Abstract
We studied the prevalence of anemia during pregnancy and its relationship with low birth weight (LBW; birth weight < 2,500 g) in Benin. We analyzed 1,508 observations from a randomized controlled trial conducted from 2005 to 2008 showing equivalence on the risk of LBW between two drugs for Intermittent Preventive Treatment of malaria during pregnancy (IPTp). Despite IPTp, helminth prophylaxis, and iron and folic acid supplementations, the proportions of women with severe anemia (hemoglobin [Hb] concentration < 80 g/L) and anemia (Hb < 110 g/L) were high throughout pregnancy: 3.9% and 64.7% during the second and 3.7% and 64.1% during the third trimester, but 2.5% and 39.6% at the onset of labor, respectively. Compared with women without anemia (Hb ≥ 110 g/L) during the third trimester, women with severe anemia (Hb < 80 g/L) were at higher risk of LBW after adjustment for potential confounding factors (prevalence ratio [PR] = 2.8; 95% confidence interval [1.4–5.6]).
Background
In developing countries, it is commonly said that over 50% of pregnant women are anemic.1 Anemia during pregnancy is a multifactorial condition. The main causes in sub-Saharan Africa are iron deficiency and malaria, followed by other micronutrient deficiencies (folic acid, vitamin A, vitamin B12) and other infections (hookworms, human immunodeficiency virus [HIV], urinary tract infections).2 Prevention programs in Africa have been implemented, especially related to malaria and helminth infections and iron and folic acid supplements. Although these interventions have shown their efficacy in clinical trials in increasing the mean hemoglobin (Hb) concentration, the effectiveness of this antenatal care (ANC) package on the prevalence of anemia has rarely been studied throughout pregnancy. Indeed, few data on the prevalence of anemia and mean Hb during pregnancy are available, according to the timing of assessment of Hb in sub-Saharan Africa. Repeated measurements by gestational age are essential because of the physiological changes of Hb during pregnancy: Hb decreases physiologically at the end of the first trimester and then increases by 40 weeks of gestation.3 As a result, assessing prevalence of anemia without taking into account timing during pregnancy may lead to biased results.
Some pregnancy complications are associated with a higher risk of low birth weight (LBW),4 but anemia during pregnancy has been inconsistently associated with an increased risk of preterm delivery and LBW.3,5 Low birth weight, which includes preterm birth and intrauterine growth restriction, results in higher risks of mortality and morbidity.6,7 This condition is highly prevalent in sub-Saharan Africa.8 Given the large prevalence of maternal anemia in sub-Saharan Africa,9 even a small increase in the risk of LBW associated with anemia during pregnancy might have an important impact in terms of public health in such settings. Fewer data are available in developing countries than in developed countries. As a result, severe anemia and some potential confounding factors like poor antenatal clinic attendance and infections including malaria10 have not been often studied. Again, studying the association between anemia and LBW without taking into account timing of Hb assessment during pregnancy may lead to biased results. Thus, it is important to understand the timing during pregnancy when the fetus may be the most vulnerable to anemia.
Our objectives were to assess the prevalence of anemia at different times during pregnancy and according to severity in three Beninese hospitals where malaria and helminth prophylaxis and iron and folic acid supplements were given as recommended by Beninese guidelines, and to study the relationships between maternal anemia and LBW at different times during pregnancy.
Methods
Ethics statement.
The study was approved by the French Comité Consultatif de Déontologie et d'Ethique and the Ethics Committee of the University of Abomey-Calavi in Benin. Informed consent was obtained from all human adult participants and from the parents or legal guardians of minors.
This secondary analysis included data extracted from a randomized controlled trial in a semi-rural town in southern Benin, located 40 km west of Cotonou. This trial comparing two Intermittent Preventive Treatments during pregnancy (IPTp) showed equivalence on the risk of LBW between (SP) and mefloquine. From July 2005 to April 2008, 1,601 pregnant women were recruited during the second trimester of pregnancy in three maternity clinics, including a tertiary referral center, after they provided signed, written informed consent. The trial's study design and maternal characteristics have been described elsewhere.11 Women of all gravidities presenting with no history of a neurologic or psychiatric disorder, and who had not either previously used SP or mefloquine or reported having adverse reactions to medications containing sulfa were eligible to participate. Among the recruited women, 44.1% had already had at least one ANC before inclusion in the trial.
We included in our analysis Hb concentration measured with a colorimeter (spectrophotometer Anadeo+, 540 nm, with Drabkine's reagent). Severe, moderate, mild, and no anemia were defined as Hb < 80 g/L, 80–99 g/L, 100–109 g/L, and ≥ 110 g/L, respectively. To have homogenous groups regarding ANC package and timing for blood assessments during pregnancy because of hemodilution, we included observations with Hb performed at inclusion for the administration of the first dose of IPTp and assessed during the second trimester (from 14 to 27 weeks of gestation), Hb performed at the second administration of IPTp during the third trimester at least 1 month after the first dose (on average 9 weeks after the first dose and after 27 weeks of gestation), and Hb performed at the onset of labor.
The IPTp was free of charge for women during the trial. From March 2006, insecticide-treated nets and a treatment of helminths were made available with low cost for all pregnant women in the study area, as part of the national program against malaria in Benin (Program National de Lutte contre le Paludisme). The treatment of helminths (mebendazole 500 mg given twice 10 days apart) was recommended during the second trimester, and during the third trimester in case of digestive symptoms. Iron and folic acid were prescribed at 200 mg/day and 5 mg/day, respectively. The supplementation was recommended during the entire pregnancy, but free of charge only during 1 month at the first dose of IPTp. Furthermore, during the trial women who were anemic or suspected to be anemic had a thick blood smear and were treated with quinine if malaria was diagnosed. Adherence treatment of quinine was not known, but a smear blood test was performed to check for absence of malaria after treatment. In addition, they received ferrous sulphate (up to 1,200 mg/day and free of charge for at least 1 month), vitamin C and mebendazole or were referred to the tertiary referral center if symptomatic and/or Hb < 70 g/L.
Malaria transmission occurs throughout the year, with two peaks during the rainy seasons (April–July and September–November). Plasmodium falciparum is the predominant species causing malaria (97%) in the study area. Malaria thick and thin blood smears were performed for parasitological examination systematically at each IPTp administration, in case of symptoms suggestive of malaria, during emergency antenatal visit and during labor. After delivery, blood smears were made from the maternal side of the placenta and the umbilical cord. Women received a 7-day quinine treatment (24 mg/kg/day) when malaria was confirmed by a thick blood smear during pregnancy or at delivery if symptomatic, but not when the diagnosis was made at the ANC visit corresponding to IPTp administration. When parasitemia was positive—at the occasion of IPTp administration, emergency ANC visit, or at delivery—quinine was given if a positive parasitemia was found again 5 days later.
We estimated gestational age at birth according to the Ballard score performed for 80% of births by a single specifically trained midwife. The Ballard Maturational Score is a validated gestational age assessment tool in newborn infants.12 When this latter was not available, gestational age was defined by the midwife's estimate at birth based on the last menstrual periods and/or uterine height. If it was not available, the midwife's estimate at the second dose of IPTp was taken, if not the one available at the first dose of IPTp. Birth weight was determined within 1 hour after birth using an electronic scale (SECA, France, ± 100 grams). Low birth weight was defined as birth weight < 2,500 g and preterm births as gestation < 37 completed weeks at birth. Women who delivered outside the study maternity clinics were interviewed if they attended the maternity clinic within a few hours after delivery; Hb, malaria parasitemia, gestational age at birth (Ballard score), and birth weight were assessed.
From May 2006, HIV testing was systematically proposed to all pregnant women and included at their first ANC visit.
Statistical analysis.
We first described the prevalence of anemia and mean Hb during the second trimester, the third trimester, and at the onset of delivery. Second, we studied the associations between known risk factors for Hb and Hb according to timing: socioeconomic variables, gravidity, supplements, malaria, and study arm. The Pearson χ2 and Student's t-test (statistics) were used to compare proportions and means, respectively. The level of statistical significance retained was P < 0.05. General linear models were used to define significant factors while adjusting for the other factors. Third, we studied the relations between anemia and LBW. Prevalence ratios (PRs) were used to study the relationships between maternal anemia and LBW with PROC GENMOD in SAS (SAS Institute, Inc., Cary, NC). The links between LBW and maternal anemia were studied alone (crude PRs)13 and then after adjustment for the following potential confounding factors based on hypothesized underlying causal relationships including known risk factors for LBW and factors associated with anemia in our data14: gravidity, body mass index (BMI) at inclusion, malaria diagnosed at the first IPTp (for the model including anemia during the second trimester), malaria diagnosed at the second IPTp (for the model including anemia during the third trimester), malaria diagnosed at delivery (including also placental malaria infection; for the model including anemia at delivery), school attendance, having electricity, having latrines, and first ANC at inclusion for Hb during the second trimester or number of ANC visits for Hb during the third trimester or at delivery. We also, first, adjusted, and second, stratified for gravidity because primigravidae are usually at higher risk of poor outcomes especially in sub-Saharan Africa. Gestational age, BMI, and maternal age were treated as continuous variables. Statistical analysis was performed with SAS 9.1.
Studying the association between anemia at delivery and LBW in both preterm and term births might produce a spurious association because of the physiological changes of Hb during pregnancy as Hb is on average lower in women delivering preterm babies than Hb in women delivering term babies, and a high proportion of these preterm babies are likely to be LBW.3 Therefore, preterm births were excluded from the model studying the association between anemia at delivery and LBW.
Results
Among 1,601 women randomized, 26 multiple pregnancies and further 37 stillbirths and spontaneous abortions were excluded. Twelve women of the 878 tested positive for HIV were excluded. Over the 1,526 remaining pregnant women, maternal Hb had never been assessed in 18 women, leaving 1,508 observations included in the analysis. Hemoglobin was assessed in 86.3% of women at the time of the first IPTp and in the second trimester (1,302 of 1,526), 73.5% at the time of the second IPTp and in the third trimester (1,122 of 1,526), and 80.1% at delivery (1,222 of 1,526). Some women for which Hb concentration was available, but not at good timing, were excluded: 5 women were assessed before the second trimester, 82 were given the first dose of IPTp during the third trimester, and 209 women were given the second dose of IPTp during the second trimester.
The median gestational age at the first and second Hb assessments was 22 and 31 weeks of gestation, respectively. Overall, the proportions of women with Hb < 80 g/L and Hb < 110 g/L were 3.9% and 64.7% during the second trimester of pregnancy, and 3.7% and 64.1% during the third trimester, whereas these proportions decreased to 2.5% and 39.6% at the onset of labor (Table 1). The mean levels of Hb during the second and third trimesters were very close (105.0 g/L [SD 13.5] versus 105.5 g/L [13.3], respectively).
Table 1.
Anemia, hemoglobin concentration (Hb), and gestational age at different stages of pregnancy*
| Hb during the 2nd trimester (1st dose of IPTp) | Hb during the 3rd trimester (2nd dose of IPTp) | Hb at delivery | ||||
|---|---|---|---|---|---|---|
| (N) | % | (N) | % | (N) | % | |
| Total | (1302) | 100.0 | (1122) | 100.0 | (1222) | 100.0 |
| Hemoglobin concentration (g/L) | (51) | 3.9 | (42) | 3.7 | (30) | 2.5 |
| < 80 (severe anemia) | (379) | 29.1 | (334) | 29.8 | (192) | 15.7 |
| 80–99 (moderate anemia) | (413) | 31.7 | (343) | 30.6 | (262) | 21.4 |
| 100–109 (mild anemia) | (459) | 35.3 | (403) | 35.9 | (738) | 60.4 |
| ≥ 110 (no anemia) | ||||||
| Hb | mean | (sd) | mean | (sd) | mean | (sd) |
| 105.0 | (13.5) | 105.5 | (13.3) | 113.7 | (16.3) | |
| median | (sd) | median | (sd) | median | (sd) | |
| Gestational age when Hb assessed | 22.0 | (3.0) | 31.0 | (2.6) | 39.0 | (1.1) |
IPTp = Intermittent Preventive Treatment during pregnancy.
Figure 1 shows the mean Hb during pregnancy according to gestational age: the mean Hb increased at the end of the third trimester after 33 weeks of gestation. Table 2 shows the variation for this mean Hb at different times according to several risk factors. The mean Hb during the second trimester was higher in multigravidae than in primigravidae (P = 0.004), but this difference was not significant anymore when women with malaria during the second trimester were excluded (P = 0.06). In the third trimester, it was the reverse: the mean Hb was higher in primigravidae than in multigravidae (P = 0.03). A low BMI at inclusion was associated with a lower mean Hb during the third trimester and at delivery. The mean Hb at inclusion during the second trimester was higher when women were taking iron before the trial compared with women with no supplementation before the study. The same trend was found with folic acid supplementation, but did not reach significance. Among the 1,003 women who declared already taking iron or folic acid at inclusion (66.5%), iron and folic acid consumption were highly linked: only eight were taking folic acid, but not iron, and five were taking iron, but not folic acid. Malaria was associated with a lower mean Hb at each blood assessment. However, the prevalence of malaria was quite low at each Hb assessment: 6.5% and 2.5% during the second and third trimesters, respectively, and 3.4% at delivery. When anemia was present, these proportions increased to 8.3% and 3.1% during the second and third trimesters, respectively, and 5.0% at delivery. A low number of antenatal visits was also significantly associated with a lower Hb at delivery (P = 0.005), but socio-economic variables were not significantly associated with the mean Hb. Having slept under a bed net the night before inclusion was not associated with the mean Hb at inclusion (P = 0.57) nor was the season of delivery with the mean Hb throughout pregnancy. The same risk factors were associated with anemia at different timing during pregnancy, significantly or at the limit of significance, except for iron and folic acid given before study that were not associated with anemia during the second trimester.
Figure 1.
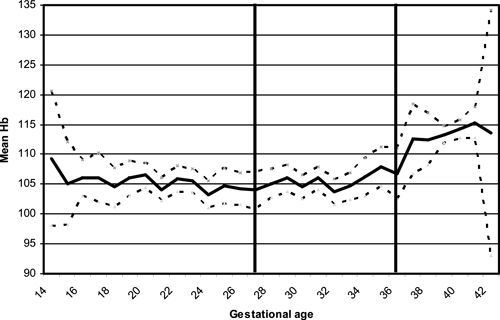
Hemoglobin concentration (Hb) during pregnancy in Benin. Mean Hb concentration and 95% confidence interval; Hb before 29 weeks of gestation (second trimester of pregnancy) referred to blood samples performed at the first administration of Intermittent Preventive Treatment during pregnancy (IPTp), Hb between 29 and 36 weeks referred to blood samples performed at the second IPTp, and Hb after 37 weeks referred to blood samples performed at the onset of delivery.
Table 2.
Mean hemoglobin (Hb) according to iron and folic acid supplementations, malaria, socio-economic status, body mass index (BMI) at inclusion and gravidity at different times during pregnancy
| Hb during the 2nd trimester | Hb during the 3rd trimester | Hb at delivery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | P | Mean (SD) | n | P | Mean (SD) | n | P | |
| Level of education | |||||||||
| None | 104.7 (14.1) | 565 | 0.50 | 105.4 (13.8) | 476 | 0.82 | 113.0 (16.3) | 544 | 0.17 |
| Some | 105.2 (13.0) | 737 | 105.6 (13.0) | 646 | 114.3 (16.3) | 678 | |||
| Having latrines | |||||||||
| No | 106.0 (14.5) | 260 | 0.24 | 104.4 (13.6) | 237 | 0.13 | 112.7 (16.7) | 264 | 0.27 |
| Yes | 104.8 (13.2) | 1042 | 105.8 (13.2) | 885 | 114.0 (16.2) | 958 | |||
| Having electricity | |||||||||
| No | 105.7 (13.7) | 514 | 0.12* | 104.7 (13.4) | 447 | 0.11 | 112.6 (16.4) | 508 | 0.03 |
| Yes | 104.6 (13.3) | 788 | 106.0 (13.2) | 675 | 114.6 (16.2) | 714 | |||
| Gravidity | |||||||||
| Primigravidae | 103.2 (14.0) | 351 | 0.004* | 106.9 (13.4) | 315 | 0.03* | 115.8 (17.2) | 309 | 0.01* |
| Multigravidae | 105.7 (13.2) | 951 | 105.0 (13.2) | 807 | 113.0 (15.9) | 913 | |||
| BMI at inclusion | |||||||||
| < 20 | 104.1 (13.6) | 250 | 0.24* | 103.7 (13.3) | 218 | 0.03* | 111.3 (15.5) | 230 | 0.01* |
| ≥ 20 | 105.2 (13.4) | 1052 | 105.9 (13.3) | 904 | 114.3 (16.4) | 992 | |||
| Iron given before study | |||||||||
| No | 103.8 (14.1) | 447 | 0.02* | 105.4 (13.4) | 369 | 0.80 | 113.6 (16.3) | 412 | 0.88 |
| Yes | 105.6 (13.1) | 855 | 105.6 (13.3) | 753 | 113.8 (16.3) | 810 | |||
| Folic acid given before study | |||||||||
| No | 104.1 (13.9) | 446 | 0.06* | 105.3 (13.5) | 368 | 0.70 | 113.6 (16.4) | 411 | 0.87 |
| Yes | 105.5 (13.2) | 856 | 105.6 (13.2) | 754 | 113.8 (16.2) | 811 | |||
| Malaria at the time of Hb assessment | |||||||||
| No | 105.5 (13.4) | 1218 | < 0.001* | 105.7 (13.3) | 1094 | 0.01* | 114.0 (16.2) | 1180 | 0.01* |
| Yes | 97.9 (12.9) | 84 | 99.3 (14.3) | 28 | 107.4 (18.3) | 42 | |||
| Number of antenatal care (ANC) visits | |||||||||
| ≤ 3 | 105.6 (13.1) | 331 | 0.92 | 111.6 (16.5) | 320 | 0.005* | |||
| ≥ 4 | 105.5 (13.4) | 791 | 114.5 (16.2) | 902 | |||||
| First ANC at inclusion | |||||||||
| No | 103.6 | 718 | 0.38 | ||||||
| Yes | 104.3 | 584 | |||||||
| Season of delivery | |||||||||
| Dry | 105.2 (13.4) | 685 | 0.75 | 105.7 (13.7) | 616 | 0.68 | 114.1 (16.3) | 697 | 0.43 |
| Rainy | 104.9 (13.6) | 582 | 105.4 (12.8) | 490 | 113.3 (16.2) | 525 | |||
| Study arm | |||||||||
| SP | 104.7 | 661 | 0.43 | 105.1 | 575 | 0.23 | 113.1 | 623 | 0.16 |
| Mefloquine | 105.3 | 641 | 106.0 | 547 | 114.4 | 599 | |||
P < 0.05 when all variables were introduced in the model with BMI considered as a continuous variable.
The proportions of LBW and preterm births were 9.1% and 1.2%, respectively (131 of 1,440 and 17 of 1,408). Severe anemia during the third trimester was associated with an increased risk of LBW compared with no anemia (Table 3).
Table 3.
Risk of low birth weight (LBW) according to maternal anemia*
| Birth weight | LBW | aPR | 95% CI | |
|---|---|---|---|---|
| Mean (SD) | (N) % | |||
| Hemoglobin concentration (Hb) in the second trimester | ||||
| < 80 | 2973 (477) | (51) 13.7 | 1.1 | (0.5–2.4) |
| 80–99 | 3013 (417) | (360) 9.4 | 0.8 | (0.5–1.2) |
| 100–109 | 3065 (414) | (390) 7.2 | 0.6 | (0.4–1.0) |
| ≥ 110 | 3023 (428) | (439) 10.0 | 1 | |
| Hb in the third trimester | ||||
| < 80 | 2960 (483) | (40) 20.0 | 2.8 | (1.4–5.6) |
| 80–99 | 3039 (405) | (325) 8.6 | 1.1 | (0.7–1.9) |
| 100–109 | 3005 (445) | (330) 9.4 | 1.2 | (0.8–2.0) |
| ≥ 110 | 3006 (403) | (389) 7.5 | 1 | |
| Hb at delivery (excluding preterm births) | ||||
| < 80 | 3008 (460) | (29) 10.3 | 1.4 | (0.6–3.5) |
| 80–99 | 3065 (401) | (183) 9.3 | 1.4 | (0.9–2.3) |
| 100–109 | 3049 (447) | (261) 8.4 | 1.2 | (0.7–1.8) |
| ≥ 110 | 3023 (404) | (729) 7.7 | 1 | |
Prevalence Ratio (PR) adjusted for malaria at the time of Hb assessment, gravidity, body mass index (BMI), having latrines, having electricity, level of education, and first antenatal care (ANC) at inclusion for Hb during the second trimester or number of ANC visits for Hb during the third trimester or at delivery.
Results were almost unchanged after adjustment for potential confounding factors. There was no significant association between anemia during the second trimester and at delivery and LBW after adjustment. The risk for LBW was not increased when hemoconcentration was present (Hb was > 130 g/L). After stratification by gravidity, even stronger associations were found in primigravidae, whereas there was no association anymore in multigravidae (Table 4), but there was no significant interaction between gravidity and severe anemia on the risk for LBW (P = 0.26). However, the confidence interval (CI) was large in primigravidae (PR = 3.8 95% CI [1.4; 10.4]). There was no significant correlation between Hb during the third trimester and birth weight when variables were considered as continuous variables among all women. There was however a significant correlation in primigravidae (P = 0.02).
Table 4.
Risk of low birth weight (LBW) according to maternal anemia during the third trimester at the time of administration of the second dose of IPTp by gravidity*
| Birth weight | LBW | Crude PR | 95% CI | aPR | 95% CI | |
|---|---|---|---|---|---|---|
| Mean (SD) | (N) % | |||||
| Among primigravidae | ||||||
| < 80 | 2719 (539) | (10) 40.0 | 4.2 | (1.6–10.8) | 3.8 | (1.4–10.4) |
| 80–99 | 2893 (316) | (72) 15.3 | 1.6 | (0.7–3.5) | 1.2 | (0.6–2.7) |
| 100–109 | 2830 (439) | (100) 18.0 | 1.9 | (0.9–3.8) | 1.6 | (0.8–3.2) |
| ≥ 110 | 2921 (365) | (115) 9.6 | 1 | 1 | ||
| Among multigravidae | ||||||
| < 80 | 3041 (444) | (30) 13.3 | 2.0 | (0.7–5.6) | 1.8 | (0.7–5.1) |
| 80–99 | 3082 (419) | (253) 6.7 | 1.0 | (0.5–1.9) | 0.9 | (0.5–1.8) |
| 100–109 | 3081 (427) | (230) 5.7 | 0.9 | (0.4–1.7) | 0.9 | (0.4–1.7) |
| ≥ 110 | 3042 (413) | (274) 6.6 | 1 | 1 | ||
Prevalence Ratio (PR) adjusted for malaria at the time of hemoglobin Hb assessment, body mass index (BMI), having electricity, level of education, and number of antenatal care (ANC) visits.
Discussion
Our study showed a very high prevalence of anemia throughout pregnancy in the Beninese hospitals included in our study despite malaria and helminth prophylaxis as well as iron and folic acid supplements. This was observed during the second and third trimesters (64%), but the prevalence also remained high at delivery (39%). In addition, we found an increased risk of LBW associated with severe anemia compared with no anemia during the third trimester.
This study represents one of the few studies of anemia throughout pregnancy and risk of LBW in sub-Saharan Africa in the context of the current ANC package. As Hb varies physiologically throughout pregnancy, an important strength of our study was to differentiate anemia according to gestational age. We also observed severity of anemia. Furthermore, we were able to take into account potential confounding factors like malaria at different times during pregnancy.
Hb during the third trimester was not recorded for about 25.6% of women because Hb was not assessed at all for 177 women or because Hb was assessed during the second trimester for other women. Women for whom we did not have this information had a lower level of education, attended less ANC visits, delivered more often baby boys, and delivered more often during the dry season. A low number of ANC visits was associated with a higher risk of LBW, but was not associated with anemia during the third trimester. Season of delivery and level of education was not associated with LBW or with anemia. In total, this should not have biased our results.
As found in developed countries,15,16 we observed that the mean Hb increased at the end of the third trimester of pregnancy. Hemoglobin is known to decrease in late second trimester and early third trimester because of plasma volume expansion.17 This is followed by the increase of red blood cells.18,19 Close results were found in some studies,15 but other studies found that the increase of Hb occurred earlier in pregnancy.3,16 Early ultrasound, which is the best method to assess gestational age, was not available as it is routinely performed in developed countries.20,21 This might have resulted in classification errors in gestational age misclassifying preterm births in term births. We found only 1% of preterm births, whereas this rate is usually higher in sub-Saharan Africa.22 However, Menendez and others23 found close incidences of LBW (11.7%) and preterm births (3.7%) where women slept under insecticide-treated nets with placebo or IPTp. Furthermore, when gestational age was defined only by the midwife's estimate at the second dose of IPTp, relationships between anemia and LBW were unchanged (data not shown). In total, a limited number of misclassifications probably occurred categorizing some preterm births as term births, but these misclassifications should not be differential according to Hb.
The association between anemia and LBW did not vary after adjustment for potential confounding factors. Anemia might have been caused by some malaria bouts that we did not register, but malaria surveillance seemed to be effective: 32 of 38 women with placental malaria infection had been previously diagnosed with malaria on thick blood smear during pregnancy or at delivery (data not shown). Because placental malaria infections are the consequence of malaria infections during pregnancy, residual confounding should be minimized. The BMI was calculated on the basis of weight at inclusion thus varying according to gestational age at inclusion, but should not have led to residual confounding because Hb did not vary widely in the second trimester. We excluded 12 infected women who were diagnosed with HIV after systematic screening was offered after May 2006, but many women were probably not aware of their status. However, the prevalence of HIV in Benin is low (around 2%) and this should not have had significant consequences on our results. We did not have information available for the following variables known to be associated with LBW: spousal violence, smoking, alcohol intake, anthropometric measures of the father.24–26 However, they should not be associated with maternal anemia and, thus, should not be confounding factors in our study. Infant's sex and adolescent pregnancies are known to be associated with LBW, but not with anemia. We found that 16.25% of pregnancies occurred before 20 years of age (data not shown). These women were at higher risk for LBW, but not for anemia during the third trimester (P = 0.50 for adolescent pregnancy; P = 0.32 for infant's sex). Thus, we did not adjust for adolescent pregnancies or infant's sex in our model.
The lack of longitudinal data for pregnant women with the same ANC package makes comparisons of prevalence of anemia difficult among studies from Africa. Menendez and others23 found a slightly higher level of mean Hb at enrollment during the second trimester of pregnancy (109.5 g/L versus 105.0 g/L in our study) and Kalilani and others27 found close prevalence of anemia at enrollment (67.5% versus 65.5% in our study), but these two studies did not mention iron and folic acid supplements before inclusion in their trial. Among women enrolled, Achidi and others28 found 69% of maternal anemia at the mean gestational age of 24 weeks and 70% at delivery in Cameroon, but as high as 34% of women with placental parasitemia. These women were given iron, folate, and pyrimethamine tablets, but not mebendazole. Marchant and others29 in Tanzania found 11% of severe anemia (Hb < 80 g/L) at a median gestational age of 30 weeks. Overall, the slightly lower level of anemia observed in our study was therefore probably mainly caused by the ANC package (IPTp, mebendazole, and iron/folic acid).
The IPTp with SP and hematinic supplementation programs have been associated with a reduced risk of maternal anemia.22,30 Thus, we expected an increase in the mean of Hb between the second and third trimesters because of free IPTp and prescribed iron and folic acid supplementation. Surprisingly, we only observed a late increase, as found physiologically in developed countries. However, most of the women were already taking iron and folic acid supplements at inclusion, and, as expected, the mean Hb was higher in women that were already taking iron before the study compared with women who were not. We also found that the mean Hb during the second trimester was higher in multigravidae than in primigravidae, but not after exclusion of women with malaria diagnosed at the same time. This probably highlights the fact that primigravidae are known to be at higher risk of malaria than multigravidae22 and indicates that anemia in primigravidae during the second trimester was highly related to malaria. Accordingly, at the second dose of IPTp, the mean Hb was higher in primigravidae compared with multigravidae, suggesting the high impact of IPTp in primigravidae. Furthermore, season of delivery was not associated with the mean Hb during the third trimester and at delivery showing that malaria was not an important risk factor for anemia at that time and the good impact of IPTp. Other risk factors not prevented or treated or a lower observance might explain the lower level of Hb in multigravidae where malaria is a cause of anemia to a lesser extent than in primigravidae. Nevertheless, despite the suggested impact of these treatments and prescribed supplements, the prevalence of anemia remained high in the third trimester and at delivery.15,16 This suggests the presence of other risk factors and/or room for improvement in the implementation and/or adherence of these interventions.
The association between maternal anemia during pregnancy and the risk of LBW remains controversial in the literature.5 Several studies in sub-Saharan Africa found an increased risk of LBW associated with maternal anemia31 or severe anemia,32 but this association was not consistent in the literature.28 Physiological plasma volume expansion might be necessary and even beneficial during pregnancy,33 but, as we found, severe anemia might have adverse effects as found by others.34–37 We found an association between severe anemia and LBW when assessing Hb in the third trimester of pregnancy. Villar and Belizan38 found that negative factors for fetus' growth around the 30th week of gestation resulted in a disproportionately growth-retarded infant. In agreement with these results, because the median gestational age for Hb assessment during the third trimester was 32 weeks, our results suggest the highest impact of severe anemia at that gestational age on LBW.
We did not find an association between moderate and mild anemia as found by others,3,34 or a significant correlation between Hb and birth weight among all women when variables were considered as continuous variables. However, we found an association between severe anemia and LBW suggesting a threshold. Moderate-to-mild anemia might represent a complex mix of pregnant women having a beneficial hemodilution and women with pathologic anemia. In these situations, moderate-to-mild anemia might not be a good indicator of poor outcome.
Because of possible misclassifications on gestational age as explained earlier, we have not been able to study the association between anemia and other relevant indicators, including indicators accounting for gestational age as others suggested.39 However, LBW is a commonly used indicator, especially in sub-Saharan Africa.
We found a higher risk of LBW associated with severe anemia in primigravidae, but not in multigravidae. The role of parity in the relationship between maternal anemia and the risk of LBW has seldom been studied in the literature.40 The mean Hb was higher in primigravidae than in multigravidae in the third trimester and at delivery. We did not have a sufficient number of women with malaria to be able to stratify by malaria. Severe anemia might reflect relatively worse conditions in primigravidae than in multigravidae, which might explain this result. However, CI was large and further studies stratifying by gravidity should be conducted to confirm this finding.
Conclusion
Our longitudinal data during pregnancy showed that, even with malaria and helminth prophylaxis, as well as iron and folic acid supplements, the prevalence of anemia throughout pregnancy remained very high. Despite these important investments to prevent and treat anemia, the goal of limited prevalence of anemia was not reached. Understanding factors involved is critical and future research on this topic is warranted. Severe anemia was associated with a higher risk of LBW in our study. This should therefore be prevented and treated, especially during the third trimester when the fetus' growth is most important. However, the adverse impact of moderate to mild anemia is unclear. Finally, the long-term outcome for children born to pregnant women with anemia is unknown and needs to be further studied.
ACKNOWLEDGMENTS
We are grateful to all the women and children who participated in the trial. We thank all the medical, laboratory, and administrative staffs of Kindji, Hopital de Zone, and Kpasse for their valuable contribution to this trial.
Footnotes
Financial support: The trial was funded by the Institut de Recherche pour le Développement. Florence Bodeau-Livinec was funded by the New York University School of Medicine and supported in part through a grant (R21-HD060524) from the National Institutes of Health.
Authors' addresses: Florence Bodeau-Livinec and Karen P Day, Department of Medical Parasitology, New York University School of Medicine, New York, NY, E-mails: bodeaf01@nyumc.org and karen.day@nyumc.org. Valerie Briand and Michel Cot, Institut de Recherche pour le Développement (IRD), Mère et Enfant face aux Infections Tropicales, Paris, France; Faculté de Pharmacie, Université Paris Descartes, Paris, France, E-mails: valerie.briand@gmail.com and michel.cot@ird.fr. Jacques Berger, Institut de Recherche pour le Développement (IRD), Montpellier, France, E-mail: jacques.berger@ird.fr. Xu Xiong, Department of Epidemiology, Tulane University, New Orleans, LA, E-mail: xxiong@tulane.edu. Achille Massougbodji, Laboratoire de Parasitologie, Faculte des Sciences de la Sante, Universite d'Abomey-Calavi, Cotonou, Benin, E-mail: massougbodjiachille@yahoo.fr.
References
- 1.WHO . Global Database on Child Growth and Malnutrition. Geneva: WHO; 1998. [Google Scholar]
- 2.van den Broek N. Anaemia in pregnancy in developing countries. Br J Obstet Gynaecol. 1998;105:385–390. doi: 10.1111/j.1471-0528.1998.tb10120.x. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff MA, Shiono PH, Berendes HW, Rhoads GG. Facts and artifacts about anemia and preterm delivery. JAMA. 1989;262:511–515. [PubMed] [Google Scholar]
- 4.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 5.Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol. 2000;17:137–146. doi: 10.1055/s-2000-9508. [DOI] [PubMed] [Google Scholar]
- 6.McCormick MC. The contribution of low birth-weight to infant-mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 7.Stanley FJ, Watson L. Trends in perinatal mortality and cerebral palsy in Western Australia, 1967 to 1985. BMJ. 1992;304:1658–1663. doi: 10.1136/bmj.304.6843.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNICEF . Low Birthweight: Country, Regional and Global Estimates. New York: UNICEF; 2004. [Google Scholar]
- 9.DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38:302–316. [PubMed] [Google Scholar]
- 10.van den Broek N. Anaemia and micronutrient deficiencies. Br Med Bull. 2003;67:149–160. doi: 10.1093/bmb/ldg004. [DOI] [PubMed] [Google Scholar]
- 11.Briand V, Bottero J, Noel H, Masse V, Cordel H, Guerra J, Kossou H, Fayomi B, Ayemonna P, Fievet N, Massoughodji A, Cot M. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis. 2009;200:991–1001. doi: 10.1086/605474. [DOI] [PubMed] [Google Scholar]
- 12.Ballard JL, Khoury JC, Wedig K, Wang L, Eilerswalsman BL, Lipp R. New Ballard Score, expanded to include extremely premature-infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 13.Spiegelman DHE. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 14.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 15.Beaton GH. Iron needs during pregnancy: do we need to rethink our targets? Am J Clin Nutr. 2000;72:265S–271S. doi: 10.1093/ajcn/72.1.265S. [DOI] [PubMed] [Google Scholar]
- 16.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284:2611–2617. doi: 10.1001/jama.284.20.2611. [DOI] [PubMed] [Google Scholar]
- 17.Xiong X, Buekens R, Fraser WD, Guo Z. Anemia during pregnancy in a Chinese population. Int J Gynaecol Obstet. 2003;83:159–164. doi: 10.1016/s0020-7292(03)00214-5. [DOI] [PubMed] [Google Scholar]
- 18.Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol. 1972;112:440. doi: 10.1016/0002-9378(72)90493-0. [DOI] [PubMed] [Google Scholar]
- 19.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–612. [PubMed] [Google Scholar]
- 20.Wingate MS, Alexander GR, Buekens P, Vahratian A. Comparison of gestational age classifications: date of last menstrual period vs. clinical estimate. Ann Epidemiol. 2007;17:425–430. doi: 10.1016/j.annepidem.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Kramer MS, Platt RW, Blonel B, Bréart G, Morin I, Wilkins R, Usher R. How does early ultrasound scan estimation of gestational age lead to higher rates of preterm birth? Am J Obstet Gynecol. 2002;186:433–437. doi: 10.1067/mob.2002.120487. [DOI] [PubMed] [Google Scholar]
- 22.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 23.Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanza S, Serra-Casas E, Macete E, Berenguera A, David C, Dobaño C, Naniche D, Mayor A, Ordi J, Mandomando I, Aponte JJ, Mabunda S, Alonso PL. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS ONE. 2008;3:e1934. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11:427–433. doi: 10.1097/00001648-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg RL, Culhane JF, Iams JD, Romero R. Preterm birth 1: epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 27.Kalilani LM, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg. 2010;104:416–422. doi: 10.1016/j.trstmh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achidi EA, Kuoh AJ, Minang JT, Ngum B, Achimbom BM, Motaze SC, Ahmadou MJ, Troye-Blomberg M. Malaria infection in pregnancy and its effects on haemoglobin levels in women from a malaria endemic area of Fako Division, South West Province, Cameroon. J Obstet Gynaecol. 2005;25:235–240. doi: 10.1080/01443610500060628. [DOI] [PubMed] [Google Scholar]
- 29.Marchant T, Schellenberg J, Edgar T, Ronsmans C, Nathan R, Abdulla S, Mukasa O, Urassa H, Lengeler C. Anaemia during pregnancy in southern Tanzania. Ann Trop Med Parasitol. 2002;96:477–487. doi: 10.1179/000349802125001221. [DOI] [PubMed] [Google Scholar]
- 30.van Eijk AM, Ayisi JG, Slutsker L, Ter Kuile FO, Rosen DH, Otieno JA, Shi YP, Kager PA, Steketee RW. Effect of haematinic supplementation and malaria prevention on maternal anaemia and malaria in western Kenya. Trop Med Int Health. 2007;12:342–352. doi: 10.1111/j.1365-3156.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 31.Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, Bulmer J, Balira R, Ross D, Mageye K, Hayes R, Mabey D. Adverse birth outcomes in United Republic of Tanzania—impact and prevention of maternal risk factors. Bull World Health Organ. 2007;85:9–18. doi: 10.2471/BLT.06.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geelhoed D, Agadzi F, Visser L, Ablordeppey E, Asare K, O'Rourke P, Van Leeuwen JS, Van Roosmalen J. Severe anemia in pregnancy in rural Ghana: a case-control study of causes and management. Acta Obstet Gynecol Scand. 2006;85:1165–1171. doi: 10.1080/00016340600672812. [DOI] [PubMed] [Google Scholar]
- 33.Goodlin RC, Dobry CA, Anderson JC, Woods RE, Quaife M. Clinical signs of normal plasma-volume expansion during pregnancy. Am J Obstet Gynecol. 1983;145:1001–1009. doi: 10.1016/0002-9378(83)90856-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol. 1998;148:998–1006. doi: 10.1093/oxfordjournals.aje.a009577. [DOI] [PubMed] [Google Scholar]
- 35.Steer P, Alam MA, Wadsworth J, Welch A. Relation between maternal hemoglobin concentration and birth weight in different ethnic groups. BMJ. 1995;310:489–491. doi: 10.1136/bmj.310.6978.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhargava M, Kumar R, Iyer PU, Ramji S, Kapani V, Bhargava SK. Effect of maternal anemia and iron depletion on fetal iron stores, birth weight and gestation. Acta Paediatr Scand. 1989;78:321–322. doi: 10.1111/j.1651-2227.1989.tb11080.x. [DOI] [PubMed] [Google Scholar]
- 37.Bondevik GT, Lie RT, Ulstein M, Kvale G. Maternal hematological status and risk of low birth weight and preterm delivery in Nepal. Acta Obstet Gynecol Scand. 2001;80:402–408. [PubMed] [Google Scholar]
- 38.Villar J, Belizan JM. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstet Gynecol Surv. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Wilcox A. On the importance-and the unimportance-of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 40.Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, Marsh K. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–778. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]


