powell. Bordeaux Institut Bergonié, France M Durand, L Mauriac. Bordet Institute, Brussels, Belgium A Di Leo, S Dolci, D Larsimont, J M Nogaret, C Philippson, M J Piccart. Bradford Royal Infirmary, UK M B Masood, D Parker, J J Price. Breast Cancer International Research Group (BCIRG) M A Lindsay, J Mackey, M Martin. Breast Cancer Study Group of the Comprehensive Cancer Centre, Limburg, Netherlands P S G J Hupperets. British Association of Surgical Oncology BASO II Trialists, London, UK T Bates, R W Blamey, U Chetty, I O Ellis, E Mallon, D A L Morgan, J Patnick, S Pinder. British Columbia Cancer Agency, Vancouver, Canada I Olivotto, J Ragaz. Cancer and Leukemia Group B, Washington DC, USA D Berry, G Broadwater, C Cirrincione, H Muss, L Norton, R B Weiss. Cancer Care Ontario, Canada H T Abu-Zahra. Cancer Research Centre of the Russian Academy of Medical Sciences, Moscow, Russia S M Portnoj. Cancer Research UK Clinical Trials Unit (CRCTU), NCRI, Birmingham, UK S Bowden, C Brookes, J Dunn, I Fernando, M Lee, C Poole, D Rea, D Spooner. Cardiff Trialists Group, UK P J Barrett-Lee, R E Mansel, I J Monypenny. Case Western Reserve University, Cleveland, OH, USA N H Gordon. Central Oncology Group, Milwaukee, WI, USA H L Davis. Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary, University of London, UK J Cuzick. Centre Léon-Bérard, Lyon, France Y Lehingue, P Romestaing. Centre Paul Lamarque, Montpellier, France J B Dubois.
Centre Regional François Baclesse, Caen, France T Delozier, B Griffon, J Mace Lesec'h. Centre René Huguenin, Paris, St Cloud, France P Rambert.
Centro Oncologico, Trieste, Italy G Mustacchi. Charles University in Prague, First Faculty of Medicine, Department of Oncology of the First Faculty of Medicine and General Teaching Hospital, Czech Republic L Petruzelka, O Pribylova. Cheltenham General Hospital, UK J R Owen. Chemo N0 Trial Group, Germany N Harbeck, F Jänicke, C Meisner, M Schmitt, C Thomssen. Chicago University, IL, USA P Meier. Chinese Academy of Medical Sciences, Beijing, People's Republic of China (in collaboration with the Oxford CTSU) Y Shan, Y F Shao, X Wang, D B Zhao (CTSU: Z M Chen, H C Pan). Christie Hospital and Holt Radium Institute, Manchester, UK A Howell, R Swindell. Clinical Trial Service Unit, Oxford, UK (ie, EBCTCG Secretariat) J A Burrett, M Clarke, R Collins, C Correa, D Cutter, S Darby, C Davies, K Davies, A Delmestri, P Elphinstone, V Evans, L Gettins, J Godwin, R Gray, C Gregory, D Hermans, C Hicks, S James, A Kerr, E MacKinnon, M Lay, P McGale, T McHugh, R Peto, J Sayer, C Taylor, Y Wang. Coimbra Instituto de Oncologia, Portugal J Albano, C F de Oliveira, H Gervásio, J Gordilho. Copenhagen Radium Centre, Denmark H Johansen, H T Mouridsen. Dana-Farber Cancer Institute, Boston, MA, USA R S Gelman, J R Harris, D Hayes, C Henderson, C L Shapiro, E Winer. Danish Breast Cancer Cooperative Group, Copenhagen, Denmark P Christiansen, B Ejlertsen, M Ewertz, M-B Jensen, S Møller, H T Mouridsen. Danish Cancer Registry, Copenhagen, Denmark B Carstensen, T Palshof. Düsseldorf University, Germany H J Trampisch. Dutch Working Party for Autologous Bone Marrow Transplant in Solid Tumours, Amsterdam & Groningen, Netherlands O Dalesio, E G E de Vries, S Rodenhuis, H van Tinteren. Eastern Cooperative Oncology Group, Boston, MA, USA R L Comis, N E Davidson, R Gray, N Robert, G Sledge, L J Solin, J A Sparano, D C Tormey, W Wood. Edinburgh Breast Unit, UK D Cameron, U Chetty, J M Dixon, P Forrest, W Jack, I Kunkler. Elim Hospital, Hamburg, Germany J Rossbach. Erasmus MC/Daniel den Hoed Cancer Center, Rotterdam, Netherlands J G M Klijn, A D Treurniet-Donker, W L J van Putten. European Institute of Oncology, Milan, Italy N Rotmensz, U Veronesi, G Viale. European Organization for Research and Treatment of Cancer, Brussels, Belgium H Bartelink, N Bijker, J Bogaerts, F Cardoso, T Cufer, J P Julien, E Rutgers, C J H van de Velde. Evanston Hospital, IL, USA M P Cunningham. Finnish Breast Cancer Group, Finland R Huovinen, H Joensuu. Fondazione Maugeri Pavia, Italy A Costa, C Tinterri. Fondazione Michelangelo, Milan, Italy G Bonadonna, L Gianni, P Valagussa. Fox Chase Cancer Center, Philadelphia, PA, USA L J Goldstein. French Adjuvant Study Group (GFEA), Guyancourt, France J Bonneterre, P Fargeot, P Fumoleau, P Kerbrat, E Luporsi, M Namer. German Adjuvant Breast Group (GABG), Frankfurt, Germany W Eiermann, J Hilfrich, W Jonat, M Kaufmann, R Kreienberg, M Schumacher. German Breast Cancer Study Group (BMFT), Freiburg, Germany G Bastert, H Rauschecker, R Sauer, W Sauerbrei, A Schauer, M Schumacher. German Breast Group (GBG), Neu-Isenburg, Germany J U Blohmer, S D Costa, H Eidtmann, B Gerber, C Jackisch, S Loibl, G von Minckwitz. Ghent University Hospital, Belgium A de Schryver, L Vakaet. GIVIO Interdisciplinary Group for Cancer Care Evaluation, Chieti, Italy M Belfiglio, A Nicolucci, F Pellegrini, M C Pirozzoli, M Sacco, M Valentini. Glasgow Victoria Infirmary, UK C S McArdle, D C Smith, S Stallard. Groote Schuur Hospital, Cape Town, South Africa D M Dent, C A Gudgeon, A Hacking, E Murray, E Panieri, ID Werner. Grupo Español de Investigación en Cáncer de Mama (GEICAM), Spain E Carrasco, M Martin, M A Segui. Gruppo Oncologico Clinico Cooperativo del Nord Est, Aviano, Italy E Galligioni. Gruppo Oncologico Dell'Italia Meridionale (GOIM), Rome, Italy M Lopez. Guadalajara Hospital de 20 Noviembre, Mexico A Erazo, J Y Medina. Gunma University, Japan J Horiguchi, H Takei. Guy's Hospital, London, UK I S Fentiman, J L Hayward, R D Rubens, D Skilton. Heidelberg University I, Germany H Scheurlen. Heidelberg University II, Germany M Kaufmann, H C Sohn. Helios Klinikum Berlin-Buch, Germany M Untch. Hellenic Breast Surgeons Society, Greece U Dafni, C Markopoulos. Hellenic Cooperative Oncology Group, Athens, Greece U Dafni, G Fountzilas. Hellenic Oncology Research Group, Greece D Mavroudis. Helsinki Deaconess Medical Centre, Finland P Klefstrom. Helsinki University, Finland C Blomqvist, T Saarto. Hospital del Mar, Barcelona, Spain M Gallen. Innsbruck University, Austria R Margreiter. Institut Claudius Regaud, Toulouse, France B de Lafontan, J Mihura, H Roché. Institut Curie, Paris, France B Asselain, R J Salmon, J R Vilcoq. Institut Gustave-Roussy, Paris, France R Arriagada, C. Bourgier, C Hill, S Koscielny, A Laplanche, M G Lê, M Spielmann. Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU, NCRI), UK R A'Hern, J Bliss, P Ellis, L Kilburn, J R Yarnold. Integraal Kankercentrum, Amsterdam, Netherlands J Benraadt, M Kooi, A O van de Velde, J A van Dongen, J B Vermorken. International Breast Cancer Study Group (IBCSG), Bern, Switzerland M Castiglione, A Coates, M Colleoni, J Collins, J Forbes, R D Gelber, A Goldhirsch, J Lindtner, K N Price, M M Regan, C M Rudenstam, H J Senn, B Thuerlimann. International Collaborative Cancer Group, Charing Cross Hospital, London, UK J M Bliss, C E D Chilvers, R C Coombes, E Hall, M Marty. International Drug Development Institute, Louvain-la-Neuve, Belgium M Buyse. International TABLE Study Group, Berlin, Germany K Possinger, P Schmid, M Untch, D Wallwiener. ISD Cancer Clinical Trials Team (incorporating the former Scottish Cancer Therapy Network), Edinburgh, UK L Foster, W D George, H J Stewart, P Stroner. Israel NSABC, Tel Aviv, Israel R Borovik, H Hayat, M J Inbar, E Robinson. Istituto Nazionale per la Ricerca sul Cancro, Genova, Italy P Bruzzi, L Del Mastro, P Pronzato, M R Sertoli, M Venturini. Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy T Camerini, G De Palo, M G Di Mauro, F Formelli, P Valagussa. Istituto Oncologico Romagnolo, Forli, Italy D Amadori. Italian Cooperative Chemo-Radio-Surgical Group, Bologna, Italy A Martoni, F Pannuti. Italian Oncology Group for Clinical Research (GOIRC), Parma, Italy R Camisa, G Cocconi, A Colozza, R Passalacqua. Japan Clinical Oncology Group–Breast Cancer Study Group, Matsuyama, Japan K Aogi, S Takashima. Japanese Foundation for Multidisciplinary Treatment of Cancer, Tokyo, Japan O Abe, T Ikeda, K Inokuchi, K Kikuchi, K Sawa. Kawasaki Medical School, Japan H Sonoo. Krakow Institute of Oncology, Poland S Korzeniowski, J Skolyszewski. Kumamoto University Group, Japan M Ogawa, J Yamashita. Leiden University Medical Center, Netherlands E Bastiaannet, C J H van de Velde, W van de Water, J G H van Nes. Leuven Akademisch Ziekenhuis, Gasthuisberg, Belgium R Christiaens, P Neven, R Paridaens, W Van den Bogaert. Ludwig-Maximilians University, Munich, Germany S Braun, W Janni. Marseille Laboratoire de Cancérologie Biologique APM, France P Martin, S Romain. Medical University Vienna – General Hospital - Department of Obstetrics and Gynaecology and Department of Medicine I, Vienna, Austria M Janauer, M Seifert, P Sevelda, C C Zielinski. Memorial Sloan-Kettering Cancer Center, New York, NY, USA T Hakes, C A Hudis, L Norton, R Wittes. Metaxas Memorial Cancer Hospital, Athens, Greece G Giokas, D Kondylis, B Lissaios. Mexican National Medical Center, Mexico City, Mexico R de la Huerta, M G Sainz. National Cancer Institute, Bethesda, MD, USA R Altemus, K Camphausen, K Cowan, D Danforth, A Lichter, M Lippman, J O'Shaughnessy, L J Pierce, S Steinberg, D Venzon, J A Zujewski. National Cancer Institute of Bari, Italy C D'Amico, M Lioce, A Paradiso. NCIC Clinical Trials Group, Kingston, Ontario, Canada J-A W Chapman, K Gelmon, P E Goss, M N Levine, R Meyer, W Parulekar, J L Pater, K I Pritchard, L E Shepherd, D Tu, T Whelan. National Kyushu Cancer Center, Japan Y Nomura, S Ohno. National Surgical Adjuvant Breast and Bowel Project (NSABP), Pittsburgh, PA, USA S Anderson, G Bass, A Brown (deceased), J Bryant (deceased), J Costantino, J Dignam, B Fisher, C Geyer, E P Mamounas, S Paik, C Redmond, S Swain, L Wickerham, N Wolmark. Nolvadex Adjuvant Trial Organisation, London, UK M Baum, I M Jackson (deceased), M K Palmer. North Central Cancer Treatment Group, Mayo Clinic, Rochester, MN, USA E Perez, J N Ingle, V J Suman. North Sweden Breast Cancer Group, Umeå, Sweden N O Bengtsson, S Emdin, H Jonsson. North-West Oncology Group (GONO), Italy L Del Mastro, M Venturini. North-Western British Surgeons, Manchester, UK J P Lythgoe, R Swindell. Northwick Park Hospital, London, UK M Kissin. Norwegian Breast Cancer Group, Oslo, Norway B Erikstein, E Hannisdal, A B Jacobsen, J E Varhaug. Norwegian Radium Hospital, Oslo, Norway B Erikstein, S Gundersen, M Hauer-Jensen, H Høst, A B Jacobsen, R Nissen-Meyer. Nottingham City Hospital, UK R W Blamey, A K Mitchell, D A L Morgan, J F R Robertson. Oita Prefectural Hospital, Japan H Ueo. Oncofrance, Paris, France M Di Palma, G Mathé (deceased), J L Misset. Ontario Clinical Oncology Group, Hamilton, Canada M Levine, K I Pritchard, T Whelan. Osaka City University, Japan K Morimoto. Osaka National Hospital, Japan K Sawa, Y Takatsuka. Oxford Radcliffe Hospitals NHS Trust, Churchill Hospital, Oxford, UK E Crossley, A Harris, D Talbot, M Taylor. PACS Adjuvant Study Group, France A L Martin, H Roché. Parma Hospital, Italy G Cocconi, B di Blasio. Petrov Research Institute of Oncology, St Petersburg, Russia V Ivanov, R Paltuev, V Semiglazov. Piedmont Oncology Association, Winston-Salem, NC, USA J Brockschmidt, M R Cooper. Pretoria University, South Africa C I Falkson. Royal Marsden NHS Trust, London and Sutton, UK R A'Hern, S Ashley, M Dowsett, A Makris, T J Powles, I E Smith, J R Yarnold. St George's Hospital, London, UK J C Gazet. St George Hospital, Sydney, Australia L Browne, P Graham. St Luke's Hospital, Dublin, Ireland N Corcoran. Sardinia Oncology Hospital A Businico, Cagliari, Sardinia N Deshpande, L di Martino. SASIB International Trialists, Cape Town, South Africa P Douglas, A Hacking, H Høst, A Lindtner, G Notter. Saskatchewan Cancer Foundation, Regina, Canada A J S Bryant, G H Ewing, L A Firth, J L Krushen-Kosloski. Scandinavian Adjuvant Chemotherapy Study Group, Oslo, Norway R Nissen-Meyer. South Sweden Breast Cancer Group, Lund, Sweden H Anderson, F Killander, P Malmström, L Rydén. South-East Sweden Breast Cancer Group, Linköping, Sweden L-G Arnesson, J Carstensen, M Dufmats, H Fohlin, B Nordenskjöld, M Söderberg. South-Eastern Cancer Study Group and Alabama Breast Cancer Project, Birmingham, AL, USA J T Carpenter. Southampton Oncology Centre, UK N Murray, G T Royle, P D Simmonds. Southwest Oncology Group, San Antonio, TX, USA K Albain, W Barlow, J Crowley, D Hayes, J Gralow, S Green, G Hortobagyi, R Livingston, S Martino, C K Osborne, P M Ravdin. Stockholm Breast Cancer Study Group, Sweden J Adolfsson, J Bergh, T Bondesson, F Celebioglu, K Dahlberg, T Fornander, I Fredriksson, J Frisell, E Göransson, M Iiristo, U Johansson, E Lenner, L Löfgren, P Nikolaidis, L Perbeck, S Rotstein, K Sandelin, L Skoog, G Svane, E af Trampe, C Wadström. Swiss Group for Clinical Cancer Research (SAKK), Bern, and OSAKO, St Gallen, Switzerland M Castiglione, A Goldhirsch, R Maibach, H J Senn, B Thürlimann. Tampere University Hospital, Finland M Hakama, K Holli, J Isola, K Rouhento, R Saaristo. Tel Aviv University, Israel H Brenner, A Hercbergs. The High-Dose Chemotherapy for Breast Cancer Study Group (PEGASE), France A L Martin, H Roché. Tokyo Cancer Institute Hospital, Japan M Yoshimoto. Toronto-Edmonton Breast Cancer Study Group, Canada A H G Paterson, K I Pritchard. Toronto Princess Margaret Hospital, Canada A Fyles, J W Meakin, T Panzarella, K I Pritchard. Tunis Institut Salah Azaiz, Tunisia J Bahi. UK Multicentre Cancer Chemotherapy Study Group, London, UK M Reid, M Spittle. UK/ANZ DCIS Trial H Bishop, N J Bundred, J Cuzick, I O Ellis, I S Fentiman, J F Forbes, S Forsyth, W D George, S E Pinder, I Sestak. UK/Asia Collaborative Breast Cancer Group, London, UK G P Deutsch, R Gray, D L W Kwong, V R Pai, R Peto, F Senanayake. University and Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy on behalf of GROCTA trialists F Boccardo, A Rubagotti. University College London, UK M Baum, S Forsyth, A Hackshaw, J Houghton, J Ledermann, K Monson, JS Tobias. University Federico II, Naples, Italy C Carlomagno, M De Laurentiis, S De Placido. University of Edinburgh, UK L Williams. University of Michigan, USA D Hayes, L J Pierce. University of Texas MD Anderson Cancer Center, Houston, TX, USA K Broglio, A U Buzdar. University of Wisconsin, USA R R Love. Uppsala-Örebro Breast Cancer Study Group, Sweden J Ahlgren, H Garmo, L Holmberg, G Liljegren, H Lindman, F Wärnberg. US Oncology, Houston, USA L Asmar, S E Jones. West German Study Group (WSG), Germany O Gluz, N Harbeck, C Liedtke, U Nitz. West of Scotland Breast Trial Group, Glasgow, UK A Litton. West Sweden Breast Cancer Study Group, Gothenburg, Sweden A Wallgren, P Karlsson, B K Linderholm. Western Cancer Study Group, Torrance, CA, USA R T Chlebowski. Würzburg University, Germany H Caffier.
Conflicts of interest
Interests of trialists in drugs or diagnostics and company support of trials (listed in publications: webappendix p 36) have not affected data availability or EBCTCG meta-analyses of these datasets. EBCTCG is funded from CRUK, BHF, and MRC core support of CTSU (http://www.ctsu.ox.ac.uk/about/). CTSU also performs trials funded by industry grants to the University of Oxford that are initiated, conducted, and interpreted independently of study funders. CTSU staff policy excludes honoraria or consultancy fees. MD has received payment from AstraZeneca through grants to his department and following lectures/expert testimony. All secretariat and other writing committee members declare no conflicts of interest.
Web Extra Material
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group . Treatment of early breast cancer: worldwide evidence, 1985–1990. Oxford University Press; Oxford: 1990. http://www.ctsu.ox.ac.uk/reports/ebctcg-1990/index_html (accessed May 20, 2011). [Google Scholar]
- 3.Dowsett M, Cuzick J, Ingle J. Meta-Analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors vs tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Bryant J, Dignam JJ. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 5.Blamey RW, Chetty U, Bates T. Radiotherapy and/or Tamoxifen after conserving surgery for breast cancers of excellent prognosis: BASO II trial. Eur J Cancer Suppl. 2008;6:55. doi: 10.1016/j.ejca.2013.02.031. A17. [DOI] [PubMed] [Google Scholar]
- 6.Paradiso A, De Lena M, Sambiasi M. Adjuvant hormonotherapy for slow-proliferating node-negative breast cancer patients. Results of the phase III trial of NCI-Bari. Breast. 2003;12:S40. P90. [Google Scholar]
- 7.Hutchins LF, Green SJ, Ravdin PM. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup protocol INT-0102. J Clin Oncol. 2005;23:8313–8321. doi: 10.1200/JCO.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Davidson NE, O'Neill AM, Vukov AM. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188) J Clin Oncol. 2005;23:5973–5982. doi: 10.1200/JCO.2005.05.551. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Anderson S, Tan Chiu E. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001;19:931–942. doi: 10.1200/JCO.2001.19.4.931. [DOI] [PubMed] [Google Scholar]
- 10.Bliss JM, Wils J, Marty M. Evaluation of the tolerability of FE50C versus FE75C in a prospective randomised trial in adjuvant breast cancer patients. Proc Annu Meet Am Soc Clin Oncol. 2002;21:A2017. [Google Scholar]
- 11.Bramwell VHC, Pritchard KI, Tu D. A randomized placebo-controlled study of tamoxifen after adjuvant chemotherapy in premenopausal women with early breast cancer (National Cancer Institute of Canada–Clinical Trials Group Trial, MA.12) Ann Oncol. 2010;21:283–290. doi: 10.1093/annonc/mdp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann M, Graf E, Jonat W. Tamoxifen versus control after adjuvant, risk-adapted chemotherapy in postmenopausal, receptor-negative patients with breast cancer: a randomized trial (GABG-IV D-93)—the German Adjuvant Breast Cancer Group. J Clin Oncol. 2005;23:7842–7848. doi: 10.1200/JCO.2005.01.3433. [DOI] [PubMed] [Google Scholar]
- 13.Colleoni M, Gelber S, Goldhirsch A. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13–93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Costa SD, Raab G. Dose-dense doxorubicin, docetaxel, and granulocyte colony-stimulating factor support with or without tamoxifen as preoperative therapy in patients with operable carcinoma of the breast: a randomized, controlled, open phase IIb study. J Clin Oncol. 2001;19:3506–3515. doi: 10.1200/JCO.2001.19.15.3506. [DOI] [PubMed] [Google Scholar]
- 15.Boccardo F, Rubagotti A, Bruzzi P. Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen receptor-positive breast cancer patients: results of a multicentric Italian study. J Clin Oncol. 1990;8:1310–1320. doi: 10.1200/JCO.1990.8.8.1310. [DOI] [PubMed] [Google Scholar]
- 16.Delozier T, Switsers O, Genot JY, et al. Late delayed adjuvant tamoxifen in breast cancer, a multicenter randomized trial. Fourth International Congress on Anti-cancer Chemotherapy 1993; Feb 2–5, 1993; Paris, France; p53.
- 17.Delozier T, Julien JP, Juret P. Adjuvant tamoxifen in postmenopausal breast cancer: Preliminary results of a randomized trial. Breast Cancer Res Treat. 1986;7:105–109. doi: 10.1007/BF01806795. [DOI] [PubMed] [Google Scholar]
- 18.Ayme Y, Spitalier JM, Amalric R, et al. Preliminary results of a three-arm randomized trial of adjuvant chemo- and/or hormone-therapy for high-risk breast cancer. Fourth EORTC breast cancer working conference 1987; June 30–July 3, 1987; Imperial College, London, UK; C2A.1.
- 19.Martin PM, Romain S, Spyratos F. Reevaluation of the indications of adjuvant hormonotherapy in high risk primary breast cancer patients. Bull Cancer. 1991;78:709–723. [PubMed] [Google Scholar]
- 20.Namer M, Fargeot P, Roche H. Improved disease-free survival with epirubicin-based chemoendocrine adjuvant therapy compared with tamoxifen alone in one to three node-positive, estrogen-receptor-positive, postmenopausal breast cancer patients: results of French Adjuvant Study Group 02 and 07 trials. Ann Oncol. 2006;17:65–73. doi: 10.1093/annonc/mdj022. [DOI] [PubMed] [Google Scholar]
- 21.Morales L, Canney P, Dyczka J. Postoperative adjuvant chemotherapy followed by adjuvant tamoxifen versus nil for patients with operable breast cancer: a randomised phase III trial of the European Organisation for Research and Treatment of Cancer Breast Group. Eur J Cancer. 2007;43:331–340. doi: 10.1016/j.ejca.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Rutqvist LE, Johansson H, Stockholm Breast Cancer Study Group Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 2007;46:133–145. doi: 10.1080/02841860601034834. [DOI] [PubMed] [Google Scholar]
- 23.Breast Cancer Trials Committee, Scottish Cancer Trials Office Adjuvant tamoxifen in the management of operable breast cancer: the Scottish trial. Lancet. 1987;330:171–175. [PubMed] [Google Scholar]
- 24.Stewart HJ, Prescott RJ, Forrest APM. Scottish Adjuvant Tamoxifen Trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93:456–462. doi: 10.1093/jnci/93.6.456. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B, Dignam J, Bryant J. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors [see comments] J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B, Costantino J, Wickerham DL. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 28.McCowan C, Shearer J, Donnan PT. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owusu C, Buist DS, Field TS. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results Program . 1973–2007 SEER data. National Cancer Institute; Washington: 2010. http://www.seer.cancer.gov (release of November, 2009, data submission) (accessed July 5, 2011). [Google Scholar]
- 31.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 32.Khoshnoud MR, Lofdahl B, Fohlin H. Immunohistochemitry compared to cytosol assays for determination of estrogen receptor and prediction of the long-term effect of adjuvant tamoxifen. Breast Cancer Res Treat. 2011;126:421–430. doi: 10.1007/s10549-010-1202-7. [DOI] [PubMed] [Google Scholar]
- 33.Badve SS, Baehner FL, Gray RP. Estrogen and progesterone receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26:2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 34.Fisher ER, Anderson S, Dean S. Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma. Cancer. 2005;103:164–173. doi: 10.1002/cncr.20761. [DOI] [PubMed] [Google Scholar]
- 35.Molino A, Micciolo R, Turazza M. Prognostic significance of estrogen receptors in 405 primary breast cancers: a comparison of immunohistochemical and biochemical methods. Breast Cancer Res Treat. 1997;45:241–249. doi: 10.1023/a:1005769925670. [DOI] [PubMed] [Google Scholar]
- 36.Hammond MEH, Hayes DF, Dowsett M. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;16:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peto R. Current misconception 3: That subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer. 2011;104:1057–1058. doi: 10.1038/bjc.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferlay J, Shin HR, Bray F. GLOBOCAN 2008 v1.2, cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet] International Agency for Research on Cancer; Lyon: 2010. http://globocan.iarc.fr (accessed July 1, 2011). [Google Scholar]
- 39.Goldhirsch A, Ingle JN, Gelber RD. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


